Abstract
Despite recent advances in the pathogenesis, treatment, and public health response to hepatitis C virus (HCV), HCV as it specifically relates to pregnancy has been a neglected condition and a markedly improved public health response to these populations is needed. HCV-monoinfected pregnant women have a 2–8% risk of viral transmission to their infant, but the mechanism and timing of mother to child transmission (MTCT) are not fully understood, nor is the natural history of the illness in pregnant women and their offspring. Recognition of HCV is relevant to infected pregnant women because of their risk of the long-term complications of infection, potential effects of infection on pregnancy, and risk of transmission to their infants. Certain risk factors for mother to child transmission (MTCT) of HCV appear similar to those for human immunodeficiency virus (HIV), however, unlike HIV, effective methods of prevention of HCV vertical transmission have not been developed. It is possible that a better understanding of HCV pathogenesis in pregnancy and MTCT of HCV infection will lead to useful prevention strategies, particularly as we enter an era where interferon-free drug cocktails may emerge as viable treatment options for HCV
Keywords: Hepatitis C Virus (HCV), Pregnancy, Mother to Child Transmission, Screening
Hepatitis C virus (HCV) infection is the most common chronic blood-borne disease in the US. Following its identification in 1989, HCV was recognized to be a major global public health problem affecting 170 million people worldwide--3% of the population1. In the United States, an estimated 4.1 million people (1.6%) have been infected, of whom 2.7 million (1.3%) have persistent viremia, the marker of chronic infection2. Though often clinically silent initially, chronic HCV infection is important because it predisposes to eventual development of liver fibrosis and hepatocellular carcinoma, as well as numerous extra-hepatic complications3. Late stage hepatic complications of HCV already compose the leading indication for liver transplantation and are anticipated continue to increase in frequency in the next decade such that by 2020 1 million Americans will be living with HCV-related cirrhosis4. Following eradication of blood-product associated HCV infections in the 1990s, intravenous drug use is now the primary route of new HCV infections in adults while mother to child transmission (MTCT) is the major route of new infections in young children in the developed world5.
More than 20 years after identification of the virus there is still no vaccine and current interferon-based treatment regimens are difficult and often unsuccessful. Further, a recent Institute of Medicine report has contended that poor control of the HCV epidemic is compounded by inadequate public health resources and a lack of knowledge of about hepatitis C among at risk populations, health care providers, and policy makers6. Nevertheless breakthroughs in drug development have raised hopes for anew era in the HCV epidemic. The first direct-acting antivirals were approved in 2011 and a host of new drug candidates are in clinical trials7. Numerous advances in the immunobiology of HCV have yielded practical tools including genetic polymorphism tests that predict likelihood of response to treatment8. Finally, recognition of the climbing rates of HCV complications and possibilities of more effective treatment regimens have led to a shift to a more assertive public health approach to HCV diagnosis. Whereas recommendations for HCV testing were previously limited to patients with certain medical or behavioral risk factors for HCV infection, in 2012 the CDC added a recommendation for universal screening of all persons born between 1945 and 1965 regardless of personal risk factors because of the higher rates of HCV in this age cohort and their likelihood of encountering complications soon9.
These advances in HCV science, therapies, and policies for adults have not been matched in pregnant populations. While it is known that HCV monoinfected pregnant women have a 2–8% risk of viral transmission to their infant, the mechanism and timing of mother to child transmission (MTCT) are not well understood, nor is the natural history of the illness in pregnant women and their offspring. To date no clear practice guidelines for pregnant women with HCV have been established. In fact a recent review for the U.S. Preventative Services Task Force failed to identify any definitive evidence based interventions for the prevention of MTCT10. HCV, as will be reviewed, does have several parallels to HIV with regard to risk factors for MTCT. Implicit to this readership is the respect for the public health coup that has nearly eliminated MTCT of HIV via antiretroviral therapy and appropriately performed cesarean delivery. Practitioners seek similar recommendations for intervention in the setting of HCV. We may even be inclined to execute similar strategies in absence of sound evidence when caring for women with HCV in an attempt to prevent MTCT. This review intends to address the current fund of knowledge that exists regarding HCV in pregnancy and will comment on screening for HCV, adverse pregnancy outcomes associated with HCV, timing and pathogenesis of MTCT, maternal disease evolution in pregnancy, and risk factors for mother to child transmission. We intend, also, to identify areas of future research, which may provide the evidence upon which to build appropriate practice patterns for management of HCV in pregnancy.
Screening
Current ACOG recommendations for HCV screening in pregnancy11 follow CDC guidelines for risk-factor based screening in the general population12. In 2012 the CDC added recommendations for universal one-time screening of all US “baby-boomers” regardless of reported risk factors9. This new recommendation was prompted by recognition of the climbing rate of HCV complications in the US, the disproportionate burden of HCV disease in this cohort, and the failure of risk-factor based screening to identify most individuals. Current CDC recommendations for HCV screening are listed in Table 1.
Table 1.
CDC and ACOG recommendations for screening
|
ACOG recommendations for HCV screening in pregnancy Source: ACOG Practice Bulletin 200711 |
|
|
CDC recommendations for HCV screening in general population Source: Recommendations for Prevention and Control of Hepatitis C Virus (HCV) Infection and HCV-Related Chronic Disease (1998)12 and Recommendations for the Identification of Chronic Hepatitis C Virus Infection Among Persons Born During 1945–1965 (2012)9 |
HCV testing is recommended for anyone at increased risk for HCV infection, including:
|
Testing of Uncertain Need
|
The rationale for screening for HCV in pregnancy
|
In practice the targeted approach to HCV screening in pregnancy has proven difficult, and it is likely that most HCV-infected pregnant women are not identified13–15. Aside from physician reluctance to discuss risk factors and testing, 40–70% of HCV-infected pregnant women do not initially report major risk factors16,17. Several of the CDC risk factors of “uncertain” significances such as a history of multiple sexual partners or sexually transmitted diseases are rather ubiquitous and of little utility for predicting maternal infection. A recent report by Delgado-Borrego et al. estimated that 85–95% of HCV-infected children in the U.S. have not been identified18, presumably due in part to the failure to identify most maternal infections.
Given the inherent inadequacies of risk-factor based screening, several have investigated whether universal screening for HCV in pregnancy would be a better approach. Patients do not seem opposed to the notion of screening for HCV. When a UK population was approached with the option of universal screening, 84% of women accepted the test and 92% felt the test should be offered to all women17. Universal screening would additionally ensure that children born to HCV infected women, a population for whom the CDC recommends screening, are properly identified and get appropriate postnatal evaluation. Plunkett and Grobman modeled universal screening in a pregnant population with 1% HCV seroprevalence and found that it was not cost effective, even when benefits of HCV diagnosis and treatment were considered for both mothers and infants and assuming that cesarean section eliminated perinatal transmission19.
An effective screening strategy utilizes an inexpensive and sensitive test to identify asymptomatic individuals at risk of a disease that has reasonably high prevalence, serious consequences if left untreated, and an effective treatment available20. HCV screening in pregnancy meets most of these criteria except, as will be reviewed, the consequences of HCV infection are often delayed for many years, there are no confirmed interventions for prevention of MTCT, and current therapies of established infection in mothers and children are expensive, associated with major side effects, and often ineffective. Without these basic tenets of screening upheld, the argument for universal screening folds. (Table 2)
Table 2.
Challenges of Universal Screening versus Risk Factor Based Screening in the Pregnant Population
| Universal Screening | Risk Factor Based Screening |
|---|---|
| Not cost effective19 | Difficult to ascertain risk factors |
| Lack of effective therapy in pregnancy | 40–70% of women with HCV do not reported identifiable risk factors16,17 |
| No evidence based intervention to limit MTCT of HCV | Current strategies miss 85–95% of infected children18 |
| No protocol to link identified women to resources for care102 |
Prevalence of Hepatitis C among Pregnant Women
The current prevalence of HCV in pregnant women in the US is unknown because the recommended practice of selectively screening for HCV among high risk patients risk misses a large proportion of infected pregnant women, and no recent large scale HCV serosurvey data are available for this population. HCV seroprevalence among women of childbearing age was estimated to be 1% (age 20–29 years) to 1.6% (age 30–39 years) in 1999–2002 NHANES2 surveys, though these data excluded homeless and imprisoned women who may have rates of HCV 20–40 times higher21–23. Seropositivity rates in pregnant women ranged from 0.24 to 4.3% in several US studies conducted between 1991 and 200324–27, but most were single sites studies and none were designed to be nationally representative. It is conceivable that the prevalence of HCV among pregnant women might be lower today than in the 1990s because fewer transfusion-associated cases would be expected. However reductions in transfusion-associated cases may be offset by increased numbers of cases acquired by intravenous drug use, related especially with the ongoing opiate abuse epidemic. Several recent state reports describe a doubling or tripling in the incidence of acute HCV in adolescents and young adults following the turn of the century28,29. Concurrently, Salihu and colleagues report climbing HCV prevalence in pregnancy. Between 1998 and 2007, the prevalence of HCV in pregnant women as determined by discharge data increased from 17/100,000 births to 125.1/100,000 births (p<0.0001)30. Thus, while no recent actual seroprevalence data are available for HCV in pregnant US women, recent data suggest that it is likely to be climbing.
Effects of pregnancy on the course of acute and chronic HCV infection
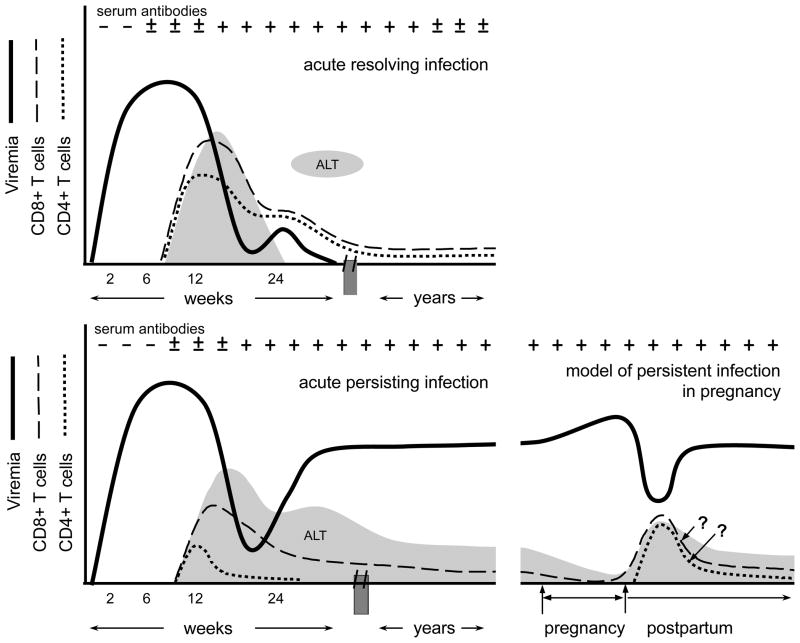
Outside of pregnancy, most acute HCV infections are asymptomatic or cause mild non-specific symptoms such as malaise and anorexia, while 10–20% lead to overt icteric hepatitis31,32. Symptoms (if present) and transaminase elevations usually follow a prolonged viremic incubation period that averages 7–8 weeks (range, 2 to 26)3 and are likely immune-mediated as they correlates temporally with appearance of functional HCV-specific CD8+ and CD4+ T-cell responses in the blood and declines in viremia (Fig 1) (reviewed in Rehermann et al.)33. In about 20–25% of acute infections, immunity prevails and viremia spontaneously resolves (Fig. 1, upper panel). In the majority however, immunity fails and lifelong infection ensues. The chronic phase of infection is characterized by exhausted cellular immunity, stable high-level viremia, and normal or elevated ALT (Fig. 1, lower left panel). Affected individuals are often asymptomatic or have only non-specific findings such as fatigue. Inability to clear viremia has been linked to an unexplained early loss of HCV-specific CD4+ T-cell immunity and development of CD8+ T-cells that are functionally exhausted or evaded by viral escape mutations33. Innate immunity is also important for control of acute infection as genetic differences in elements controlling IL28B (interferon-λ) and receptors governing NK cell activation have been linked to the outcome of infection34,35 and numerous viral mechanisms to block innate immune signaling have been identified33.
Figure 1.
Patterns of viremia and cellular immunity in acute resolving infection, acute persisting infection, and a model of chronic HCV infection during pregnancy. (Upper left) Following a prolonged incubation period, viremia declines when HCV-specific CD8+ and CD4+ T-cells appear in the blood. Concurrent spikes in alanine aminotransferase (ALT) levels likely reflect immune-mediated hepatocyte injury. Successful T-cell responses do not fully contract into memory populations until after viremia is resolved. (Bottom left) In persisting infections viremia may be temporarily contained but eventually rebounds as HCV-specific CD4+ T-cell responses are lost and CD8+ T-cell responses become ineffective. (Bottom right) In some women, viral levels rise during pregnancy and then decline significantly in the postpartum period, together with inverse changes in ALT. In this model we hypothesize that these viral load and ALT fluctuations reflect further suppression of the already dysfunctional HCV-specific T-cell responses during pregnancy followed by a sharp and unusual rebound in T-cell function after delivery.
How the immune changes of pregnancy affect the course of acute hepatitis C infection is not well understood as less than a dozen cases have been described (reviewed in in Gonzalez et al.)36,37. Interestingly, jaundice is present in the majority of reported cases of pregnant women with acute HCV, but this may reflect a diagnostic or publication bias rather than evidence of more severe disease in pregnancy36,37. Indeed, asymptomatic acute HCV infection in pregnancy is difficult to diagnose unless there is a distinct exposure that permits accurate timing of seroconversion. Whether pregnancy alters the outcome of acute HCV infection is unknown, but it conceivable that the immunomodulation of pregnancy could favor viral persistence rather than clearance37. At the same time it is unknown if acute infection during pregnancy is more likely to result in MTCT or adverse pregnancy outcomes than chronic infection. Premature delivery was described in 3 of 8 reported cases of HCV, but this again could represent a bias for publication of more severe cases36,37.
Several key studies of viral levels in chronically infected pregnant women have documented a modest increase (0.2 to 0.8 log IU/ml) in average viral load over the course of pregnancy, concurrent with declines in ALT to levels that are lower than would be expected from hemodilution alone38–40. Because ALT elevation is thought to reflect immune mediated hepatocyte injury rather than toxicity from viral replication per se, several authors have speculated that these viral load and ALT changes in pregnancy reflect suppression of cellular immunity by the maternofetal tolerance mechanisms of pregnancy that prevent rejection of the HLA semi-mismatched fetus38,39, though no published studies have tested this hypothesis. Interestingly, some women sustain unusually sharp decreases in viremia 1–3 months after delivery41, and a few go on to actually resolve their chronic viremia in the postpartum period42, an otherwise rare phenomenon43. We have observed that women with significant postpartum declines in viremia have broader HCV-specific T-cells interferon-γ producing responses44 suggesting these revived cells might be responsible for postpartum viral declines (Fig. 1, lower right panel). Efforts are ongoing to better characterize this unusual postpartum restoration of HCV-specific T-cell responses and define the associated cytokine environment. These observations have lead to intriguing suggestions that the immunologic milieu of the postpartum time period may be a strategic time for maternal antiviral treatment5,45.
Adverse Pregnancy Outcomes Associated with HCV
While it is clear that pregnancy affects immune control of HCV, it has been less clear how chronic HCV affects pregnancy outcomes. Only recently have studies been sufficiently powered to distinguish the contribution of HCV to adverse pregnancy outcomes from tobacco, alcohol, and drug use and other confounding variables that disproportionately affect HCV-positive women46. Small studies conducted in the first years after discovery of the virus did not identify adverse obstetric or fetal outcomes statistically associated with HCV infection24,47 though several noted an increased rate of cesarean delivery25,47,48 which authors speculated could reflect inability of obstetricians to use fetal scalp electrodes for reassurance of fetal status48. Since then larger population-based and case-control studies have inconsistently uncovered independent associations of maternal HCV infection with gestational diabetes49,50, preterm delivery46, low birth weight46,49, small for gestational age49, and cholestasis of pregnancy51–53 (Table 3).
Table 3.
Adverse pregnancy outcomes associated with maternal hepatitis C infection following adjustment for demographic factors including tobacco and drug use.
| Authors | Source | # HCV ab + | Comparator group | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| Gestational DM | Pre-maturity | LBW | SGA | Unique Findings | ||||
| Connell et al.46 | Florida discharge data 1998–2007 | 999 | 1,669,370 | NS | OR 1.40 (1.15–1.72) | OR 1.39 (1.11–1.74) | OR 1.19 (0.97–1.46) | Congenital anomalies OR 1.55 (1.14–2.11) |
| Reddick et al.50 | NIS 1995–2005 discharge data | 555 | 296,218 | OR 1.61 (1–2.59) | OR 1.22 (0.94–1.52) | |||
| Pergam et al.49 | Washington state birth certificate 2003–2005 | 506 | 2,024 random selected | If excess wt gain OR 2.51 (1.04–6.03) | OR 1.54 (0.97–2.43) | OR 2.17 (1.24–3.80) | OR 1.46 (1–2.13) | NICU OR 2.91 (1.86–4.55) Neonatal assisted ventilation OR 2.37 (1.46–3.85) |
| 124 drug using | 1,439 drug using | QNS | OR 1.03 (0.66–1.61) | OR 1.19 (0.74–1.91) | OR 0.97 (0.57–1.64) | NICU OR 2.80 (1.83–4.29) Neonatal assisted ventilation OR 1.82 (1.03–3.22) |
||
| Berkley et al.51 | Univ. New Mexico pregnancy drug treatment program | 159 drug using | 141 drug using HCV neg. | Not tested | NS | 33% vs 17%, adj P = 0.06 | Cholestasis of pregnancy (6.3% vs 0%, P = 0.002) Neonatal abstinence syndrome if mother on methadone (88.4% vs 36.4%, P< 0.001) |
|
The increased risk of gestational diabetes among HCV infected pregnant women is plausible given that chronic HCV infection increases the risk of insulin resistance and diabetes mellitus54 in non-pregnant persons. Mechanisms for prematurity, low birth weight/small for gestational age outcomes in HCV are not known – and could reflect inadequately controlled effects of maternal substance use, but one study of placental immunity in HCV infection suggested that alterations such as increased cytotoxicity of placental NKT cells could potentially account for the increased risk of preterm delivery55.
Pathogenesis of MTCT of HCV
The timing and route by which HCV passes from mother to child, and the host defense mechanisms that govern this transmission, remain poorly understood. As with HIV, estimates of the timing of transmission have relied on the presence or absence of viral RNA in the neonate at delivery, and both intrauterine and peripartum transmission events have been described. Mok et al. reported that 31% of 54 HCV infected children were HCV-RNA PCR positive in the first 3 days after birth, consistent with intrauterine transmission. The remainder were assumed to have late intrauterine or intrapartum transmission on the basis of a negative PCR in the first 3 days of life and a subsequent positive PCR months later56. Other studies substantiate the likelihood that both intrauterine and late intrauterine/intrapartum transmission events can occur57,58.
As recently reviewed by Le Campion et al.45, there are several routes by which HCV could conceivably cross the placenta to accomplish in utero transmission, including transcytosis, trafficking in maternal mononuclear cells59,60, receptor mediated entry into and possibly infection of trophoblasts61,62, or other injury to the placental barrier. With an estimated 1013 to 1014 virions circulating through the placental vascular bed daily63, it is likely that some viral particles enter the fetal circulation even if the mechanism of transplacental passage is inefficient. In cases of intra-partum transmission, it is conceivable that transmission occurs by leakage of maternal blood into fetal circulation across the placenta during labor or via cutaneous injuries (e.g. fetal scalp electrode).
Given the probability that some viral particles enter the fetal circulation in most pregnancies, it is rather remarkable that the rate of HCV MTCT is as low as it is (2–8% for HCV versus 25% for HIV). This conundrum has prompted investigation into the immune mechanisms that limit HCV MTCT. Starting at the maternal-fetal interface, Hurtado and colleagues characterized innate immune cells in the decidua, placenta, and cord blood, comparing HCV-infected women to healthy controls. Relative to cord blood, placental tissues had greater frequencies of NKT and γδ T-cells and greater cytotoxicities of NKT and NK cells, suggesting that an antiviral immune gradient exists at the interface. HCV infection further enhanced placental NKT and γδ T-cell frequencies and placental NKT cytotoxicity while at the same time reducing expression of NK cell activation markers in both compartments. Given the role of NK cells in immunity to acute HCV infection in adults, these findings suggest that placental immune cells may play an active antiviral role in limiting MTCT of HCV55.
Hypothesizing that some viral particles enter the fetal circulation in most pregnancies, Babik and colleagues examined cellular adaptive immunity and plasma inflammatory markers in the cord blood of 7 neonates born to HCV-positive women, compared to 8 neonates born to HCV-negative women. Although no HCV-specific T-cell responses were detected in the exposed neonates, relative to controls these exposed neonates had suppression of T-cell activation markers and plasma pro-inflammatory markers that was counterbalanced by increased capacity of T-cells to produce interferon-γ upon polyclonal stimulation63. These distinct changes in the fetal immune system were interpreted as evidence that the fetal immune system had come into contact with HCV. Several studies have examined HCV-specific T-cell responses in older aviremic children born to HCV-infected women64,65. HCV-specific T-cells were detected in some of the children, but it is not yet clear whether these responses were primed following in utero or later household exposure66, nor is it clear whether they contributed to immune protection or were simply a marker of past exposure. Genetic studies of HLA types have identified several candidate class II alleles associated with protection from or predisposition to vertical transmission, supporting a role of fetal antigen-specific CD4+ T-cell immunity in governing MTCT, but most candidate class II alleles have not been corroborated in subsequent studies67–69.
Infection, or at least association, of HCV with maternal peripheral blood mononuclear cells (PBMCs) has been linked to an increased risk of MTCT in studies by Azzari, Indolfi, Resti, and colleagues59,60,70. The authors reasoned that if maternal PBMCs are a conduit for HCV MTCT, then fetal alloimmune responses that target maternal cells may be protective against transmission, and mother/child pairs with concordance of HLA alleles (the primary molecules targeted by fetal alloimmune responses against leukocytes) should be at higher risk of MTCT. Concordance of class I molecules was not shown to be associated with increased risk of transmission71, but in support of their theory concordance of class II HLA molecules did in fact significantly increase the risk of vertical transmission67.
Additional genetic factors known to be determinants of infection outcome in adults have been examined for a role in the risk of HCV MTCT, including polymorphisms in IL-28B promotor34,72, mannose binding lectin, tumor necrosis factor-α, interferon-γ, IL-10, and transforming growth factor-β67. None of these were associated with altered risk of HCV MTCT, though IL-28B genotype was predictive of viral clearance in the infant once transmission had occurred72. Finally, fetal gender has been identified as a possible risk factor for MTCT, as a European collaborative study identified that females were twice as likely to be affected by MTCT than males (adjusted odds ratio [OR], 2.07 [95% CI, 1.23–3.48]; P=. 006)73.
Clinical Risk Factors for MTCT
Clinical risk factors for MTCT echo those seen in HIV: demonstrable viral load, exposure to maternal blood, prolonged rupture of membranes, and internal fetal monitoring. Maternal coinfection with HIV also increases this risk. (Table 5).
Viral Load
Much data exists regarding the risk that maternal viremia poses to MTCT. It is generally accepted that transmission does not occur in the setting of undetectable viral loads26,58,74,75, as there are only a few cases of such transmission in the literature potentially explained by lack of sensitivity of RNA testing to detect lower levels of viremia or transient viremia 76–78. Mast and colleagues submit that further studies of MTCT be limited to those who are RNA positive or at least stratified by RNA status26.
There appears to be an increased risk of MTCT with increasing levels of viremia. Steininger and colleagues demonstrated that in HCV-infected women undergoing vaginal delivery, viral loads were higher among transmitters than non-transmitters (8.1X 105 vs. 1.4 X 104, p =0.056)58, and Mast el al. had similar findings in HCV monoinfected mothers26. Dal Molin et al. found that women with markedly elevated levels above 107IU/ml appeared to be at particularly high risk of transmission (OR 10.2, p=0.03)75. Nevertheless, no lower threshold of viremia below which transmission does not occur has been determined. As a matter of practicality, obtaining viral load serves its best purpose in counseling patients who demonstrate absence of viremia.
Exposure to Maternal Blood
Steininger determined that children who delivered vaginally to mothers who sustained a vaginal or perineal laceration had 6 fold higher risks of becoming HCV-infected58. Exposure to maternal blood can be extrapolated in the setting of twins affected discordantly. Boxall et al. report on a 4 sets of twins where MTCT was discordant, and in 3 of the 4 sets, the second twin was the one infected. There were no episiotomy or internal monitors used in any case. They postulate that placental separation allows inoculation of the second twin, but it may be additionally considered that exposure to maternal blood after the delivery of the first twin is increased with the delivery of the second79.
Prolonged Rupture of Membranes and Internal Fetal Monitoring
The obstetric factors of prolonged monitoring and internal fetal monitoring (scalp electrodes and IUPC) have been shown to increase risk of MTCT. In cases where membrane rupture occurs >6 hours before delivery, higher rates of MTCT have been demonstrated.80,81. Mast and colleagues conducted a multivariate analysis restricted to HCV RNA–positive mothers and determined membrane rupture >6 h to be significantly associated with MTCT (odds ratio [OR], 9.3 [95% CI, 1.5–179.7]). Their data additionally identify use of internal fetal monitors to be similarly associated (OR, 6.7 [95% CI, 1.1–35.9])26.
Amniocentesis
Though Minola et al., 2001, acknowledge that amniocentesis may contribute to the risk of MTCT82, a prospective study of 22 HCV positive women were followed and compared to 11 controls. All women underwent amniocentesis in the 4th month of pregnancy. Of the 22 women, 16 demonstrated viremia, and in only 1 of the viremic women (6.3%) was HCV virus detected. This did not result in HCV infection in the offspring83. Additionally, as the single case of identification of HCV in the amniotic fluid occurred in the presence of anterior placentation, the possibility of contamination from transplacental passage does exist. The data on amniocentesis is limited in pregnancy, but is not thought to significantly increase the risk of MTCT.
Maternal Disease Severity
In a study among HCV-RNA positive mothers, elevated pre-pregnancy ALT levels were associated with an increased risk of vertical transmission, though quantitative viral load measurements were not available to distinguish if hepatocyte injury was an independent predictor of MTCT84.
HIV Coinfection
Maternal HIV coinfection has been consistently associated with an increased risk of HCV MTCT (OR 2.8 (95% CI, 1.17–6.81) upon meta-analysis).85 Reasons for this increased risk are unclear, but could relate to higher levels of HCV in maternal plasma86 or PBMC compartments,87 altered immunity at the placental barrier, or physical disruption of the placenta due to HIV infection of trophoblasts (reviewed in Le Campion et al.).45 To our knowledge, no studies have addressed whether suppression of HIV in the HAART era ameliorates this increased risk of HCV transmission in coinfected women.
Cesarean Delivery
Those interested in HCV MTCT see the inherent similarities to HIV with regard to obstetric risk factors for transmission of a blood-borne virus. Therefore, it is not difficult to extrapolate that interventions such as cesarean delivery might effectively eliminate peripartum transmission. Much interest was piqued when Gibb and colleagues published their work in 2000, examining the impact of mode of delivery on MTCT. In their study, 441 mother-infant pairs from the UK and Ireland were included. Overall vertical transmission rate was 6.7%, 3.8 times higher in HIV positive women. Transmission rates were 7.7% among 339 women undergoing vaginal delivery, 5.9% among 54 women undergoing emergency cesarean section, and 0% among 31 women undergoing elective cesarean section57. Though Paccagnini et al. suggested similar promise in their cohort88, the benefit of elective cesarean has yet to be confirmed. Many studies have found no association between mode of delivery and decreased MTCT rates73,89,90. One analysis found that elective cesarean section would need to substantially reduce the risk of perinatal transmission in order to be cost effective, e.g. a 77% reduction in transmission would be required if the background perinatal transmission rate were 7.7%91. Current evidence does not support the use of elective cesarean section to prevent MTCT. The Cochrane Database solidifies the recommendations, stating, “Currently there is no evidence from randomised controlled trials upon which to base any practice recommendations regarding planned caesarean section versus vaginal delivery for preventing mother to infant Hepatitis C Virus transmission”92.
Breastfeeding
Until recently the risk associated with breastfeeding in the setting of maternal infection with HCV has been controversial. While HCV virus is demonstrated in the human milk and colostrum93,94, the quantity is too low to infect the newborn. Other theories imply that gastric secretions may inactivate the virus57,80,94–96. Both the AAP and ACOG endorse safe breastfeeding as defined by absence of cracked, damaged or bleeding nipples. In the setting of HIV coinfection, recommendations remain to avoid breastfeeding 97.
Treatment of HCV during Pregnancy
Combined pegylated interferon-alfa (IFN-α) and ribavirin for 24–48 weeks have been standard therapy for HCV. However, this regimen results in a sustained virologic response (SVR, undetectable viral load through 6 months after completing treatment) in only about 50% of persons infected with the most prevalent genotype, and it is associated with a host of constitutional, psychiatric, hematologic and other side effects98. Treatment in pregnancy is not an option because of safety concerns. Ribavirin in particular is contraindicated (FDA pregnancy category X) because of teratogenic effects in numerous animal studies at low doses99. Ribavirin has along half-life and avoidance is recommended for women and their male partners starting 6 months prior to conception99. Nevertheless, preliminary data from a ribavirin birth registry have not corroborated teratogenic effects in humans99. Pegylated IFN-α is FDA pregnancy category C and experience is limited in pregnancy, though a recent review of IFN-α exposed pregnancies did not identify adverse fetal outcomes100. Therapy in the postpartum period has not been widely utilized because it is contraindicated in breastfeeding, and even for the non-breastfeeding mother therapy is not likely practical while caring for a young infant given the significant side effects of this regimen. Nevertheless breakthroughs in HCV drug development have raised hope for a new era of HCV therapy. A flurry of new drug candidates termed direct acting antivirals (DAAs) have been identified, including compounds that that target the HCV NS3/4A protease, the NS5B polymerase, the NS5A protein, as well as host proteins critical for HCV replication7. In 2011, two protease inhibitors telaprevir and boceprevir were FDA approved for genotype 1 infection. These drugs increase SVR rates for treatment naïve genotype 1 infections from 38–44% to 66–74%101. Viral resistance mutations emerge readily to these protease inhibitors necessitating their use as adjuncts to standard pegylated IFN-α and ribavirin therapy, but the hope is that new multi-drug cocktails in development will replace interferon.
Improved efficacy and tolerability of new drug regimens may open possibilities for treatment of HCV in pregnancy or the postpartum period. Antiviral treatment during pregnancy such as done for HIV may help prevent HCV MTCT, but it is not clear that this will be justified given that pediatric HCV may also be more readily curable with new drug regimens and pediatric HCV lacks the early consequences of pediatric HIV5. Antiviral treatment in the postpartum period however may prove to be a strategic approach for multiple reasons, including possible improved efficacy from a synergistic effect with rebounding immunity in this timeframe5,44,45, recent engagement of the HCV-infected mother in the health care system, and the potential to prevent future pregnancies complicated by maternal HCV. Further, improved efficacy of new drug regimens will require reassessment of the utility of universal screening for HCV in pregnant women. The cost-effectiveness analysis of universal HCV screening in pregnancy by Plunkett19 assumed treatment efficacy of 54% (range 50–58%), whereas it is conceivable that new regimens will have efficacy > 90%. Finally, as public health goals shift toward towards more aggressive screening of HCV in the general population to reduce HCV morbidity9 and eventually eliminate adult and pediatric HCV, universal HCV screening in pregnancy may prove to be a logical step.
Concluding Remarks
HCV as it relates to pregnancy is poorly understood, and practice patterns for optimal management of pregnancies affected by HCV have not been established. Obstetric risk factors for mother to child transmission of HCV share some similarities with the vertical transmission mode of human immunodeficiency virus. However, unlike HIV, effective methods of prevention of vertical transmission have not been developed for HCV. It is possible that a better understanding of MTCT of HCV infection will lead to useful prevention strategies, especially as we enter an era where interferon-free drug therapies are emerging as viable treatment options for HCV. If effective, they may represent pharmacotherapy appropriate for use during pregnancy, nullifying the arguments against comprehensive screening strategies in pregnancy.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network has prioritized a multi-center observational cohort study to further define risk factors for MTCT and adverse pregnancy outcomes associated with HCV in a prospective trial. It is hoped that this large clinical trial will not only address Institute of Medicine priorities outlined earlier, but in conjunction with the basic science efforts described in this paper, also help establish optimal practice patterns for women and their children affected by HCV.
Table 4.
Clinical Risk Factors for MTCT
| Maternal Risk Factor | Risk of MTCT |
|---|---|
| HCV PCR-Positive103–105 | 3–40% |
| HCV PCR-Negative56 | 0% |
| Breastfeeding58, 94, 95 | 0–3.7% |
| HIV Positive58, 86, 106, 107 | 15–26% |
| HIV Negative57, 86, 88, 108 | 0–12% |
| IV Drug Abuse and Transfusion104 | 7–12% |
| IV Drug Abuse95 | 8.6% |
| Ruptured Membranes >6 hours, PCR Positive26 | 8.6%, OR 9.3 (95%CI 1.5–179.7) |
| Internal Fetal Monitoring, PCR Positive26 | 18.8%, OR 6.7 (95% CI 1.1–35.9) |
Contributor Information
Mona R Prasad, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, Wexner Medical Center at The Ohio State University, 395 W 12th Avenue, Columbus, Ohio 43210.
Jonathan R. Honegger, Department of Pediatrics, The Ohio State University College of Medicine, Center for Vaccine and Immunity, The Research Institute at Nationwide Children’s Hospital.
References
- 1.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. The Lancet infectious diseases. 2005;5:558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Annals of internal medicine. 2006;144:705–14. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 3.Lauer GM, Walker BD. Hepatitis C virus infection. The New England journal of medicine. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 4.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 138:513–21. 21, e1–6. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 5.Arshad M, El-Kamary SS, Jhaveri R. Hepatitis C virus infection during pregnancy and the newborn period - are they opportunities for treatment? Journal of viral hepatitis. 2011;18:229–36. doi: 10.1111/j.1365-2893.2010.01413.x. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell AE, Colvin HM, Palmer Beasley R. Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology. 2010;51:729–33. doi: 10.1002/hep.23561. [DOI] [PubMed] [Google Scholar]
- 7.Poordad F, Dieterich D. Treating hepatitis C: current standard of care and emerging direct-acting antiviral agents. Journal of viral hepatitis. 2012;19:449–64. doi: 10.1111/j.1365-2893.2012.01617.x. [DOI] [PubMed] [Google Scholar]
- 8.Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. doi: 10.1038/nature08309. [DOI] [PubMed] [Google Scholar]
- 9.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 2012;61:1–32. [PubMed] [Google Scholar]
- 10.Cottrell EB, Chou R, Wasson N, Rahman B, Guise JM. Reducing risk for mother-to-infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Annals of internal medicine. 2012 doi: 10.7326/0003-4819-158-2-201301150-00575. [DOI] [PubMed] [Google Scholar]
- 11.ACOG Practice Bulletin No. 86: Viral hepatitis in pregnancy. Obstetrics and gynecology. 2007;110:941–56. doi: 10.1097/01.AOG.0000263930.28382.2a. [DOI] [PubMed] [Google Scholar]
- 12.Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recommendations and reports : Morbidity and mortality weekly report Recommendations and reports / Centers for Disease Control. 1998;47:1–39. [PubMed] [Google Scholar]
- 13.Giles M, Hellard M, Sasadeusz J. Hepatitis C and pregnancy: an update. Aust N Z J Obstet Gynaecol. 2003;43:290–3. doi: 10.1046/j.0004-8666.2003.00083.x. [DOI] [PubMed] [Google Scholar]
- 14.Blasig A, Wagner EC, Pi D, et al. Hepatitis C infection among pregnant women in British Columbia: reported prevalence and critical appraisal of current prenatal screening methods. Canadian journal of public health Revue canadienne de sante publique. 2011;102:98–102. doi: 10.1007/BF03404155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinto CS, Martins RM, Andrade SM, Stief AC, Oliveira RD, Castro AR. Hepatitis C virus infection among pregnant women in Central-Western Brazil, 2005–2007. Revista de saude publica. 2011;45:974–6. doi: 10.1590/s0034-89102011005000053. [DOI] [PubMed] [Google Scholar]
- 16.Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology. 2000;31:751–5. doi: 10.1002/hep.510310328. [DOI] [PubMed] [Google Scholar]
- 17.Ward C, Tudor-Williams G, Cotzias T, Hargreaves S, Regan L, Foster GR. Prevalence of hepatitis C among pregnant women attending an inner London obstetric department: uptake and acceptability of named antenatal testing. Gut. 2000;47:277–80. doi: 10.1136/gut.47.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delgado-Borrego A, Smith L, Jonas MM, et al. Expected and actual case ascertainment and treatment rates for children infected with hepatitis C in Florida and the United States: epidemiologic evidence from statewide and nationwide surveys. The Journal of pediatrics. 2012;161:915–21. doi: 10.1016/j.jpeds.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Plunkett BA, Grobman WA. Routine hepatitis C virus screening in pregnancy: a cost-effectiveness analysis. American journal of obstetrics and gynecology. 2005;192:1153–61. doi: 10.1016/j.ajog.2004.10.600. [DOI] [PubMed] [Google Scholar]
- 20.Wilson JM, Jungner YG. Public Health Paper. 1968. Principles and practices of screening for disease. [Google Scholar]
- 21.Nyamathi AM, Dixon EL, Robbins W, et al. Risk factors for hepatitis C virus infection among homeless adults. J Gen Intern Med. 2002;17:134–43. doi: 10.1046/j.1525-1497.2002.10415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox RK, Currie SL, Evans J, et al. Hepatitis C virus infection among prisoners in the California state correctional system. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2005;41:177–86. doi: 10.1086/430913. [DOI] [PubMed] [Google Scholar]
- 23.Macalino GE, Dhawan D, Rich JD. A missed opportunity: hepatitis C screening of prisoners. Am J Public Health. 2005;95:1739–40. doi: 10.2105/AJPH.2004.056291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bohman VR, Stettler RW, Little BB, Wendel GD, Sutor LJ, Cunningham FG. Seroprevalence and risk factors for hepatitis C virus antibody in pregnant women. Obstetrics and gynecology. 1992;80:609–13. [PubMed] [Google Scholar]
- 25.Silverman NS, Jenkin BK, Wu C, McGillen P, Knee G. Hepatitis C virus in pregnancy: seroprevalence and risk factors for infection. American journal of obstetrics and gynecology. 1993;169:583–7. doi: 10.1016/0002-9378(93)90627-u. [DOI] [PubMed] [Google Scholar]
- 26.Mast EE, Hwang LY, Seto DS, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. The Journal of infectious diseases. 2005;192:1880–9. doi: 10.1086/497701. [DOI] [PubMed] [Google Scholar]
- 27.Bradley JS, Graham S, Picchio GR, Vugia DJ, Kharrazi M. Prevalence of hepatitis C virus antibody in newborn infants in southern California in 2003. The Pediatric infectious disease journal. 2011;30:618–20. doi: 10.1097/INF.0b013e318214dc4d. [DOI] [PubMed] [Google Scholar]
- 28.Notes from the field: risk factors for hepatitis C virus infections among young adults--Massachusetts, 2010. MMWR Morbidity and mortality weekly report. 2011;60:1457–8. [PubMed] [Google Scholar]
- 29.Notes from the field : hepatitis C virus infections among young adults--rural Wisconsin, 2010. MMWR Morbidity and mortality weekly report. 2012;61:358. [PubMed] [Google Scholar]
- 30.Salihu HM, Connell L, Salemi JL, August EM, Weldeselasse HE, Alio AP. Prevalence and temporal trends of hepatitis B, hepatitis C, and HIV/AIDS co-infection during pregnancy across the decade, 1998–2007. J Womens Health (Larchmt) 2012;21:66–72. doi: 10.1089/jwh.2011.2979. [DOI] [PubMed] [Google Scholar]
- 31.Alter MJ, Margolis HS, Krawczynski K, et al. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. The New England journal of medicine. 1992;327:1899–905. doi: 10.1056/NEJM199212313272702. [DOI] [PubMed] [Google Scholar]
- 32.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21–9. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 33.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. The Journal of clinical investigation. 2009;119:1745–54. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas DL, Thio CL, Martin MP, et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature. 2009;461:798–801. doi: 10.1038/nature08463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khakoo SI, Thio CL, Martin MP, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–4. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 36.Kogure T, Ueno Y, Kanno N, et al. Sustained viral response of a case of acute hepatitis C virus infection via needle-stick injury. World journal of gastroenterology : WJG. 2006;12:4757–60. doi: 10.3748/wjg.v12.i29.4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez F, Medam-Djomo MA, Lucidarme D, et al. Acute hepatitis C during the third trimester of pregnancy. Gastroenterologie clinique et biologique. 2006;30:786–9. doi: 10.1016/s0399-8320(06)73316-4. [DOI] [PubMed] [Google Scholar]
- 38.Wejstal R, Widell A, Norkrans G. HCV-RNA levels increase during pregnancy in women with chronic hepatitis C. Scand J Infect Dis. 1998;30:111–3. doi: 10.1080/003655498750003456. [DOI] [PubMed] [Google Scholar]
- 39.Gervais A, Bacq Y, Bernuau J, et al. Decrease in serum ALT and increase in serum HCV RNA during pregnancy in women with chronic hepatitis C. Journal of hepatology. 2000;32:293–9. doi: 10.1016/s0168-8278(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 40.Paternoster DM, Santarossa C, Grella P, et al. Viral load in HCV RNA-positive pregnant women. The American journal of gastroenterology. 2001;96:2751–4. doi: 10.1111/j.1572-0241.2001.04135.x. [DOI] [PubMed] [Google Scholar]
- 41.Lin HH, Kao JH. Hepatitis C virus load during pregnancy and puerperium. Bjog. 2000;107:1503–6. doi: 10.1111/j.1471-0528.2000.tb11675.x. [DOI] [PubMed] [Google Scholar]
- 42.Hattori Y, Orito E, Ohno T, et al. Loss of hepatitis C virus RNA after parturition in female patients with chronic HCV infection. Journal of medical virology. 2003;71:205–11. doi: 10.1002/jmv.10471. [DOI] [PubMed] [Google Scholar]
- 43.Watanabe H, Saito T, Shinzawa H, et al. Spontaneous elimination of serum hepatitis C virus (HCV) RNA in chronic HCV carriers: a population-based cohort study. Journal of medical virology. 2003;71:56–61. doi: 10.1002/jmv.10448. [DOI] [PubMed] [Google Scholar]
- 44.Honegger J, Prasad M, Walker C. Broadened Virus-Specific T-cell Responses are Associated with Postpartum Declines in Hepatitis C Viremia, Abstract 1225. Infectious Diseases Society of America Annual Meeting; San Diego. 2012. [Google Scholar]
- 45.Le Campion A, Larouche A, Fauteux-Daniel S, Soudeyns H. Pathogenesis of hepatitis C during pregnancy and childhood. Viruses. 2012;4:3531–50. doi: 10.3390/v4123531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Connell LE, Salihu HM, Salemi JL, August EM, Weldeselasse H, Mbah AK. Maternal hepatitis B and hepatitis C carrier status and perinatal outcomes. Liver international : official journal of the International Association for the Study of the Liver. 2011;31:1163–70. doi: 10.1111/j.1478-3231.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- 47.Floreani A, Paternoster D, Zappala F, et al. Hepatitis C virus infection in pregnancy. British journal of obstetrics and gynaecology. 1996;103:325–9. doi: 10.1111/j.1471-0528.1996.tb09736.x. [DOI] [PubMed] [Google Scholar]
- 48.Hillemanns P, Dannecker C, Kimmig R, Hasbargen U. Obstetric risks and vertical transmission of hepatitis C virus infection in pregnancy. Acta obstetricia et gynecologica Scandinavica. 2000;79:543–7. [PubMed] [Google Scholar]
- 49.Pergam SA, Wang CC, Gardella CM, Sandison TG, Phipps WT, Hawes SE. Pregnancy complications associated with hepatitis C: data from a 2003–2005 Washington state birth cohort. American journal of obstetrics and gynecology. 2008;199:38, e1–9. doi: 10.1016/j.ajog.2008.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reddick KL, Jhaveri R, Gandhi M, James AH, Swamy GK. Pregnancy outcomes associated with viral hepatitis. Journal of viral hepatitis. 2011;18:e394–8. doi: 10.1111/j.1365-2893.2011.01436.x. [DOI] [PubMed] [Google Scholar]
- 51.Berkley EM, Leslie KK, Arora S, Qualls C, Dunkelberg JC. Chronic hepatitis C in pregnancy. Obstetrics and gynecology. 2008;112:304–10. doi: 10.1097/AOG.0b013e318180a4f3. [DOI] [PubMed] [Google Scholar]
- 52.Locatelli A, Roncaglia N, Arreghini A, Bellini P, Vergani P, Ghidini A. Hepatitis C virus infection is associated with a higher incidence of cholestasis of pregnancy. British journal of obstetrics and gynaecology. 1999;106:498–500. doi: 10.1111/j.1471-0528.1999.tb08305.x. [DOI] [PubMed] [Google Scholar]
- 53.Paternoster DM, Fabris F, Palu G, et al. Intra-hepatic cholestasis of pregnancy in hepatitis C virus infection. Acta obstetricia et gynecologica Scandinavica. 2002;81:99–103. [PubMed] [Google Scholar]
- 54.White DL, Ratziu V, El-Serag HB. Hepatitis C infection and risk of diabetes: a systematic review and meta-analysis. Journal of hepatology. 2008;49:831–44. doi: 10.1016/j.jhep.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hurtado CW, Golden-Mason L, Brocato M, Krull M, Narkewicz MR, Rosen HR. Innate immune function in placenta and cord blood of hepatitis C--seropositive mother-infant dyads. PloS one. 2010;5:e12232. doi: 10.1371/journal.pone.0012232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mok J, Pembrey L, Tovo PA, Newell ML. When does mother to child transmission of hepatitis C virus occur? Arch Dis Child Fetal Neonatal Ed. 2005;90:F156–60. doi: 10.1136/adc.2004.059436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gibb DM, Goodall RL, Dunn DT, et al. Mother-to-child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet. 2000;356:904–7. doi: 10.1016/s0140-6736(00)02681-7. [DOI] [PubMed] [Google Scholar]
- 58.Steininger C, Kundi M, Jatzko G, Kiss H, Lischka A, Holzmann H. Increased risk of mother-to-infant transmission of hepatitis C virus by intrapartum infantile exposure to maternal blood. The Journal of infectious diseases. 2003;187:345–51. doi: 10.1086/367704. [DOI] [PubMed] [Google Scholar]
- 59.Azzari C, Moriondo M, Indolfi G, et al. Higher risk of hepatitis C virus perinatal transmission from drug user mothers is mediated by peripheral blood mononuclear cell infection. Journal of medical virology. 2008;80:65–71. doi: 10.1002/jmv.21023. [DOI] [PubMed] [Google Scholar]
- 60.Azzari C, Resti M, Moriondo M, Ferrari R, Lionetti P, Vierucci A. Vertical transmission of HCV is related to maternal peripheral blood mononuclear cell infection. Blood. 2000;96:2045–8. [PubMed] [Google Scholar]
- 61.Nie QH, Gao LH, Cheng YQ, et al. Hepatitis C virus infection of human cytotrophoblasts cultured in vitro. Journal of medical virology. 2012;84:1586–92. doi: 10.1002/jmv.23380. [DOI] [PubMed] [Google Scholar]
- 62.Mostafavi A, Arshad M, Qiang G, Bradrick S, Jhaveri R. Examining Cells of Trophoblastic Origin for Permissiveness for Hepatitis C virus Replication, Abstract 1338. Infectious Diseases Society of America Annual Meeting; San Diego. 2012. [Google Scholar]
- 63.Babik JM, Cohan D, Monto A, Hartigan-O’Connor DJ, McCune JM. The human fetal immune response to hepatitis C virus exposure in utero. The Journal of infectious diseases. 2011;203:196–206. doi: 10.1093/infdis/jiq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-Kamary SS, Hashem M, Saleh DA, et al. Hepatitis C Virus-Specific Cell-Mediated Immune Responses in Children Born to Mothers Infected with Hepatitis C Virus. The Journal of pediatrics. 2013;162:148–54. doi: 10.1016/j.jpeds.2012.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Della Bella S, Riva A, Tanzi E, et al. Hepatitis C virus-specific reactivity of CD4+-lymphocytes in children born from HCV-infected women. Journal of hepatology. 2005;43:394–402. doi: 10.1016/j.jhep.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 66.Hashem M, El-Karaksy H, Shata MT, et al. Strong Hepatitis C Virus (HCV)-specific Cell-mediated Immune Responses in the Absence of Viremia or Antibodies Among Uninfected Siblings of HCV Chronically Infected Children. The Journal of infectious diseases. 2011;203:854–61. doi: 10.1093/infdis/jiq123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bevilacqua E, Fabris A, Floreano P, et al. Genetic factors in mother-to-child transmission of HCV infection. Virology. 2009;390:64–70. doi: 10.1016/j.virol.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Bosi I, Ancora G, Mantovani W, et al. HLA DR13 and HCV vertical infection. Pediatric research. 2002;51:746–9. doi: 10.1203/00006450-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 69.Martinetti M, Pacati I, Cuccia M, et al. Hierarchy of baby-linked immunogenetic risk factors in the vertical transmission of hepatitis C virus. International journal of immunopathology and pharmacology. 2006;19:369–78. doi: 10.1177/039463200601900213. [DOI] [PubMed] [Google Scholar]
- 70.Indolfi G, Resti M. Perinatal transmission of hepatitis C virus infection. Journal of medical virology. 2009;81:836–43. doi: 10.1002/jmv.21437. [DOI] [PubMed] [Google Scholar]
- 71.Azzari C, Indolfi G, Betti L, et al. Vertical hepatitis C virus transmission is not related to mother-child class-1 HLA concordance. International journal of immunopathology and pharmacology. 2007;20:827–31. doi: 10.1177/039463200702000419. [DOI] [PubMed] [Google Scholar]
- 72.Ruiz-Extremera A, Munoz-Gamez JA, Salmeron-Ruiz MA, et al. Genetic variation in interleukin 28B with respect to vertical transmission of hepatitis C virus and spontaneous clearance in HCV-infected children. Hepatology. 2011;53:1830–8. doi: 10.1002/hep.24298. [DOI] [PubMed] [Google Scholar]
- 73.A significant sex--but not elective cesarean section--effect on mother-to-child transmission of hepatitis C virus infection. The Journal of infectious diseases. 2005;192:1872–9. doi: 10.1086/497695. [DOI] [PubMed] [Google Scholar]
- 74.Giacchino R, Tasso L, Timitilli A, et al. Vertical transmission of hepatitis C virus infection: usefulness of viremia detection in HIV-seronegative hepatitis C virus-seropositive mothers. The Journal of pediatrics. 1998;132:167–9. doi: 10.1016/s0022-3476(98)70507-4. [DOI] [PubMed] [Google Scholar]
- 75.Dal Molin G, D’Agaro P, Ansaldi F, et al. Mother-to-infant transmission of hepatitis C virus: rate of infection and assessment of viral load and IgM anti-HCV as risk factors. Journal of medical virology. 2002;67:137–42. doi: 10.1002/jmv.2202. [DOI] [PubMed] [Google Scholar]
- 76.Granovsky MO, Minkoff HL, Tess BH, et al. Hepatitis C virus infection in the mothers and infants cohort study. Pediatrics. 1998;102:355–9. doi: 10.1542/peds.102.2.355. [DOI] [PubMed] [Google Scholar]
- 77.Resti M, Azzari C, Galli L, et al. Maternal drug use is a preeminent risk factor for mother-to-child hepatitis C virus transmission: results from a multicenter study of 1372 mother-infant pairs. The Journal of infectious diseases. 2002;185:567–72. doi: 10.1086/339013. [DOI] [PubMed] [Google Scholar]
- 78.Dore GJ, Kaldor JM, McCaughan GW. Systematic review of role of polymerase chain reaction in defining infectiousness among people infected with hepatitis C virus. BMJ. 1997;315:333–7. doi: 10.1136/bmj.315.7104.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boxall E, Baumann K, Price N, Sira J, Brown M, Kelly D. Discordant outcome of perinatal transmission of hepatitis C in twin pregnancies. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2007;38:91–5. doi: 10.1016/j.jcv.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 80.Spencer JD, Latt N, Beeby PJ, et al. Transmission of hepatitis C virus to infants of human immunodeficiency virus-negative intravenous drug-using mothers: rate of infection and assessment of risk factors for transmission. Journal of viral hepatitis. 1997;4:395–409. doi: 10.1046/j.1365-2893.1997.00073.x. [DOI] [PubMed] [Google Scholar]
- 81.Thomas SL, Newell ML, Peckham CS, Ades AE, Hall AJ. A review of hepatitis C virus (HCV) vertical transmission: risks of transmission to infants born to mothers with and without HCV viraemia or human immunodeficiency virus infection. International journal of epidemiology. 1998;27:108–17. doi: 10.1093/ije/27.1.108. [DOI] [PubMed] [Google Scholar]
- 82.Minola E, Maccabruni A, Pacati I, Martinetti M. Amniocentesis as a possible risk factor for mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;33:1341–2. doi: 10.1053/jhep.2001.0103305le02. [DOI] [PubMed] [Google Scholar]
- 83.Delamare C, Carbonne B, Heim N, et al. Detection of hepatitis C virus RNA (HCV RNA) in amniotic fluid: a prospective study. Journal of hepatology. 1999;31:416–20. doi: 10.1016/s0168-8278(99)80031-2. [DOI] [PubMed] [Google Scholar]
- 84.Indolfi G, Azzari C, Moriondo M, Lippi F, de Martino M, Resti M. Alanine transaminase levels in the year before pregnancy predict the risk of hepatitis C virus vertical transmission. Journal of medical virology. 2006;78:911–4. doi: 10.1002/jmv.20640. [DOI] [PubMed] [Google Scholar]
- 85.Polis CB, Shah SN, Johnson KE, Gupta A. Impact of maternal HIV coinfection on the vertical transmission of hepatitis C virus: a meta-analysis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;44:1123–31. doi: 10.1086/512815. [DOI] [PubMed] [Google Scholar]
- 86.Zanetti AR, Tanzi E, Paccagnini S, et al. Mother-to-infant transmission of hepatitis C virus. Lombardy Study Group on Vertical HCV Transmission Lancet. 1995;345:289–91. doi: 10.1016/s0140-6736(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 87.Blackard JT, Smeaton L, Hiasa Y, et al. Detection of hepatitis C virus (HCV) in serum and peripheral-blood mononuclear cells from HCV-monoinfected and HIV/HCV-coinfected persons. The Journal of infectious diseases. 2005;192:258–65. doi: 10.1086/430949. [DOI] [PubMed] [Google Scholar]
- 88.Paccagnini S, Principi N, Massironi E, et al. Perinatal transmission and manifestation of hepatitis C virus infection in a high risk population. The Pediatric infectious disease journal. 1995;14:195–9. doi: 10.1097/00006454-199503000-00005. [DOI] [PubMed] [Google Scholar]
- 89.Airoldi J, Berghella V. Hepatitis C and pregnancy. Obstetrical & gynecological survey. 2006;61:666–72. doi: 10.1097/01.ogx.0000238671.13495.33. [DOI] [PubMed] [Google Scholar]
- 90.Roberts EA, Yeung L. Maternal-infant transmission of hepatitis C virus infection. Hepatology. 2002;36:S106–13. doi: 10.1053/jhep.2002.36792. [DOI] [PubMed] [Google Scholar]
- 91.Plunkett BA, Grobman WA. Elective cesarean delivery to prevent perinatal transmission of hepatitis C virus: a cost-effectiveness analysis. American journal of obstetrics and gynecology. 2004;191:998–1003. doi: 10.1016/j.ajog.2004.05.062. [DOI] [PubMed] [Google Scholar]
- 92.McIntyre PG, Tosh K, McGuire W. Caesarean section versus vaginal delivery for preventing mother to infant hepatitis C virus transmission. Cochrane database of systematic reviews (Online) 2006:CD005546. doi: 10.1002/14651858.CD005546.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kumar RM, Shahul S. Role of breast-feeding in transmission of hepatitis C virus to infants of HCV-infected mothers. Journal of hepatology. 1998;29:191–7. doi: 10.1016/s0168-8278(98)80003-2. [DOI] [PubMed] [Google Scholar]
- 94.Lin HH, Kao JH, Hsu HY, et al. Absence of infection in breast-fed infants born to hepatitis C virus-infected mothers. The Journal of pediatrics. 1995;126:589–91. doi: 10.1016/s0022-3476(95)70356-x. [DOI] [PubMed] [Google Scholar]
- 95.Yeung LT, King SM, Roberts EA. Mother-to-infant transmission of hepatitis C virus. Hepatology. 2001;34:223–9. doi: 10.1053/jhep.2001.25885. [DOI] [PubMed] [Google Scholar]
- 96.Effects of mode of delivery and infant feeding on the risk of mother-to-child transmission of hepatitis C virus. European Paediatric Hepatitis C Virus Network. BJOG. 2001;108:371–7. [PubMed] [Google Scholar]
- 97.ACOG. Practice Bulletin 86: Viral Hepatitis in Pregnancy. 2007. [DOI] [PubMed] [Google Scholar]
- 98.Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roberts SS, Miller RK, Jones JK, et al. The Ribavirin Pregnancy Registry: Findings after 5 years of enrollment, 2003–2009. Birth defects research Part A, Clinical and molecular teratology. 2010;88:551–9. doi: 10.1002/bdra.20682. [DOI] [PubMed] [Google Scholar]
- 100.Yazdani Brojeni P, Matok I, Garcia Bournissen F, Koren G. A systematic review of the fetal safety of interferon alpha. Reprod Toxicol. 2012;33:265–8. doi: 10.1016/j.reprotox.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 101.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433–44. doi: 10.1002/hep.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Medicine Io. Hepatitis and Liver Cancer: A National Stategy for Prevention and Control of Hepatitis B and C. 2010. [PubMed] [Google Scholar]
- 103.La Torre A, Biadaioli R, Capobianco T, et al. Vertical transmission of HCV. Acta obstetricia et gynecologica Scandinavica. 1998;77:889–92. [PubMed] [Google Scholar]
- 104.Resti M, Azzari C, Mannelli F, et al. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV-1. Tuscany Study Group on Hepatitis C Virus Infection. BMJ. 1998;317:437–41. doi: 10.1136/bmj.317.7156.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ohto H, Terazawa S, Sasaki N, et al. Transmission of hepatitis C virus from mothers to infants. The Vertical Transmission of Hepatitis C Virus Collaborative Study Group. The New England journal of medicine. 1994;330:744–50. doi: 10.1056/NEJM199403173301103. [DOI] [PubMed] [Google Scholar]
- 106.Tovo PA, Palomba E, Ferraris G, et al. Increased risk of maternal-infant hepatitis C virus transmission for women coinfected with human immunodeficiency virus type 1. Italian Study Group for HCV Infection in Children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1997;25:1121–4. doi: 10.1086/516102. [DOI] [PubMed] [Google Scholar]
- 107.Ferrero S, Lungaro P, Bruzzone BM, Gotta C, Bentivoglio G, Ragni N. Prospective study of mother-to-infant transmission of hepatitis C virus: a 10-year survey (1990–2000) Acta obstetricia et gynecologica Scandinavica. 2003;82:229–34. doi: 10.1034/j.1600-0412.2003.00107.x. [DOI] [PubMed] [Google Scholar]
- 108.Fischler B, Lindh G, Lindgren S, et al. Vertical transmission of hepatitis C virus infection. Scandinavian journal of infectious diseases. 1996;28:353–6. doi: 10.3109/00365549609037918. [DOI] [PubMed] [Google Scholar]