Highlights
► This case is an exceptionally rare primary neuroendocrine carcinoma of the fallopian tube, with a clinical presentation as torsion. ► TTF1 immunoreactivity can be found in neuroendocrine carcinomas originating from the fallopian tube.
Keywords: Neuroendocrine carcinoma, Fallopian tube, Epithelial tumor
Introduction
Neuroendocrine carcinomas (NECs) originate from endocrine cells of the diffuse neuroendocrine system, arising mainly in the gastrointestinal tract, lungs, and pancreas (Pinchot et al., 2008). They are rarely seen in the female genital system, and when they are, they are found most commonly in the ovaries and cervix (Eichhorn and Young, 2001). Very exceptionally, NEC occurs in another part of the genital tract (e.g., the endometrium or vagina), where it can present the pathologist with a diagnostic dilemma and the gynecologist with a therapeutic challenge. Only two previous cases of primary NEC in the fallopian tube have been reported to date (Neumann et al., 2010; Dursun et al., 2004). Here we present a new case and discuss its unique clinical presentation, pathological findings, and therapeutic management.
Case report
A 59-year-old postmenopausal woman was admitted through the emergency department for acute pelvic pain. Her medical history was unremarkable. She had one uncomplicated vaginal delivery.
She presented with an intense pelvic pain, and medical examination showed no other symptoms. Ultrasound and computed tomography (CT) examinations showed a highly dense left adnexal mass of 7 cm in diameter (Fig. 1). During the exploratory laparoscopy, we observed a torsion with necrosis at the distal part of the left fallopian tube. We observed no other abnormal findings in the genital tract or the entire peritoneal cavity during the procedure. Peritoneal washing was performed for cytologic analysis. A left salpingectomy was performed and removed with an endobag.
Fig. 1.
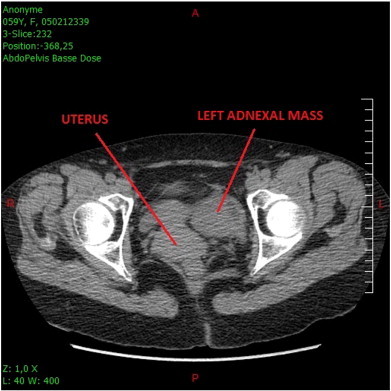
Emergency Pelvic CT scan (left adnexal mass).
Histopathologic examination revealed a tumor of 2 cm in diameter with complete infiltration and rupture of the fallopian tube wall. The peritoneal washing was positive. The neoplasm was composed of a dense and monomorphic population of small undifferentiated cells (Fig. 2), with high mitotic activity (Ki 67: 80%). Immunostaining showed that the tumor was neuroendocrine, as it was positive for pan-cytokeratin AE1–AE3 (polyclonal), chromogranin A (clone DAK-A3), synaptophysin (clone SY38), neuron-specific enolase (BBS/NcV1-H14) and CD56 (1B6) (Fig. 3). Tumor cells also exhibited nuclear immunoreactivity with TTF1 (8G7G3/1).
Fig. 2.
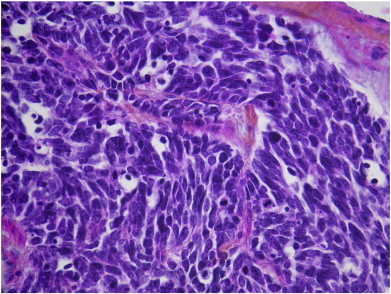
Dense monomorphic population of small undifferentiated cells.
Fig. 3.
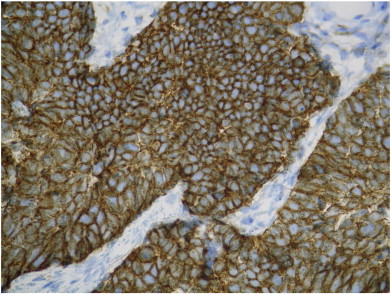
Homogeneous positive immunostaining for CD 56.
An additional laparotomy was performed 3 weeks later: staging, total hysterectomy, right salpingo-oophorectomy, left oophorectomy, omentectomy and pelvic and para-aortic lymphadenectomy. There was no evidence of macroscopic peritoneal carcinomatosis detected during the procedure. The pathology examination found multiple neoplastic foci located in the right adnexa and omentum. Metastasis was found in two out of the 44 lymph nodes removed. These two metastatic nodes were located in the para-aortic area.
Imaging performed before the laparotomy (abdominal and thoracic CT and PET-FDG) and postoperatively (bone scintigraphy, spinal MRI) found no distant metastasis.
Adjuvant chemotherapy was then administered. The patient tolerated 6 cycles of carboplatin/etoposide well and is alive 9 months after the initial diagnosis, with no evidence of recurrence or relapse.
Comment
NECs originate from endocrine cells of the diffuse neuroendocrine system, which in turn consists of a variety of cells present in the central and peripheral nervous systems and in several endocrine organs. These cells can produce many biologically active amines and peptides able to act as neurotransmitters, hormones, or paracrine regulators. Neuroendocrine cells have been identified within normal epithelium throughout the female genital tract by a number of techniques (Eichhorn and Young, 2001), but their presence in the fallopian tube has not been clearly demonstrated. Some authors suggest that NECs of the female genital tract arise from mullerian epithelial stem cells in the uterus (Sivridis et al., 1989). Other potential sources of NEC in the female genital tract include migrational errors of neuroendocrine cells, their implantation during previous surgery, neuroendocrine differentiation of uncommitted stromal cells, or metastasis (Neumann et al., 2010).
The clinical presentation of this case is unique, since the symptoms suggested adnexal torsion rather than the fairly improbable ruptured salpingian tumor that was found. The dense adnexal mass revealed by the CT scan did not indicate the unusual nature of this tumor. Pelvic pain was the main symptom in the previous cases reported, but its onset was progressive, and the clinical examination revealed a palpable adnexal mass. Surgical resection was achieved with negative microscopic margins in both cases (Neumann et al., 2010; Dursun et al., 2004).
Most NECs of the female genital tract are small-cell carcinoma, as here. Neumann et al., however, reported a case of primary NEC of the fallopian tube composed of medium to large cells (Neumann et al., 2010). Fallopian tube NECs appear to be aggressive neoplasms, like other NEC in the gynecologic tract. High mitotic activity was similarly described in the two previous case reports (Neumann et al., 2010; Dursun et al., 2004).
NEC diagnosis requires immunohistochemical analysis. The most commonly used nonhormonal immunohistochemical markers are chromogranin A, synaptophysin, cytokeratin, and CD56. NSE and Leu-7 lack specificity and may not be conclusive for neuroendocrine differentiation when other stains are negative (Eichhorn and Young, 2001). In our case, the immunostaining was consistent with the histopathologic examination and confirmed the neuroendocrine nature of the tumor. Immunostaining for amine (serotonin) and peptide (somatostatin, glucagon, calcitonin, vasoactive intestinal peptide) hormonal markers was negative, consistent with the patient's lack of endocrine symptoms.
The TTF1 immunoreactivity observed raises the issue of the origin of the neoplasm. This marker is a widely used and useful immunohistochemical marker of thyroid and pulmonary neoplasms, including primary pulmonary adenocarcinomas and small-cell carcinomas (Travis, 2010). However, TTF1 positivity is extremely common and may be a useful marker of primary neuroendocrine carcinomas of other organs as well. It is of no value in ruling out a primary pulmonary NEC, and cases of NECs from the female genital tract positive for TTF1 have been reported (McCluggage et al., 2010). In this case, the absence of pulmonary symptoms and the normal thoracic images further points in favor of a primary fallopian tube NEC. Our thorough search of the literature indicates that this is only the third primary monomorphic NEC of the fallopian tube to be reported. Two other cases of mixed tumors of the fallopian tube with either a mullerian tumor or a teratoma associated with a NEC have been described (Astall et al., 2000).
Because these tumors are so rare, their optimal treatment has not been established. Combination therapy might be useful because of their usually aggressive course. The most logical approach might be surgical treatment similar to that of other fallopian tube carcinomas, followed by an adjuvant chemotherapy protocol as proposed for neuroendocrine tumors. In our case, poor prognosis factors included the preoperative ruptured tumor, the high mitotic activity and metastatic implants found in the right adnexa, omentum and para-aortic nodes.
In conclusion, primary fallopian tube neuroendocrine carcinoma is very rare with our case only the third to be reported. Its diagnosis requires simultaneous consideration of the pathological, immunohistochemical and radiological findings and discarding differential diagnoses. Treatment is not standardized. However the use of chemotherapy commonly used for treating NEC (carboplatin/etoposide) appears to be a reasonable option. The prognosis of NEC tumor is poor though our patient is without evidence of disease after 9 months.
Conflict of interest statement
We declare that we have no conflicts of interest.
References
- Astall E.C., Brewster J.A., Lonsdale R. Malignant carcinoid tumour arising in a mature teratoma of the fallopian tube. Histopathology. 2000;36(3):282–283. doi: 10.1046/j.1365-2559.2000.0872c.x. [DOI] [PubMed] [Google Scholar]
- Dursun P., Salman M.C., Taskiran C., Usubutun A., Ayhan A. Primary neuroendocrine carcinoma of the fallopian tube: a case report. Am. J. Obstet. Gynecol. 2004;190(2):568–571. doi: 10.1016/j.ajog.2003.07.030. [DOI] [PubMed] [Google Scholar]
- Eichhorn J.H., Young R.H. Neuroendocrine tumors of the genital tract. Am. J. Clin. Pathol. 2001;115:S94–S112. doi: 10.1309/64CW-WKGK-49EF-BYD1. (Suppl.) [DOI] [PubMed] [Google Scholar]
- McCluggage W.G., Kennedy K., Busam K.J. An immunohistochemical study of cervical neuroendocrine carcinomas: neoplasms that are commonly TTF1 positive and which may express CK20 and P63. Am. J. Surg. Pathol. 2010;34(4):525–532. doi: 10.1097/PAS.0b013e3181d1d457. [DOI] [PubMed] [Google Scholar]
- Neumann F., Diedhiou A., Sainte-Rose D., Duvillard P. Primary neuroendocrine tumor of the fallopian tube: a case report. Ann. Pathol. 2010;30(1):33–35. doi: 10.1016/j.annpat.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Pinchot S.N., Holen K., Sippel R.S., Chen H. Carcinoid tumors. Oncologist. 2008;13(12):1255–1269. doi: 10.1634/theoncologist.2008-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivridis E.G., Gerhenson D., Soeige N., Brock W.A., Saul P., Copeland L.J. Small cell carcinoma of the utérine cervix: pathology and prognostic factors. Surg. Pathol. 1989;2:105–115. [Google Scholar]
- Travis W.D. Advances in neuroendocrine lung tumors. Ann. Oncol. 2010;21(Suppl. 7):vii65–vii71. doi: 10.1093/annonc/mdq380. [DOI] [PubMed] [Google Scholar]


