Abstract
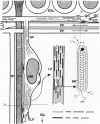
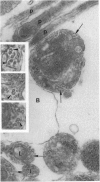
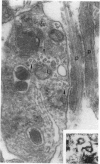
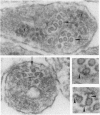
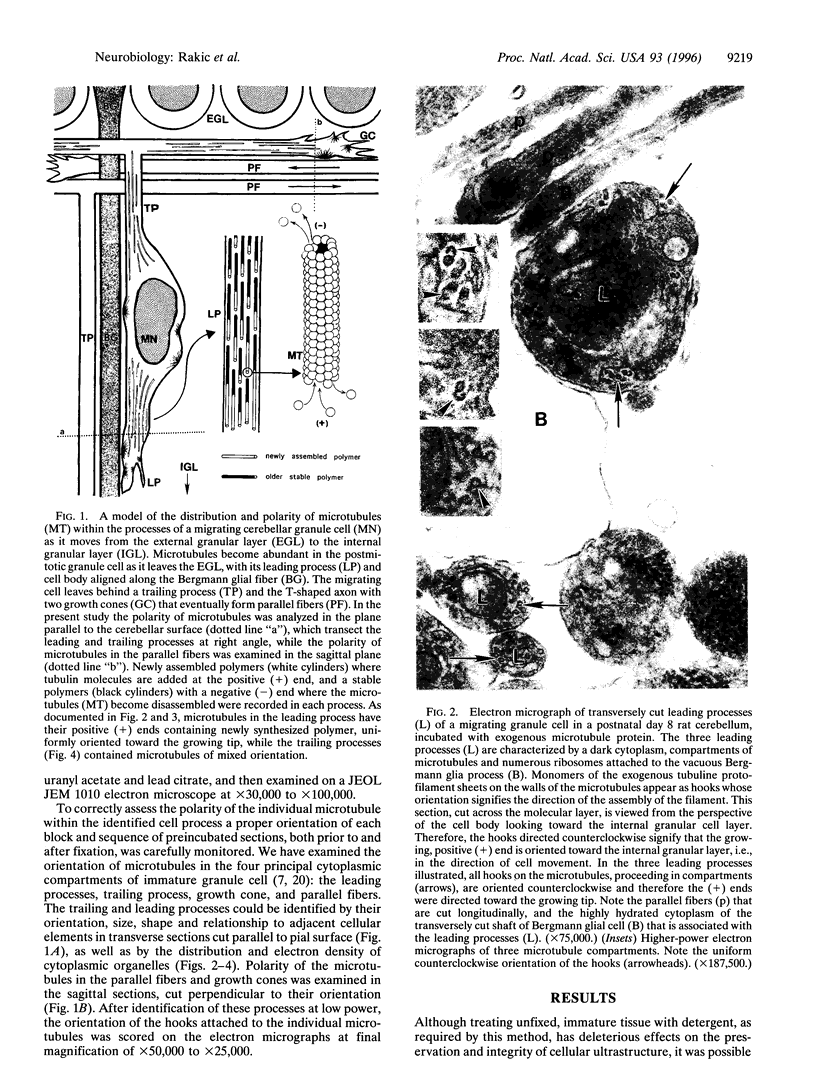
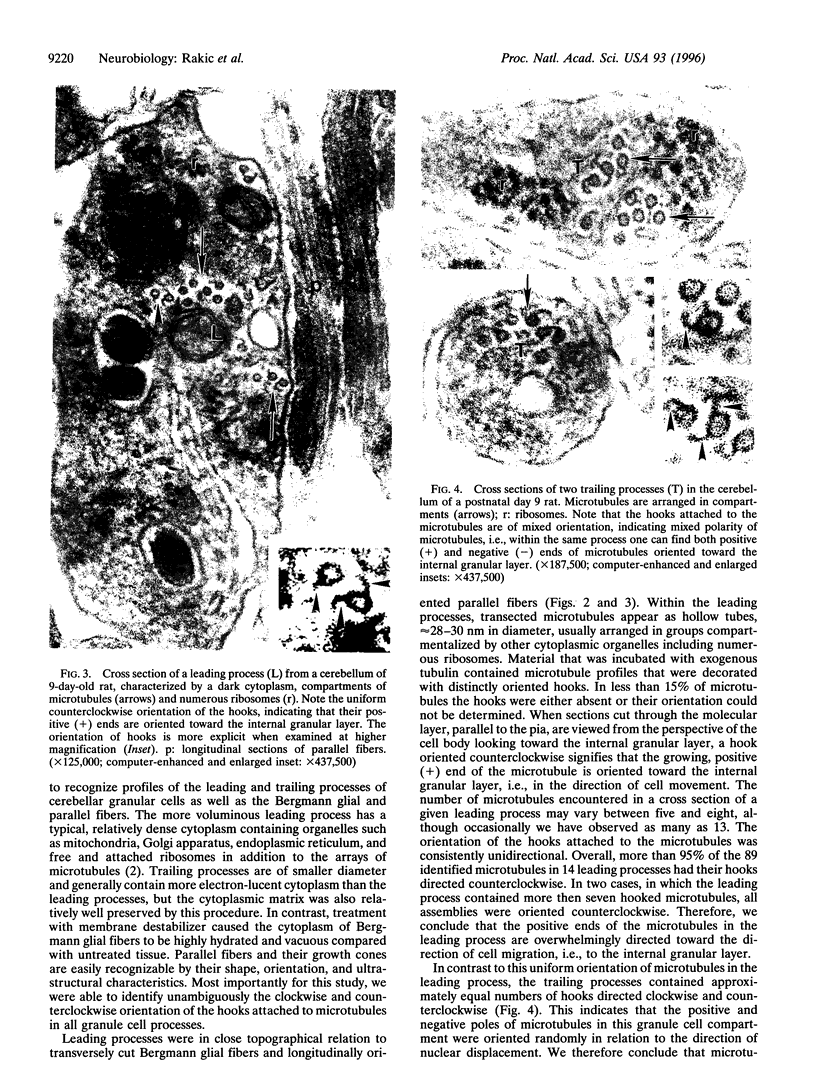
The active migration of neurons from their sites of origin to their final destinations requires the unidirectional translocation of the nuclei and somatic cytoplasm within the growing leading processes. To explore the cellular machinery underlying this translocation, we determined the polarity of microtubules situated within the leading and trailing processes of migrating cerebellar granule cells in situ. Our analysis reveals that the newly assembled positive ends of the microtubules in the leading process uniformly face the growing tip, while their disintegrating negative ends face the nucleus. In the trailing process, by contrast, microtubule arrays are of mixed polarity. We suggest that the dynamics of slow polymerization in combination with fast disintegration of oriented microtubules create "push" and "pull" forces that contribute to the piston-like saltatory displacement of the nucleus and cytoplasm within the membrane cylinder of the leading process of the migrating neuron.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad F. J., Pienkowski T. P., Baas P. W. Regional differences in microtubule dynamics in the axon. J Neurosci. 1993 Feb;13(2):856–866. doi: 10.1523/JNEUROSCI.13-02-00856.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amos L. A., Baker T. S. The three-dimensional structure of tubulin protofilaments. Nature. 1979 Jun 14;279(5714):607–612. doi: 10.1038/279607a0. [DOI] [PubMed] [Google Scholar]
- Anton E. S., Cameron R. S., Rakic P. Role of neuron-glial junctional domain proteins in the maintenance and termination of neuronal migration across the embryonic cerebral wall. J Neurosci. 1996 Apr 1;16(7):2283–2293. doi: 10.1523/JNEUROSCI.16-07-02283.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Deitch J. S., Black M. M., Banker G. A. Polarity orientation of microtubules in hippocampal neurons: uniformity in the axon and nonuniformity in the dendrite. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8335–8339. doi: 10.1073/pnas.85.21.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Slaughter T., Brown A., Black M. M. Microtubule dynamics in axons and dendrites. J Neurosci Res. 1991 Sep;30(1):134–153. doi: 10.1002/jnr.490300115. [DOI] [PubMed] [Google Scholar]
- Barth P. G. Disorders of neuronal migration. Can J Neurol Sci. 1987 Feb;14(1):1–16. doi: 10.1017/s031716710002610x. [DOI] [PubMed] [Google Scholar]
- Black M. M., Baas P. W. The basis of polarity in neurons. Trends Neurosci. 1989 Jun;12(6):211–214. doi: 10.1016/0166-2236(89)90124-0. [DOI] [PubMed] [Google Scholar]
- Brown A., Slaughter T., Black M. M. Newly assembled microtubules are concentrated in the proximal and distal regions of growing axons. J Cell Biol. 1992 Nov;119(4):867–882. doi: 10.1083/jcb.119.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron R. S., Rakic P. Identification of membrane proteins that comprise the plasmalemmal junction between migrating neurons and radial glial cells. J Neurosci. 1994 May;14(5 Pt 2):3139–3155. doi: 10.1523/JNEUROSCI.14-05-03139.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L., Pryer N. K., Salmon E. D. Real-time observations of microtubule dynamic instability in living cells. J Cell Biol. 1988 Dec;107(6 Pt 1):2223–2231. doi: 10.1083/jcb.107.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P., Walsh F. S. Cell adhesion molecules, second messengers and axonal growth. Curr Opin Neurobiol. 1992 Oct;2(5):595–601. doi: 10.1016/0959-4388(92)90024-f. [DOI] [PubMed] [Google Scholar]
- Garcia-Segura L. M., Rakic P. Differential distribution of intermembranous particles in the plasmalemma of the migrating cerebellar granule cells. Brain Res. 1985 Nov;355(1):145–149. doi: 10.1016/0165-3806(85)90014-8. [DOI] [PubMed] [Google Scholar]
- Gilbert S. P., Webb M. R., Brune M., Johnson K. A. Pathway of processive ATP hydrolysis by kinesin. Nature. 1995 Feb 23;373(6516):671–676. doi: 10.1038/373671a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten M. E., Mason C. A. Mechanisms of glial-guided neuronal migration in vitro and in vivo. Experientia. 1990 Sep 15;46(9):907–916. doi: 10.1007/BF01939383. [DOI] [PubMed] [Google Scholar]
- Heidemann S. R. Microtubule polarity determination based on formation of protofilament hooks. Methods Enzymol. 1991;196:469–477. doi: 10.1016/0076-6879(91)96040-x. [DOI] [PubMed] [Google Scholar]
- Hirose K., Fan J., Amos L. A. Re-examination of the polarity of microtubules and sheets decorated with kinesin motor domain. J Mol Biol. 1995 Aug 18;251(3):329–333. doi: 10.1006/jmbi.1995.0437. [DOI] [PubMed] [Google Scholar]
- Hoenger A., Sablin E. P., Vale R. D., Fletterick R. J., Milligan R. A. Three-dimensional structure of a tubulin-motor-protein complex. Nature. 1995 Jul 20;376(6537):271–274. doi: 10.1038/376271a0. [DOI] [PubMed] [Google Scholar]
- Jefferson A. B., Schulman H. Phosphorylation of microtubule-associated protein-2 in GH3 cells. Regulation by cAMP and by calcium. J Biol Chem. 1991 Jan 5;266(1):346–354. [PubMed] [Google Scholar]
- Kidd G. J., Andrews S. B., Trapp B. D. Organization of microtubules in myelinating Schwann cells. J Neurocytol. 1994 Dec;23(12):801–810. doi: 10.1007/BF01268092. [DOI] [PubMed] [Google Scholar]
- Komuro H., Rakic P. Dynamics of granule cell migration: a confocal microscopic study in acute cerebellar slice preparations. J Neurosci. 1995 Feb;15(2):1110–1120. doi: 10.1523/JNEUROSCI.15-02-01110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro H., Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993 Apr 2;260(5104):95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- Komuro H., Rakic P. Selective role of N-type calcium channels in neuronal migration. Science. 1992 Aug 7;257(5071):806–809. doi: 10.1126/science.1323145. [DOI] [PubMed] [Google Scholar]
- Lafont F., Burkhardt J. K., Simons K. Involvement of microtubule motors in basolateral and apical transport in kidney cells. Nature. 1994 Dec 22;372(6508):801–803. doi: 10.1038/372801a0. [DOI] [PubMed] [Google Scholar]
- McIntosh J. R., Euteneuer U. Tubulin hooks as probes for microtubule polarity: an analysis of the method and an evaluation of data on microtubule polarity in the mitotic spindle. J Cell Biol. 1984 Feb;98(2):525–533. doi: 10.1083/jcb.98.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T., Evans L., Schulze E., Kirschner M. Sites of microtubule assembly and disassembly in the mitotic spindle. Cell. 1986 May 23;45(4):515–527. doi: 10.1016/0092-8674(86)90283-7. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Rakic P., Cameron R. S., Komuro H. Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr Opin Neurobiol. 1994 Feb;4(1):63–69. doi: 10.1016/0959-4388(94)90033-7. [DOI] [PubMed] [Google Scholar]
- Rakic P. Defects of neuronal migration and the pathogenesis of cortical malformations. Prog Brain Res. 1988;73:15–37. doi: 10.1016/s0079-6123(08)60494-x. [DOI] [PubMed] [Google Scholar]
- Rakic P., Komuro H. The role of receptor/channel activity in neuronal cell migration. J Neurobiol. 1995 Mar;26(3):299–315. doi: 10.1002/neu.480260303. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol. 1971 Mar;141(3):283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- Reiner O., Albrecht U., Gordon M., Chianese K. A., Wong C., Gal-Gerber O., Sapir T., Siracusa L. D., Buchberg A. M., Caskey C. T. Lissencephaly gene (LIS1) expression in the CNS suggests a role in neuronal migration. J Neurosci. 1995 May;15(5 Pt 2):3730–3738. doi: 10.1523/JNEUROSCI.15-05-03730.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinsch S. S., Mitchison T. J., Kirschner M. Microtubule polymer assembly and transport during axonal elongation. J Cell Biol. 1991 Oct;115(2):365–379. doi: 10.1083/jcb.115.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas R. J., Hatten M. E. Motility and cytoskeletal organization of migrating cerebellar granule neurons. J Neurosci. 1995 Feb;15(2):981–989. doi: 10.1523/JNEUROSCI.15-02-00981.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorke L. B. A perspective: the role of disordered genetic control of neurogenesis in the pathogenesis of migration disorders. J Neuropathol Exp Neurol. 1994 Mar;53(2):105–117. doi: 10.1097/00005072-199403000-00001. [DOI] [PubMed] [Google Scholar]
- Sablin E. P., Fletterick R. J. Crystallization and preliminary structural studies of the ncd motor domain. Proteins. 1995 Jan;21(1):68–69. doi: 10.1002/prot.340210108. [DOI] [PubMed] [Google Scholar]
- Saxton W. M., Stemple D. L., Leslie R. J., Salmon E. D., Zavortink M., McIntosh J. R. Tubulin dynamics in cultured mammalian cells. J Cell Biol. 1984 Dec;99(6):2175–2186. doi: 10.1083/jcb.99.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y. H., Mandelkow E. The anatomy of flagellar microtubules: polarity, seam, junctions, and lattice. J Cell Biol. 1995 Jan;128(1-2):81–94. doi: 10.1083/jcb.128.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp K. S., Meade L. B., LaVail J. H. Microtubule polarity in the peripheral processes of trigeminal ganglion cells: relevance for the retrograde transport of herpes simplex virus. J Neurosci. 1994 Jan;14(1):318–325. doi: 10.1523/JNEUROSCI.14-01-00318.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]