Highlights
-
•
We report a rare case of EGIST in rectovaginal septrum.
-
•
Adolescent patient who died due to an aggressive EGIST
-
•
Infrequent type of pelvic EGIST and literature review
Keywords: Gastronintestinal stromal tumor, Antineoplastic agent resistance, Adolescent, Imatinib, Pelvic exenteration, Treatment failure
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract (Trent and Benjamin, 2006). Sixty percent of GISTs arises from the stomach, 25% from the small intestine, and 10% from the colon or rectum (Trent and Benjamin, 2006). The remaining 5% arises from other intraabdominal locations—for example, the omentum, mesentery, retroperitoneal space, or urinary bladder (Trent and Benjamin, 2006). GISTs arising from such atypical sites are called extragastrointestinal stromal tumors (EGISTs) (Reith et al., 2000). Because EGISTs seldom produce symptoms, 75% of EGISTs are larger than 10 cm at the time of detection (Reith et al., 2000). EGISTs arising from the omentum appear to have a better prognosis than EGISTs arising in the mesentery and retroperitoneum (Reith et al., 2000). About 95% of GISTs and EGISTs express C-Kit receptor (CD117) and CD34 on immunohistochemical staining (Trent and Benjamin, 2006). Surgery and tyrosine kinase inhibitors are the current treatment options for these tumors (Trent and Benjamin, 2006). We previously reported our experience with treating GISTs discovered as incidental findings in patients with suspected ovarian masses. Here, we report a case of an EGIST arising in the rectovaginal septum.
Case report
A 15-year-old female, gravida 0, para 0, presented to an orthopedic surgeon in February 2008 complaining of sudden-onset sacral pain. The patient's past medical history was unremarkable. The orthopedic physical examination was normal. Lumbar radiography was performed, and the findings were reported as normal. One month later, the patient presented to her pediatrician complaining of constipation. A digital rectal examination was performed, and the findings were normal. In May 2008, the patient experienced rectal pressure and consulted a colorectal surgeon, who palpated a 2-cm rubbery mass on the impinging on the anterior rectal wall 6 cm from the anal margin. Rectosigmoidoscopy was performed and confirmed a 2-cm mass compressing the anterior wall of the rectum. The lesion was completely resected with negative margins via transrectal resection and sent for pathologic evaluation. There were no complications associated with the resection.
The gross appearance of the lesion was that of a sarcomatous tumor; microscopically, the tumor was composed of a proliferation of mildly pleomorphic spindle cells with elongated nuclei and eosinophilic cytoplasm. Tumor necrosis was present, and up to 40 mitotic figures per 50 high-power fields were identified (Fig. 1). Immunohistochemical staining demonstrated positive staining for C-Kit protein (CD117) and DOG 1.1 (discovered on GIST) and negative staining for CD34, smooth muscle actin, desmin, S-100, h-caldesmon, and a keratin cocktail (AE1/AE3) (Fig. 2). Margins were reported as negative. The diagnosis of an EGIST was made.
Fig. 1.
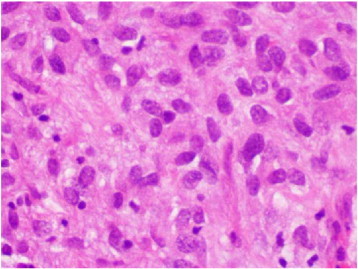
H&E 40 ×. Proliferation of spindle cells with mild pleomorphism. Few mitotic figures were noted.
Fig. 2.
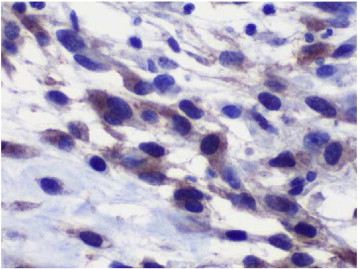
CD117. 40 ×. Neoplastic cells demonstrating strong diffuse cytoplasmic staining.
Treatment with tyrosine kinase inhibitors was recommended because of the high mitotic rate, but the patient, in consultation with her family, opted not to undergo adjuvant therapy. Four months after surgery, in September 2008, the patient presented with a rectal mass, tenesmus, and pain. A computed tomography (CT) scan showed a mass compressing on the anterior rectal wall approximately 5 cm in longest diameter. The patient was treated with imatinib 400 mg daily for 1 month. Given the short treatment interval there was no change in the size of the mass. The recurrent tumor was then completely excised via a rectal approach. Histologically, the tumor was similar to the initial one and was diagnosed as an EGIST. One month after surgery, in October 2008, sunitinib 50 mg daily was started. At evaluation 3 months after surgery, there were no signs or symptoms of disease. In May 2009, a CT scan was performed and showed evidence of recurrence at the rectovaginal septum. A team of gynecologic oncologists and surgical oncologists performed a posterior exenteration. All margins were negative. The patient and her family opted not to undergo radiation therapy because EGISTs have a low rate of response to such therapy.
In July 2009, the patient was restarted on imatinib because she did not tolerate sunitinib and because imatinib was the only available treatment in Colombia at that time. A positron emission tomography (PET)/CT scan performed 3 months later did not show any evidence of disease. In November 2009, however, the patient had another recurrence of her disease as documented on PET/CT, which revealed a 27-cm pelvic mass as well as multiple other masses in the retroperitoneum, urinary bladder, and anterior abdominal wall.
Supportive care and hospice care were recommended given the patient's persistent disease despite surgery and treatment with tyrosine kinase inhibitors. The patient died from multiple organ failure 6 days after diagnosis of the widespread recurrent disease.
Discussion
GISTs are rare neoplasms and EGISTs are even less common. Fewer than 10% of all GISTs are located on or near the rectum (Trent and Benjamin, 2006). These tumors may present as a rectovaginal mass and may be discovered on routine vaginal examination. GISTs may arise from the anterior rectal wall, in which case they are true GISTs, or directly from the rectovaginal septum or the posterior wall of the vagina, in both cases they are classified as EGISTs. These latter tumors (EGISTs) are usually misdiagnosed as myosarcomas or liposarcomas given the lack of awareness by surgeons, gynecologist and even by pathologists of EGISTs and their uncommon sites of appearance (Reith et al., 2000) since they both have the same pattern arising from stromal gastrointestinal cells in tissues outside the gastrointestinal tract.
Pathologically, GISTs, as well as EGISTs, may be as small as a few millimeters or as large as 35 cm or more. GISTs on the intestinal serosa are frequently found as a single, well-circumscribed nodule, and GISTs arising from other locations are frequently bulky lesions. GISTs are composed of a proliferation of spindle cells that mimics sarcoma. The neoplastic cells express C-Kit (CD117) and CD34 and, to a lesser degree, h-caldesmon, smooth muscle actin, and S-100 (Fletcher et al., 2002). The DOG 1.1 marker is a fragment of cDNA encoding a protein whose function is unknown. This marker may be helpful when a suspected GIST is negative for C-Kit; DOG 1.1 has high sensitivity (94.4%) in the diagnosis of GISTs and is positive in 30% of cases that are negative for C-Kit (Trent and Benjamin, 2006).
As is the case for patients with GISTs, the prognosis for patients with EGISTs is predicted by tumor size, mitotic rate, and site of origin (Reith et al., 2000). In the case of an EGIST, the tumor location often means that patients do not have any symptoms until the tumor is already very large. Reith et al. (2000) reported that a mitotic rate of over 2 mitoses per 50 high-power fields, high cellularity, and the presence of necrosis indicates more aggressive EGISTs.
The current treatment for localized disease for GISTs and EGISTs is to perform a complete surgical resection (Trent and Benjamin, 2006). However, even after tumor resection with negative margins, high-risk EGISTs usually recur locally, and local recurrence usually occurs before distant metastasis becomes evident (Trent and Benjamin, 2006). Standard systemic chemotherapy agents, such as doxorubicin, epirubicin, docetaxel, and gemcitabine, and radiation therapy have proven to be of no value in the treatment of GISTs (Trent and Benjamin, 2006). Neoadjuvant or adjuvant therapy for GISTs and EGISTs is based on tyrosine kinase inhibitors, either imatinib of 400 mg per day (first choice) or sunitinib of 50 mg per day in cycles of 4 weeks on and 2 weeks off (second choice). Each of these regimens for at least 1 year has been shown to improve disease-free and overall survival (Trent and Benjamin, 2006). Studies have documented a 1-year relapse-free survival rate after adequate treatment with surgery and TKI's of 97%, a 5-year disease-free survival rate of 65%, and a 5-year disease-specific survival rate of 68% (Trent and Benjamin, 2006).
Contrast-enhanced CT is the standard imaging modality for the evaluation of GISTs. The typical findings are a heterogeneously enhancing exophytic mass. Newer modalities such as fluorodeoxyglucose-PET help differentiate active tumor from necrotic tissue because the metabolic rate of viable tumor cells (in terms of the standardized uptake value), which have higher glucose uptake, is higher than the metabolic rate of necrotic tissue. We reviewed the literature and found reports of 10 patients with rectovaginal EGIST (Nasu et al., 2004; Ceballos et al., 2004; Weppler and Gaertner, 2005; Takano et al., 2006; Lam et al., 2006; Nagase et al., 2007; Zhang et al., 2009). The mean age of these 10 patients was 52.8 years (range, 36–75). The mean tumor size was 6.25 cm (range, 3.5–8.5). Five patients were treated with surgery alone, which ranged from local excision to abdominoperineal resection of the rectum with posterior vaginal wall resection. One patient had surgery plus adjuvant therapy with imatinib, and another patient had imatinib as the only treatment. For three patients, the report did not specify the treatment. All tumors were positive for CD117 and CD34, but only two were positive for smooth muscle actin. Three patients had recurrent disease, one at 2 years after unspecified initial treatment, one at 7.5 years after local excision, and one at 10 years after unspecified initial treatment. No deaths were reported (Table 1).
Table 1.
Reported cases of rectovaginal extragastrointestinal stromal tumor.
| First author, reference | No. of pts. | Age, years | Tumor size, cm | Treatment | No. of mitosis per 50 HPF | Necrosis | Cd117 | Cd34 | SMA | Ki-67 | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nasu et al. (2004) | 1 | 54 | 8.5 | Surgery | 5–10 | + | + | + | + | N/A | Alive at 13 months |
| Ceballos et al. (2004) | 1 | 75 | 4.5 | Local excision | 12–15 | + | + | + | − | N/A | Recurrence at 7.5 years |
| Weppler and Gaertner (2005) | 1 | 66 | 8 | Imatinib | > 5 | − | + | + | − | N/A | N/A |
| Takano et al. (2006) | 1 | 38 | 7 | Surgery | 1–2 | N/A | + | + | + | N/A | Alive at 12 months |
| Lam et al. (2006) | 3 | 36 | 4 | N/A | 15 | − | + | + | − | N/A | Recurrence at 2 years |
| 48 | 6 | N/A | 12 | + | + | + | − | N/A | Recurrence at 10 years | ||
| 61 | 8 | N/A | 16 | − | + | + | − | N/A | N/A | ||
| Nagase et al. (2007) | 2 | 42 | 3.5 | Local excision | < 1 | + | + | + | − | 20–30 | Alive at 4 years |
| 66 | 5 | Surgery + imatinib | 2–3 | − | + | + | − | 30 | Alive at 6 months | ||
| Zhang et al. (2009) | 1 | 42 | 8 | Local excision | 10 | + | + | + | − | 3 | Alive, no time report |
| Muñoz (present case) | 1 | 15 | 2 | Surgery + TKIs* | 40 | + | + | − | − | N/A | Recurrence at 4 months, 12 months, and 18 months; DOD |
HPF, high-power field; N/A, not available; SMA, smooth muscle actin; TKIs, tyrosine kinase inhibitors.
The patient in this case report is the youngest patient reported to date with an EGIST. The patient had a poor response to imatinib and sunitinib. Her tumor was positive for the new DOG 1.1 marker as well as C-Kit (CD117) and CD34, confirming the diagnosis of EGIST, but despite appropriate diagnosis and treatment, the tumor was refractory to tyrosine kinase inhibitors, and the patient died of the disease. Better understanding of EGISTs and further investigation of the behavior of these uncommon tumors is needed to achieve accurate early diagnosis and thereby permit more effective and focused therapy for patients with this disease.
Written informed consent was obtained from the patient's guardian for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgments
The authors thank Dr. Christopher Fletcher for his collaboration in the pathological analysis and immunohistochemistry for the diagnosis of this case.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Mario Muñoz, Email: mariomed69@gmail.com.
Carolina Echeverri, Email: echeverri_carolina@hotmail.com.
Pedro T. Ramirez, Email: peramire@mdanderson.org.
Lina Echeverri, Email: Linae10@hotmail.com.
Luis Rene Pareja, Email: reneparejafranco@yahoo.com.
References
- Ceballos K.M., Francis J.A., Mazurka J.L. Gastrointestinal stromal tumor presenting as a recurrent vaginal mass. Arch. Pathol. Lab. Med. 2004;128:1442–1444. doi: 10.5858/2004-128-1442-GSTPAA. [DOI] [PubMed] [Google Scholar]
- Fletcher C.D., Berman J.J., Corless C. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum. Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- Lam M.M., Corless C.L., Goldblum J.R. Extragastrointestinal stromal tumors presenting as vulvovaginal/rectovaginal septal masses: a diagnostic pitfall. Int. J. Gynecol. Pathol. 2006;25:288–292. doi: 10.1097/01.pgp.0000215291.22867.18. [DOI] [PubMed] [Google Scholar]
- Nagase S., Mikami Y., Moriya T. Vaginal tumors with histologic and immunocytochemical feature of gastrointestinal stromal tumor: two cases and review of the literature. Int. J. Gynecol. Cancer. 2007;17:928–933. doi: 10.1111/j.1525-1438.2007.00892.x. [DOI] [PubMed] [Google Scholar]
- Nasu K., Ueda T., Kai S. Gastrointestinal stromal tumor arising in the rectovaginal septum. Int. J. Gynecol. Cancer. 2004;14:373–377. doi: 10.1111/j.1048-891x.2004.014230.x. [DOI] [PubMed] [Google Scholar]
- Reith J.D., Goldblum J.R., Lyles R.H. Extragastrointestinal (soft tissue) stromal tumors: an analysis of 48 cases with emphasis on histologic predictors of outcome. Mod. Pathol. 2000;13:577–585. doi: 10.1038/modpathol.3880099. [DOI] [PubMed] [Google Scholar]
- Takano M., Saito K., Kita T. Preoperative needle biopsy and immunohistochemical analysis for gastrointestinal stromal tumor of the rectum mimicking vaginal leiomyoma. Int. J. Gynecol. Cancer. 2006;16:927–930. doi: 10.1111/j.1525-1438.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- Trent J.C., Benjamin R.S. New developments in gastrointestinal stromal tumor. Curr. Opin. Oncol. 2006;18:386–395. doi: 10.1097/01.cco.0000228747.02660.e2. [DOI] [PubMed] [Google Scholar]
- Weppler E.H., Gaertner E.M. Malignant extragastrointestinal stromal tumor presenting as a vaginal mass: report of an unusual case with literature review. Int. J. Gynecol. Cancer. 2005;6:1169–1172. doi: 10.1111/j.1525-1438.2005.00269.x. [DOI] [PubMed] [Google Scholar]
- Zhang W., Peng Z., Xu L. Extragastrointestinal stromal tumor arising in the rectovaginal septum: report of an unusual case with literature review. Gynecol. Oncol. 2009;113:399–401. doi: 10.1016/j.ygyno.2009.02.019. [DOI] [PubMed] [Google Scholar]


