Abstract
Nut consumption reduces cardiovascular risk, and reductions in blood pressure and peripheral vascular resistance may be important mediators of this relationship. We evaluated effects of pistachios on flow-mediated dilation and blood pressure response to acute stress. Twenty-eight adults with dyslipidemia completed a randomized, crossover, controlled-feeding study. All of the meals were provided and calories were controlled. After 2 weeks on a typical Western diet (35% total fat and 11% saturated fat), test diets were presented in counterbalanced order for 4 weeks each, a low-fat control diet (25% total fat and 8% saturated fat), a diet containing 10% of energy from pistachios (on average, 1 serving per day; 30% total fat and 8% saturated fat), and a diet containing 20% of energy from pistachios (on average, 2 servings per day, 34% total fat and 8% saturated fat). None of the resting hemodynamic measures significantly differed from pretreatment values. When resting and stress levels were included in the repeated-measures analysis, average reductions in systolic blood pressure were greater after the diet containing 1 serving per day versus 2 servings per day of pistachios (mean change in systolic blood pressure, −4.8 vs −2.4 mm Hg, respectively; P<0.05). After the higher dose, there were significant reductions in peripheral resistance (−62.1 dyne·s×cm−5) and heart rate (−3 bpm) versus the control diet (P<0.0001). These changes were partially offset by increases in cardiac output. There was no effect of diet on fasting flow-mediated dilation. Reductions in peripheral vascular constriction and the resulting decrease in hemodynamic load may be important contributors to lower risk in nut consumers.
Keywords: diet, stress, blood pressure, endothelium, total peripheral resistance, nuts, pistachios
Nut consumption is associated with significant reductions in cardiovascular disease (CVD) risk and all-cause mortality.1–5 In the Nurses' Health Study, women who consumed >2 servings of nuts per week had an 18% reduction in cardiac death compared with women who did not eat nuts regularly.5 In short-term, randomized trials, almonds,6,7 walnuts,8–10 and pistachios11,12 significantly reduced low-density lipoprotein (LDL) cholesterol and total cholesterol, when compared with a typical Western diet or diets low in saturated fat (SFA; for review, see Sabate et al13). We have shown previously that including 1 or 2 servings per day of pistachios in a healthy diet reduced LDL cholesterol by 9% to 12%.11 The effects of pistachios on the ratio of LDL cholesterol to high-density lipoprotein cholesterol were dose dependent, with larger improvements in LDL cholesterol/high-density lipoprotein cholesterol11 and greater reductions in oxidized LDL14 when participants consumed 2 servings per day.
In 1 epidemiological study, nut intake was associated with lower blood pressure (BP) and lower risk of hypertension.15 However, the protective effect of nuts was limited to lean individuals, and sodium was not considered (for review, see Casas-Agustench et al16). Relatively few clinical studies of nuts have reported BP data, and findings have been inconsistent, with some studies reporting significant reductions in BP when nuts are consumed,17,18 whereas others report no significant change.6,9 Little is known about the mechanism(s) that may underlie the relationship between nut consumption and BP, and dose-response relationships have not been studied. Based on our previous work with walnuts,18 we hypothesized that lower peripheral vascular resistance may mediate the relationship between nut consumption and BP. Furthermore, given the putative role of stress in the development of hypertension,19,20 it is important to confirm whether reductions in BP persist during exposure to acute stress. Three previous studies have shown significant improvements in flow-mediated dilation (FMD), a measure of endothelial function, after walnut consumption.21–23 One unrandomized trial suggests that pistachios also increase FMD.24 We hypothesized that adding pistachios to a healthy diet would lower BP at rest and during stress, and that this change would be mediated by reductions in peripheral vascular resistance and increases in FMD.
Methods
Study Design and Participants
We measured changes in vascular reactivity in healthy, nonsmoking men (n= 10) and women (n= 18) with elevated LDL cholesterol who completed a 3-period, randomized, crossover, controlled-feeding study examining the effects of pistachios on risk factors of CVD.11 Participant demographics have been described previously.11 Participants had LDL cholesterol ≥2.86 mmol/L, triglyceride <3.94 mmol/L, BP <160/90 mm Hg, body mass index between 21 and 35 kg/m2, and fasting blood glucose ≤6.9 mmol/L. Exclusion criteria included the following: BP or cholesterol-lowering medication; use of nutritional supplements; pregnancy; weight loss ≥10% of body weight in the previous 6 months; vegetarian or weight-loss diets; and history of liver, kidney, autoimmune, or vascular disease. Approval was given by the institutional review board at Pennsylvania State University, and all of the participants provided signed informed consent. One participant was unable to comply with the protocol and withdrew from the study.
All of the meals were provided, and calorie levels were customized to maintain body weight (Table 1). The diet design and detailed nutrient profile have been published previously.11 After 2 weeks on a typical Western diet (run-in diet, 35% total fat [TF], 11% SFA], test diets were presented in counterbalanced order for 4 weeks each, including a low-fat control diet (25% TF, 8% SFA), a diet containing 10% of total energy from pistachios (30% TF, 8% SFA, 1 serving per day), and a diet containing 20% of total energy from pistachios (34% TF, 8% SFA, 2 servings per day). Table 1 shows that, as pistachio intake increased, fiber, monounsaturated fatty acids, polyunsaturated fatty acids, potassium, and protein increased, whereas sodium decreased modestly. Calories from carbohydrates were replaced with calories from pistachios. Salted, roasted pistachios (50% of the daily dose) were consumed as snacks, in place of baked potato chips and pretzels. Unsalted pistachios were incorporated into recipes. The median calorie intake was 2200 kcal/d.
Table 1. Nutrient profile of the test diets.
| Nutrient | Pretreatment (Run-In Diet) | Control Diet | 1 Serving per d of Pistachios | 2 Servings per d of Pistachios |
|---|---|---|---|---|
| Total fat, % kcal | 35.1 | 25.4 | 29.6 | 34.3 |
| Saturated fat, % kcal | 11.2 | 7.8 | 7.7 | 7.7 |
| Polyunsaturated fat, % kcal | 8.0 | 4.5 | 5.8 | 7.7 |
| Monounsaturated fat, % kcal | 13.1 | 9.1 | 12.0 | 15.3 |
| Protein, % kcal | 16.5 | 15.4 | 16.7 | 16.9 |
| Carbohydrate, % kcal | 49.8 | 62.7 | 57.6 | 53.5 |
| Fiber, g | 21.4 | 32.8 | 33.3 | 35.9 |
| Sodium, mg | 3166 | 3207 | 2805 | 2646 |
| Potassium, mg | 2514 | 2819 | 2995 | 3164 |
Foods were prepared in a metabolic kitchen, and calorie levels were customized to individual needs to prevent changes in body weight. Values were determined by using Nutritionist PRO (Axxya Systems, LLC, Stafford, TX) approximations. Full details of the diet design have been published previously.11
Hemodynamic Measures
Systolic BP (SBP) and diastolic BP were measured by an automated, oscillometric device (Dinamap Pro 100 Monitor, GE Medical Systems) at 1- to 4-minute intervals, as participants were seated with their arm at heart level. Cardiac output (CO; L/min) and total peripheral resistance (TPR; dyne·s×cm−5) were measured via a Hutcheson Impedance Cardiograph, a tetrapolar band electrode array, and the Cardiac Output Program (Bio-Impedance Technology, Inc, Chapel Hill, NC).25,26 After a 20-minute rest period, participants engaged in a standardized mental arithmetic task (the Paced Auditory Serial Addition Task27) for 5 minutes. After a 10-minute recovery, participants underwent the cold pressor test. They immersed their foot, up to the ankle, in an ice bath for 2.5 minutes and then rested for 10 minutes. Hemodynamic measurements were collected throughout, and task averages were calculated. Participants were required to fast for 2 hours to avoid postmeal spikes in glucose. Instructions were given so that time since last meal was held constant for each individual.
Flow-Mediated Dilation
Details of our protocol and reliability statistics have been published previously.28 After a 12-hour fast, the brachial artery in the upper arm was imaged at end diastole, via high-frequency ultrasound and a 10-MHz linear-array transducer, during rest (1 minute), arterial occlusion via a forearm cuff (5 minutes), and reactive hyperemia (2 minutes). Diameters were measured with edge-detection software (Brachial Analyzer, MIA, Iowa City, IA). FMD was measured as the maximum percentage of change in brachial artery diameter after hyperemia.
Data Analysis
After confirming normality, effects of diet were analyzed as change scores, calculated as the end of treatment minus end of the baseline (prerandomization/stabilization) diet. Treatment differences were analyzed with mixed models (SAS version 8, Cary, NC). Diet, treatment period (first, second, etc), task (rest, math, recovery1, cold pressor, and recovery2), and the diet × task interaction were entered as fixed effects; subject was a random effect. Diet by task and diet by period interactions were uniformly nonsignificant (no evidence of carryover). The prerandomization (baseline) value was entered as a covariate, and it was statistically significant for all of the variables. Tukey tests were used to adjust for multiple comparisons. To facilitate comparison with other studies of nuts, the analysis was repeated with only the resting baseline values. Finally, a separate mixed-models analysis was conducted to examine the effects of acute stress on hemodynamic variables during the prerandomization testing session, with task as a fixed effect and subject as a random effect. This study was powered to detect a 10% change in TPR, a 5% change in SBP, and a 20% change in FMD (with power=0.80 and α=0.05).28 α≤0.05 was considered statistically significant. We report adjusted (least-squares) means±SEs.
Results
Effects of Acute Stress on Systemic Hemodynamics Before Randomization
The math task increased SBP, diastolic BP, heart rate, and CO compared with the resting period (main effects of task, P≤0.0001; Tukey P≤0.0003; Table 2). The cold pressor increased SBP, diastolic BP, and TPR relative to baseline rest (P≤0.0001).
Table 2. Effects of acute stress and diet on hemodynamic variables measured ≥2 h after eating a meal.
| Vascular Measurement | Task | Pretreatment Mean (End Run-In) | Change After Control Diet | Change After 1 Serving per d of Pistachios | Change After 2 Servings per d of Pistachios | P Value for Diet Effect |
|---|---|---|---|---|---|---|
| Systolic BP, mm Hg | Rest | 111.9±2.5 | −5.2±1.9 | −6.2±1.9 | −5.0±1.9 | |
| Math | 131.1 ±2.5 | −1.0±1.9 | −3.5±1.9 | −0.9±1.9 | ||
| Recovery | 116.8±2.5 | −2.9±1.8 | −6.2±1.8 | −3.3±1.8 | ||
| Cold | 130.4±2.5 | −1.3±1.9 | −2.1 ±1.9 | 1.8±1.9 | ||
| Recovery | 117.2±2.5 | −1.5±1.8 | −5.8±1.8 | −4.7±1.8 | ||
| Average | −1.8±1.2 | −4.8±1.2* | −2.4±1.2† | 0.007 | ||
| Diastolic BP, mm Hg | Rest | 69.5±1.2 | −2.2±1.0 | −1.7±1.0 | −2.3±1.0 | |
| Math | 78.6±1.2 | −1.2±1.0 | −2.6±1.0 | −1.7±1.0 | ||
| Recovery | 72.2±1.2 | −2.3±1.0 | −3.6±1.0 | −2.8±1.0 | ||
| Cold | 79.5±1.2 | −0.3±1.1 | −0.8±1.1 | −1.4±1.1 | ||
| Recovery | 72.0±1.2 | −1.8±1.0 | −2.5±1.0 | −3.3±1.0 | ||
| Average | −1.6±0.7 | −2.2±0.7 | −2.3±0.7 | 0.30 | ||
| Heart rate, bpm | Rest | 70.5±1.5 | −3.1±1.1 | −3.4±1.1 | −5.0±1.1 | |
| Math | 76.7±1.5 | −0.2±1.1 | −0.5±1.1 | −1.3±1.1 | ||
| Recovery | 71.9±1.5 | −1.7±1.1 | −3.2±1.1 | −3.5±1.1 | ||
| Cold | 72.6±1.5 | −0.1±1.1 | −1.0±1.1 | −1.9±1.1 | ||
| Recovery | 70.1 ±1.5 | −1.9±1.1 | −3.1±1.1 | −3.5±1.1 | ||
| Average | −1.3±0.8 | −2.2±0.8 | −3.0±0.8* | 0.006 | ||
| Stroke volume, | Rest | 68.3±3.0 | 2.5±2.0 | 2.1 ±2.0 | 7.0±2.0*† | |
| mL per beat | Math | 68.1 ±3.0 | −1.8±2.0 | −2.7±2.0 | 2.6±2.0 | |
| Recovery | 68.0±3.0 | 0.9±2.0 | 2.1 ±2.0 | 4.4±2.0 | ||
| Cold | 69.3±3.0 | −0.2±2.0 | 1.7±2.0 | 4.1 ±2.0 | ||
| Recovery | 69.2±3.0 | −0.8±2.0 | 1.8±2.0 | 5.0±2.0 | ||
| Average | 0.4±1.6 | 1.0±1.6 | 4.6±1.6*† | 0.0001 | ||
| Cardiac output, L/min | Rest | 4.74±0.19 | −0.12±0.13 | −0.16±0.13 | 0.07±0.13 | |
| Math | 5.22±0.19 | −0.01 ±0.13 | −0.11 ±0.13 | 0.23±0.13 | ||
| Recovery | 4.80±0.19 | −0.07±0.13 | −0.11 ±0.13 | 0.04±0.13 | ||
| Cold | 5.95±0.19 | −0.01 ±0.13 | 0.07±0.13 | 0.18±0.13 | ||
| Recovery | 4.76±0.19 | −0.10±0.13 | −0.15±0.13 | 0.02±0.13 | ||
| Average | −0.1 ±0.1 | −0.1 ±0.1 | 0.2±0.1*† | 0.0001 |
BP indicates blood pressure. Data are presented as least-squares means±SEs from the SAS MIXED procedure, with subject entered as a random effect. SEs for repeated measurements are identical using this method. At the pretreatment visit, there were significant task effects for all variables except stroke volume (P<0.0001). Treatment effects are presented as change scores (end of treatment – end of baseline run-in). Analyses are adjusted for the prerandomization value. When only resting values were analyzed, stroke volume was the only variable with a significant treatment effect (P=0.01).
Significant difference vs the control diet using Tukey test (P<0.05).
Significant difference vs 1 serving per day using Tukey test (P<0.05).
Effects of Diet on BP and Heart Rate
When only the resting values were considered, there was no effect of treatment on BP or heart rate (Table 2). When all of the measurements were tested with rest, task, and recovery periods in a repeated-measures analysis, SBP and heart rate differed by diet (P≤0.007; Table 2). Average reductions in SBP were significantly greater when the diet containing 1 serving per day of pistachios was consumed (mean change in SBP of −4.8 mm Hg) compared with the diet containing 2 servings per day of pistachios (−2.4 mmHg; Tukey P<0.05) or the control diet (−1.8 mmHg; Tukey P<0.05). SBP response to the higher dose of pistachios did not differ from control. Heart rate decreased to a greater extent after 2 servings per day versus the control diet (Tukey P=0.0001); the response to 1 serving per day was intermediate and did not differ from the other diets. Diastolic BP was reduced by ≈2 mm Hg after all 3 of the diets (P≤0.02); there was no difference in the magnitude of this effect across diets (P=0.30).
Effects of Diet on Peripheral Vasodilation and Myocardial Response
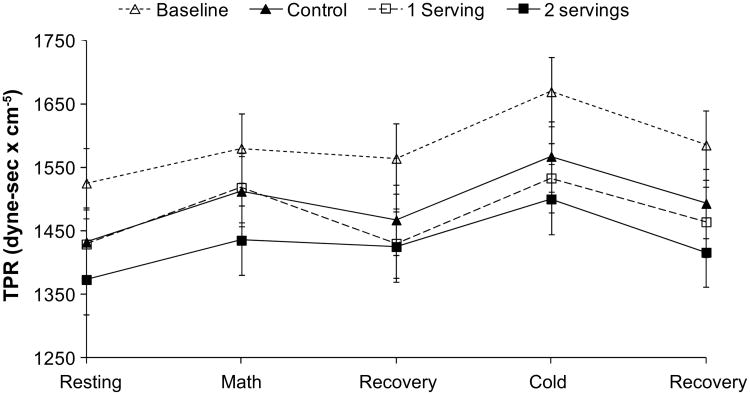
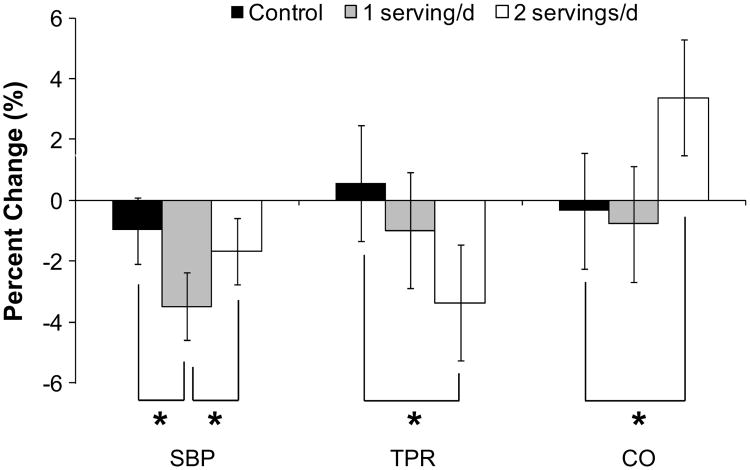
Resting values of CO (Table 2) and TPR (Figure 1) did not differ by treatment (Table 2). Resting stroke volume was significantly higher after 2 servings per day. When all of the task periods were included in the analysis, there were significant effects of treatment on stroke volume, CO, and TPR (P≤0.007; Table 2 and Figure 1). Reductions in TPR were greater after the diet providing 2 servings per day of pistachios versus control (Tukey P<0.0001; Figure 1). TPR change after 1 serving per day was intermediate and did not differ from control. CO and stroke volume showed the opposite pattern, with higher values after the diet containing 2 servings per day versus the control (Tukey P=0.0001; Table 2) and the diets containing 1 serving per day (Tukey P<0.05). Figure 2 shows percentage of change in SBP, CO, and TPR to allow visual comparison of the hemodynamic shifts across the 3 diets. There were no effects of diet on basal brachial artery diameter, postdeflation peak diameter, or FMD (percentage of change in artery diameter; Table 3).
Figure 1.
Effects of diets on total peripheral resistance (TPR). The diet containing 2 servings per day of pistachios lowered TPR to a greater extent than control and 1 serving per day (main effect of diet, P<0.0001). ∆, baseline; ▲, control; □, 1 serving; ■, 2 servings.
Figure 2.
Percentage of change from pretreatment in mean systolic blood pressure (SBP), total peripheral resistance (TPR), and cardiac output (CO). Mean changes are calculated by averaging across the rest, stress, and recovery periods to depict the main effect of diet. *Tukey P<0.05. ■, control; ▓, 1 serving per day; □, 2 servings per day.
Table 3. Ultrasound measurements of brachial arterial diameter and flow-mediated dilation (assessed after a 12-h fast).
| Vascular Response | Pretreatment Mean (End Run-In) | Change After Control Diet | Change After 1 Serving per d of Pistachios | Change After 2 Servings per d of Pistachios | P Value for Diet Effect |
|---|---|---|---|---|---|
| Basal diameter, mm | 3.92±0.14 | 0.01 ±0.05 | 0.01 ±0.05 | −0.06±0.05 | 0.39 |
| Peak diameter, mm | 4.15±0.14 | 0.02±0.06 | 0.02±0.05 | −0.04±0.05 | 0.51 |
| FMD, % Δ | 5.71 ±0.63 | 0.61 ±0.62 | 0.28±0.60 | 0.83±0.60 | 0.65 |
FMD indicates flow-mediated dilation. Data are presented as least-squares means±SEs from the SAS MIXED procedure, with subject entered as a random effect. SEs for repeated measurements are identical using this method. Treatment effects are presented as change scores (end of treatment–end of baseline run-in). Analyses are adjusted for the prerandomization value.
Discussion
In the present randomized, controlled-feeding study, BP-lowering effects of a pistachio-supplemented diet were dose dependent, with the more moderate diet (1 serving per day of pistachios) eliciting greater average reductions in average SBP. This pattern was evident during exposure to acute stress tests in the laboratory, whereas resting levels of BP and heart rate were unchanged. Analysis of the underlying hemodynamics suggests an explanation for the pattern of BP changes. As shown in Figures 1 and 2, significant reductions in average TPR were observed only after the diet that provided 2 servings per day of pistachios. One would expect larger decreases in BP to accompany the diet with the largest decrease in peripheral vascular resistance. However, there was an increase in CO when participants consumed 2 servings per day. The increase in CO may be a compensatory response to peripheral vasodilation, although the temporal dynamics of this response are unknown.
The finding that resting BP is unchanged with nut consumption is in agreement with some6,9,13 but not all17 clinical studies of nuts. In this study, the significant diet effects emerged only when repeated observations collected during rest, stress, and recovery are included. Although it is tempting to speculate that the diet effects are more pronounced during stress or recovery, interactions of task and treatment were not significant for any variable. Furthermore, statistical power is significantly enhanced by including repeated measurements across the testing session, and significant differences may result from having more data points. This hypothesis could be easily tested in future studies. A recent review of the effects of nuts on BP recommended that future studies include ambulatory BP monitoring.16 This technique is powerful because it involves dozens of repeated measurements in the same individual, it is closely tied to end organ damage, and it reflects BP during the experience of daily life.
Another limitation of this study is that we cannot attribute the significant shifts in hemodynamics to a specific bioactive compound or nutrient in pistachios. With increasing doses of pistachios, TF increased from 25% kcal on the control diet to 34% kcal on the diet containing 2 servings per day. As pistachios replaced carbohydrates in the diet, intake of fiber, unsaturated fatty acids, lutein, zeaxanthin, γ-tocopherol, and potassium increased (and sodium decreased).14 It is unlikely that modest changes in sodium and potassium were sufficient to fully account for the BP change reported herein. Despite disparities in sodium and potassium content, the control diet and the diet containing 2 servings per day of pistachios elicited equivalent BP reductions. Furthermore, the diet that produced the largest BP reduction (1 serving per day) was not the diet with the lowest sodium and highest potassium content. Future studies of nuts and hemodynamic measures should carefully match diets for sodium, potassium, and magnesium content when possible.
Given the energy density of nuts and the need to control for body weight in studies in which BP is an outcome, controlled-feeding studies must always vary by ≥2 macronutrients to prevent changes in weight. In the present study, calories from carbohydrates were reduced as increasing doses of pistachios were incorporated into the meals, and future studies should address whether changes in hemodynamics observed in this study are independent of changes in the macronutrient or micronutrient profile.
In spite of the significant reductions in peripheral vasoconstriction with the higher dose of pistachios, brachial artery FMD was not significantly changed. We observed the same pattern in a previous study in which walnuts and walnut oil were included in the diet.18 We note that peripheral vascular resistance is regulated by multiple, overlapping (and sometimes oppositional) regulatory systems, including endothelial function, sympathetic and parasympathetic activity, and myogenic stimulation, as well as endocrine, autocrine, and paracrine factors. Future work will be required to identify the mechanism(s) responsible for the reduction in peripheral vascular resistance. In the present study, FMD measurements occurred in the fasted state; thus, we cannot rule out an acute increase in vascular reactivity. Future studies should measure FMD (and other measures of endothelial function, eg, adhesion molecules and NO metabolites) in the postprandial state, to determine whether there is an acute effect of pistachio consumption on vascular reactivity. Two of the 3 positive studies of walnuts conducted their vascular assessments in the postprandial state,21,22 which may explain, in part, the discrepant results between the FMD effects of pistachios and walnuts. It also is possible that α-linolenic acid contained in walnuts is driving their effect on FMD or that differences in processing are responsible (walnuts are typically eaten raw, whereas pistachios are roasted).
Current dietary guidelines place an emphasis on foods that improve multiple CVD risk factors.29 Taken together with significant reductions in LDL cholesterol and oxidized LDL cholesterol observed in study participants,11,14 decreases in peripheral vascular resistance and SBP after the pistachio diets would be expected to lower CVD risk.
Perspectives
Epidemiological studies show that individuals who regularly consume nuts are at lower risk of cardiovascular morbidity and mortality compared with individuals who do not eat nuts regularly.1–5 The American Heart Association recommends consuming ≥4 servings per week of nuts, legumes, and seeds.23,29 The 2010 Dietary Guidelines for Americans advises consumption of a variety of high-protein plant foods, including unsalted nuts and seeds.30 Taken together with improvements in dyslipidemia and antioxidant activity reported previously, reductions in peripheral vascular constriction (and the resulting decrease in hemodynamic load) may be important contributors to lower CVD risk in nut consumers. Future studies should examine the underlying mechanisms for peripheral vasodilation reported here and also seek to identify the bioactive components in pistachios that mediate this response. Furthermore, given the cost of certain nuts and the energy density of nuts, additional research is needed to establish the lowest required dose to achieve a reduction in left ventricular workload.
Novelty and Significance.
What is new?
Moderate fat diets containing pistachios reduced BP and vascular resistance during acute stress.
Endothelial function was not affected by the pistachio diets
What is relevant?
Reductions in hemodynamic load during stress may be a mechanism through which nut consumption confers cardioprotection.
It is possible to integrate nuts into the habitual diet without weight gain, although this requires reducing intake of other high-fat/high-calorie foods in exchange for nuts.
Summary
Taken together with improvements in dyslipidemia and antioxidant activity reported previously, reductions in peripheral vascular constriction (and the resulting decrease in hemodynamic load) may be important contributors to lower CVD risk in nut consumers.
Acknowledgments
The services provided by the General Clinical Research Center of the Pennsylvania State University are appreciated.
Sources of Funding: Primary funding was provided by California Pistachio Commission of Fresno California and the Western Pistachio Association (now the American Pistachio Growers). The study was partially supported by National Institutes of Health grant M01 RR 10732. C.D.K. was supported by a postdoctoral fellowship from the Natural Sciences and Engineering Research Council of Canada.
Footnotes
Disclosures: S.G.W. and P.M.K.E. have received research grants and travel support from the Western Pistachio Association (now the American Pistachio Growers).
Contributor Information
Sheila G. West, Department of Biobehavioral Health, Pennsylvania State University, University Park, PA; Department of Nutritional Sciences, Pennsylvania State University, University Park, PA
Sarah K. Gebauer, Department of Nutritional Sciences, Pennsylvania State University, University Park, PA
Colin D. Kay, Department of Biobehavioral Health, Pennsylvania State University, University Park, PA; Department of Nutritional Sciences, Pennsylvania State University, University Park, PA
David M. Savastano, Department of Biobehavioral Health, Pennsylvania State University, University Park, PA
Christopher Diefenbach, Department of Biobehavioral Health, Pennsylvania State University, University Park, PA.
Deborah M. Bagshaw, Department of Nutritional Sciences, Pennsylvania State University, University Park, PA
Penny M. Kris-Etherton, Department of Nutritional Sciences, Pennsylvania State University, University Park, PA
References
- 1.Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease: the Adventist Health Study. Arch Intern Med. 1992;152:1416–1424. [PubMed] [Google Scholar]
- 2.Hu FB, Stampfer MJ, Manson JE, Rimm EB, Colditz GA, Rosner BA, Speizer FE, Hennekens CH, Willett WC. Frequent nut consumption and risk of coronary heart disease in women: prospective cohort study. BMJ. 1998;317:1341–1345. doi: 10.1136/bmj.317.7169.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellsworth JL, Kushi LH, Folsom AR. Frequent nut intake and risk of death from coronary heart disease and all causes in postmenopausal women: the Iowa Women's Health Study. Nutr Metab Cardiovasc Dis. 2001;11:372–377. [PubMed] [Google Scholar]
- 4.Kris-Etherton PM, Zhao G, Binkoski AE, Coval SM, Etherton TD. The effects of nuts on coronary heart disease risk. Nutr Rev. 2001;59:103–111. doi: 10.1111/j.1753-4887.2001.tb06996.x. [DOI] [PubMed] [Google Scholar]
- 5.Baer HJ, Glynn RJ, Hu FB, Hankinson SE, Willett WC, Colditz GA, Stampfer M, Rosner B. Risk factors for mortality in the Nurses' Health Study: a competing risks analysis. Am J Epidemiol. 2010;173:319–329. doi: 10.1093/aje/kwq368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wien M, Bleich D, Raghuwanshi M, Gould-Forgerite S, Gomes J, Monahan-Couch L, Oda K. Almond consumption and cardiovascular risk factors in adults with prediabetes. J Am Coll Nutr. 2010;29:189–197. doi: 10.1080/07315724.2010.10719833. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins DJ, Kendall CW, Marchie A, Parker TL, Connelly PW, Qian W, Haight JS, Faulkner D, Vidgen E, Lapsley KG, Spiller GA. Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein (a), homocysteine, and pulmonary nitric oxide–a randomized, controlled, crossover trial. Circulation. 2002;106:1327–1332. doi: 10.1161/01.cir.0000028421.91733.20. [DOI] [PubMed] [Google Scholar]
- 8.Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary α-linolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134:2991–2997. doi: 10.1093/jn/134.11.2991. [DOI] [PubMed] [Google Scholar]
- 9.Sabate J, Fraser GE, Burke K, Knutsen SF, Bennett H, Lindsted KD. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med. 1993;328:603–607. doi: 10.1056/NEJM199303043280902. [DOI] [PubMed] [Google Scholar]
- 10.Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr. 2009;90:56–63. doi: 10.3945/ajcn.2009.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gebauer SK, West SG, Kay CD, Alaupovic P, Bagshaw D, Kris-Etherton PM. Effects of pistachios on cardiovascular disease risk factors and potential mechanisms of action: a dose-response study. Am J Clin Nutr. 2008;88:651–659. doi: 10.1093/ajcn/88.3.651. [DOI] [PubMed] [Google Scholar]
- 12.Sheridan MJ, Cooper JN, Erario M, Cheifetz CE. Pistachio nut consumption and serum lipid levels. J Am Coll Nutr. 2007;26:141–148. doi: 10.1080/07315724.2007.10719595. [DOI] [PubMed] [Google Scholar]
- 13.Sabate J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170:821–827. doi: 10.1001/archinternmed.2010.79. [DOI] [PubMed] [Google Scholar]
- 14.Kay CD, Gebauer SK, West SG, Kris-Etherton PM. Pistachios increase serum antioxidants and lower serum oxidized-ldl in hypercholesterolemic adults. J Nutr. 2010;140:1093–1098. doi: 10.3945/jn.109.117366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djousse L, Rudich T, Gaziano JM. Nut consumption and risk of hypertension in us male physicians. Clin Nutr. 2009;28:10–14. doi: 10.1016/j.clnu.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casas-Agustench P, Lopez-Uriarte P, Ros E, Bullo M, Salas-Salvado J. Nuts, hypertension and endothelial function. Nutr Metab Cardiovasc Dis. 2011;21(suppl 1):S21–S33. doi: 10.1016/j.numecd.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, Fiol M, Gomez-Gracia E, Lopez-Sabater MC, Vinyoles E, Aros F, Conde M, Lahoz C, Lapetra J, Saez G, Ros E. Effects of a mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 18.West SG, Krick AL, Klein LC, Zhao G, Wojtowicz TF, McGuiness M, Bagshaw DM, Wagner P, Ceballos RM, Holub BJ, Kris-Etherton PM. Effects of diets high in walnuts and flax oil on hemodynamic responses to stress and vascular endothelial function. J Am Coll Nutr. 2010;29:595–603. doi: 10.1080/07315724.2010.10719898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55:1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 20.Matthews KA, Katholi CR, McCreath H, Whooley MA, Williams DR, Zhu S, Markovitz JH. Blood pressure reactivity to psychological stress predicts hypertension in the cardia study. Circulation. 2004;110:74–78. doi: 10.1161/01.CIR.0000133415.37578.E4. [DOI] [PubMed] [Google Scholar]
- 21.Cortes B, Nunez I, Cofan M, Gilabert R, Perez-Heras A, Casals E, Deulofeu R, Ros E. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol. 2006;48:1666–1671. doi: 10.1016/j.jacc.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 22.Ros E, Nunez I, Perez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2004;109:1609–1614. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y, Njike VY, Millet J, Dutta S, Doughty K, Treu JA, Katz DL. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care. 2010;33:227–232. doi: 10.2337/dc09-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sari I, Baltaci Y, Bagci C, Davutoglu V, Erel O, Celik H, Ozer O, Aksoy N, Aksoy M. Effect of pistachio diet on lipid parameters, endothelial function, inflammation, and oxidative status: a prospective study. Nutrition. 2010;26:399–404. doi: 10.1016/j.nut.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 25.West SG, Likos-Krick A, Brown P, Mariotti F. Oral L-arginine improves hemodynamic responses to stress and reduces plasma homocysteine in hypercholesterolemic men. J Nutr. 2005;135:212–217. doi: 10.1093/jn/135.2.212. [DOI] [PubMed] [Google Scholar]
- 26.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJP. Committee report: methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 27.Tombaugh TN. A comprehensive review of the paced auditory serial addition test (PASAT) Arch Clin Neuropsych. 2006;21:53–76. doi: 10.1016/j.acn.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 28.West SG, Wagner P, Schoemer SL, Hecker KD, Hurston KL, Likos Krick A, Boseska L, Ulbrecht J, Hinderliter AL. Biological correlates of day-to-day variation in flow-mediated dilation in individuals with type 2 diabetes: a study of test-retest reliability. Diabetologia. 2004;47:1625–1631. doi: 10.1007/s00125-004-1502-8. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic impact goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 30.US Department of Agriculture and US Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th. Washington, DC: U.S. Government Printing Office; 2010. [Google Scholar]