Highlights
-
•
Rare case of PSTT limited to the vagina presenting eight years after last pregnancy and four years after hysterectomy
-
•
Differential diagnosis with other vaginal tumors can be challenging but it is critical because behavior and management are different.
-
•
Stage-adapted management is proposed and surgery is the mainstay treatment for localized disease.
Keywords: PSTT, Vaginal mass, Recurrence, Immunohistochemistry, Hysterectomy
Introduction
Gestational trophoblastic neoplasia (GTN) comprises a spectrum of malignant tumors derived from the trophoblast including the invasive mole (IM), the choriocarcinoma (CH), the placental site trophoblastic tumor (PSTT) and the epithelioid trophoblastic tumor (ETT).
PSTT is a rare entity with less than 300 cases reported and accounts for 1–2% of GTN. Kurman et al. (1976) first described this pathology in 1976 and named it trophoblastic pseudotumor. The malignant potential of PSTT was recognized later, leading to the current nomenclature. It is derived from the intermediate cytotrophoblast.
PSTT most often presents with amenorrhea or abnormal bleeding together with uterine enlargement. However, a wide range of other presenting symptoms has been reported such as abdominal pain, uterine rupture and galactorrhea (Gillespie and Hancock, 2009). This slow-growing tumor tends to stay initially in the uterus but more than 30% of patients have metastatic disease at the time of presentation (Schmid et al., 2009). The sites of metastases include the peritoneum, the lung, the liver and the brain. PSTT may follow any gestational event, normal or pathological pregnancy. In a series of 55 cases (Baergen et al., 2006), the diagnosis was made within an interval of 34 months following a previous gestation, mainly affecting women of childbearing age.
The prognosis of PSTT depends on the disease's extent (Schmid et al., 2009; Baergen et al., 2006; Feltmate et al., 2001). The outcome of patients treated for early-stage diseases is usually favorable. Hysterectomy is the preferred treatment for women with localized disease. For selected cases, partial myometrectomy may be performed to preserve fertility (Saso et al., 2012). The prognosis drops rapidly when metastatic disease is present. For advanced-stage diseases, a combination of surgery and polychemotherapy is required.
We report the case of a patient who develops a recurrence of an ignored PSTT at the vaginal vault diagnosed 8 years after her last pregnancy and 4 years after a hysterectomy for benign condition.
Case
A 46-year-old woman, gravida 4, para 3, presented to her gynecologist complaining of abdominal pain and purulent vaginal discharge. Her past medical history included 3 cesarean sections. The last one was 8 years prior to her presentation and a tubal ligation was performed. Four years later, she underwent a total hysterectomy for recurrent and infected hematocolpos in semi-urgent clinical conditions with no further histological or radiological preoperative work-up. Histological report showed an incomplete healing of the uterine cesarean scar and a high-grade cervical intraepithelial neoplasia.
Clinical and ultrasound examination revealed an ovarian mass consistent with an adnexal abscess of the left ovary and an indurated vaginal vault. Laparoscopic adnexal drainage and vaginal vault biopsy were undertaken. The infectious episode resolved promptly but the vaginal biopsy revealed an unexpected vaginal invasive squamous cell carcinoma.
The patient was therefore referred to a gynecologic oncologist. Clinical examination, pelvic MRI and PET-CT identified suspicious bilateral multilocular ovarian cysts and a 2 cm mass limited to the vaginal vault without parametrial extension. The patient underwent resection of the vaginal lesion, bilateral salpingo-oophorectomy and pelvic lymphadenectomy. The macroscopic examination of the vaginal tumor reveals an ill-defined nodular growth measuring 3.5 × 3.5 × 3.8 cm. The mass is pearly white color with areas of hemorrhage (Fig. 1, A). On microscopic examination, the tumor consists of a pleomorphic population of polygonal intermediate trophoblast cells with amphophilic to clear cytoplasm. These cells have often a single irregular highly atypical nucleus with focally prominent nucleoli, but some are multinucleate, resembling to syncytiotrophoblastic giant cells (Fig. 1, B&C). Mitotic figures (2/10 high power field), extensive necrosis, hemorrhage and vascular invasion are observed. The immunohistochemical pattern is ambiguous (Fig. 2). The staining of hCG, hPL and inhibine is only focally positive while the staining of placental alkaline phosphatase (PLAP) is more diffuse. The staining of CKAE1/AE3 and CK7 is positive and the staining of CK5/6 and P63 is negative. The staining of CD30 and alpha-fetoprotein (AFP) is also negative. Finally, Ki-67 is expressed in 60–70% of the tumor cells. The pathological diagnosis of PSTT is proposed and confirmed by the expert panel of pathologists of the Belgian Referent Center of GTD. The oophorectomy and the lymph node status are negative. In this context, the pathology slides of the hysterectomy performed 4 years previously were reviewed and showed no sign of PSTT.
Fig. 1.
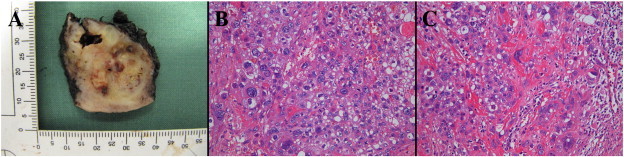
A. Gross appearance of sectioned vaginal vault mass. B&C. Histological features of PSTT. Pleomorphic population of trophoblastic tumors cells focally intermixed in interstitial fibrinoid material. Note mitotic figures (C) (HE, 200 ×).
Fig. 2.
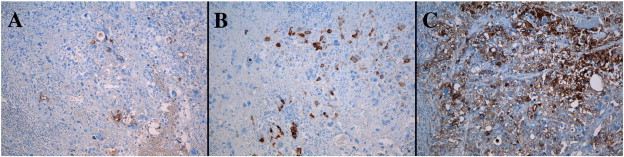
Immunophenotype of tumor cells (HIS, 100 ×). A, B & C. The staining of hCG (A) and hPL (B) is focally positive. The staining of PLAP (C) is more diffuse. In PSTT, the staining of hPL is usually strong and diffuse while expression of PLAP is negative or focal.
The serum hCG concentration was elevated to 41.0 UI/L the day after the surgery. Serum hPL level was not measured. An extended radiological work-up did not show evidence of metastasis.
Treatment options including adjuvant chemotherapy versus observation were discussed. Considering that surgery remains the preferred treatment for patients with localized PSTT, that total surgical resection of the unique lesion was achieved and that PSTT tends to be resistant to chemotherapy, observation has been elected. After a follow-up of 10 months, the patient is still in clinical, biological (β-hCG) and radiological (pelvic MRI) remission.
Discussion
As shown in this case report, clinical and pathological presentation of PSTT can be extremely variable. It can be challenging to distinguish PSTT from other forms of GTN or nontrophoblastic tumors. The distinction is critical because behavior, staging system and management are different. The review of the pathology by experts in this field is of paramount importance as demonstrated by the experience from several European referral centers.
Laboratory examinations can be helpful to confirm the diagnosis. In contrast to other forms of GTN, PSTT arises from the implantation-site intermediate trophoblast. Given that hCG is produced by syncytiotrophoblasts, the serum hCG levels in PSTT do not correlate with the tumor burden neither with the malignant behavior and thus have no predictive value (Hassadia et al., 2005). By contrast, the hPL level is elevated in most cases. Furthermore, the hCG-free ß-subunit is the main form of hCG in PSTT, as reported recently by Cole et al., and could be used to distinguish PSTT from other trophoblastic neoplasia and nontrophoblastic tumors that produce hCG (Cole et al., 2006). Immunochemistry is a useful diagnostic method. The classical immunophenotypic pattern of PSTT in comparison of choriocarcinoma and nontrophoblastic tumor is illustrated in Table 1 (Mazur, 2005). However, as observed in the present case, there is some overlapping between diagnostic criteria for PSTT and choriocarcinoma.
Table 1.
Characteristic immunohistochemical profiles of PSTT, choriocarcinoma and nontrophoblastic tumors.
| PSTT | Choriocarcinoma | Nontrophoblastic tumor | |
|---|---|---|---|
| hCG | +/− | +++ | +/− |
| hPL | +++ | + | − |
| PLAP | − | +/− | +/− |
| Inhinin-α | ++ | ++ | − |
| Mel-CAM (CD 146) | +++ | ++ | − |
| CK AE1/3, CK 18 | +++ | +++ | +++a |
| EMA | ++ | + | ++a |
| P63 | − | + | + |
| Ki67b | 10–30% | > 50% | Variable |
Mel-CAM : Melanoma cell adhesion molecule; CK : Cytokeratine; EMA : epithelial membrane antigen.
CK and EMA only immunostaining for carcinomas.
Marker of proliferative activity.
Information about optimal management strategy is restricted because the condition is rare and the natural course varies widely. The revised 2000 FIGO staging system (Oncology, 2002) has a prognostic and therapeutic usefulness for GTN. PSTT should be considered separately. Indeed, studies show that prognostic factors of the GTN staging system do not apply accurately to PSTT. The FIGO anatomical staging system is the strongest predictor of patient outcome presenting with PSTT (Schmid et al., 2009; Baergen et al., 2006). A retrospective review of 62 patients with PSTT treated over a 30-year period indicates that the probability of 10-year overall survival for those with stage I, II or III/IV disease was respectively 90, 52 and 49%. This further supports the importance of an early diagnosis (Schmid et al., 2009). Other factors associated with an unfavorable outcome include patient's age (older than 35 years), prolonged intervals from previous pregnancy (over 48 months), prior term pregnancy, high hCG level (over 1000 mIU/ml), tumor size, depth of myometrial invasion, tumor necrosis, high mitotic count (over 5 per 10 HPF) and clear cytoplasm. It is interesting to note that the prognostic factors are derived from pathological analysis of the uterus and therefore are not fully applicable for the present case. Management of recurrence is generally based on data derived from metastatic disease treatment. Schmid et al. (2009) report that the prognosis of patients developing recurrent or refractory disease is poor and that only four (22%) patients achieved long-term survival beyond 60 months. To our knowledge, specific data related to the optimum management of local recurrence are limited.
Authors propose a stage-adapted management. Surgery is advocated for stage I disease. They recommend adjuvant chemotherapy for patients who have risk factors of recurrence or persistent raised postoperative serum hCG level or both. Patients with stage II, III or IV PSTT are usually managed with combined surgery and chemotherapy. There is no definitive evidence of benefit of adjuvant chemotherapy for stage II disease. Given that PSTT is less chemotherapy responsive than other types of GTN, polychemotherapy is recommended. Although there is no conclusive difference between platinum-based versus non-platinum based regimens, a platinum-containing multidrug chemotherapy is generally preferred such as EP/EMA (Schmid et al., 2009).
Conclusion
The relevance of this case lies in its atypical clinical and pathological presentation. Gynecologists confronted with vaginal lesion must consider besides primary vaginal tumors, secondary vaginal implants even if the patient underwent a hysterectomy. Indeed, 84% of vaginal cancers are metastases and arise not only from GTN but also from other gynecologic malignancies, melanoma, hypernephroma, bladder cancer, breast cancer and colorectal adenocarcinoma.
Any gynecologist who has to deal with PSTT faces a challenge not only in terms of diagnosis but also in terms of therapy. Although several authors try to establish management strategy, a management algorithm remains controversial because of the lack of data due to the low incidence of the disease and its unpredictable course. Patients should ideally be referred to a center with expertise in gestational trophoblastic disease to assure their optimal care.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Baergen R.N., Rutgers J.L., Young R.H., Osann K., Scully R.E. Placental site trophoblastic tumor: a study of 55 cases and review of the literature emphasizing factors of prognostic significance. Gynecol. Oncol. 2006;100:511–520. doi: 10.1016/j.ygyno.2005.08.058. [DOI] [PubMed] [Google Scholar]
- Cole L.A., Khanlian S.A., Muller C.Y., Giddings A., Kohorn E., Berkowitz R. Gestational trophoblastic diseases: 3. Human chorionic gonadotropin-free ß-subunit, a reliable marker of placental site trophoblastic tumors. Gynecol. Oncol. 2006;102:160–164. doi: 10.1016/j.ygyno.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Feltmate C.M., Genest D.R., Wise L., Bernstein M.R., Goldstein D.P., Berkowitz R.S. Placental site trophoblastic tumor: a 17-year experience at the New England Trophoblastic Disease Center. Gynecol. Oncol. 2001;82:415–419. doi: 10.1006/gyno.2001.6265. [DOI] [PubMed] [Google Scholar]
- Gillespie A.M., Hancock B.W. Placental site trophoblastic tumour. In: Hancock B.W., Seckl M.J., Berkowitz R.S., Cole L.A., editors. Gestational Trophoblastic Disease. 3rd ed. International Society for the Study of Trophoblastic Diseases; London: 2009. pp. 420–429. ( www.isstd.org/gtd/index.html) [Google Scholar]
- Hassadia A., Gillespie A., Tidy J., Everard R.G.N.J., Wells M., Coleman R. Placental site trophoblastic tumour: clinical features and management. Gynecol. Oncol. 2005;99:603–607. doi: 10.1016/j.ygyno.2005.06.054. [DOI] [PubMed] [Google Scholar]
- Kurman R.J., Scully R.E., Norris H.J. Trophoblastic pseudotumor of the uterus. Cancer. 1976;38:1214–1226. doi: 10.1002/1097-0142(197609)38:3<1214::aid-cncr2820380323>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Mazur M.T. Gestational trophoblastic disease. In: Mazur M.T., Kurman R.J., editors. Diagnosis of Endometrial Biopsies and Curettings: A Practical Approach. Springer; New-York: 2005. pp. 67–99. [Google Scholar]
- Oncology F.I.G.O. Committee: FIGO staging for gestational trophoblastic neoplasia 2000. FIGO Oncology Committee. Int. J. Gynaecol. Obstet. 2002;77:285–287. doi: 10.1016/s0020-7292(02)00063-2. [DOI] [PubMed] [Google Scholar]
- Saso S., Haddad J., Ellis P., Lindsay I., Sebire N.J., McIndoe A. Placental site trophoblastic tumours and the concept of fertility preservation. BJOG. 2012;119:369–374. doi: 10.1111/j.1471-0528.2011.03230.x. [DOI] [PubMed] [Google Scholar]
- Schmid P., Nagai Y., Agarwal R., Hancock B., Savage P.M., Sebire N.J. Prognostic markers and long-term outcome of placental-site trophoblastic tumours: a retrospective observational study. Lancet. 2009;374:48–55. doi: 10.1016/S0140-6736(09)60618-8. [DOI] [PubMed] [Google Scholar]


