Abstract
Perceptual temporal order judgments require an individual to determine the relative timing of two spatially separate events. Here we reveal the brain regions involved with this task. We had participants observe perceptually identical visual stimuli while conducting two different tasks: discriminating temporal order or discriminating spatial properties. By contrasting the functional magnetic resonance imaging signals during these tasks, we were able to isolate regions specifically engaged by each task. Participants observed two briefly presented rectangles. In one task, participants were instructed to report which appeared first, and, in the other, they were requested to report which rectangle was squarer. A potential confound of this study is that the temporal order judgment (TOJ) task required processing of brief events (onsets), whereas the shape task did not require temporal selectivity. To address this, we conducted a second study in which both tasks required discriminating brief events concurrent with the object onsets. The stimuli were similar to the first experiment, except a gray line was briefly superimposed on each rectangle at onset. Participants reported either which rectangle appeared first (TOJ) or which rectangle had a slightly wider gray line (shape). The first study found that the TOJ task resulted in greater bilateral activation of the temporal parietal junction (TPJ). The second revealed TOJ activation in the TPJ of the left hemisphere. This suggests that TPJ activation increases when we need to temporally sequence information. This finding supports the notion that the TPJ may be a crucial component of the “when” pathway.
Introduction
Umpires need to sequence the relative timing of two spatially disparate events: did the runner's foot touch the plate before the ball striking the glove? Science explores this issue with the temporal order judgment (TOJ) task, in which participants report the onset sequence of two events. Despite the popularity of this paradigm, no previous study has directly investigated the brain regions involved.
The TOJ is a powerful tool for investigating perception, revealing both an individual's point of subjective simultaneity (PSS) (a measure of bias) as well as their just noticeable difference (JND) (a measure of sensitivity). This task proves that our subjective experience is often at odds with objective reality. For example, a foveal item can be perceived before a peripheral item that was actually presented earlier in time (Rutschmann, 1966), and, although dim items are generally perceived as appearing later than brighter items, in certain circumstances, this effect can be reversed (Bachmann et al., 2004). In addition to these bottom-up factors, the TOJ is also influenced by spatial attention (Spence et al., 2001).
Husain and Rorden (2003) suggested that the temporal parietal junction (TPJ) encodes temporal information. More recently, Battelli et al. (2007) argued that the right TPJ forms the brain's “when” system for visual stimuli, complementing the more ventral object recognition (“what”) and more dorsal motor transformation (“how”) systems.
Indeed, a number of studies report that patients with right (Rorden et al., 1997; Robertson et al., 1998; Baylis et al., 2002; Snyder and Chatterjee, 2004; Eramudugolla et al., 2007; Sinnett et al., 2007) and left (Baylis et al., 2002) hemisphere injury exhibit biased performance on the TOJ. Battelli et al. (2003) report that patients with right hemisphere injury are impaired at detecting whether an item is flickering out-of-phase with its neighbors. Although many of these studies describe injuries that include the TPJ, none of these studies have conducted an objective lesion mapping analysis and therefore do not conclusively address the brain areas responsible for temporal processing.
There is evidence that briefly disrupting the right parietal lobe can disrupt spatial processing. Grossman et al. (2005) found that stimulating right superior temporal sulcus interfered with perception of biological motion. Recently, Woo et al. (2009) reported that disrupting right but not left parietal cortex led to biased TOJ performance, similar to the effects reported in neurological patients.
These brain disruption studies suggest that the TOJ may require the TPJ, but note that most emphasize biased performance (changes in PSS) rather than impaired performance (changes in JND). However, none of these previous studies rigorously tested the anatomy involved. Our aim was to use brain activation methods to identify the regions involved with this task. Specifically, we compared brain activation during periods when participants were making visual temporal order judgments to periods when they were making shape discriminations while observing identical stimuli. This contrast provides a pure measure of task-related differences, uncontaminated by differences in low-level perception or motoric response. We hypothesized that the TPJ is involved with the TOJ.
Materials and Methods
Participants.
Twelve students from the University of South Carolina volunteered for each experiment. In experiment 1, three were male and nine were female, and two of the females were left-handed. Experiment 2 also tested 12 participants, 10 of which participated in experiment 1. Other details, including handedness and proportion of females, were the same as in experiment 1. Note that we also analyzed the data from this experiment in which we excluded the left-handed individuals and found no substantial differences. All volunteers had normal vision or wore contact lenses. Participants volunteered for the 1 h session and were compensated $20 for their time. All participants gave written informed consent, and the local ethics committee approved the study.
Apparatus.
Stimuli presentation and data collection used a Windows XP computer (Microsoft). Images were displayed using a 1024 × 768 pixel resolution digital light-processing projector with a long-throw lens that was located outside the Faraday cage of the scanner. The image was directed through a wave guide and reflected off of a front-silvered mirror, which in turn reflected the images onto a back-projection screen that could be observed from another mirror mounted on the head coil of the scanner. A magnetic resonance imaging (MRI)-compatible response glove was used for making stimulus judgments in the scanner.
Behavioral procedure.
Each participant completed a brief color calibration task. This was followed by a session in which they switched between blocks of temporal order judgments and shape discrimination trials, with these tasks separated by rest periods. All of these tasks were practiced outside of the scanner and then repeated inside the scanner.
The participants completed a color calibration to ensure that the red, green, and gray colors used in the subsequent task were adjusted to have similar luminance, ensuring that behavioral responses were not biased by object salience. Color calibration was achieved using a simple flicker task, in which the display showed four quadrants, with the top left and bottom right showing red and the other two quadrants displaying green; then these colors were rapidly switched back and forth while the observer adjusted luminance (using the thumb and index fingers to increase or decrease luminance). Initially, the intensity of green was adjusted to match the red value, and then the display changed to show red and gray quadrants, with the gray manually adjusted to match the red. Throughout, red was held to a constant value of 200 (on a 0–255 scale) while the intensity of green or gray was adjusted until the participant reported perceiving minimum flicker by pressing their middle finger. Participants completed four adjustments of both the green and gray intensities, with subsequent displays using the average intensity rating of these four samples.
Next, the participant began the interleaved TOJ and shape judgment tasks. Throughout this session, the display background was gray, with a central fixation circle. Each trial began with the sequential presentation of two rectangles, one red and one green (Fig. 1, top). The rectangles were presented in diagonally opposite corners of the display with onsets separated by 33 or 67 ms. Diagonal presentation was used to encourage central fixation (e.g., if targets always appeared at the same location to the left and right of fixation, the participant might be tempted to foveate one of the peripheral target locations to improve performance on the shape task).
Figure 1.

A visual representation of the stimuli presented in the order of their presentation in each experiment. Top, Experiment 1. A fixation is followed by the presentation of the first rectangle (here red), which can appear in any one of the four corners. This is followed by the presentation of a second (here slightly taller) rectangle (green) that appears in the opposite corner. The size, onset, and location of the two rectangles presented in each trial were randomized. In this example, the fixation mark is yellow, noting that the participant should report which item appeared first (TOJ); trials with a blue fixation prompted the reporting of which rectangle was more square (shape). Bottom, Experiment 2. Identical except that the rectangles are the same shape and initially have gray lines in them of differing widths. In this experiment, the shape discrimination was based on these brief gray bars. Importantly, the durations of the shape and TOJ tasks in experiment 2 were equivalent.
In experiment 1, each trial included one rectangle with a standard size (2.04 × 2.72° of visual angle) on one side of the display contrasted with a rectangle on the opposite side that differed by a small (2.10 × 2.66° or 2.01 × 2.78°) or large (2.16 × 2.57° or 1.92 × 2.87°) amount (pilot testing had suggested that these easy and difficult shape differences matched the difficulty of discriminating the onset asynchronies). The duration from the onset of the first rectangle until the offset of both stimuli was 300 ms. Each trial lasted for 2.5 s, with trials clustered into blocks of six trials each. Each of these blocks was followed by 7.5 s of rest (in which only the fixation was visible). The persistent fixation point changed color at the start of each rest block, alternating between blue (if a shape discrimination block was impending) and yellow (indicating the TOJ task for the upcoming block). If the fixation was blue, participants indicated which of the rectangles was more square (shape task), the red one or the green one. If the fixation was yellow, participants indicated which of the stimuli came on first (temporal order task), the red one or the green one. Other than fixation color, stimuli were identical between shape and TOJ tasks. In both tasks, the middle finger of the right-hand was pressed if the green stimulus was the correct answer, and the index finger of that hand was pressed if the red stimulus was the correct answer.
A few modifications were made for experiment 2. The red and green rectangles were now 2.4° squares. At onset, a horizontal gray bar was superimposed on each square. One bar was always 1.2 × 0.4°, and the other was either shorter (0.8°) or wider (1.8°, sizes were again selected through pilot testing to have similar performance to the TOJ task). These gray bars only stayed on for 33 or 83 ms. The bottom of Figure 1 illustrates the time course for these trials. As before, the red and green items were presented for 300 ms (from onset of first item until both items vanished). The squares were presented for this extended exposure because brief displays make the TOJ task substantially easier. Specifically, brief displays and synchronous offsets make the two stimuli have very different durations and therefore different salience, whereas brief onsets and asynchronous offsets provide two temporal order signals. In experiment 1, during the shape task, the participants made judgments regarding the squareness of the rectangles. In contrast, in experiment 2, the participants discriminated between the width of the brief gray bars, a very brief stimulus that was relatively identical in duration to the TOJ information. The stimulus blocks were 40 s long (with 16 trials per block). This did not affect the length of the experiment run.
Imaging procedure and analysis.
All functional MRI (fMRI) data were collected on a Siemens 3T Trio scanner with a 12-channel radio frequency head coil. During the first 10 min in the scanner, the participant performed the color calibration and practiced the tasks. Next, the participant completed the two sessions of the tasks during continuous fMRI acquisition. Each session lasted 14 min, with a T2* echo planar imaging pulse sequence using the following parameters: repetition time, 2.2 s; echo time, 30 ms; flip angle, 90°; 64 × 64 matrix; 192 × 192 mm field of view; 36 ascending 3-mm-thick slices with 20% slice gap, resulting in voxels with an effective distance of 3 × 3 × 3.6 mm between voxel centers. Between the two fMRI sessions, a gradient echo field map (echo time, 5.19 and 7.65 ms) was acquired while the participant rested, with identical alignment and spatial properties as the fMRI protocol.
fMRI data processing was performed on each participant's data using FSL 4.1 (Smith et al., 2004). Data preprocessing included motion correction, brain extraction, spatial undistortion of the fMRI images using the field maps, spatial smoothing using a 8 mm full-width half-maximal Gaussian kernel, as well as a low-pass (σ = 2.8 s) and high-pass (σ = 42.5 s) temporal filter. Individual statistical analysis included local autocorrelation correction, followed by a second-level analysis to combine data across the two scanning sessions. The undistorted fMRI images were normalized to the Montreal Neurological Institute (MNI) 152 template image use the linear routines of FLIRT (FMRIB's Linear Image Registration Tool), allowing analysis between individuals. A higher-level mixed-effects analysis was computed for the entire group, with the subsequent statistical maps thresholded at Z > 2.3, followed by a cluster significance threshold of p = 0.05 adjusted for multiple comparisons (Worsley, 2001).
Results
Behavioral results
For experiment 1, the accuracy (and mean response time, in milliseconds) for the easy TOJ trials was 84.1% (1054) and 67.9% (1146) for the hard TOJ trials. In contrast, performance for the easy shape trials was 84.4% (1154) and 66.5% (1197) for the hard trials. This suggests that the pilot matching of difficulty across the two tasks was only partially successful. Specifically, accuracy was highly similar between TOJ and shape tasks [F(1,11) = 0.069; mean squared error (MSE) = 0.0054]. The reaction times, although approximately similar, did come out statistically different (F(1,11) = 35.13; p = 0.0002; MSE = 2193). Fortunately, for our hypothesis, the response time was longer in the shape task (suggesting that this task was more difficult), and thus, any activation in TOJ judgments over shape judgments was not attributable to increased task difficulty of the TOJ task.
For experiment 2, the accuracy (and mean response time, in milliseconds) for the easy TOJ trials was 86.9% (1050) and 74.3% (1115) for the hard TOJ trials. In contrast, performance for the easy shape trials was 81.8% (1153) but 68.8% (1205) for the hard trials. In this study, the pilot matching of difficulty across the two tasks was not as successful as in experiment 1. Specifically, accuracy was worse for shape than TOJ judgments (F(1,11) = 5.31; p = 0.042; MSE = 0.006). The reaction times also confirm this (F(1,11) = 18.23; p = 0.001; MSE = 6067). As in experiment 1, the shape task was the more difficult than the TOJ task, and therefore any additional activation in TOJ task relative to the shape task is unlikely to reflect general task difficulty.
Imaging results
The results for experiment 1 are shown in Figure 2 (blue overlay), with details in Table 1. In line with our hypothesis, we observed bilateral activation of the TPJ for the TOJ more than shape discrimination contrast. Additionally, we observed task-specific activation in the right inferior frontal gyrus, a region that has been associated along with the TPJ as involved in temporal processing (Husain and Rorden, 2003). In addition, this analysis detected activity in the right superior frontal gyrus (which we refer to as frontal eye fields). For completeness, we also conducted a final statistical contrast to identify regions that showed more activation in the shape task relative to the TOJ task. No regions were detected by this shape > TOJ contrast.
Figure 2.

Brain renderings and axial slices displaying the Z-scores of clusters of fMRI voxels more significantly activated by the TOJ than shape tasks for experiment 1 (blue to gray to white gradient) and experiment 2 (red to yellow to white gradient). Top left, Sagittal rendering of the left hemisphere. Top middle, Rendering from a superior–posterior viewpoint. Top right, Sagittal rendering of the right hemisphere. Brain slices are axial and displayed on a template created by averaging the brains of the 12 participants from experiment 2. They are presented under their MNI coordinates (blue text), and a sagittal slice is included with blue lines, indicating the positions of each axial slice. All Z-scores presented are >2.3 and have survived a corrected cluster threshold (p < 0.05).
Table 1.
Clusters in which greater activation was observed during the TOJ task than during the shape task for experiment 1
| Cluster | Z-mean volume | Z-score | MNI coordinates (x, y, z) | Location | Brodmann area |
|---|---|---|---|---|---|
| 1 | 3.2125 43.64 cc | 5.36 | 62, −40, 36 | Right intraparietal sulcus | 40 |
| 4.74 | 52, −62, 8 | Right posterior inferior temporal cortex | 37 | ||
| 4.33 | 64, −50, 14 | Right temporal parietal junction | 21 | ||
| 4.3 | 66, −44, 20 | Right temporal parietal junction | 22 | ||
| 4.25 | 60, −52, 16 | Right temporal parietal junction | 21 | ||
| 4.2 | 58, −40, 24 | Right temporal parietal junction | 48 | ||
| 2 | 3.2107 34.94 cc | 5.97 | −58, −54, 6 | Left temporal parietal junction | 21 |
| 5 | −44, −46, 52 | Left intraparietal sulcus | 40 | ||
| 4.46 | −50, −44, 38 | Left supramarginal sulcus | 40 | ||
| 4.02 | −60, −42, 34 | Left supramarginal sulcus | 40 | ||
| 3.47 | −48, −46, 24 | Left temporal parietal junction | 41 | ||
| 3.45 | −46, −44, 20 | Left temporal parietal junction | 41 | ||
| 3 | 2.8246 19.18 cc | 4.12 | 28, 48, 2 | Right inferior frontal lobe | 47 |
| 4.09 | 50, 40, −10 | Right inferior frontal lobe | 47 | ||
| 3.66 | 44, 32, −6 | Right inferior frontal lobe | 47 | ||
| 3.59 | 44, 20, 6 | Right inferior frontal gyrus | 45 | ||
| 3.5 | 36, 58, 12 | Right middle frontal gyrus | 46 | ||
| 3.19 | 20, 54, 12 | Right frontal pole | 10 | ||
| 4 | 2.8169 12.91 cc | 3.65 | 26, 14, 54 | Right frontal eye field | 8 |
| 3.55 | 42, 6, 40 | Right supplementary motor area | 6 | ||
| 3.51 | 26, 8, 42 | Right supplementary motor area | 6 | ||
| 3.5 | 26, 10, 46 | Right supplementary motor area | 6 | ||
| 3.49 | 26, 2, 62 | Right supplementary motor area | 6 | ||
| 3.48 | 22, 4, 62 | Right supplementary motor area | 6 |
These results reflect the group analysis from 12 participants. The mean Z-score and volume are reported for each cluster. The information for peaks within each cluster are also reported, including the Z-score of the peak, MNI coordinates (x, y, z referring to the three spatial dimensions), anatomical location, and approximate Brodmann area.
The results for experiment 2 are shown in Figure 2 (red overlay), with details in Table 2. The statistical contrast for more activation in the TOJ task relative to the shape task resulted in a large cluster of activation in the left TPJ. We also observed task-related activation in the right inferior parietal lobe (supramarginal gyrus), right inferior frontal gyrus, and bilateral frontal eye fields. The statistical contrast for more activation during the shape task relative to TOJ task resulted in no significantly activated voxels.
Table 2.
Clusters in which greater activation was observed during the TOJ task than during the shape task for experiment 2
| Cluster | Z-mean volume | Z-score | MNI coordinates (x, y, z) | Location | Brodmann area |
|---|---|---|---|---|---|
| 1 | 2.784 72.45 cc | 4.24 | 36, −8, 54 | Right intraparietal sulcus | 40 |
| 4.12 | −44, 6, 44 | Left frontal eye field | 6 | ||
| 3.95 | −40, −42, 54 | Left intraparietal sulcus | 40 | ||
| 3.92 | 16, 4, 70 | Right frontal eye field | 6 | ||
| 3.74 | 20, 16, 46 | Right frontal eye field | 8 | ||
| 3.64 | −36, 6, 56 | Left frontal eye field | 6 | ||
| 2 | 3.1152 27.66 cc | 5.16 | −50, −48, 10 | Left temporal parietal junction | 21 |
| 4.41 | −50, −42, 20 | Left temporal parietal junction | 41 | ||
| 4.22 | −54, −36, 34 | Left supramarginal gyrus | 2 | ||
| 3.82 | −66, −38, 24 | Left temporal parietal junction | 42 | ||
| 3.52 | −66, −40, 10 | Left temporal parietal junction | 22 | ||
| 3.39 | −34, −48, 12 | Left temporal parietal junction | 41 | ||
| 3 | 2.7099 16.95 cc | 4.43 | 44, 0, −12 | Right inferior frontal lobe | 48 |
| 4.38 | 52, 20, 12 | Right inferior frontal lobe | 48 | ||
| 4.23 | 52, 22, 12 | Right inferior frontal gyrus | 45 | ||
| 3.85 | 54, 36, −4 | Right inferior frontal gyrus | 45 | ||
| 3.61 | 52, 38, −10 | Right inferior frontal lobe | 47 | ||
| 3.52 | 2, −16, −10 | Thalamus |
The values are the same as described for Table 1.
We also conducted a region-of-interest analysis for experiment 2. The previously described voxelwise statistical test (in which an independent test is computed for each three-dimensional voxel of the brain image) has inherently low statistical power and therefore can often fail to detect real effects. This is attributable to many factors, including the noisy nature of individual voxels and the need to control for multiple comparisons. Therefore, we also conducted region-of-interest analysis, using the TPJ clusters identified in experiment 1. Note that these are large clusters, which include both the TPJ and surrounding regions (as shown in Fig. 2, Table 1). For each individual, we examined the Z-score map for the TOJ > shape contrast (in which negative values indicate regions with numerically more activity in the shape vs TOJ task, whereas positive values reflect numerically more activity for the TOJ vs shape task), with the dependent variable equal to the difference between the top 10% of voxels compared with the bottom 10% of voxels (in other words, if the minimum decile had a mean Z = −2 and the maximum decile had a mean Z = +3, then the computed value would be +1). The use of deciles helps apply data from a group-based statistical map to single individuals (identifying an individual's cortical modules). A one-tailed single-sample t test revealed that both the left and right hemisphere clusters were significantly greater than zero (left, t(11) = 4.05, p < 0.00095; right, t(11) = 2.22, p < 0.0242), suggesting that this cluster was more activated during the TOJ than shape task. A one-tailed within-subjects t test revealed a small trend for a greater effect in the left relative to the right cluster (t(11) = 1.54, p < 0.075). This provides some evidence that the right hemisphere TPJ cluster was more active for the TOJ task than the shape task.
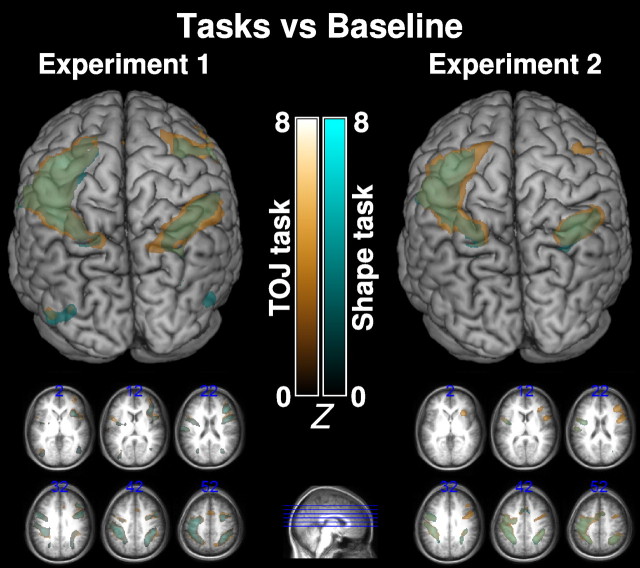
We also conducted a statistical contrast to identify regions more active during the task than for both experiments. These findings are shown in Figure 3, with the TOJ > rest contrast shown in gold and the shape > rest contrast shown in cyan. Note that we observed similar activations for both the shape and TOJ task when compared with rest. Specifically, we detected activation in the classical attention network regions (Cabeza and Nyberg, 2000): the frontal eye fields, lateral occipital cortex, and supramarginal gyrus. These contrasts also identified regions corresponding to the movement and sensation of the right hand (which was used for motoric responses). Note that, because rest periods had shorter duration than the tasks, these contrasts have less statistical power than comparison between tasks, which likely explains the failure of either contrast to highlight the TPJ. Throughout our experiments, the participants made responses with their right hand. This explains unilateral activation in the left primary motor and sensory cortices for the comparisons between either task and the baseline resting periods. However, because the same hand (and same fingers) were used in both tasks, this laterality is unlikely to have any influence on the comparison between these two tasks (because this lateralized activity should be cancelled out). However, future research could control for this, for example, by counterbalancing the hand used for response.
Figure 3.
Brain renderings and axial slices displaying the Z-scores of clusters of fMRI voxels more significantly activated by TOJ task than baseline (gradient of dark gold to light gold corresponding to Z-scores of 0–8) and shape task more than baseline (gradient of dark cyan to light cyan corresponding to Z-scores of 0–8). Left, Results from experiment 1. Right, Results from experiment 2. Top, Rendering from a superior–posterior viewpoint. Bottom, Axial slices displaying the activation maps. Slices are displayed on a template created by averaging the brains of the 12 participants from each corresponding experiment (with slice coordinates shown as blue text, and blue lines on the sagittal slice illustrating slice positioning). All Z-scores presented are >2.3 and have survived a corrected cluster threshold (p < 0.05).
Discussion
Our experiments reveal that the temporoparietal junction is more activated when individuals make temporal order judgments than when they make shape judgments. The statistical contrast for more activity during the TOJ task than the shape task in experiment 1 resulted in bilateral clusters, including the TPJ, a cluster of activity extending from the posterior right inferior frontal gyrus through the right insula and into the orbitofrontal cortex, as well as a third cluster near the frontal eye fields. However, a potential confound with this contrast is the fact that the TOJ task required attending to stimulus onsets, whereas the shape task did not require this form of temporal selectivity. Given that the findings of Shulman et al. (2003) suggest that the TPJ mediates the termination of visual search, it is possible to explain the results of our first experiment as merely reflecting differences in the likely duration of visual search performed by participants across our two conditions. In experiment 2, we altered our stimuli to control for this, such that both tasks required making decisions about events that occurred during the onset of the stimuli. Interestingly, the same statistical contrast revealed TPJ activation only in the left hemisphere (along with the right middle and inferior frontal gyri and the anterior supramarginal gyrus bilaterally). Crucially, the location of this cluster was effectively identical to those observed in experiment 1.
Our first experiment identified bilateral TPJ activation, whereas the second study only observed statistically significant activation in the left hemisphere. Our findings of left hemisphere involvement seem at odds with the brain disruption literature. However, we want to stress that the main empirical finding of our study is the strong evidence for left hemisphere activation rather than the failure to find TOJ-modulated activation of the right hemisphere in our second experiment. Interpreting null results from voxelwise brain analysis is hazardous, because these methods have inherently low statistical power (as described previously). In addition, there are several studies that show right-lateralized TPJ activation and deactivation during tasks that require visual attention (Shulman et al., 2007). Additional evidence for this lateralization comes from visual extinction, in which neurological patients are able to detect a single item at any location (demonstrating that they are not physically blind) but only report the ipsilesional item when two targets are presented simultaneously. Crucially, the TPJ is the most common anatomical substrate for this disorder (Karnath et al., 2003), and this deficit is more common after right rather than left hemisphere injury (Becker and Karnath, 2007). Therefore, the right TPJ activation may be modulated by other functions during both temporal and shape tasks (reducing the effective signal-to-noise in our statistical contrast), whereas the function of the left TPJ is more specific to temporal order. Indeed, our region-of-interest analysis did provide some evidence that the right TPJ was more active during the TOJ task than the shape task. Definitive evidence for this hypothesis requires brain disruption techniques.
Although brain disruption studies have focused on the right hemisphere, this may reflect a selection bias. First, patients with damage to the posterior left hemisphere often have language comprehension deficits, making consenting and testing somewhat more difficult. Indeed, it is clear that many previous TOJ studies have only tested individuals with right hemisphere injury. Second, right hemisphere patients often have more severe and prolonged spatial deficits, which might lead to biases in the TOJ task. The strongest evidence that the right but not left hemisphere is crucial for detection of temporal events is the previously described flicker paradigm reported by Battelli et al. (2003), who found flicker deficits in three patients with right parietal injury but relatively normal performance in three individuals who had left parietal injury. However, it is possible that none of these left hemisphere patients had injury to the putative left hemisphere temporal order module. Indeed, Baylis et al. (2002) do report that some individuals with left hemisphere injury also show deficits on the TOJ task.
Likewise, reviewing the transcranial magnetic stimulation (TMS) literature, it appears that the majority of previous studies have focused exclusively on the influence of disrupting the right hemisphere (Battelli et al., 2007). Although these studies clearly demonstrate that the right hemisphere plays a role, they do not provide direct information regarding the role of the left hemisphere. One important exception to this study is the recent work by Woo et al. (2009), who found biased performance on the TOJ task after right but not left hemisphere stimulation (stimulating the P4 and P3 sites of the 10–20 system, respectively). We do believe there is a clear explanation for reconciling our findings with those of Woo et al., in that they stimulated a more dorsal region than we observed in our study. It is possible that their left hemisphere stimulation site simply did not influence the crucial portions of the left hemisphere. In this respect, TMS and fMRI can provide complementary roles, with the ability of fMRI to sample the whole brain allowing scientists to determine the locus of different neural signals, and with follow-up TMS studies demonstrating whether these sites are crucial for the task or merely involved with the task. We note that the coordinates from our fMRI study are based on the average observed in a group of individuals, and the precise locus for this function is likely to have some individual variability. However, our coordinates could provide a starting estimate for a functional localizer, in which different cortical sites are stimulated until a location is found that specifically modulates performance on the TOJ task.
Another important point regarding the findings of Woo et al. and many of the patient findings is the observation that brain disruption causes a bias in the TOJ task (with individuals perceiving an item on the disrupted side as preceding an objectively simultaneous item on the opposite side). However, many of these studies do not show problems in sensitivity on these tasks (individuals do not show a change in their JND, rather only a bias in their PSS). This evidence could be accounted for as reflecting a bias in top-down attentional selection rather than a direct role in computing the temporal order. Alternatively, normal performance on these tasks may rely on both the left and right TPJ operating together, effectively operating as comparators. Our data provide a clear anatomical target for future brain disruption techniques (such as TMS), allowing them to compare unilateral with bilateral disruption. In particular, bilateral comparator models predict that unilateral stimulation results in changes of PSS, whereas bilateral disruption impairs the JND. It should be noted that changes in PSS could in theory be influenced by a low-level impairment (reduced salience of one stimulus), a high-level impairment (biased attention toward one location), as well as a specific disruption to the temporal-order selection mechanism. Therefore, definitive evidence for a TOJ mechanism requires a disruption study that can demonstrate shifts in JND. Specifically, we suggest that future disruption studies use nonlateralized stimuli (e.g., judging whether an upper or lower item appeared first), because unilateral stimulation is likely to elicit lateralized low-level and high-level biases in PSS.
Our hypotheses specifically focused on the role of TPJ in the TOJ task, although it is noteworthy that additional regions were also detected. Both studies identified the right middle and inferior frontal gyri as well as both the left and right anterior supramarginal gyrus, regions that Husain and Rorden (2003) associated along with the TPJ as being involved with encoding salience in space and time. It is unclear whether these regions form a unified network. In addition, it is unclear whether disruption of these regions results in unique symptoms. These questions can be directly addressed using disruption methods.
Our findings provide compelling evidence that the TPJ is specifically engaged during the visual TOJ task. The power of the paradigm we use is that it controls for low-level effects of stimuli and response, allowing a direct comparison of task-related modulation. We believe that this paradigm can be adapted to further our understanding of the human brain, specifically advancing the current work focused on visual perception using the TOJ task. Future work can help resolve whether the same brain region is involved regardless of modality. There is evidence that top-down attentional strategies influence TOJ responses across vision and touch (Wada, 2003) as well as vision and audition (Zampini et al., 2003). It is also worth noting that the neurological biases observed on this task appear to be cross-modal. For example, Eramudugolla et al. (2007) found a strong correlation between visual and auditory analogs of this task (but see Sinnett et al., 2007). Indeed, there is substantial evidence that the TPJ is involved in integrating information from both vision and audition (Calvert et al., 2001). We argue that the TPJ is critical for integrating stimuli over time, and therefore this area becomes even more active in a task that requires reporting temporal properties compared with a task that requires describing spatial properties (Downar et al., 2000). Future work could directly test this hypothesis by examining brain activation during nonvisual TOJ tasks.
Footnotes
This work was supported by National Institutes of Health Grant R01 NS054266.
References
- Bachmann et al., 2004.Bachmann T, Põder E, Luiga I. Illusory reversal of temporal order: the bias to report a dimmer stimulus as the first. Vision Res. 2004;44:241–246. doi: 10.1016/j.visres.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Battelli et al., 2003.Battelli L, Cavanagh P, Martini P, Barton JJ. Bilateral deficits of transient visual attention in right parietal patients. Brain. 2003;126:2164–2174. doi: 10.1093/brain/awg221. [DOI] [PubMed] [Google Scholar]
- Battelli et al., 2007.Battelli L, Pascual-Leone A, Cavanagh P. The “when” pathway of the right parietal lobe. Trends Cogn Sci. 2007;11:204–210. doi: 10.1016/j.tics.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis et al., 2002.Baylis GC, Simon SL, Baylis LL, Rorden C. Visual extinction with double simultaneous stimulation: what is simultaneous? Neuropsychologia. 2002;40:1027–1034. doi: 10.1016/s0028-3932(01)00144-0. [DOI] [PubMed] [Google Scholar]
- Becker and Karnath, 2007.Becker E, Karnath HO. Incidence of visual extinction after left versus right hemisphere stroke. Stroke. 2007;38:3172–3174. doi: 10.1161/STROKEAHA.107.489096. [DOI] [PubMed] [Google Scholar]
- Cabeza and Nyberg, 2000.Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Calvert et al., 2001.Calvert GA, Hansen PC, Iversen SD, Brammer MJ. Detection of audio-visual integration sites in humans by application of electrophysiological criteria to the BOLD effect. Neuroimage. 2001;14:427–438. doi: 10.1006/nimg.2001.0812. [DOI] [PubMed] [Google Scholar]
- Downar et al., 2000.Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3:277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Eramudugolla et al., 2007.Eramudugolla R, Irvine DR, Mattingley JB. Association between auditory and visual symptoms of unilateral spatial neglect. Neuropsychologia. 2007;45:2631–2637. doi: 10.1016/j.neuropsychologia.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Grossman et al., 2005.Grossman ED, Battelli L, Pascual-Leone A. Repetitive TMS over posterior STS disrupts perception of biological motion. Vision Res. 2005;45:2847–2853. doi: 10.1016/j.visres.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Husain and Rorden, 2003.Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect. Nat Rev Neurosci. 2003;4:26–36. doi: 10.1038/nrn1005. [DOI] [PubMed] [Google Scholar]
- Karnath et al., 2003.Karnath HO, Himmelbach M, Küker W. The cortical substrate of visual extinction. Neuroreport. 2003;14:437–442. doi: 10.1097/01.wnr.0000059778.23521.88. [DOI] [PubMed] [Google Scholar]
- Robertson et al., 1998.Robertson IH, Mattingley JB, Rorden C, Driver J. Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature. 1998;395:169–172. doi: 10.1038/25993. [DOI] [PubMed] [Google Scholar]
- Rorden et al., 1997.Rorden C, Mattingley JB, Karnath HO, Driver J. Visual extinction and prior entry: impaired perception of temporal order with intact motion perception after unilateral parietal damage. Neuropsychologia. 1997;35:421–433. doi: 10.1016/s0028-3932(96)00093-0. [DOI] [PubMed] [Google Scholar]
- Rutschmann, 1966.Rutschmann R. Perception of temporal order and relative visual latency. Science. 1966;152:1099–1101. doi: 10.1126/science.152.3725.1099. [DOI] [PubMed] [Google Scholar]
- Shulman et al., 2003.Shulman GL, McAvoy MP, Cowan MC, Astafiev SV, Tansy AP, d'Avossa G, Corbetta M. Quantitative analysis of attention and detection of signals during visual search. J Neurophysiol. 2003;90:3384–3397. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- Shulman et al., 2007.Shulman GL, Astafiev SV, McAvoy MP, d'Avossa G, Corbetta M. Right TPJ deactivation during visual search: functional significance and support for a filter hypothesis. Cereb Cortex. 2007;17:2625–2633. doi: 10.1093/cercor/bhl170. [DOI] [PubMed] [Google Scholar]
- Sinnett et al., 2007.Sinnett S, Juncadella M, Rafal R, Azañón E, Soto-Faraco S. A dissociation between visual and auditory hemi-inattention: evidence from temporal order judgements. Neuropsychologia. 2007;45:552–560. doi: 10.1016/j.neuropsychologia.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Smith et al., 2004.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Snyder and Chatterjee, 2004.Snyder JJ, Chatterjee A. Spatial-temporal anisometries following right parietal damage. Neuropsychologia. 2004;42:1703–1708. doi: 10.1016/j.neuropsychologia.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Spence et al., 2001.Spence C, Shore DI, Klein RM. Multisensory prior entry. J Exp Psychol. 2001;130:799–832. doi: 10.1037//0096-3445.130.4.799. [DOI] [PubMed] [Google Scholar]
- Wada, 2003.Wada Y. Crossmodal attention between vision and touch in temporal order judgment task. Shinrigaku Kenkyu. 2003;74:420–427. doi: 10.4992/jjpsy.74.420. [DOI] [PubMed] [Google Scholar]
- Woo et al., 2009.Woo SH, Kim KH, Lee KM. The role of the right posterior parietal cortex in temporal order judgment. Brain Cogn. 2009;69:337–343. doi: 10.1016/j.bandc.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Worsley, 2001.Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: an introduction to methods. New York: Oxford UP; 2001. pp. 251–270. [Google Scholar]
- Zampini et al., 2003.Zampini M, Shore DI, Spence C. Audiovisual temporal order judgments. Exp Brain Res. 2003;152:198–210. doi: 10.1007/s00221-003-1536-z. [DOI] [PubMed] [Google Scholar]