Abstract
Background
Polyunsaturated fatty acids (PUFA) have beneficial effects on cardiovascular risk, although the mechanisms are incompletely understood. In a previous article, we showed significant reductions in low-density lipoprotein cholesterol and several markers of inflammation with increasing intake of alpha-linolenic acid (ALA) from walnuts and flax.
Objective
To examine effects of ALA on cardiovascular responses to acute stress, flow-mediated dilation (FMD) of the brachial artery, and blood concentrations of endothelin-1 and arginine-vasopressin (AVP).
Design
Using a randomized, crossover study design, cardiovascular responses to acute stress were assessed in 20 hypercholesterolemic subjects, a subset of whom also underwent FMD testing (n = 12). Participants were fed an average American diet (AAD) and 2 experimental diets that varied in the amount of ALA and linoleic acid (LA) that they contained. The AAD provided 8.7% energy from PUFA (7.7% LA, 0.8% ALA). On the LA diet, saturated fat was reduced, and PUFA from walnuts and walnut oil provided 16.4% of energy (12.6% LA, 3.6% ALA). On the ALA diet, walnuts, walnut oil, and flax oil provided 17% energy from PUFA (10.5% LA, 6.5% ALA).
Results
The ALA and LA diets significantly reduced diastolic blood pressure (−2 to −3 mm Hg) and total peripheral resistance (−4%), and this effect was evident at rest and during stress (main effect of diet, p < 0.02). FMD increased (+34%) on the diet containing additional ALA. AVP also increased by 20%, and endothelin-1 was unchanged.
Conclusions
These results suggest novel mechanisms for the cardioprotective effects of walnuts and flax, and further work is needed to identify the bioactives responsible for these effects.
Keywords: alpha-linolenic acid, walnuts, flax, psychological stress, blood pressure, endothelial function, total peripheral resistance, omega-3 fatty acids
INTRODUCTION
Nut consumption significantly reduces cardiovascular disease (CVD) risk [1–4]. In addition, increased dietary polyunsaturated fatty acids (PUFA) from oils and fish are also cardioprotective [4,5]. Controlled clinical studies suggest that improvements in the lipid profile may partially explain these effects. For example, diets containing walnuts reduce total and low-density lipoprotein cholesterol (LDL-C) by 11%–16% [6–8]. We previously reported that diets high in PUFA from walnuts and flax significantly reduce C-reactive protein (CRP) and several markers of inflammation and endothelial dysfunction in adults with high cholesterol [6]. Ros and colleagues [9] reported that adding walnuts (45–65 g/d) to a Mediterranean diet increased endothelial function (as measured by flow-mediated dilation [FMD] of the brachial artery) by 63% versus a Mediterranean diet with olive oil, but without nuts. This team also has shown that adding walnuts to a high-fat meal attenuates its effect on endothelial function [10]. Thus, there is growing evidence that PUFA-rich foods such as walnuts beneficially affect multiple CVD risk factors, and in this article, we describe their effects on cardiovascular responses to stress.
Walnuts are a concentrated source of alpha-linolenic acid (ALA), an omega-3 fatty acid that may be responsible for improvements in lipids [11] and endothelial function [12,13]. There is controversy about whether ALA has lasting effects on CVD risk [14,15]. In some epidemiologic studies, higher consumption of ALA is associated with lower diastolic blood pressure [16,17] and lower risk of ischemic heart disease [11,18]. Several small studies have reported significant reductions in neuroendocrine [18–20] and cardiovascular [21–23] responses to psychological stress after treatment with omega-3 fatty acids from marine [18–20] or plant [21–23] sources. We [24] and others [25,26] have shown that exaggerated cardiovascular response to acute stress is a significant predictor of CVD risk, independent of traditional risk factors. Thus, attenuated cardiovascular reactivity to stress may be an additional mechanism through which ALA reduces coronary risk.
In this article, we measured the diet-related change in blood pressure and total peripheral resistance responses to standardized laboratory stressors, serum fatty acid concentrations, and vasoactive hormones in 20 adults with elevated LDL-C enrolled in a randomized, controlled feeding study in which the ALA levels were manipulated [6]. The FMD of the brachial artery was assessed at the end of each diet period in a subset of patients. We examined whether the magnitude of vascular response was dependent on lipid, CRP, or hormonal responses to the diets. We hypothesized that increasing the intake of PUFA from walnuts and flax would improve vascular reactivity to stress and endothelial function and that changes in vasoactive hormones (endothelin-1 and arginine vasopressin [AVP]) may explain these effects.
MATERIALS AND METHODS
Participants
The inclusion criteria included total cholesterol between 5.2 and 6.2 mmol/L; LDL-C between the 40th and 90th percentile; no current tobacco use; body mass index between 25 and 35 kg/m2; no current use of nutritional supplements or medications for hypercholesterolemia, hypertension, or inflammatory disease; and no history of CVD, hypertension, diabetes, or other systemic disease. The controlled feeding study required 5 visits per week to the diet center and a commitment to consume all of the food provided during 18 weeks of the intervention. The participants in this study (n = 20) are a subset of those involved in the study by Zhao et al. [6], plus an additional 5 participants who consumed identical diets and completed the same outcome assessments. As soon as the required equipment, personnel, and ethics approval were available, all remaining participants underwent hemodynamic testing, and the last 12 participants completed the ultrasound protocol. Thus, although there is considerable overlap between this sample and the one reported in our previous article, the samples are not identical. Most importantly, this is the first presentation of the data on endothelial function and hemodynamics. Participant characteristics are shown in Table 1.
Table 1.
Baseline Characteristics of Participants1 (n = 20)
| Age, y | 49.3 ± 1.7 |
| BMI, kg/m2 | 28.8 ± 0.8 |
| Weight, kg | 89.6 ± 2.6 |
| Total cholesterol, mmol/L | 5.8 ± 0.1 |
| LDL-C, mmol/L | 3.9 ± 0.1 |
| HDL-C, mmol/L | 1.2 ± 0.1 |
| Triglycerides, mmol/L | 1.4 ± 0.1 |
| Glucose, mg/dL | 87.1 ± 1.3 |
| C-reactive protein, mg/L | 3.1 ± 0.7 |
| Systolic blood pressure, mm Hg | 112.5 ± 2.3 |
| Diastolic blood pressure, mm Hg | 72.7 ± 1.1 |
| Heart rate, bpm | 69.3 ± 1.6 |
| Total peripheral resistance, dyne-sec × cm−5 | 1285.6 ± 43.4 |
| Cardiac output, L/min | 5.5 ± 0.2 |
| Stroke volume, mL/beat | 79.8 ± 3.1 |
Blood pressure and other hemodynamic variables are average resting values collected at the end of the control diet. All other variables were measured at screening. For total peripheral resistance, cardiac output, stroke volume, and heart rate, n = 19.
BMI = body mass index, LDL-C = low-density lipoprotein cholesterol, HDL-C = high-density lipoprotein cholesterol.
Study Design
As described previously [6], we employed a randomized, 3-period, crossover, controlled-feeding study design. All meals and snacks were provided. Diets included an average American diet (AAD) that served as the control (based on typical U.S. intake of macronutrients [19]) and 2 experimental diets high in total PUFA and low in saturated fat. One test diet was higher in ALA (the ALA diet), and one test diet was higher in linoleic acid (the LA diet). Subjects were assigned via a randomization table to a counterbalanced sequence of 3 diets (6 weeks each) by a diet study center manager who was not involved in outcome assessment. Technicians who measured outcome variables were blinded to treatment assignment. However, the use of whole nuts in the 2 walnut diets prevented complete blinding of participants.
The protocol was approved by the Institutional Review Board of Pennsylvania State University and was conducted in accord with institutional guidelines. Subjects gave written informed consent. Recruitment began in August 2000, and the last participant finished the study in December 2002. All measurements and blood samples were collected in clinics and laboratories on the Penn State campus. Diet-related change in hemodynamic response to stress was the primary outcome. Secondary outcomes included FMD, endothelin-1, and AVP.
Experimental Diets
Diets provided similar amounts of total fat, carbohydrate, protein, and cholesterol, and nutrient profiles of the meals were validated by biochemical analyses (Table 2). Diets were tailored to individual calorie needs to maintain body weight. Walnuts replaced major food sources of protein (meats and full-fat dairy foods) in the average American diet. For the high-PUFA diets, half of the total fat was derived from plant sources of n-3 fatty acids. The LA:ALA ratio on the LA diet was set at 3.5:1. This value is the relative proportion of these fatty acids in walnuts. It is also the ratio that was shown to be protective in the Lyon Diet Heart Study [20].
Table 2.
Results of Direct Measurement of Macronutrient Composition for the 3 Diets1
| Nutrient | AAD | LA Diet | ALA Diet |
|---|---|---|---|
| Carbohydrate, % en | 49.8 | 46.8 | 46.3 |
| Protein, % en | 15.7 | 16.1 | 16.1 |
| Total fat, % en | 34.5 | 37.1 | 37.6 |
| SFA | 12.7 | 8.5 | 8.2 |
| MUFA | 13.2 | 12.2 | 12.3 |
| PUFA | 8.7 | 16.4 | 17.2 |
| LA | 7.7 | 12.6 | 10.5 |
| ALA | 0.8 | 3.6 | 6.5 |
| LA/ALA | 9.5/1 | 3.5/1 | 1.6/1 |
| Cholesterol, mg/d | 311 | 304 | 305 |
Cholesterol was estimated using Nutritionist V software. Other nutrients were directly assayed. The addition of walnuts to the ALA and LA diets increased fiber intake by ~2.5 g/d for individuals consuming 2100 kcal/d.
AAD = average American diet, LA = linoleic acid, ALA = alpha-linolenic acid, SFA = saturated fatty acids, MUFA = monounsaturated fatty acids, PUFA = polyunsaturated fatty acids.
Walnuts and walnut oil were used because they are concentrated sources of LA and ALA (100 g of walnuts provides ~38 g of LA and 9 g of ALA; 100 g of walnut oil provides 53 g of LA and 10 g of ALA). On the ALA and LA diets, daily consumption of walnuts and walnut oil were similar (37 g and 15 g, respectively, at an energy intake of 10,032 kJ/d). In addition, the ALA diet contained 19 g of flaxseed oil (55 g of ALA /100 g oil). Walnuts and the oils were used in baked goods, salad dressings, pesto, and so forth, and half the daily dose of nuts was consumed as a snack. No adverse events were reported.
Clinical and Biochemical Analyses
Fatty acid compositions in total serum lipid were measured following extraction, transmethylation, and capillary gas-liquid chromatography [21]. AVP and endothelin-1 were measured via enzyme immunoassay using commercially available kits (Assay Designs, Ann Arbor, MI).
Hemodynamic Measures
Systemic hemodynamics were assessed during the last week of each diet via a Hutcheson Impedance Cardiograph-2000 and the Cardiac Output Program (Bio-impedance Technology, Inc., Chapel Hill, NC) [22]. Participants were instrumented with 4 band electrodes and 3 spot electrodes. Blood pressure was measured while participants were seated, with the right arm at heart level, using an oscillometric device (Dinamap Pro 100 Monitor, GE Medical Systems, Jupiter, FL) and appropriate cuff size. Cardiac output and total peripheral resistance were calculated using standard formulae [23,24].
We selected 2 standardized stress tasks that have been extensively validated in the literature on stress and cardiovascular disease [25,26]. Test sessions included a resting baseline (30 minutes), a speech stressor (5 minutes), recovery (10 minutes), and the foot cold pressor task (2.5 minutes). During the speech, participants prepared (2 minutes) and delivered (3 minutes) a speech on a hypothetical disagreement (e.g., being falsely accused of shoplifting). Speech topics were changed at the second and third visits. The cold pressor required participants to place one foot in 4°C water for 2.5 minutes. Averages of 2 or more readings were calculated within each event for blood pressure and other hemodynamic measures.
Flow-Mediated Dilation
Only a subset of our participants (n = 12) underwent ultrasound testing because of delay in gaining access to the equipment. Measurements of brachial artery diameter and blood flow volume/velocity were made by a single, well-trained sonographer (P.W.) [27] using an Acuson 128XP ultrasound imaging system (Siemens AG, USA) with a 10-MHz linear-array transducer. We have shown excellent test-retest reliability with these measurements [27]. Longitudinal, 2-dimensional images of the brachial artery were measured above the elbow of the right arm. The sonographer identified a relatively straight segment of artery in which there was no bifurcation, and measurements were taken to ensure correct placement on a subsequent visit. Diameters were measured at end diastole during the following events: rest (1 minute), arterial occlusion (5 minutes) via inflation of a cuff on the forearm (distal to the target artery) to 200 mm Hg, and reactive hyperemia (2 minutes). We employed edge-detection software (Brachial Analyzer, MIA, Iowa City, IA), with manual review by a technician blinded to treatment. Peak diameter was taken as the largest average diameter in the 2-minute deflation sequence (typically observed 40–70 seconds postdeflation). FMD was measured as the percentage change (Δ%) in arterial diameter after hyperemia. One subject exhibited mild arrhythmia at rest, and his data were not included in analyses of FMD and blood flow.
Data Analysis
Data were tested for normality and transformed where appropriate. Treatment effects were examined using mixed models (SAS version 8, Cary, NC) using intention-to-treat analysis. Models included diet, period, and treatment order as fixed effects and subject as a random effect. Models for hemodynamic variables also included task (baseline, speech preparation, speech, recovery, and cold pressor) and the diet × task interaction. Based on our previous work [27], this study was powered to detect a 10% change in total peripheral resistance, a 5% change in diastolic blood pressure, and a 35% change in FMD (with power = 0.80 and alpha = 0.05).
Statistically significant effects (p ≤ 0.05) were evaluated with Tukey post hoc tests, and we report least squares means ± SE. Interrelationships between the variables were estimated by Pearson or Spearman correlations, as appropriate. Correlations were also conducted on change scores (e.g., experimental diet – control diet), and these analyses were performed separately for the ALA and LA diets.
RESULTS
Effects of Acute Stress on Systemic Hemodynamics
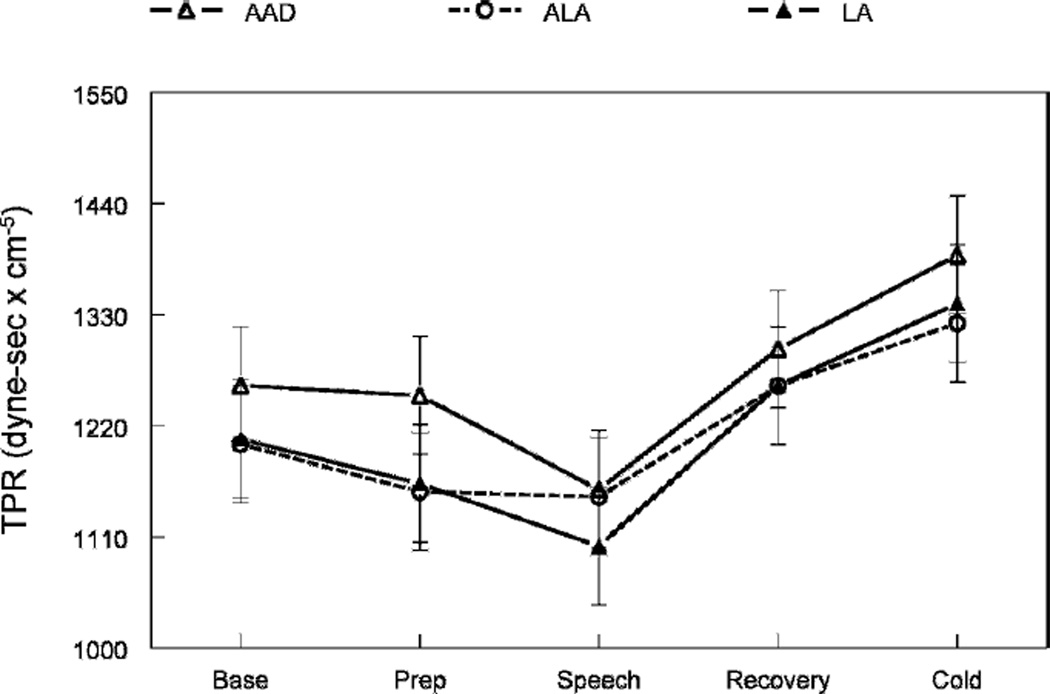
As expected, the speech task significantly increased blood pressure, heart rate, stroke volume, and cardiac output and reduced total peripheral resistance (main effects of task period, p ≤ 0.01; Table 3 and Figs. 1 and 2). The cold pressor significantly increased blood pressure, heart rate, and total peripheral resistance (p ≤ 0.001).
Table 3.
Effects of Treatment on Cardiovascular Variables at Rest and during Stress (n = 20)1
| Task | AAD | ALA Diet | LA Diet |
p Value for Diet Effect |
ALA Diet – AA |
LA Diet – AA |
ALA Diet – LA Diet |
|---|---|---|---|---|---|---|---|
| Systolic BP, mm Hg | |||||||
| Base | 112.2 ± 3.8 | 111.2 ± 3.8 | 113.2 ± 3.8 | ||||
| Prep | 132.4 ± 3.8 | 131.3 ± 3.8 | 137.5 ± 3.8 | ||||
| Speech | 140.2 ± 3.8 | 137.2 ± 3.8 | 138.2 ± 3.8 | ||||
| Recovery | 121.1 ± 3.8 | 118.4 ± 3.8 | 121.2 ± 3.8 | ||||
| Cold | 137.4 ± 3.9 | 134.6 ± 3.8 | 135.9 ± 3.9 | ||||
| Average | 128.7 ± 3.3 | 126.6 ± 3.3 | 129.2 ± 3.3 | 0.15 | 0.31 | 0.92 | 0.15 |
| Heart rate, bpm | |||||||
| Base | 69.7 ± 2.4 | 66.4 ± 2.4 | 68.4 ± 2.4 | ||||
| Prep | 79.3 ± 2.4 | 79.0 ± 2.4 | 81.3 ± 2.4 | ||||
| Speech | 81.4 ± 2.4 | 80.0 ± 2.4 | 81.9 ± 2.4 | ||||
| Recovery | 69.5 ± 2.4 | 68.0 ± 2.4 | 67.8 ± 2.4 | ||||
| Pressor | 74.9 ± 2.4 | 72.1 ± 2.4 | 74.0 ± 2.4 | ||||
| Average | 74.9 ± 2.0 | 73.1 ± 2.0 | 74.7 ± 2.0 | 0.11 | 0.13 | 0.95 | 0.23 |
| Stroke volume, mL/beat | |||||||
| Base | 81.2 ± 10.1 | 87.9 ± 10.1 | 88.1 ± 10.1 | ||||
| Prep | 87.1 ± 10.1 | 88.2 ± 10.1 | 91.5 ± 10.1 | ||||
| Speech | 91.4 ± 10.1 | 92.2 ± 10.1 | 96.3 ± 10.1 | ||||
| Recovery | 84.6 ± 10.1 | 88.2 ± 10.1 | 90.2 ± 10.1 | ||||
| Cold | 82.6 ± 10.1 | 89.0 ± 10.1 | 86.0 ± 10.1 | ||||
| Average | 85.4 ± 9.9 | 89.1 ± 9.9 | 90.4 ± 9.9 | 0.002 | 0.03 | 0.002 | 0.63 |
| Cardiac output, L/min | |||||||
| Base | 5.7 ± 0.5 | 5.8 ± 0.5 | 6.0 ± 0.5 | ||||
| Prep | 6.9 ± 0.5 | 7.0 ± 0.5 | 7.6 ± 0.5 | ||||
| Speech | 7.4 ± 0.5 | 7.3 ± 0.5 | 7.9 ± 0.5 | ||||
| Recovery | 5.9 ± 0.5 | 6.0 ± 0.5 | 6.1 ± 0.5 | ||||
| Cold | 6.2 ± 0.5 | 6.4 ± 0.5 | 6.3 ± 0.5 | ||||
| Average | 6.4 ± 0.5 | 6.5 ± 0.5 | 6.8 ± 0.5 | 0.02 | 0.79 | 0.02 | 0.10 |
Data are least squares means ± SE from the SAS MIXED procedure. The SEs for repeated measurements are identical when using this method.
AAD = average American diet, ALA = alpha-linolenic acid, BP = blood pressure, LA = linoleic acid.
Fig. 1.
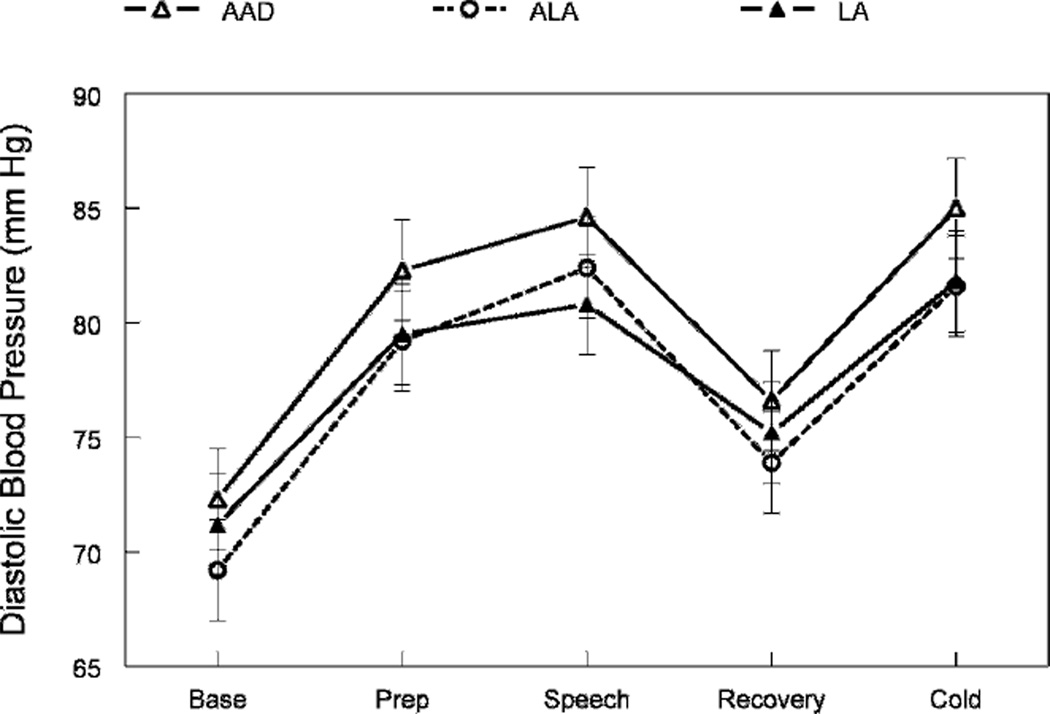
Effects of diets on diastolic blood pressure at rest and during stress. The figure shows the main effect of treatment (p = 0.0002). Diastolic blood pressure was significantly lower than the ALA and LA diets compared to control (AAD).
Fig. 2.
Effects of diets on total peripheral resistance at rest and during stress. The figure shows the main effect of treatment (p = 0.02). Peripheral vascular resistance was significantly lower after the ALA and LA diets compared to control (AAD), indicating vasodilatation.
Effects of Diet on Hemodynamic Responses to Stress
There were significant differences across the diets in diastolic blood pressure, total peripheral resistance, stroke volume, and cardiac output (p ≤ 0.05; Table 3 and Figs. 1 and 2). Relative to the control diet, the 2 high-PUFA diets reduced diastolic blood pressure by 2–3 mm Hg (p ≤ 0.001; Fig. 1) and total peripheral resistance by 4.0% (p ≤ 0.05; Fig. 2). Stroke volume increased by 4% on the ALA diet (p ≤ 0.01) and 6% on the LA diet (p ≤ 0.01). Cardiac output significantly increased during the LA diet only (+0.4 L/min, p = 0.05). Heart rate and systolic blood pressure were unchanged (p ≥ 0.10). This pattern of hemodynamic changes was equally evident at rest and during acute stress (e.g., there were no interactions of task and diet, p ≥ 0.80).
Effects of Diet on Other Measures of Coronary Risk
During the ALA diet only, FMD increased by 34% relative to the control diet (p = 0.05; Table 4). Doppler-derived measures of arterial blood flow velocity and volume (measured at rest and during hyperemia) were not significantly affected by the diets. Table 5 shows that the LA diet significantly increased serum concentrations of omega-6 PUFA (predominantly LA), while the ALA diet significantly increased serum concentrations of omega-3 PUFA (ALA, eicosapentaenoic acid [EPA], and docosapentaeneoic acid [DPA]). AVP increased by 17% on the ALA diet only (Table 4; p < 0.05 vs. control diet). There were no changes in high-density lipoprotein cholesterol, triglycerides, endothelin-1, or body weight.
Table 4.
Effects of Treatment on Vascular Parameters (Flow Volume, Flow-Mediated Dilation, Hormones) and Body Weight1
| AAD | ALA Diet | LA Diet |
p Value for Diet Effect |
ALA – AAD |
LA – AAD |
ALA – LA |
|
|---|---|---|---|---|---|---|---|
| Vascular ultrasound measures (n = 12) | |||||||
| Basal artery diameter, mm | 4.3 ± 0.2 | 4.2 ± 0.2 | 4.3 ± 0.2 | 0.10 | 0.56 | 0.49 | 0.08 |
| Peak deflation diameter, mm | 4.5 ± 0.2 | 4.5 ± 0.2 | 4.6 ± 0.2 | 0.34 | 0.98 | 0.38 | 0.46 |
| Flow-mediated dilation, % Δ | 6.1 ± 1.1 | 8.2 ± 1.0 | 6.7 ± 1.0 | 0.02 | 0.02 | 0.66 | 0.10 |
| Basal blood flow, mL/min | 150.6 ± 15.2 | 157.8 ± 14.7 | 160.2 ± 14.6 | 0.89 | 0.94 | 0.89 | 0.99 |
| Peak deflation blood flow, mL/min | 855.5 ± 135.9 | 891.2 ± 133.3 | 977.7 ± 133.1 | 0.35 | 0.91 | 0.35 | 0.54 |
| Change in blood flow, % Δ | 568.3 ± 86.2 | 556.5 ± 81.1 | 603.3 ± 80.7 | 0.88 | 0.99 | 0.94 | 0.88 |
| Vasoactive hormones (n = 20) | |||||||
| Endothelin-1, pg/mL | 2.0 ± 0.2 | 1.9 ± 0.2 | 1.7 ± 0.2 | 0.40 | 0.89 | 0.38 | 0.63 |
| AVP, pg/mL | 8.3 ± 0.4 | 9.7 ± 0.4 | 9.1 ± 0.4 | 0.05 | 0.04 | 0.36 | 0.44 |
| Body weight, kg | 89.7 ± 3.2 | 89.8 ± 3.2 | 90.0 ± 3.2 | 0.81 | 0.88 | 0.99 | 0.8 |
Data are means ± SE. p values are from Tukey post hoc tests.
AAD = average American diet, ALA = alpha-linolenic acid, AVP = arginine vasopressin, LA = linoleic acid.
Table 5.
Effects on Treatment on Individual Fatty Acid Concentrations in Serum Lipid (Expressed as % by Weight of Total Fatty Acids) for All Study Participants (n = 20)1
| AAD | ALA Diet | LA Diet |
p Value for Overall Diet Effect |
ALA Diet vs. AAD |
LA Diet vs. AAD |
ALA Diet vs. LA Diet |
|
|---|---|---|---|---|---|---|---|
| Myristic | 5.9 ± 1.1 | 5.5 ± 1.1 | 6.1 ± 1.1 | 0.23 | — | — | — |
| Palmitic | 19.5 ± 0.4 | 17.9 ± 0.4 | 17.5 ± 0.4 | 0.0001 | 0.0001 | 0.0001 | — |
| Palmitoleic | 1.6 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 | 0.0001 | 0.003 | 0.0001 | — |
| Stearic | 6.9 ± 0.1 | 6.8 ± 0.1 | 6.6 ± 0.1 | 0.1 | — | 0.09 | — |
| LA | 33.3 ± 0.6 | 34.3 ± 0.6 | 36.4 ± 0.6 | 0.0001 | — | 0.0001 | 0.002 |
| ALA | 0.6 ± 0.2 | 3.2 ± 0.2 | 2.1 ± 0.2 | 0.0001 | 0.0001 | 0.0001 | 0.008 |
| GLA | 0.5 ± 0.0 | 0.4 ± 0.0 | 0.4 ± 0.0 | 0.0001 | 0.0001 | 0.0001 | 0.08 |
| Arachidonic | 8.7 ± 0.4 | 7.7 ± 0.4 | 7.8 ± 0.4 | 0.0001 | 0.0001 | 0.0004 | — |
| EPA (n-3) | 0.6 ± 0.1 | 1.3 ± 0.1 | 0.9 ± 0.1 | 0.0001 | 0.0001 | 0.0005 | 0.0001 |
| DPA (n-3) | 0.6 ± 0.0 | 0.8 ± 0.0 | 0.7 ± 0.0 | 0.0001 | 0.0001 | — | 0.007 |
| DHA (n-3) | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.8 ± 0.1 | 0.58 | — | — | — |
| MUFA | 20.8 ± 0.9 | 19.5 ± 0.9 | 18.7 ± 0.9 | 0.0002 | 0.02 | 0.0001 | — |
| n-3 PUFA | 3.7 ± 0.3 | 7.0 ± 0.3 | 5.5 ± 0.3 | 0.0001 | 0.0001 | 0.0001 | 0.0004 |
| n-6 PUFA | 43.4 ± 0.8 | 43.0 ± 0.8 | 45.4 ± 0.8 | 0.0005 | — | 0.005 | 0.0007 |
| LA:ALA ratio | 48.6 ± 2.2 | 13.8 ± 2.2 | 18.9 ± 2.2 | 0.0001 | 0.0001 | 0.0001 | — |
| n-6:n-3 ratio | 11.9 ± 0.4 | 6.5 ± 0.4 | 8.4 ± 0.4 | 0.0001 | 0.0001 | 0.0001 | 0.0008 |
| SFA | 32.0 ± 0.8 | 30.4 ± 0.8 | 30.4 ± 0.8 | 0.005 | 0.01 | 0.01 | — |
Data are means ± SE. p values are from Tukey post hoc tests.
AAD = average American diet, ALA = alpha-linolenic acid, LA = linoleic acid, GLA = gamma linoleic acid, EPA = eicosapentaneoic acid, DPA = docosapentaenoic acid, DHA = docosahexaenoic acid, MUFA = monounsaturated fatty acids, PUFA = polyunsaturated fatty acids, SFA = saturated fatty acids.
Correlates of Hemodynamic Response to the High PUFA Diets
Changes in serum fatty acids were correlated with changes in blood pressure. On the ALA diet, subjects with the largest increases in serum DPA (C22:5 n-3) showed the largest reductions in diastolic blood pressure (r = −0.46, p = 0.05). Similarly, DPA response to the LA diet was inversely correlated with change in systolic (r = −0.49, p < 0.05) and diastolic blood pressure (r = −0.52, p <0.05). Change in systolic blood pressure on the LA diet was inversely correlated with change in serum EPA (C20:5 n3, r = −0.49, p < 0.05). However, changes in serum ALA and LA were not significantly correlated with changes in hemodynamics.
Correlates of FMD Response to the Diets
In our previous publication from this study, we reported a significant reduction in CRP on the ALA diet. Here, we examined whether the decrease in CRP on the ALA diet was correlated with the significant increase in FMD. Participants with larger decreases in systemic inflammation had larger improvements in endothelial function (r = −0.67, p < 0.05). There was no relationship between diet-related change in lipids, vasoactive hormones, or cell adhesion molecules and change in FMD.
DISCUSSION
This study is the first to show significant reductions in systemic vascular resistance with diets high in both omega-3 and omega-6 fatty acids. In healthy adults with elevated LDL-C, the LA diet (which contained walnuts and walnut oil, but no flax oil) significantly reduced blood pressure and vascular resistance, both at rest and during acute stress. In a previous report from this sample [6], we showed that the LA diet significantly reduced LDL-C and total cholesterol. Adding more ALA from flax did not further improve the lipid profile, and the present study shows that blood pressure and peripheral resistance were significantly reduced by both of the walnut-rich diets. However, reductions in CRP and increases in FMD were observed only on the higher ALA diet. Furthermore, the magnitude of changes was correlated, and this suggests that improvements in vascular endothelial function may result from the anti-inflammatory effects of ALA or other constituents of flax oil. Additional studies are required to confirm this result.
The effects of walnuts on systemic vascular tone and lipids may result from their high content of ALA and LA or from combined effects of fiber, gamma-tocopherol, and antioxidant polyphenols [28]. Our findings suggest that the effects of plant-derived ALA on endothelial function and CRP are dose dependent, although dose and the source of ALA (walnuts vs. flax) were confounded in this study. It is not clear whether increasing the dose of walnuts and walnut oil would yield improvements in FMD, although 2 previous studies found significant improvements in endothelial function after acute [10] and chronic [9] walnut consumption. Some previous clinical studies have concluded that walnuts have no effect on blood pressure [9,29]. In contrast, ALA consumption is linked to lower blood pressure in epidemiologic studies [17,30], and the PREDIMED investigators reported substantial reductions in blood pressure when nuts were added to a Mediterranean diet [31]. Our study design differs from the previous studies because we measured blood pressure repeatedly during a prolonged rest period and under conditions of sympathetic activation. Future studies should include repeated measures of blood pressure to increase statistical power.
Reductions in peripheral resistance and increases in FMD were not correlated with changes in the lipid profile, and this suggests that lipid lowering is not the primary mechanism for vasodilatory effects in the present study. Nuts are a concentrated source of the nitric oxide precursor L-arginine, a nutrient that has been shown to enhance vasodilation and reduce blood pressure responses to stress at doses of 8–30 g/d [32,33]. However, the 2 walnut diets provided <1 g/d of additional L-arginine, and this change is unlikely to explain these effects. Although the bioactives responsible for peripheral dilation could not be determined in this study, peripheral artery constriction is an important determinant of cardiac workload.
After both of the experimental diets, reductions in diastolic blood pressure were proportional to the increase in serum concentrations of DPA (C22:5 n3). On the LA diet only, there was an inverse correlation between change in serum EPA (C20:5 n3) and change in systolic blood pressure. Changes in serum ALA and the LA-ALA ratio were not significantly correlated with vascular or blood pressure responses to the diets. These results suggest that conversion of ALA to the longer-chain omega-3 fatty acids may be critical to its vasodilatory effects. However, an in vitro study from our group [34] showed that exposure to ALA reduces the production of inflammatory cytokines in cultured monocytes. Taken together with the results from the present study, it appears that the anti-inflammatory effects of ALA are not entirely dependent on conversion to long-chain omega-3 fatty acids.
There is also evidence that ALA improves endothelial function acutely. For example, we showed that a high-fat meal containing ALA improved FMD by 53% in patients with type 2 diabetes and high triglycerides [12]. The magnitude of FMD response to ALA was very similar to that observed after consumption of marine-derived omega-3 fatty acids. Taken together with results from Ros et al. [9,10], we conclude that foods and oils containing ALA have significant beneficial effects on endothelial function that are independent of changes in endothelin-1. It is surprising that AVP was increased by the ALA diet (and we note a similar trend for the LA diet). We speculate that this may be a compensatory response to systemic vasodilation. In other words, the modest increase in AVP may be a response to lower blood pressure, and it may also explain why stroke volume significantly increased on the intervention diets.
In the present study, calorie intake and the macronutrient profile of the diet were held constant to carefully examine the effects of changing the fatty acid profile on vascular reactivity. However, to match the meals for calories and total fat content, substitution of saturated fat for unsaturated fats was necessary. Thus, we cannot directly address whether the reduction in saturated fat or the increase in polyunsaturated fats (or changes in other bioactives present in flax and walnuts) is the primary mechanism for vasodilation observed in this study, and this is a limitation of the present study. Placebo-controlled trials of omega-3 supplements in free-living subjects support our conclusion that these effects are independent of changes in saturated fatty acids. Spence et al. [35] instructed subjects to add 30 g/d of flax seed to their habitual diet and found significant reductions in blood pressure at rest and during a mental stress task, despite the fact that saturated fatty acids were not directly manipulated. In contrast to the present study, none of the 3 flax cultivars tested by Spence et al. significantly lowered total peripheral resistance [35]. However, in the Spence et al. study, background diet was not controlled and no placebo treatment was used for comparison. In the present study, we show that the reductions in blood pressure following a diet high in ALA result from significant reductions in systemic vascular resistance. We provide the first evidence that changes in systemic hemodynamics are observed following diets high in walnuts and walnut oil, and sustained reductions in total peripheral vascular resistance may have important beneficial effects on the myocardium [36]. Furthermore, we showed that subjects with larger increases in serum concentrations of PUFA exhibited larger decreases in diastolic blood pressure.
CONCLUSION
This article identifies novel mechanisms for the cardiovascular benefits of PUFA contained in walnuts and flax. Taken together with reductions in LDL-C and inflammation reported previously [6], these improvements in endothelial function and cardiovascular responses to stress would be expected to significantly reduce the risk of CVD.
Acknowledgments
Primary funding was provided by the California Walnut Commission of Sacramento, California. Additional support came from the Heart and Stroke Foundation of Ontario. The services provided by the General Clinical Research Center of The Pennsylvania State University are appreciated. The study was supported by NIH grant M01 RR 10732.
Abbreviations
- AAD
average American diet
- ALA
alpha linolenic acid
- BMI
body mass index
- CRP
C-reactive protein
- CVD
cardiovascular disease
- DPA
docosapentaeneoic acid
- EPA
eicosapentaenoic acid
- FMD
flow mediated dilation
- LA
linoleic acid
- LDL-C
low density lipoprotein cholesterol
- PUFA
polyunsaturated fatty acids
Footnotes
These data were presented at the following scientific conferences: 2004, American Heart Association, Council on Nutrition Physical Activity and Metabolism; 2005, Gerontological Association of America; 2006, World Nutra Conference; 2008, American Psychosomatic Society.
REFERENCES
- 1.Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease. The Adventist Health Study. Arch Intern Med. 1992;152:1416–1424. [PubMed] [Google Scholar]
- 2.Hu FB, Stampfer MJ, Manson JE, Rimm EB, Colditz GA, Rosner BA, Speizer FE, Hennekens CH, Willett WC. Frequent nut consumption risk of coronary heart disease in women: prospective cohort study. BMJ. 1998;317:1341–1345. doi: 10.1136/bmj.317.7169.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellsworth JL, Kushi LH, Folsom AR. Frequent nut intake and risk of death from coronary heart disease and all causes in postmenopausal women: the Iowa Women’s Health Study. Nutr Metab Cardiovasc Dis. 2001;11:372–377. [PubMed] [Google Scholar]
- 4.Kris-Etherton PM, Zhao G, Binkoski AE, Coval SM, Etherton TD. The effects of nuts on coronary heart disease risk. Nutr Rev. 2001;59:103–111. doi: 10.1111/j.1753-4887.2001.tb06996.x. [DOI] [PubMed] [Google Scholar]
- 5.Wijendran V, Hayes KC. Dietary n-6 and n-3 fatty acid balance and cardiovascular health. Annu Rev Nutr. 2004;24:597–615. doi: 10.1146/annurev.nutr.24.012003.132106. [DOI] [PubMed] [Google Scholar]
- 6.Zhao G, Etherton TD, Martin KR, West SG, Gillies PJ, Kris-Etherton PM. Dietary alphalinolenic acid reduces inflammatory and lipid cardiovascular risk factors in hypercholesterolemic men and women. J Nutr. 2004;134:2991–2997. doi: 10.1093/jn/134.11.2991. [DOI] [PubMed] [Google Scholar]
- 7.Sabate J, Fraser GE, Burke K, Knutsen SF, Bennett H, Lindsted KD. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med. 1993;328:603–607. doi: 10.1056/NEJM199303043280902. [DOI] [PubMed] [Google Scholar]
- 8.Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors a meta-analysis and systematic review. Am J Clin Nutr. 2009;90:56–63. doi: 10.3945/ajcn.2009.27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ros E, Nunez I, Perez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2004;109:1609–1614. doi: 10.1161/01.CIR.0000124477.91474.FF. [DOI] [PubMed] [Google Scholar]
- 10.Cortes B, Nunez I, Cofan M, Gilabert R, Perez-Heras A, Casals E, Deulofeu R, Ros E. Acute effects of high-fat meals enriched with walnuts or olive oil on postprandial endothelial function. J Am Coll Cardiol. 2006;48:1666–1671. doi: 10.1016/j.jacc.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 11.Bemelmans WJ, Broer J, Feskens EJ, Smit AJ, Muskiet FA, Lefrandt JD, Bom VJ, May JF, Meyboom-de Jong B. Effect of an increased intake of alphalinolenic acid and group nutritional education on cardiovascular risk factors:the Mediterranean Alphalinolenic Enriched Groningen Dietary Intervention (MARGARIN) study. Am J Clin Nutr. 2002;75:221–227. doi: 10.1093/ajcn/75.2.221. [DOI] [PubMed] [Google Scholar]
- 12.West SG, Hecker KD, Mustad VA, Nicholson S, Schoemer SL, Wagner P, Hinderliter AL, Ulbrecht J, Ruey P, Kris-Etherton PM. Acute effects of monounsaturated fatty acids with and without omega-3 fatty acids on vascular reactivity in individuals with type 2 diabetes. Diabetologia. 2005;48:113–122. doi: 10.1007/s00125-004-1600-7. [DOI] [PubMed] [Google Scholar]
- 13.Steer P, Vessby B, Lind L. Endothelial vasodilatory function is related to the proportions of saturated fatty acids and alpha-linolenic acid in young men, but not in women. Eur J Clin Invest. 2003;33:390–396. doi: 10.1046/j.1365-2362.2003.01147.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n-3 Fatty acids from fish or fishoil supplements, but notalpha-linolenic acid, benefit cardiovascular disease outcomes in primary- and secondary-prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. doi: 10.1093/ajcn/84.1.5. [DOI] [PubMed] [Google Scholar]
- 15.Woo KS, Chook P, Lolin YI, Sanderson JE, Metreweli C, Celermajer DS. Folic acid improves arterial endothelial function in adults with hyperhomocystinemia. J Am Coll Cardiol. 1999;34:2002–2006. doi: 10.1016/s0735-1097(99)00469-6. [DOI] [PubMed] [Google Scholar]
- 16.Bemelmans WJ, Muskiet FA, Feskens EJ, de Vries JH, Broer J, May JF, Jong BM. Associations of alpha-linolenic acid and linoleic acid with risk factors for coronary heart disease. Eur J Clin Nutr. 2000;54:865–871. doi: 10.1038/sj.ejcn.1601102. [DOI] [PubMed] [Google Scholar]
- 17.Ueshima H, Stamler J, Elliott P, Chan Q, Brown IJ, Carnethon MR, Daviglus ML, He K, Moag-Stahlberg A, Rodriguez BL, Steffen LM, Van Horn L, Yarnell J, Zhou B. Food omega-3 fatty acid intake of individuals (total, linolenic acid, long-chain) and their blood pressure INTERMAP study. Hypertension. 2007;50:313–319. doi: 10.1161/HYPERTENSIONAHA.107.090720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu FB, Stampfer MJ, Manson JE, Rimm EB, Wolk A, Colditz GA, Hennekens CH, Willett WC. Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr. 1999;69:890–897. doi: 10.1093/ajcn/69.5.890. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Department of Agriculture ARS. “Nutrient Intakes from Food: Mean Amounts and Percentages of Calories from Protein, Carbohydrate, Fat, and Alcohol, One Day, 2005–2006.”. Belts-ville, MD: USDA ARS; 2008. Accessed at: www.ars.usda.gov/ba/bhnrc/fsrg. [Google Scholar]
- 20.de Lorgeril MSP, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet traditional risk factors the rate of cardiovascular complications after myocardial infarctionfinal report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 21.Stark KD, Holub BJ. Differential eicosapentaenoic acid elevations and altered cardiovascular disease risk factor responses after supplementation with docosahexaenoic acid in postmenopausal women receiving and not receiving hormone replacement therapy. Am J Clin Nutr. 2004;79:765–773. doi: 10.1093/ajcn/79.5.765. [DOI] [PubMed] [Google Scholar]
- 22.West SG, Hinderliter AL, Wells EC, Girdler SS, Light KC. Transdermal estrogen reduces vascular resistance and serum cholesterol in postmenopausal women. Am J Obstet Gynecol. 2001;184:926–933. doi: 10.1067/mob.2001.112104. [DOI] [PubMed] [Google Scholar]
- 23.Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJP. Committee report: methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. Accessed at: http://www.sprweb.org/articles/Sherwood90.pdf. [DOI] [PubMed] [Google Scholar]
- 24.Ventura HO, Taler SJ, Strobeck JE. Hypertension as a hemody-namic diseasethe role of impedance cardiography in diagnostic, prognostic, and therapeutic decision making. Am J Hypertens. 2005;18:26S–43S. doi: 10.1016/j.amjhyper.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Everson SA, Lynch JW, Kaplan GA, Lakka TA, Sivenius J, Salonen JT. Stress-induced blood pressure reactivity and incident stroke in middle-aged men. Stroke. 2001;32:1263–1270. doi: 10.1161/01.str.32.6.1263. [DOI] [PubMed] [Google Scholar]
- 26.Gianaros PJ, Bleil ME, Muldoon MF, Jennings JR, Sutton-Tyrrell K, McCaffery JM, Manuck SB. Is cardiovascular reactivity associated with atherosclerosis among hypertensives? Hypertension. 2002;40:742–747. doi: 10.1161/01.hyp.0000035707.57492.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West SG, Wagner P, Schoemer SL, Hecker KD, Hurston KL, Likos Krick A, Boseska L, Ulbrecht J, Hinderliter AL. Biological correlates of day-to-day variation in flow-mediated dilation in individuals with type 2 diabetes:a study of test-retest reliability. Diabetologia. 2004;47:1625–1631. doi: 10.1007/s00125-004-1502-8. [DOI] [PubMed] [Google Scholar]
- 28.Kay CD, Kris-Etherton PM, West SG. Effects of antioxidant-rich foods on vascular reactivity: review of the clinical evidence. Curr Atheroscler Rep. 2006;8:510–522. doi: 10.1007/s11883-006-0027-7. [DOI] [PubMed] [Google Scholar]
- 29.Iwamoto M, Sato M, Kono M, Hirooka Y, Sakai K, Takeshita A, Imaizumi K. Walnuts lower serum cholesterol in Japanese men and women. J Nutr. 2000;130:171–176. doi: 10.1093/jn/130.2.171. [DOI] [PubMed] [Google Scholar]
- 30.Paschos GK, Magkos F, Panagiotakos DB, Votteas V, Zampelas A. Dietary supplementation with flaxseed oil lowers blood pressure in dyslipidaemic patients. Eur J Clin Nutr. 2007;61:1201–1206. doi: 10.1038/sj.ejcn.1602631. [DOI] [PubMed] [Google Scholar]
- 31.Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, Fiol M, Gomez-Gracia E, Lopez-Sabater MC, Vinyoles E, Aros F, Conde M, Lahoz C, Lapetra J, Saez G, Ros E. Effects of a Mediterranean-style diet on cardiovascular risk factors:a randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 32.West SG, Likos-Krick A, Brown P, Mariotti F. Oral L-arginine improves hemodynamic responses to stress and reduces plasma homocysteine in hypercholesterolemic men. J Nutr. 2005;135:212–217. doi: 10.1093/jn/135.2.212. [DOI] [PubMed] [Google Scholar]
- 33.Brown AA, Hu FB. Dietary modulation of endothelial function:implications for cardiovascular disease. Am J Clin Nutr. 2001;73:673–686. doi: 10.1093/ajcn/73.4.673. [DOI] [PubMed] [Google Scholar]
- 34.Zhao G, Etherton TD, Martin KR, Vanden Heuval JP, Gillies PJ, West SG, Kris-Etherton PM. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem Biophys Res Commun. 2005;336:909–917. doi: 10.1016/j.bbrc.2005.08.204. [DOI] [PubMed] [Google Scholar]
- 35.Spence JD, Thornton T, Muir AD, Westcott ND. The effect of flax seed cultivars with differing content of alpha-linolenic acid and lignans on responses to mental stress. J Am Coll Nutr. 2003;22:494–501. doi: 10.1080/07315724.2003.10719327. [DOI] [PubMed] [Google Scholar]
- 36.Watabe D, Hasimoto J, Htanaka R, Hanazawa T, Ohba H, Ohkubo T, Kuikuya M, Totsune K, Imai Y. Electrocardiographic left ventricular hypertrophy and arterial stiffness: the Ohasama study. Am J Hypertens. 2006;19:1199–1205. doi: 10.1016/j.amjhyper.2006.05.001. [DOI] [PubMed] [Google Scholar]