Highlights
► Nintedanib is an anti-angiogenic agent that has demonstrated activity in relapsed ovarian cancer. ► Our patient had prolonged response to nintedanib, allowing her to have potentially curative surgery 6 years after her diagnosis. ► The relationship between angiogenesis and BRCA mutation is worth exploring in ovarian cancer.
Keywords: Nintedanib, BIBF 1120, Ovarian cancer, Angiogenesis, VEGF, BRCA
Background
Management of advanced ovarian cancer consists of radical surgery and systemic chemotherapy. Despite a good initial response to chemotherapy, most women relapse and eventually develop treatment resistance. As such there is a need for novel therapeutic agents, and one promising approach is to target tumor angiogenesis. Nintedanib (BIBF 1120) is an orally administered potent blocker of the receptors of vascular endothelial growth factor (VEGFR-1–3), platelet-derived growth factor (PDGFR-α/β) and fibroblast growth factor (FGFR-1–3). It has recently been shown to have activity as maintenance treatment for relapsed ovarian cancer in a randomized phase II trial (Ledermann et al., 2011). In this article, we present one of the patients in the trial, with a germline BRCA1 mutation who appeared to have experienced therapeutic benefit to nintedanib for an exceptionally prolonged time.
Case presentation
A 38-year-old BRCA1-positive lady presented in January 2006 at 30 weeks pregnancy with breathlessness and abdominal swelling. Investigations revealed a large pleural effusion, ascites and widespread peritoneal disease and omental cake. Ascitic fluid was found to contain malignant serous papillary cells that stained positive for cytokeratin 7 and CA-125, but negative for cytokeratin 20 and calretinin. Her serum CA-125 was more than 12,000 U/ml.
She was given two cycles of carboplatin, after which she gave birth to a healthy baby by spontaneous vaginal delivery in March 2006. Two weeks later she restarted chemotherapy with two cycles of carboplatin and paclitaxel and her CA-125 came down to 264 U/ml. Optimal debulking surgery was performed in May 2006 and this was followed by four more cycles of carboplatin and paclitaxel, which she completed in July 2006. Her CA-125 was within the normal range and contrast-enhanced computed tomography (CT) showed no evidence of residual disease. In January 2007, after a 5-month treatment-free interval her CA-125 increased to 100 U/ml and a CT scan showed a 2 cm node in the common iliac chain (Fig. 1A). Based on these findings, she was given second-line chemotherapy with five cycles of epirubicin, cisplatin and capecitabine. She completed her treatment in June 2007, by then her CA-125 had normalized and she had a radiological complete response (CR). In the same month she was entered into the phase II trial comparing post-chemotherapy maintenance nintedanib (starting at 250 mg twice daily) with placebo. Upon unblinding of the trial for analysis of primary endpoint (progression-free survival — PFS) at 9 months, it turned out she was receiving the active drug. Since there was a maintained clinical benefit, treatment with nintedanib was continued. She tolerated the treatment well, with only grade 1 toxicities of fatigue, anorexia, nausea and diarrhea. A dose reduction of nintedanib to 200 mg was required in April 2008, and again in May 2009 to 150 mg due to fatigue and diarrhea. Her Eastern Cooperative Oncology Group performance status was zero throughout the treatment and she was able to remain in fulltime employment.
Fig 1.
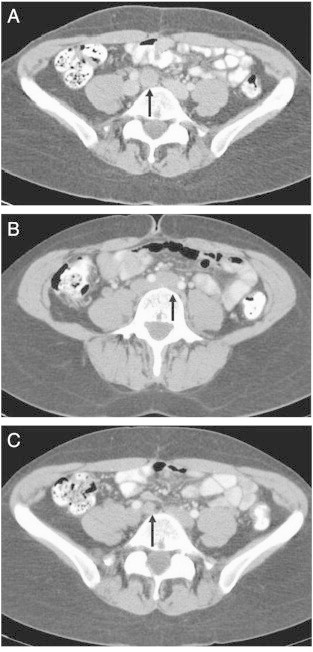
(A) Axial contrast-enhanced CT image demonstrating an enlarged lymph node in the common iliac chain, immediately beneath the confluence of the common iliac veins, 5 months after first-line chemotherapy. (B) Enlarged lymph nodes immediately beneath the aortic bifurcation and (C) in the right common iliac chain, 1 year after second-line chemotherapy and starting nintedanib.
In June 2008 her CA-125 went up from 10 to 24 U/ml and a CT scan showed enlarged lymph nodes immediately beneath the aortic bifurcation and in the right common iliac chain, suggesting disease recurrence (Fig. 1B and C). She continued with nintedanib as the trial continuation protocol explicitly allowed for the option to remain on therapy in case of equivocal progressive disease if clinical benefit was maintained.
Her disease remained stable for another 30 months. A CT scan in December 2010 showed a slightly more pronounced enlargement of the previously identified lymph nodes (CA-125 was 28 U/ml). 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) in February 2011 showed 18FDG uptake in the sub-aortic region and the right common iliac area (Fig. 2). Again, nintedanib was continued in view of the perceived slow tumor growth, not warranting an immediate change of therapy. A repeat FDG-PET in November 2011 showed that uptake was still restricted to these lymph nodes, although these had increased in size (CA-125 was 51 U/ml). Given the long interval from relapse and the continued absence of disseminated disease elsewhere, a decision was made to resect the involved lymph nodes. After pausing nintedanib for 5 weeks, a laparotomy was performed in January 2012. Six lymph nodes were removed, four showing mainly fibrous tissue with interspersed malignant cells (Fig. 3), and the remaining two were tumor-free.
Fig. 2.
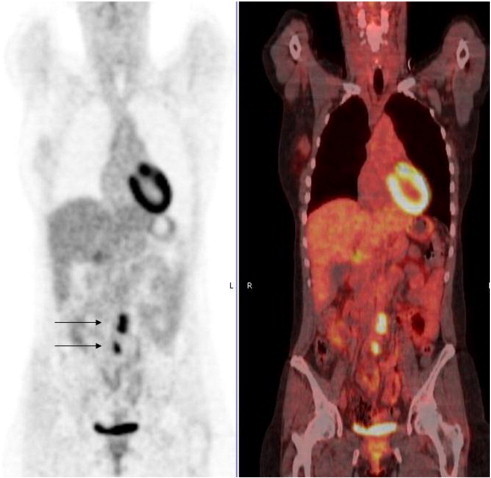
Coronal 18FDG-PET and fused 18FDG-PET-CT images demonstrating avid 18FDG uptake in lymph nodes in the sub-aortic region and the right common iliac area.
Fig. 3.
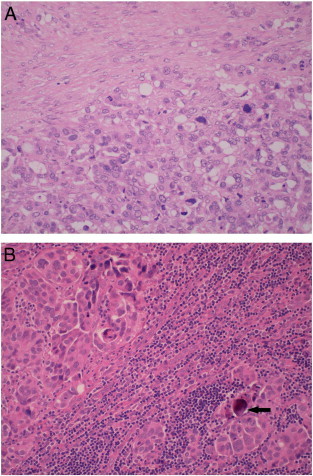
Microscopic images of dissected lymph nodes 4.5 years after starting nintedanib (× 200 magnification). (A) Most of the lymph nodes showed mainly fibrous tissue (top) with interspersed malignant cells (bottom). (B) Normal lymphoid cells (center) were interrupted by high grade serous carcinoma (upper left and lower right) with a classical psammoma body (arrow).
At the time of surgery she had been on nintedanib for nearly 4.5 years. After surgery had restored the CR status of the patient, oral nintedanib was restarted 4 weeks later, and to date still continues.
Discussion
Angiogenesis has a major role in ovarian cancer progression. When tumors outgrow their blood supply, hypoxia causes the stabilization of hypoxia-inducible factor (HIF)-1α, resulting in the upregulation of pro-angiogenic factors, particularly VEGF. A number of anti-angiogenic agents have been tested in phase II trials and have shown single-agent activity in relapsed ovarian cancer, including the oral multikinase inhibitors cediranib and pazopanib (Tagawa et al., 2012). A recent phase II trial of nintedanib for patients who had just completed chemotherapy for relapsed ovarian cancer has shown a promising impact on PFS at 36 weeks compared to placebo (Ledermann et al., 2011). This drug was well tolerated with no significant toxicities except for higher rate of abnormal liver function tests. Nintedanib is currently being evaluated in a first-line phase III AGO-OVAR 12/LUME-OVAR 1 trial in combination with carboplatin and paclitaxel and for up to 2 years maintenance therapy.
In the GOG-0218 and ICON7 trials (Burger et al., 2011; Perren et al., 2011), the addition of bevacizumab (a humanized monoclonal antibody against VEGF) to carboplatin and paclitaxel as the first-line treatment after debulking surgery considerably improved PFS, particularly for patients at high risk of progression (Perren et al., 2011). Furthermore, the OCEANS phase III trial showed that the addition of maintenance bevacizumab to gemcitabine and carboplatin in the second-line setting for platinum-sensitive disease resulted in significant improvement in PFS (Aghajanian et al., 2012). More recently, the AURELIA trial also reported improvement in PFS with the addition of bevacizumab to chemotherapy in platinum-resistant recurrent ovarian cancer (Pujade-Lauraine et al., 2012). In this case report, the patient relapsed within 6 months after first-line therapy, and after achieving CR to second-line chemotherapy, the interval to reappearance of disease under long-term anti-angiogenic monotherapy was much prolonged, suggesting a tumor-suppressive effect of nintedanib in the absence of visible disease. The cytostatic rather than tumoricidal nature of anti-angiogenic agents means that long-term maintenance therapy is likely to be needed, and an orally-administered drug such as nintedanib would be preferable provided its efficacy is confirmed in larger studies.
Anti-tumor therapies are usually discontinued at signs of progression, but it has been suggested that continuation of anti-angiogenic agents may still afford clinical benefit even in the presence of progressive disease. The concept of continuation of anti-angiogenic treatment through progression has been further corroborated by data from colorectal cancer patients, and is currently being tested in phase III studies such as the TML trial. In this case report, nintedanib was maintained despite the reappearance of visible disease, due to a perception of continued clinical benefit. Tumor growth and the rise in CA125 were slow in the presence of this drug. The multimodal approach, to also perform surgical removal of slow-growing disease while anti-angiogenic therapy is continued thereafter, has been able to restore her CR status.
Could the perceived clinical benefit of this patient be related to nintedanib in addition to her BRCA1 mutation? BRCA1/2 mutations in ovarian cancer patients are associated with better prognosis. This could be related to the perturbed DNA repair mechanisms in BRCA-deficient cancer cells, making them more sensitive to DNA targeting agents and radiotherapy. The relationship between BRCA mutation and angiogenesis has been explored by Kawai et al. (2002), who demonstrated that mutated BRCA1 protein in breast cancer cells was unable to suppress VEGF expression and secretion as a result of failure of BRCA1 to interact with estrogen receptor α. Depletion of BRCA1 in breast cancer cells in vivo could lead to higher levels of VEGF, enhanced vascularization and reduced cell death (Navaraj et al., 2009). This suggests that anti-angiogenic strategy may be particularly effective in treating BRCA1-deficient tumors. Other studies, however, have shown that BRCA1 mutation is correlated with reduced VEGF levels in breast cancer patients (Tarnowski et al., 2004), via the regulation of HIF-1α stability by BRCA1 (Kang et al., 2006). Given these conflicting findings, the relationship between BRCA mutation and angiogenesis in ovarian cancer is worth exploring in order to optimize future targeted therapies in this group of patients.
Conclusion
Our patient demonstrated a protracted course of disease stabilization whilst on nintedanib following early relapse after platinum-based chemotherapy. The disease was contained within the involved lymph nodes that had associated fibrosis, for 4.5 years from starting single-agent maintenance treatment within the phase II trial. This patient is currently free of disease following surgical resection of residual disease 6 years from diagnosis. A number of trials have demonstrated the effectiveness of anti-angiogenic agents in the treatment of relapsed ovarian cancer. Continuous maintenance treatment with these drugs, even through disease progression, may have an important role in disease control. The relationship between angiogenesis and BRCA mutation is unknown at present and is also worth exploring.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflict of interest statement
JAL received travel grant from Boehringer-Ingelheim to attend international meeting; JAL also took part in educational conference and has advisory role. MM is employed by Boehringer-Ingelheim.
Contributor Information
Han Hsi Wong, Email: han.wong@addenbrookes.nhs.uk.
Christine Parkinson, Email: christine.parkinson@addenbrookes.nhs.uk.
Jonathan A. Ledermann, Email: j.ledermann@ucl.ac.uk.
James D. Brenton, Email: james.brenton@cancer.org.uk.
Michael Merger, Email: michael.merger@boehringer-ingelheim.com.
Ashley Shaw, Email: ashley.shaw@addenbrookes.nhs.uk.
Aileen Patterson, Email: aileen.patterson@addenbrookes.nhs.uk.
Mahmood Shafi, Email: mahmood.shafi@addenbrookes.nhs.uk.
Helena M. Earl, Email: hme22@cam.ac.uk.
References
- Ledermann J.A., Hackshaw A., Kaye S., Jayson G., Gabra H., McNeish I. Randomized phase II placebo-controlled trial of maintenance therapy using the oral triple angiokinase inhibitor BIBF 1120 after chemotherapy for relapsed ovarian cancer. J. Clin. Oncol. 2011;29:3798–3804. doi: 10.1200/JCO.2010.33.5208. [DOI] [PubMed] [Google Scholar]
- Tagawa T., Morgan R., Yen Y., Mortimer J. Ovarian cancer: opportunity for targeted therapy. J. Oncol. 2012;2012:682480. doi: 10.1155/2012/682480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R.A., Brady M.F., Bookman M.A., Fleming G.F., Monk B.J., Huang H. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- Perren T.J., Swart A.M., Pfisterer J., Ledermann J.A., Pujade-Lauraine E., Kristensen G. A phase 3 trial of bevacizumab in ovarian cancer. N. Engl. J. Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- Aghajanian C., Blank S.V., Goff B.A., Judson P.L., Teneriello M.G., Husain A. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. 2012;30:2039–2045. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujade-Lauraine E., Hilpert F., Weber B., Reuss A., Poveda A., Kristensen G. AURELIA: a randomized phase III trial evaluating bevacizumab (BEV) plus chemotherapy (CT) for platinum (PT)-resistant recurrent ovarian cancer (OC) [abstract] J. Clin. Oncol. 2012;30:LBA5002. doi: 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- Kawai H., Li H., Chun P., Avraham S., Avraham H.K. Direct interaction between BRCA1 and the estrogen receptor regulates vascular endothelial growth factor (VEGF) transcription and secretion in breast cancer cells. Oncogene. 2002;21:7730–7739. doi: 10.1038/sj.onc.1205971. [DOI] [PubMed] [Google Scholar]
- Navaraj A., Finnberg N., Dicker D.T., Yang W., Matthew E.M., El-Deiry W.S. Reduced cell death, invasive and angiogenic features conferred by BRCA1-deficiency in mammary epithelial cells transformed with H-Ras. Cancer Biol. Ther. 2009;8:2417–2444. doi: 10.4161/cbt.8.24.10850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarnowski B., Chudecka-Glaz A., Gorski B., Rzepka-Górska I. Vascular endothelial growth factor (VEGF) levels and mutation of the BRCA1 gene in breast cancer patients. Breast Cancer Res. Treat. 2004;88:287–288. doi: 10.1007/s10549-004-0779-0. [DOI] [PubMed] [Google Scholar]
- Kang H.J., Kim H.J., Rih J.K., Mattson T.L., Kim K.W., Cho C.H. BRCA1 plays a role in the hypoxic response by regulating HIF-1alpha stability and by modulating vascular endothelial growth factor expression. J. Biol. Chem. 2006;281:13047–13056. doi: 10.1074/jbc.M513033200. [DOI] [PubMed] [Google Scholar]


