Abstract
Background
The common adverse effects of linezolid for treating septic patients with gram-positive cocci is anemia and thrombocytopenia, which limit its clinical application.
Objectives
We determined the effects of vitamin B6 adjunctive therapy on linezolid-associated cytopenias, and retrospectively studied 75 septic patients who received at least 7 days of linezolid treatment.
Methods
Patients were divided into a linezolid treatment group (LTG; n = 41) that received linezolid only and a combination treatment group (CTG; n = 34) that received both linezolid and vitamin B6. Each group was further subdivided into those with sepsis and those with severe sepsis. Each patient had red blood cell (RBC), hemoglobin (Hb), hematocrit (Hct), and platelet (PLT) measurements at baseline (day 0) and every other day for 2 weeks during treatment; these parameters were compared between the groups and assessed for time-dependent trends.
Results
For patients in the LTG, RBC, Hb, and Hct values showed statistically significant reductions over time, and these values were lower compared with the values in the CTG. The CTG also showed downward trends, except on the first day of treatment. The PLT count also decreased in both groups. Patients with severe sepsis had lower PLT counts in both treatment groups compared with the septic patients.
Conclusions
Septic patients who received a combination treatment of linezolid and vitamin B6 might show positive effects for linezolid-associated reductions in some hematologic parameters (RBC, Hb, and Hct). This combined treatment might also slow PLT reduction, which was more evident in patients with severe sepsis. ClinicalTrials.gov identifier: NCT01295801.
Key words: linezolid, linezolid-associated cytopenias, sepsis, vitamin B6
Introduction
Sepsis occurs frequently, has a high mortality rate, incurs high treatment costs, and is 1 of the leading causes of death in an intensive care unit (ICU). Thus, it is of great importance to expand the range of treatment options for patients with severe sepsis to achieve higher survival rates. The primary pathogens involved in sepsis infections are gram-positive bacteria. However, for ICU patients, the number of infections due to drug-resistant, gram-positive bacteria is increasing, especially those due to Staphylococcus aureus, coagulase-negative staphylococci and enterococci, and methicillin-resistant Staphylococcus aureus (MRSA)1; these infections pose a pressing problem in the treatment of patients with sepsis.
Vancomycin and linezolid are among the first treatment choices for MRSA infections.2 However, a number of vancomycin-resistant strains of MRSA have been reported.1 The high efficacy of linezolids, a new line of antibacterial drugs that was introduced within the past decade, has made valuable contributions to the treatment of vancomycin-resistant strains of MRSA.3 However, as linezolids have become more widely used, their adverse effects on the circulatory system,4 including anemia and thrombocytopenia,5 have become more prominent, thus limiting their clinical applications. However, Spellberg et al6 reported that combination therapy with vitamin B6 could reverse linezolid-induced thrombocytopenia and prevent these adverse events.
Thus, the purpose of this retrospective study was to further explore how the application of vitamin B6 as an adjuvant treatment affects linezolid-associated cytopenias in septic patients.
Materials and Methods
Patients
Patients for this study were selected from the respiratory care unit and the surgical ICU at the PLA General Hospital in Beijing, China, from January 2010 to March 2011. All of the patients signed an informed consent form. Patients with sepsis were divided into different groups based on either a clear or highly suspected infection focus and at least 2 of the following criteria: (1) body temperature either >38oC or <36oC; (2) heart rate >90 beats/min; (3) either respiratory rate >20 times/min or partial pressure of carbon dioxide in the arteries <32 mm Hg; or (4) peripheral blood white blood cell count >12.0 × 109/L or <4.0 × 109/L or immature neutrophils >10%.7 Severe sepsis was associated with organ dysfunction, hypoperfusion, and hypotension. Patients with severe sepsis exhibited at least 1 of the following: (1) hypoxemia (oxygenation index partial pressure of oxygen in the arteries/fraction of inspired oxygen <300); (2) acute renal injury as evidenced by either oliguria (urine output <0.5 mL/kg/h for ≥2 hours) or a serum creatinine increase of >0.5 mg/dL (44.2 μmol/L); (3) coagulation abnormalities, in which activated partial thromboplastin time was >60 seconds, international normalized ratio was >1.5 seconds, or platelet (PLT) count was <100 × 109/L; (4) hyperbilirubinemia, in which total bilirubin was either >4 mg/dL or 70 mmol/L; (5) metabolic acidosis, in which either pH was <7.3 or lactic acid levels were >2 times higher than normal (lactic acidosis); and (6) infection-associated hypotension, in which systolic blood pressure was <80 mm Hg, mean arterial pressure (MAP) was <70 mm Hg, or systolic blood pressure had a decrease of >40 mm Hg.8
The study exclusion criteria were the following: (1) <18 years of age; (2) AIDS; (3) neutral neutropenia (polymorphonuclear neutrophil count of <500 μL–1); (4) linezolid treatment course of <7 days, and (5) use of chloramphenicol and thiamphenicol for treatment.
Patient Groups and Treatments
All patients received either intravenous or oral linezolid for at least 7 days for a gram-positive bacterial infection. We included 75 patients with sepsis in this study. One group received linezolid only (linezolid treatment group [LTG]; n = 41) and another group received a combined treatment of linezolid and vitamin B6 as an adjuvant therapy (combined treatment group [CTG]; n = 34). We further subdivided the LTG and the CTG into 2 subgroups: sepsis and severe sepsis. For the CTG, there were 18 patients with sepsis (CTG1) and 16 patients with severe sepsis (CTG2). For the LTG, there were 26 patients with sepsis (LTG1) and 15 patients with severe sepsis (LTG2).
For linezolid treatment, we used either linezolid intravenous injections (300 mL [600 mg]/bag, lot number 09K20Z40; Fresenius Kabi Norge AS, Oslo, Norway) or linezolid tablets (600 mg/tablet, batch number C100082; Pfizer Pharmaceuticals LLC, New York, New York). The linezolid injection was 300 mL (600 mg) administered intravenously, and the linezolid tablets were 600 mg, taken orally once every 12 hours. For the vitamin B6 adjuvant treatment, we used an injection of amino acids (1 mL [50 mg]/support, production lot number approved by the state H12020522 products; Tianjin Jingyao Amino Acid Co, Ltd, Tianjin, China). Vitamin B6 treatment was administered at 2 mL (50 mg) by intravenous injection, to a total of 100 mg/d.6,9
Study Measurements
The study was approved by the local ethics committee. For each patient, we recorded age, sex, days of drug compliance, Acute Physiology and Chronic Health Evaluation (APACHE II) scores, instances of a transfusion of blood products during treatment, underlying diseases and mortality, and other general information. We used routine clinical laboratory methods to determine red blood cell (RBCs) counts, hemoglobin (Hb), hematocrit (Hct), and PLT counts. These 4 indicators were determined before medication administration (baseline, day 0) and on days 1, 3, 5, 7, 9, 11, 13, and 15 during the treatment course.
Statistical Methods
Data analysis was performed using SPSS (version 16.0 statistical software; IBM, Armonk, New York). Results for continuous variables are presented as means (SD) and were compared using Student’s t-test. Results for categorical variables are given as the number of cases (percentage) and compared using a χ2 test. We used repeated-measures ANOVA and independent sample t-tests to compare the groups for differences at different time points and by medication given before and during treatment for laboratory test result changes in RBCs, Hb, Hct, and PLT. A P value <0.05 was considered statistically significant.
Results
Patient Demographic Characteristics
Our CTG included 34 patients, and the LTG included 41 patients. As shown in Table I, patients in the CTG took their medication for 15.9 (8.2) days, whereas those in the LTG took their medication for 14.2 (6.2) days; these durations were not significantly different (P > 0.05). There were also no significant differences between these groups in age, sex, APACHE II scores, the number of instances of a transfusion of blood products during treatment, blood filtration conditions, underlying disease conditions, or mortality (P > 0.05).
Table I.
Clinical characteristics of septic patients receiving various treatments.
| Variable | Linezolid + Vitamin B6 (n = 34) | Linezolid (n = 41) | P | |
|---|---|---|---|---|
| Age, y, mean (SD) | 56.8 (21.9) | 61.8 (18.5) | 0.082 | |
| Gender, n (%) | 0.395 | |||
| Male | 20 (68.8) | 28 (68.3) | ||
| Female | 14 (41.2) | 13 (31.7) | ||
| Duration of linezolid, d, mean (SD) | 15.9 (8.2) | 14.2 (6.2) | 0.379 | |
| APACHE II, mean (SD) | 15.7 (5.8) | 19.2 (7.4) | 0.194 | |
| Blood product transfusion, n (%) | 22 (64.7) | 24 (58.5) | 0.585 | |
| Hemofiltration, n (%) | 6 (17.6) | 5 (12.2) | 0.506 | |
| Underlying disease | ||||
| Hypertension , n (%) | 7 (20.6) | 11 (26.8) | 0.529 | |
| COPD, n (%) | 4 (11.8) | 6 (14.6) | 0.716 | |
| Chronic renal disease, n (%) | 8 (23.5) | 11 (26.8) | 0.744 | |
| Diabetes, n (%) | 3 (8.8) | 8 (19.5) | 0.193 | |
| Immune suppression, n (%) | 4 (11.8) | 4 (9.8) | 0.779 | |
| Nervous system disease, n (%) | 5 (14.7) | 5 (12.2) | 0.698 | |
| Coronary heart disease, n (%) | 11 (32.4) | 14 (34.1) | 0.870 | |
| No underlying disease | 10 (29.4) | 7 (17.1) | 0.209 | |
| Death, n (%) | 14 (41.2) | 13 (31.7) | 0.312 | |
APACHE, Acute Physiology and Chronic Health Evaluation; COPD, chronic obstructive pulmonary disease.
Trends in Hematology Results during the Treatment of Septic Patients
Red Blood Cell Counts
Figure 1A shows the trends in RBCs for septic patients in the LTG and the CTG at different times during their treatments (Table II). Compared with baseline (day 0) values, the RBC levels for septic patients in the LTG showed a significant reduction with time starting on day 3. By comparison, patients in the CTG did not exhibit any obvious downward trend in RBCs over time, except for the first day of treatment, on which their RBC levels were slightly lower than those before treatment (P < 0.05). Starting on day 9, the RBC results of the CTG and the LTG were significantly different (P < 0.05). This illustrated that patients treated with linezolid alone had a more significant downward trend in RBC levels compared with patients receiving linezolid treatment in combination with vitamin B6.
Figure 1.
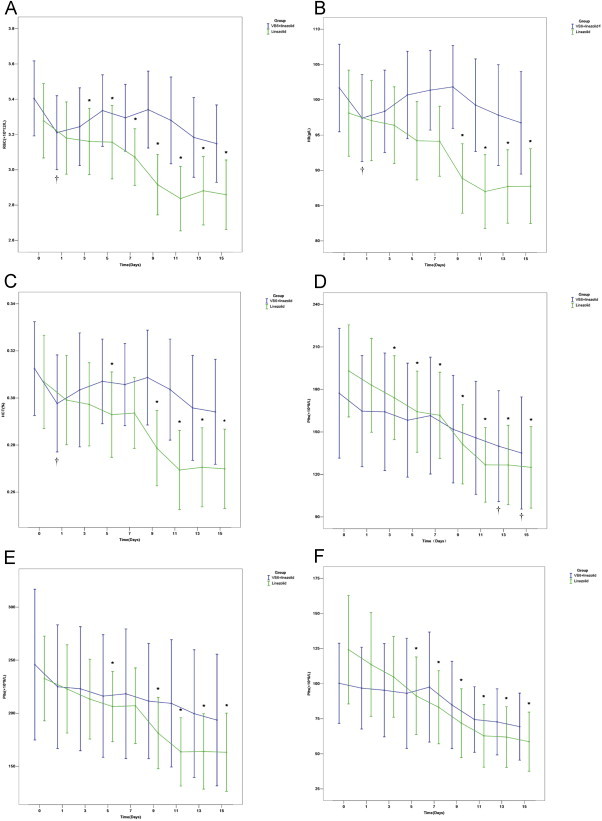
Trends in laboratory parameters with treatment time. (A) Red blood cell (RBC), (B) hemoglobin (Hb), (C) hematocrit (Hct), (D) platelet (PLT), (E) PLTs for septic patients, and (F) PLTs for severe septic patients. ⁎P < 0.05 compared with the linezolid treatment group before treatment. †P < 0.05 compared with the combined treatment group before treatment. VB6, vitamin B6.
Table II.
Repeated measures of the ANOVA results for red blood cell counts (RBCs), hemoglobin (Hb), hematocrit (Hct), and platelet counts (PLTs).
| Time Points of Observation, d | RBCs |
P | Hb |
P | Hct |
P | PLTs |
P | PLTs in Nonsepsis Patients |
P | PLTs in Severe Sepsis Patients |
P | ||||||
| LTG | CTG | LTG | CTG | LTG | CTG | LTG | CTG | LTG | CTG | LTG | CTG | |||||||
| 0⁎ | 3.29 (0.71) | 3.34 (0.59) | 0.751 | 98.10 (19.31) | 101.68 (17.77) | 0.410 | 0.31 (0.06) | 0.31 (0.06) | 0.505 | 193.05 (102.88) | 177.32 (131.27) | 0.563 | 232.77 (98.60) | 245.83 (142.67) | 0.721 | 124.20 (69.80) | 100.25 (53.58) | 0.291 |
| 1st | 3.18 (0.67) | 3.18 (0.58) | 0.995 | 97.05 (18.06) | 97.41 (17.68) | 0.930 | 0.30 (0.06) | 0.30 (0.06) | 0.831 | 183.78 (107.01) | 155.82 (112.63) | 0.470 | 222.92 (102.79) | 225.00 (117.22) | 0.951 | 113.60 (66.65) | 96.81 (54.60) | 0.448 |
| 3rd | 3.16 (0.63) | 3.18 (0.54) | 0.841 | 96.39 (17.24) | 98.35 (16.14) | 0.620 | 0.30 (0.06) | 0.30 (0.07) | 0.356 | 174.17 (93.57) | 164.21 (118.82) | 0.686 | 214.19 (89.13) | 225.44 (126.66) | 0.728 | 104.80 (51.97) | 95.31 (62.33) | 0.650 |
| 5th | 3.09 (0.69) | 3.29 (0.50) | 0.168 | 94.20 (17.61) | 100.68 (17.76) | 0.118 | 0.29 (0.06) | 0.31 (0.05) | 0.117 | 164.29 (90.65) | 158.29 (115.26) | 0.802 | 206.39 (81.96) | 216.22 (116.00) | 0.743 | 91.33 (49.90) | 93.13 (73.63) | 0.938 |
| 7th | 3.10 (0.62) | 3.27 (0.50) | 0.163 | 94.12 (15.69) | 101.35 (16.18) | 0.054 | 0.29 (0.05) | 0.31 (0.05) | 0.130 | 161.76 (96.32) | 161.53 (118.26) | 0.993 | 207.12 (88.08) | 218.39 (122.73) | 0.724 | 83.13 (47.00) | 97.56 (73.51) | 0.523 |
| 9th | 2.95 (0.58) | 3.31 (0.56) | 0.008 | 88.85 (15.58) | 101.82 (16.82) | 0.001 | 0.28 (0.05) | 0.31 (0.06) | 0.006 | 141.27 (88.63) | 151.88 (108.61) | 0.643 | 181.31 (83.16) | 211.50 (109.14) | 0.304 | 71.87 (44.24) | 84.81 (58.21) | 0.493 |
| 11th | 2.88 (0.63) | 3.26 (0.66) | 0.015 | 87.00 (15.58) | 99.24 (18.76) | 0.004 | 0.27 (0.05) | 0.30 (0.06) | 0.004 | 126.68 (83.35) | 145.85 (114.17) | 0.406 | 163.65 (79.60) | 209.33 (120.56) | 0.137 | 62.80 (40.30) | 74.44 (43.90) | 0.449 |
| 13th | 2.88 (0.63) | 3.18 (0.65) | 0.042 | 87.71 (16.48) | 97.82 (20.47) | 0.020 | 0.27 (0.05) | 0.30 (0.06) | 0.037 | 126.68 (88.60) | 139.94 (112.03) | 0.569 | 164.04 (87.95) | 199.67 (120.90) | 0.264 | 61.93 (39.10) | 72.75 (44.16) | 0.477 |
| 15th | 2.86 (0.62) | 3.15 (0.63) | 0.050 | 87.76 (16.81) | 96.74 (20.81) | 0.042 | 0.27 (0.05) | 0.29 (0.06) | 0.046 | 125.00 (91.23) | 135.15 (113.48) | 0.669 | 163.27 (91.33) | 193.61 (124.65) | 0.356 | 58.67 (38.06) | 69.38 (44.79) | 0.480 |
P values represent the statistical differences between two groups at various time points of observation.
CTG, combined treatment group; LTG, linezolid treatment group.
0 indicates the initial day before treatment.
Hemoglobin
Figure 1B shows that treatment with linezolid alone (LTG) resulted in a significant decrease in Hb levels over time compared with the Hb levels before treatment (Table II). This slow decline became apparent on day 5. For patients in the CTG, a reduction in Hb levels was not obvious, except that the Hb levels on the first day of treatment compared with those before treatment were lower (P = 0.018). The Hb levels of the CTG and the LTG differed significantly from the ninth day onward, with lower Hb levels in the LTG.
Hematocrit
The trends for the Hct are shown in Figure 1C (Table II). Figure 1C shows that the Hct of the LTG appeared to decrease on the fifth day compared with patients in the CTG, and on the ninth day, Hct was significantly lower. However, in the CTG, a decline in Hct was not obvious, with the exception that Hct on the first day of treatment was significantly decreased compared with baseline (day 0). With regard to the Hct, patients in the LTG had a significant downward trend compared with those in the CTG.
Platelet Counts
As shown in Figure 1D (Table II), septic patients in both the LTG and CTG showed downward trends in their PLT counts during treatment, although this time-dependent downward trend was only statistically significant in the LTG. However, there were no significant differences between these 2 groups at the different observation times (P < 0.05).
Platelet Counts in Patients with Nonsevere Sepsis and Severe Sepsis
To further evaluate the declines in the PLT during treatment, each patient group was further divided into subgroups of nonsevere sepsis and severe sepsis. The time-dependent trends for nonsevere sepsis patients are shown in Figure 1E (Table II) and for severe sepsis patients in Figure 1F (Table II). As shown in Figure 1E for the 2 sepsis groups, there were no significant differences in the PLT count between these groups (P > 0.05). In the CTG, the PLT values did not statistically differ from baseline. For patients with nonsevere sepsis in the LTG, during the course of treatment, between 3 and 7 days, there was no significant difference compared with samples taken before treatment. However, starting on day 9, there was a significant downward trend in the PLT count.
As shown in Figure 1F for the 2 severe sepsis subgroups (LTG2 and CTG2), there were no statistically significant differences at any of the observation points. In the CTG, although there appeared to be a downward trend in PLTs, this was not statistically significant. For severely septic patients in the LTG, statistically significant differences from baseline were observed from the fifth day onward during the course of treatment. It should be noted that, overall, the PLT values were lower for severely septic patients (Figure 1F) compared with patients with nonsevere sepsis (Figure 1E) regardless of the treatment regimen.
Discussion
Sepsis is a condition caused by systemic inflammation and is 1 of the leading causes of death in the ICU. Although in-depth studies have been conducted on both the pathogenic mechanisms of sepsis and its pathophysiology in recent years and more treatments have been discovered, the high morbidity and mortality associated with infection remain difficult to counteract.10 In an epidemiological survey of surgical ICUs in 10 teaching hospitals located in 6 Chinese provinces, 53.8% of patients were treated for gram-positive infections, 45.9% of patients were treated for gram-negative bacterial infections, and 22% of patients were treated for invasive fungal infections.11 Gram-positive strains were the major pathogenic strains associated with sepsis.
Linezolid was the first oxazolidinone antibiotic to be approved for treating gram-positive bacterial infections.3 It not only exhibits excellent therapeutic effects against multiple resistant gram-positive bacteria such as vancomycin-resistant Enterococcus faecalis, MRSA, and multidrug-resistant strains,12 but it also has antibacterial activities against anaerobic bacteria and atypical mycobacteria.13,14 However, as the use of linezolid has become more widespread in clinical practice, several adverse outcomes have been observed in patients who received long-term linezolid treatments.
After using the drug for >2 weeks, adverse outcomes primarily affecting the hematopoietic system were reported, including anemia and thrombocytopenia.4 In a study of disseminated mycobacterial infections in which patients received both linezolid and vitamin B6, Spellberg et al5 observed that vitamin B6 might reduce or even prevent linezolid-related thrombocytopenia. Subsequently, in a retrospective study of 24 patients with bone marrow infections who received both linezolid and vitamin B6, the opposite conclusion was found, in that adjuvant treatment with vitamin B6 for sepsis in combination with linezolid did not reduce linezolid-related hematologic adverse outcomes.9 In a study by Youssef et al,15 who used linezolid and vitamin B6 as an adjuvant therapy for 31 cancer patients complicated by infections, vitamin B6 reduced secondary anemia, but it did not prevent the reduction in PLT and leukocyte counts.
Our retrospective clinical study found that patients who were treated for sepsis with linezolid had reductions in RBC counts, Hb, Hct, and PLT counts. On the first day of observation for the linezolid and vitamin B6 treatment group, the RBC, Hb, and Hct levels were significantly lower than baseline, but throughout the remainder of the treatment course used in this study the downward trend observed just after treatment began did not significantly change. Septic patients who received vitamin B6 combined with linezolid also showed similar effects for PLT counts, although the downward trend in PLTs was only statistically significant after the first 13 days of treatment. Thus, it could be inferred that vitamin B6 used in adjuvant therapy could prevent linezolid-related reductions in blood cells to some extent, and although it was highly effective for red cells, its effects on thrombocytopenia might not be as obvious.
Hb is comprised of heme and globin, and vitamin B6 is essential for hemoglobin synthesis. It participates in the synthesis of a heme precursor, -δ-amino-γ-keto acid, and is a δ-amino-γ-keto acid synthesis enzyme cofactor.16 Sideroblastic anemia is defined as a vitamin B6 genetic factor heme synthesis pathway disorder. This Hb gene mutation may occur in cell anemia, small cell anemia, and megaloblastic anemia; thus, vitamin B6 treatment is useful for such patients.17 Additionally, linezolid inhibits mitochondrial protein synthesis and blocks mitochondrial respiration, which may cause sideroblastic anemia, because RBCs produce an important factor to counteract this effect.18
In our CTG, the RBCs, Hb, and Hct were only significantly lower on the first day after starting treatment. Concurrently, the LTG exhibited significant reductions in the RBC counts over time, indicating that linezolid might cause a temporary RBC gene mutation and temporarily inhibit mitochondrial respiration, which would result in this type of anemia in these patients. Vitamin B6 could promote the synthesis of hemoglobin, which could restore the RBCs in the later periods of treatment and maintain the levels observed before treatment. It could be inferred that linezolids might play a critical role by affecting RBCs and reducing the RBC pigment metabolic pathway.
One report suggested that linezolid treatment-induced thrombocytopenia might be immune-mediated,19 which was similar to the mechanism associated with quinine and/or quinidine-like immune-mediated thrombocytopenia. This immune-related thrombocytopenia is due to either a drug or its metabolites binding to either the PLT membrane glycoprotein 1b/IX or glycoprotein IIb/IIIa, which produces an immunogenic compound of immunoglobulin-G and a Fab fragment. Immunoglobulin-G and an Fc fragment can combine with macrophages and be cleared by the reticuloendothelial system, resulting in thrombocytopenia.20,21
In this study, patients treated with both linezolid and vitamin B6 did show changes in PLTs during the first 9 days of medication use, but these changes in PLTs were not statistically significant until 13 days after initiation of treatment compared with the samples taken before treatment began. In the sepsis subgroups CTG1 and LTG1, there was a downward trend despite the fact that the CTG and 2 patients in the LTG did not show significant differences at each observation point. In particular, the decline observed in the CTG was obviously slower. In the severe sepsis subgroups CTG2 and LTG2, the downward trend in the group treated with linezolid alone was clearly more evident, although the PLT counts were not significantly different at each observation point. We speculated that vitamin B6 given to septic patients with linezolid-related thrombocytopenia might slow the loss of PLTs, but this was not conclusive.
It was reported that linezolid blood levels might affect hematopoietic function and that clearance of linezolid was closely related to Hb levels.22 We speculated that this latter mechanism involved alterations in PLT dynamics by vitamin B6. This might due to the maintenance of Hb levels, which could increase the clearance of linezolid and reduce its accumulation in the body, thus reducing the chance of immune complex formation when linezolid binds to either the membrane glycoprotein 1b/IX or glycoprotein IIb/IIIa. Thus, there would not be a significant reduction in PLT counts. However, with an extended treatment time, there was a clear reduction in the PLT count, which suggested that there was no significant effect on the ability of vitamin B6 to reduce PLT counts.
This retrospective study had some limitations. First, this was a single-center retrospective study; thus, the experimental controls were not ideal. Second, because the overall sample size was small, the power of the statistical tests might have been reduced, so the probability of significant reductions might have been low. Third, the patients only received medication for approximately 14 days, which might have caused some limitations with regard to medication use duration. Finally, this study used both oral and intravenous administration; the route of administration for different drugs could affect the occurrence of adverse reactions because these depend on bioavailability and pharmacokinetic parameters.23 We could speculate that the bioavailability and pharmacokinetic parameters of intravenously infused linezolid might be different from those of orally administered linezolid. In this retrospective study, we did not distinguish between intravenous and oral administration. Thus, we could not rule out that a different route of linezolid administration might reduce the observed effects on the measured blood cell parameters.
In summary, patients who received linezolid for extended periods could develop linezolid-related cytopenias. Thus, before and during treatment, we should closely monitor changes in hematologic parameters. Care should be taken during symptomatic treatment to stop administration of the medicine when reductions in blood cell counts are noted. In this preliminary retrospective study, we found that administering linezolid, along with adjuvant treatment with vitamin B6, to patients with sepsis either prevented or delayed the reductions in the RBC counts to a certain extent, whereas no improvement was observed in the development of thrombocytopenia. Vitamin B6 is a low-cost supplement with few side effects. Thus, its use in conjunction with linezolid could be regarded as an effective method to reduce blood cell counts. These observations will require either additional studies with larger sample sizes or a prospective study to confirm the effects noted here. Furthermore, studies must also be conducted regarding the mechanisms involved with vitamin B6 to prevent and treat linezolid-related pancytopenia.
Conflicts of Interest
The authors have indicated that they have no conflicts of interest regarding the content of this article.
Acknowledgments
Financial support for this study was provided by the National Science and Technology Support Program (Item number 2009BAI86B03). This study was approved by the Committee on Ethics of the Chinese PLA General Hospital (No.20091027-001) and by the ClinicalTrials.gov identifier: NCT01295801. Drs. Deng and Su acquired the data, managed the data analysis, and drafted the manuscript. Dr. Z.-x. Liang, Ms. L.-l. Liang, Mr. Yan, Ms. Jia, and Dr. Zhang contributed to the data collection. Dr. Feng assisted in the data analysis and the use of medical statistics. Dr. Xie designed the study and was responsible for protocol revisions, data analysis, and final draft revision. All authors have read and approved the final manuscript.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Fraimow H.S., Tsigrelis C. Antimicrobial resistance in the intensive care unit: mechanisms, epidemiology, and management of specific resistant pathogens. Crit Care Clin. 2011;27:163–205. doi: 10.1016/j.ccc.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Lowy F.D. Staphylococcal infections. In: Fauci A.S., Braunwald E., Kasper D.L., editors. Harrison's Principles of Internal Medicine. 17th ed. McGraw-Hill; New York: 2008. pp. 872–881. [Google Scholar]
- 3.Moellering R.C. Linezolid: the first oxazolidinone antimicrobial. Ann Internal Med. 2003;138:135–142. doi: 10.7326/0003-4819-138-2-200301210-00015. [DOI] [PubMed] [Google Scholar]
- 4.Birmingham M.C., Rayner C.R., Meagher A.K. Linezolid for the treatment of multidrug-resistant, gram-positive infection: experience from a compassionate-use program. Clin Infect Dis. 2003;36:159–168. doi: 10.1086/345744. [DOI] [PubMed] [Google Scholar]
- 5.Senneville E., Legout L., Valette M. Risk factors for anaemia in patients on prolonged linezolid therapy for chronic osteomyelitis: a case-control study. J Antimicrob Chemother. 2004;54:798–802. doi: 10.1093/jac/dkh409. [DOI] [PubMed] [Google Scholar]
- 6.Spellberg B., Yoo T., Bayer A.S. Reversal of linezolid-associated cytopenias, but not peripheral neuropathy, by administration of vitamin B6. J Antimicrob Chemother. 2004;54:832–835. doi: 10.1093/jac/dkh405. [DOI] [PubMed] [Google Scholar]
- 7.Bone R.C., Balk R.A., Cerra F.B. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 8.Levy M.M., Fink M.P., Marshall J.C. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 9.Plachouras D., Giannitsioti E., Athanassia S. Effect of pyridoxine on the incidence of myelosuppression during prolonged linezolid treatment. Clin Infect Dis. 2006;43:e89–e91. doi: 10.1086/508280. [DOI] [PubMed] [Google Scholar]
- 10.Andreu Ballester J.C., Ballester F., González Sánchez A. Epidemiology of sepsis in the Valencian Community (Spain), 1995-2004. Infect Control Hosp Epidemiol. 2008;29:630–634. doi: 10.1086/589583. [DOI] [PubMed] [Google Scholar]
- 11.Cheng B., Xie G., Yao S. Epidemiology of severe sepsis in critically ill surgical patients in ten university hospitals in China. J Crit Care Med. 2007;35:2538–2546. doi: 10.1097/01.CCM.0000284492.30800.00. [DOI] [PubMed] [Google Scholar]
- 12.Yanagihara K., Kaneko Y., Sawai T. Efficacy of linezolid against methicillin-resistant or vancomycin-insensitive Staphylococcus aureus in a model of hematogenous pulmonary infection. Antimicrob Agents Chemother. 2002;46:3288–3291. doi: 10.1128/AAC.46.10.3288-3291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown-Elliott B.A., Crist C.J., Mann L.B. In vitro activity of linezolid against slowly growing nontuberculous mycobacteria. Antimicrob Agents Chemother. 2003;47:1726–1738. doi: 10.1128/AAC.47.5.1736-1738.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Behra-Miellet J., Calvet L., Dubreuil L. Activity of linezolid against anaerobic bacteria. Int J Antimicrob Agents. 2003;22:28–34. doi: 10.1016/s0924-8579(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 15.Youssf S., Hachem R., Chemaly R.F. The role of vitamin B6 in the prevention of haematological toxic effects of linezolid in patients with cancer. J Antimicrob Chemother. 2008;61:421–424. doi: 10.1093/jac/dkm506. [DOI] [PubMed] [Google Scholar]
- 16.Young L.S. Hematologic effects of linezolid versus vancomycin. Clin Infect Dis. 2004;38:1065–1066. doi: 10.1086/382364. [DOI] [PubMed] [Google Scholar]
- 17.Harris J.W. X-linked, pyridoxine-responsive sideroblanstic anemia. N Engl J Med. 1994;330:709–711. doi: 10.1056/NEJM199403103301011. [DOI] [PubMed] [Google Scholar]
- 18.Dawson M.A., Davis A., Elliott P., Cole-Sinclair M. Linezolid-induced dyserythropoiesis: chloramphenicol toxicity revisited. Intern Med J. 2005;35:626–628. doi: 10.1111/j.1445-5994.2005.00912.x. [DOI] [PubMed] [Google Scholar]
- 19.Berndtein W.B., Trotta R.F., Rector J.T. Mechanisms for linezolid-induced anemia and thrombocytopenia. Ann Pharmacother. 2003;37:517–520. doi: 10.1345/aph.1C361. [DOI] [PubMed] [Google Scholar]
- 20.George J.N., Aster R.H. Drug-induced thrombocytopenia: pathogenesis, evaluation, and management. Hematology Am Soc Hematol Educ Program. 2009:153–158. doi: 10.1182/asheducation-2009.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chong B.H., Du X.P., Berndt M.C. Characterization of the binding domains on platelet glycoproteins Ib-IX and IIb/IIIa complexes for the quinine/quinindine-dependent antibodies. 1991;l77:2190–2199. [PubMed] [Google Scholar]
- 22.Hiraki Y., Tsuji Y., Matsumoto K. Influence of linezolid clearance on the induction of thrombocytopenia and reduction of hemoglobin. Am J Med Sci. 2011;342:456–460. doi: 10.1097/MAJ.0b013e318218cf18. [DOI] [PubMed] [Google Scholar]
- 23.Stalker D.J., Jungbluth G.L., Hopkins N.K., Batts D.H. Pharmacokinetics and tolerance of single- and multiple-dose oral or intravenous linezolid, an oxazolidinone antibiotic, in healthy volunteers. J Antimicrob Chemother. 2003;51:1239–1246. doi: 10.1093/jac/dkg180. [DOI] [PubMed] [Google Scholar]


