Highlights
► Lynch syndrome involves many rare cancers, such as urothelial carcinoma. ► The case describes incidental urothelial carcinoma associated with Lynch syndrome. ► Clinical suspicion of rare cancers must be maintained in certain patients.
Introduction
Cystoscopy can be performed during hysterectomy for many reasons, especially for suspicion of urologic injury (Ribeiro et al., 1999). Though it is debated whether routine cystoscopy should be performed at every hysterectomy, especially with regard to cost-effectiveness, routine cystoscopy is still commonly performed.
Although not cost-effective in the general population, another potential use of routine cystoscopy is to evaluate for non-iatrogenic ureteral abnormalities. Additionally, some surgeons at large academic institutions take advantage of universal or selective post-operative cystoscopy as an opportunity to teach trainees this important surgical skill. At the time of hysterectomy, most urologic findings (e.g. hematuria or lack of ureteral efflux) are due to bladder and/or ureteral injuries. Many of these findings are due to idiopathic and self-resolving causes (Wilson and Merkur, 2008). However, urologic findings in certain gynecologic oncology patients should raise concern about the possibility of synchronous malignancies. For instance, Lynch syndrome (hereditary non-polyposis colorectal cancer, HNPCC) is a rare but significant risk factor for ureteral transitional cell carcinoma (TCC). Whereas there have been reports of intraoperatively-found incidental lymphoepithelial ureteral carcinomas (Ma et al., 2008), a Pubmed search using the terms “transitional cell carcinoma” (or ureteral tumor), “incidental” and “hysterectomy” revealed no prior reports of incidentally-found ureteral TCCs associated with and leading to the diagnosis of Lynch syndrome.
Case
A 73-year-old G5P5 Caucasian presented to her primary physician for postmenopausal bleeding. Patient's weight was normal (body mass index 24.7 kg/m2). Review of systems was otherwise unremarkable. Family history yielded breast cancer in a maternal aunt and ovarian cancer in a maternal cousin. Ultrasound showed an enlarged uterus and a 15.6 mm endometrial stripe. Endometrial biopsy demonstrated a FIGO grade 1 endometrioid endometrial adenocarcinoma. She underwent total laparoscopic hysterectomy, bilateral salpingo-oophorectomy with pelvic and paraaortic lymph node dissection. Routine cystoscopy after the procedure revealed potent urinary efflux through the left ureteral orifice, and a potent and persistent hematuric jet from the right. An attempt at passing a 5 Fr whistle-tip stent catheter through the distal right ureter was unsuccessful. Urology was consulted and the decision was made to explore the pelvis via a Pfannenstiel incision. The distal right pelvic ureter was found to be non-dilated and non-injured. The patient was switched to a fluoroscopy-compatible bed, and retrograde ureteronephrogram showed a filling defect at the L5 level. There was proximal dilatation above the mid-right ureter with significant tortuosity (Fig. 1). It was impossible to advance a retrograde ureteral stent. Therefore, an angiographic catheter was left in place distal to the lesion for repeat attempt by interventional radiology. Postoperatively, an attempt was made to ascertain history of prior hematuria, which the patient denied. Postoperative computed tomography urogram confirmed filling defects in the right ureter, with associated proximal hydroureter and mild hydronephrosis (Fig. 2). Despite distal ureteral access and digital subtraction radiologic equipment, it remained impossible to advance a stent retrogradely. A percutaneous nephrostomy tube was placed and brush sampling of the mid-ureteral lesion yielded no evidence of atypia or malignancy.
Fig. 1.
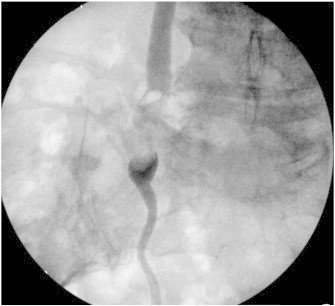
Intraoperative retrograde ureteronephrogram demonstrating right-sided filling defect accompanied by proximal ureteral dilation.
Fig. 2.
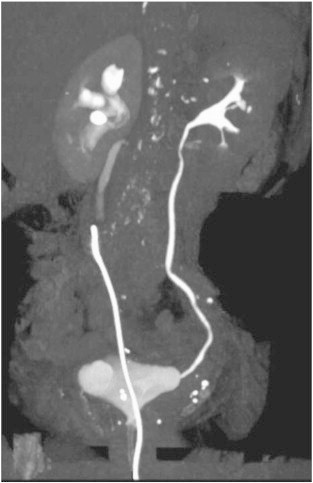
Computed tomography urogram confirming the presence of right-sided ureteral filling defect and hydronephrosis immediately proximal to the tip of a retrograde ureteral access catheter. Evaluation for enhancement is limited due to streak artifact and volume averaging caused by the foreign body.
Pathological evaluation of the endometrium revealed a stage IA grade 1 endometrioid endometrial adenocarcinoma. The tumor invaded superficially with evidence of lymphovascular space invasion, and 14 pelvic and periaortic lymph nodes were negative for malignancy. The tumor showed loss of nuclear MSH6 expression (Fig. 3A).
Fig. 3.
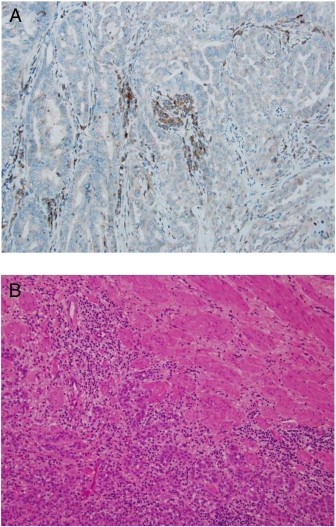
Panel A: Immunohistochemistry demonstrates lack of MSH6 protein (stromal expression serves as internal positive control). Panel B: H&E, 40 × photomicrograph demonstrates muscle invasive ureteral papillary transitional cell carcinoma.
Due to strong clinical suspicion for ureteral neoplasm, the patient was taken back to the operating room 8 weeks after her initial procedure and underwent right subtotal ureterectomy (13.5 cm), Boari flap reconstruction, and indwelling double-J ureteral stent placement. The specimen contained a 4 × 0.8 cm solid, white-tan intraluminal mass extending along the axis of the ureter. Microscopic exam confirmed a low-grade papillary transitional cell carcinoma with invasion to the superficial muscularis propria (Fig. 3B). Margins of resection were negative. The patient recovered uneventfully. A 2-week postoperative cystogram showed postsurgical changes without evidence of leaks; the patient's urethral catheter and stent were removed. At the time of this report, the patient is receiving adjuvant vaginal cuff brachytherapy and MSH6 genotyping efforts are ongoing.
Comment
Ureteral TCCs are extremely uncommon in the general population. The lifetime risk of these lesions is greatly increased with a history of Lynch syndrome (Watson et al., 2008).
A salient point from this case is that clinical suspicion of a urothelial neoplasm is heightened with additional findings such as hematuria, Lynch syndrome-associated cancers, and low BMI. Indeed, the mean BMI of patients with microsatellite-unstable and Lynch-related tumors is significantly less than those with microsatellite-stable tumors (Cohn et al., 2008). Thus, in this patient, endometrial cancer with superimposed hematuria raised concerns about an occult TCC in the setting of Lynch syndrome.
There is lack of consensus regarding potential screening tools for urologic neoplasms in patients with Lynch and other familial cancer susceptibility syndromes. The National Comprehensive Cancer Network (NCCN) guidelines on screening for different Lynch-associated cancers are vague and lack supportive data. For urinary tract cancers, an annual urinalysis is suggested. However, there is no definitive recommendation regarding targeted age groups or potential use of screening urinary cytology (Kohlmann and Gruber, 2004). The incidence of urinary tract cancers, even in Lynch patients, is very low under the age of thirty. Nonetheless, one study suggests that 21% of newly-diagnosed urothelial carcinomas may be associated with Lynch syndrome (Audenet et al., in press). Further research is needed to define optimal screening guidelines, to ensure multifaceted active surveillance in Lynch syndrome patients.
In the absence of adequate screening guidelines, the use of sound clinical judgment becomes imperative. In the present case, despite inconclusive initial imaging and negative cytology the decision to actively pursue workup for hematuria ended up revealing the TCC. Thus, controversial but minimally-morbid procedures such as postoperative cystoscopy after hysterectomy might provide additional benefits to patients. Taken together, this report underscores the need for being “clinically alert” in suspecting rare cancers. Often, the notion of rare cancers is dismissed and not further worked up when initial tests prove unrevealing. Neglecting a thorough workup risks allowing potentially early lesions to evolve (Fairley et al., 1998). The risk may supersede cost-effectiveness in the management of this and other uncommon tumors.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgment
Siteman Cancer Center is supported by NCI Cancer Center Support Grant P30 CA091842.
References
- Audenet, F., Colin, P., Yates, D.R., Ouzzane, A., Pignot, G., Long, J-A, et al., in press. A proportion of hereditary upper urinary tract urothelial carcinomas are misclassified as sporadic according to a multi-institutional database analysis: proposal of patient-specific risk identification tool. BJU Int. http://dx.doi.org/10.1111/j.1464-410X.2012.11298.x. [DOI] [PubMed]
- Cohn D.E., Pavelka J.C., Frankel W.L., Morrison C.D., Hampel H., Copeland L.J. Correlation between patient weight and defects in DNA mismatch repair: is this the link between an increased risk of previous cancer in thinner women with endometrial cancer? Int. J. Gynecol. Cancer. 2008;18:136–140. doi: 10.1111/j.1525-1438.2007.00964.x. [DOI] [PubMed] [Google Scholar]
- Fairley J.H., Douglas T.H., Mcleod D.G., Harrison C.R. Ureteral carcinoma presenting as a complex pelvic mass in a postmenopausal patient. Gynecol. Oncol. 1998;70:134–136. doi: 10.1006/gyno.1998.5052. [DOI] [PubMed] [Google Scholar]
- Kohlmann W., Gruber S.B. Lynch syndrome. In: Pagon R.A., Bird T.D., Dolan C.R., editors. GeneReviews [Internet] University of Washington; Seattle (WA): 2004. Feb 5 [Updated 2011 Aug 11] (1993-. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1211/. Retrieved July 5, 2012) [Google Scholar]
- Ma P., Leonard T., Trussel J.C. Lymphoepithelioma-like carcinoma of the ureter discovered intraoperatively during a hysterectomy. Can. J. Urol. 2008;15:4421–4424. [PubMed] [Google Scholar]
- Ribeiro A., Reich H., Rosenberg J., Guglielminetti E., Vidali A. The value of intra-operative cystoscopy at the time of laparoscopic hysterectomy. Hum. Reprod. 1999;14:1727–1729. doi: 10.1093/humrep/14.7.1727. [DOI] [PubMed] [Google Scholar]
- Watson P., Vasen H.F., Mecklin J.P., Bernstein I., Aarnio M., Jarvinen H.J. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int. J. Cancer. 2008;123:444–449. doi: 10.1002/ijc.23508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M., Merkur H. Hematuria at laparoscopic hysterectomy: a 9-year review at Sydney West Advanced Pelvic Surgery, Australia. J. Minim. Invasive Gynecol. 2008;15:146–151. doi: 10.1016/j.jmig.2007.12.007. [DOI] [PubMed] [Google Scholar]


