Abstract
Transient global ischemia in rats induces delayed death of hippocampal CA1 neurons. Early events include caspase activation, cleavage of anti-death Bcl-2 family proteins and large mitochondrial channel activity. However, a causal role of these events in ischemia-induced neuronal death is unclear. Unexpectedly, we found that the Bcl-2/Bcl-xL inhibitor ABT-737, which enhances death of tumor cells, protects rats against neuronal death in a clinically relevant model of brain ischemia. Bcl-xL is prominently expressed in adult neurons and can be cleaved by caspases to generate a pro-death fragment ΔN-Bcl-xL. We found that ABT-737 administered before or after ischemia inhibited ΔN-Bcl-xL-induced mitochondrial channel activity and neuronal death. To establish a causal role for ΔN-Bcl-xL, we generated knockin mice expressing caspase-resistant Bcl-xL. The knockin mice exhibit strikingly reduced mitochondrial channel activity and reduced vulnerability to ischemia-induced neuronal death. These findings point to truncated Bcl-xL as a potentially important therapeutic target in ischemic brain injury.
Transient global or forebrain ischemia, arising as a consequence of cardiac arrest or cardiac surgery in humans or induced experimentally in animals, causes selective, delayed death of hippocampal CA1 pyramidal neurons and cognitive deficits at 3-7 days after insult1-3. Early events are disruption of the functional integrity of the outer mitochondrial membrane by the formation of large ion channels, mitochondrial release of cytochrome c, and activation of caspases4-6. Although it is clear that caspases are engaged in response to global ischemia, a full understanding of the role of caspase substrates in global ischemia-induced death is still unknown 7.
Bcl-2 family proteins regulate apoptotic cell death by controlling the permeability of the outer mitochondrial membrane. The prevailing view is that anti-apoptotic Bcl-2 family members such as BcL-xL prevent homo-oligomerization of pro-apoptotic family members Bax and Bak in the outer mitochondrial membrane, thereby preventing the release of cytochrome c to promote caspase activation and apoptotic cell death. Bcl-2/Bcl-xL act by directly inhibiting Bax/Bak and by inhibiting the activators of Bax/Bak8, 9. The cancer chemotherapeutic agent, ABT-737 mimics the BH3 domain of pro-apoptotic family proteins and binds Bcl-xL, Bcl-2, and Bcl-w with high affinity to inhibit their anti-apoptotic activity 10-13
Bcl-xL not only influences neuronal survival, but also modulates neuronal activity under physiological conditions. Introduction of recombinant Bcl-xL protein into the presynaptic terminal of the squid giant axon potentiates transmitter release and vesicle recycling following intense synaptic activity 14, and injection of the Bcl-2/Bcl-xL inhibitor ABT-737 slows recovery of synaptic responses. In contrast, in hypoxic conditions, ABT-737 increases synaptic transmission, preventing hypoxia-induced synaptic rundown at the squid giant synapse15. Moreover, caspase activation is critical to induction of long term depression at Schaffer collateral to CA1 synapses 16. This raises the unexpected possibility that Bcl-xL and/or other targets of ABT-737 can have opposing effects on synaptic strength, depending on whether the synapse is hypoxic. One way in which this could occur is through proteolytic cleavage of BcL-xL to generate its pro-death fragment ΔN-Bcl-xL17.
Bcl-2 family proteins are substrates for caspases and other proteases; cleavage generally elicits pro-death activitiy18,19, 20. Application of recombinant ΔN-Bcl-xL, the C-terminal cleavage product of Bcl-xL, activates large conductance channel activity21 that mimics channel activity in mitochondria of postischemic neurons6,21. The present study provides evidence for a causal role of caspase-cleaved Bcl-xL in formation of mitochondrial channel activity. We show that treatment of animals with the Bcl-xL inhibitor ABT-737 prior to or after induction of global ischemia in vivo markedly inhibits ischemia-induced formation of large channel activity in mitochondria and neuronal death. Mutation of the caspase cleavage sites in Bcl-xL in mice attenuates ischemia-induced neuronal death. Our findings indicate that ABT-737 prevents cleavage of Bcl-xL and inhibits the activity of cleaved Bcl-xL, thereby affording protection against ischemia-induced neuronal death.
RESULTS
ABT-737 attenuates ischemia-induced neuronal death in rats
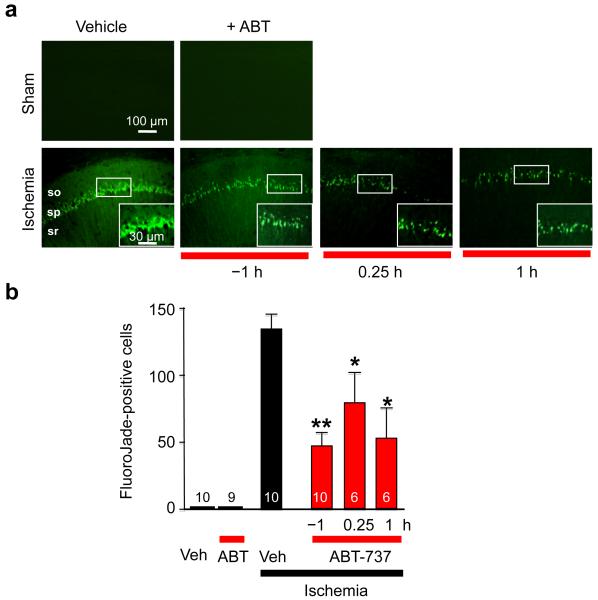
Transient global ischemia in rats, induced by the 4-vessel occlusion model (10 min), followed by reperfusion, mimics global ischemia arising in human brain following cardiac arrest5, 6, 22. Although the entire brain becomes hypoxic, neurons throughout the brain depolarize, ATP is depleted and a massive rise in intracellular Ca2+ occurs, global ischemia elicits highly selective, delayed death primarily of hippocampal CA1 pyramidal neurons. We first sought to determine whether anti-apoptotic Bcl-xL, protein, which is abundantly expressed in adult hippocampal neurons23, protects neurons from ischemia-induced death. Surprisingly, pretreatment of animals with ABT-737 (1 μM final, a concentration sufficient to inhibit Bcl-xL in vivo,10 injected unilaterally into the right ventricle stereotactically at 1 h prior to transient global ischemia), significantly inhibited neuronal death in the hippocampal CA1, assessed by the number of neurons that are positive for Fluoro-Jade (FJ), a marker of degenerating neurons (Fig. 1a, b) and by neuronal counts of toluidine blue-stained sections at 6 d postischemia relative to that of vehicle-injected animals subjected to ischemia (Supplementary Fig. 1a, b). To test ABT-737 in a more clinically relevant scenario, ABT-737 was administered at 15 min or 1 h following reperfusion. Under these conditions, ABT-737 afforded robust neuroprotection against ischemia-induced neuronal death in the hippocampal CA1 (Fig. 1a, b). In addition, ABT-737 attenuated activated caspase-3 as late as 5 d after ischemia (Supplementary Fig. 2). This is a novel and unexpected finding, given the extensive evidence that caspase-3 activity is increased as early as 1-3 h after ischemia5, 21, 24, 25 and peaks at approximately 24 h after ischemia5, 24 and that ultrastructural studies26 provide compelling evidence that postischemic neurons exhibit morphological features of necrotic injury. These findings, together with our previous findings, demonstrate that ABT-737 suppresses a pro-death activity over an extensive time period.
Figure 1. Treatment with the Bcl-xL inhibitor ABT-737 protects against ischemia-induced neuronal death in the CA1.
(a) High magnification images taken at 5 d after ischemia of Fluoro-Jade-labeled brain sections at the level of the dorsal hippocampus from animals treated with vehicle or ABT-737 (icv, 125 μM) at three times (1 h before ischemia (−1 h), 15 min after ischemia (+15 m), and 1 h after ischemia (+1 h)) and subjected to sham operation or global ischemia. (b) Histograms ± S.E.M (in all Figs.) showing number of degenerating neurons per region of interest within the hippocampal CA1 pyramidal cell layer. n = 4 sections per animal; number of animals per treatment group as indicated on bars, ttest *, P < 0.05. **, P < 0.01. ***, P < 0.001. “Veh”, vehicle, “ABT”, ABT-737.
Pretreatment of rats with ABT-737 attenuates ischemia-induced mitochondrial channel activity
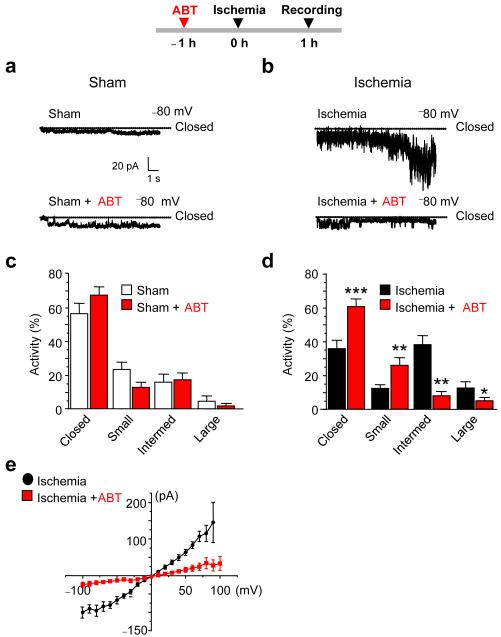
Transient global ischemia in vivo induces large channel openings in the outer mitochondrial membrane of whole mitochondria isolated from brain6, 21. We next examined whether ABT-737 attenuates large channel activity in mitochondria by performing patch clamp recordings of whole mitochondria isolated from the hippocampal CA1 at 1 h after ischemia or sham surgery. ABT-737 (administered at 1 h prior to ischemia) had little or no effect on sham-operated animals, but markedly attenuated the appearance of ischemia-induced large (>760 pS) and intermediate (180-760 pS) conductance mitochondrial channel activity, and increased the closed-time and prevalence of small (<180 pS) channel activity (Fig. 2a-d). ABT-737 reduced the peak conductance of channel openings assessed by current-voltage analysis (Fig. 2e) and peak conductance at a single voltage (ABT-737-treated mitochondria, 118 ± 24 pS, n = 5 mitochondria, 3 animals; vehicle treated mitochondria, 922 ± 76 pS. n = 7 mitochondria, 3 animals, P < 0.0001). Thus, ABT-737 attenuates ischemia-induced induction of mitochondrial channel activity.
Figure 2. The Bcl-xL inhibitor ABT-737 blocks ischemia-induced mitochondrial channel formation.
(a,b) Sample recordings from mitochondria isolated from the hippocampus at 1 h after ischemia. Animals were pretreated with ABT-737 (icv, 125 μM) or vehicle 1 prior to induction of global ischemia. Organelle-attached patches were recorded at Vh = −80 mV. (c,d) Histograms showing channel activity for recordings like those illustrated in (a,b). Channel activity was classified as follows: closed, small: < 180 pS, intermediate: 180 to 760 pS, and large: > 760 pS. For sham-operated vehicle-treated n=5 mitochondria, 35 traces (10 s per trace); ABT-737-treated, n=8 mitochondria, 51 traces. For ischemia, vehicle-treated, n = 10 mitochondria, 68 traces; ABT-737, n = 9 mitochondria, 62 traces. 6 animals were used to pool mitochondria for each condition. (e) Current–voltage relations for recordings from mitochondria as in a, b. Vehicle, n = 7 mitochondria from 3 animals; ABT-737, n = 4 mitochondria from 3 animals. Pretreatment of animals with ABT-737 prior to ischemia decreased the slope of the I-V relationship, indicative of decreased conductance through the mitochondrial outer membrane. ttest **, P < 0.001. ***, P < 0.0001. “Intermed”, Intermediate.
ABT-737 directly attenuates ischemia-induced mitochondrial channel activity
To examine the impact of ABT-737 delivered to intact animals on mitochondrial channel activity, ABT-737 was applied in vitro to mitochondria isolated from hippocampal CA1 at 1 h after global ischemia. Application of ABT-737 via the patch pipette and bath perfusate markedly reduced ischemia-induced channel activity in isolated post-ischemic mitochondria (Fig. 3a, b). Moreover, ABT-737 reduced the peak conductance of channel openings, assessed by current-voltage analysis (Fig. 3c) and by determination of channel openings at a single voltage (ABT-737, 398 ± 62 pS, n = 8; vehicle, 966 ± 241 pS, n = 11, P < 0.05).
Figure 3. ABT-737 directly attenuates channel activity in post-ischemic mitochondria.
(a) Representative sample recordings from mitochondria isolated from the CA1 of the hippocampus of experimental animals at 1 h after ischemia and treated in vitro in the absence or presence of ABT-737 (5 μM, applied via patch pipette and bath perfusate). Organelle-attached patches were recorded at Vh −100 mV. (b) Histograms showing closed, small, intermediate and large channel activity for mitochondrial recordings as in a. Channel activity was classified as described in the legend to Figure 2. Ischemia, n = 10 independent mitochondria recorded from 3 animals; ischemia + ABT-737, n = 9 independent mitochondria, recorded from 3 animals (c) Current–voltage relations for recordings from mitochondria as in a; ischemia, n = 11 mitochondria; ischemia + ABT, n = 7 mitochondria). ttest ***, P < 0.0001; **, P < 0.005; *, P < 0.05.
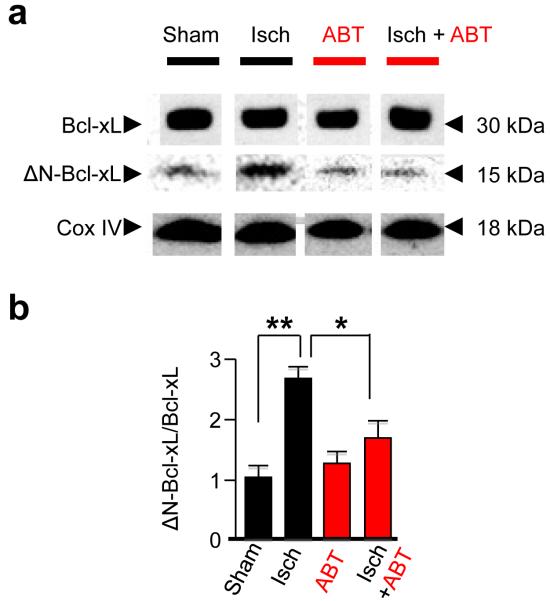
Because ABT-737 does not inhibit pro-apoptotic Bcl-2 family members Bax and Bak 10, these proteins are presumably not the targets of ABT-737 relevant to protection against ischemic injury. However, Bcl-xL, a known target of ABT-73710 can be proteolytically-processed to release pro-apoptotic C-terminal cleavage fragments17, 27. We examined the impact of ischemia on the appearance of cleaved Bcl-xL in CA1. Under physiological conditions, ΔN61-Bcl-xL was present at low abundance (Fig. 4a, b), consistent with the possibility that caspases may have physiological, as well as a pathological, functions16. Ischemia increased ΔN61-Bcl-xL abundance, assessed by Westerns probed with an antibody specific for ΔN61-Bcl-xL (n = 5 independent experiments). Ischemia promoted formation of cleaved Bcl-xL evident at 1 h and at 24 h, but levels declined to near control values by 48 h, a time when histologically-detectable cell death is first apparent (Fig. 4a,b, Supplementary Fig. 3)21. In contrast, Bcl-2 and Bcl-w were unchanged (Supplementary Fig. 3).
Figure 4. Pretreatment of animals with ABT-737 attenuates ischemia-induced cleavage of Bcl-xL.
(a) Western blot analysis of protein samples from the mitochondrial fraction of the hippocampus taken at 1 hr after surgery from animals pretreated with vehicle or ABT-737 (icv, 125 μM) and subjected to sham operation or global ischemia. Pretreatment of animals with ABT-737 markedly attenuated appearance of ΔN-Bcl-xL, the cleavage fragment of Bcl-xL. (b) Summary data showing Bcl-xL and ΔN-Bcl-xL abundance. The abundance of ΔN-Bcl-xL was normalized to that of full-length Bcl-xL by means of ImageJ software. Sham, n = 5; ischemia, n = 7; sham+ABT-737, n = 5; ischemia+ABT-737, n = 6 animals; ttest *, P < 0.05, **, P < 0.005. “Isch”, Ischemia. Western blot images illustrated in panel a have been cropped. Full-length blots are presented in Supplementary Fig. 11.
ABT-737 inhibits ΔN-Bcl-xL–elicited channel activity of mammalian brain mitochondria
In squid giant synapse, injection of the Bcl-2/Bcl-xL inhibitor ABT-737 into the presynaptic terminal slows the recovery of synaptic responses after repetitive synaptic activity, but ameliorates hypoxia-induced synaptic rundown15, raising the unexpected possibility that Bcl-xL and/or other targets of ABT-737 can produce opposing effects on synaptic strength. In response to injurious stimuli, endogenous Bcl-xL can be cleaved into two cleavage fragments, ΔN61-Bcl-xL or ΔN76-Bcl-xL, each of which elicits cell death in cultured cells17, 27. We tested the effects of recombinant ΔN61-Bcl-xL and ΔN76-Bcl-xL, introduced via the patch pipette, on the induction of channel activity under control (non-ischemic) conditions. Both proteins induced discrete intermediate- and large-conductance channel openings in response to a wide range of amplitudes (Fig. 5a,b, Supplementary Fig. 4a,b)15, 21. Although application of ABT-737 alone decreased the appearance of small conductance channel activity (Fig. 5a-c), application of ABT-737 together with recombinant cleaved Bcl-xL via the patch pipette prevented the appearance of large and intermediate conductance channel activity (Fig. 5a-c, Supplementary Fig. 4a,b) and decreased the ΔN61-Bcl-xL-elicited peak conductance (ΔN61-Bcl-xL, 911 ± 81 pS; ΔN61-Bcl-xL+ABT-737, 384 ± 58 pS, n = 25 of each, P < 0.01). Application of ABT-737 to mitochondria produced a greater inhibition of ΔN61-Bcl-xL-than ΔN76-Bcl-xL-elicited channel activity (Fig. 5a-c, Supplementary Fig. 4a,b). Whereas ΔN61-/76-Bcl-xL elicited channel activity similar to that reported for the pro-apoptotic Bcl-2 family protein Bax21, 28, 29, ABT-737 does not bind or inhibit Bax30. Therefore, Bax is not a likely candidate for the ischemia-induced large conductance channel activity. For subsequent experiments, we focused on ΔN61-Bcl-xL (hereafter termed ΔN-Bcl-xL).
Figure 5. ABT-737 attenuates ΔN61-Bcl-xL-elicited channel activity and cytochrome c release.
(a) Sample mitochondrial recordings from control hippocampus performed in the absence and presence of ABT-737. Organelle-attached patches recorded at Vh = +60 mV. (b) Sample mitochondrial recordings from control hippocampus performed in the absence and presence of ΔN61-Bcl-xL (30 μg/ml) or ΔN61-Bcl-xL+ABT-737 (5 μM, applied via the patch pipette). Organelle-attached patches recorded at Vh = +60 mV. (c) Histograms showing closed, small, intermediate and large channel activity for recordings like those illustrated in a and b. Control, 8 (X10sec) traces per mitochondrion, N=4 mitochondria; control + ABT-737, 30 traces n = 3 mitochondria; ΔN61-Bcl-xL, n = 25 traces, 5 mitochondria; ΔN61-Bcl-xL + ABT-737, n = 25 traces, 5 mitochondria. (d, e) Mitochondria were treated with recombinant ΔN61-Bcl-xL at concentrations as indicated. Mitochondrial pellet and supernatant were analyzed for cytochrome c, COX IV, SMAC and VDAC. The concentration of cytochrome c in the mitochondrial fraction was normalized to cytochrome c in the cytoplasmic fraction. (f) Summary data from d, e showing cytochrome c release from the mitochondria (n = 3-6 samples per treatment). (g, h) Mitochondria were treated with recombinant ΔN61-Bcl-xL (1 μM) in the presence or absence of 5 μM ABT-737 (n = 3-4 samples per treatment). Mitochondrial pellet and supernatant were analyzed for cytochrome c and VDAC. (i) Summary data from g, h showing that ABT-737 prevents cytochrome c released from the mitochondria. (n = 6-9; 2-4 samples per treatment group per experiment; 3 independent experiments). ttest *, P < 0.05; **, P < 0.01. Western blot images illustrated in panels d, e, g and h have been cropped. Full-length blots are presented in Supplementary Fig. 11.
Caspase cleavage of Bcl-2/Bcl-xL has been reported to promote release of cytochrome c from lipid vesicles and from mitochondria31, 32. Although recombinant ΔN61-Bcl-xL promoted only limited release of cytochrome c from hippocampal mitochondria, this effect was reversed by 1 μM ABT-737 (Fig. 5d-i, Supple. Fig. 5), suggesting that ABT-737 can inhibit the direct effects of ΔN61-Bcl-xL.
ΔN-Bcl-xL induces cell death in neurons and in cells lacking Bax and Bak
ΔN-Bcl-xL could act directly to promote neuronal death, or could act as an activator of Bax or Bak to promote death. To examine whether ΔN-Bcl-xL elicits cell death in neurons in a Bax- or Bak-independent manner, Bax/Bak double knockout mouse embryonic fibroblasts (MEFs) were transfected with ΔN-Bcl-xL. Transfected cells were marked with co-transfected eGFP and viability was assessed by MTS assay (see Methods) at 18 h after transfection. Under these conditions, ΔN61-Bcl-xL induced cell death in cells lacking Bax and Bak, relative to that of cells expressing eGFP alone (which itself induced little or no cell death). Application of ABT-737 to cells at the time of transfection markedly inhibited ΔN-Bcl-xL-elicited cell death (Fig. 6a). These findings indicate that ΔN-Bcl-xL is sufficient to trigger cell death, even in the absence of Bax and Bak. In contrast, experiments performed on single knockout MEFs lacking either Bax or Bak suggest that Bax and Bak can participate in ΔN-Bcl-xL-induced cell death, and that ABT-737 is ineffective at inhibiting this death (Supplementary Fig. 6), presumably due to the effects of Bax and/or Bak alone.
Figure 6. ΔN61-Bcl-xL induces cell death in hippocampal neurons and Bax−/− Bak−/− MEFs.
(a) ABT-737 attenuates ΔN61-Bcl-xL-elicited cell death in double knock out MEFs. Double knockout MEFs were transfected with ΔN61-Bcl-xL plus eGFP or eGFP alone. Summary data show percent cell survival as assayed by MTS solution (see methods) in the absence and presence of 200 nM ABT-737 (Bax−/− Bak−/− MEFs: control, n = 9; ABT-737, n = 10; ΔN61-Bcl-xL, n = 10; ΔN61-Bcl-xL + ABT-737, n = 6; Results represent 5 independent experiments (b) Hippocampal neurons at DIV 14 expressing eGFP with ΔN61-Bcl-xL or eGFP alone (green, eGFP; yellow arrowheads an example of a transfected neuron) at 24 h after transfection were assayed for caspase-3-like activity (red). ΔN61-Bcl-xL, had increased cell death as compared to eGFP control. (c) ABT-737 attenuates ΔN61-Bcl-xL-elicited cell death in hippocampal neurons. Hippocampal neurons were transfected with eGFP and indicated constructs and maintained in the absence or presence of ABT-737 (1 μM, applied in the medium). At 4 d after transfection, DIV 18, the number of dead cells was detected by propidium iodide uptake and abnormal morphology and expressed as a percent of total transfected cells (eGFP, n = 22 coverslips; eGFP+ABT-737, n = 10 coverslips; eGFP+Bax, n = 10 coverslips; eGFP+Bax+ABT-737, n = 10 coverslips; eGFP+ΔN61-Bcl-xL, n = 16 coverslips; eGFP+ΔN61-Bcl-xL+ABT-737, n = 11 coverslips from 3 independent experiments; P < 0.001, ΔN61-Bcl-xL vs. ΔN61-Bcl-xL+ABT-737; P < 0.001, ΔN61-Bcl-xL vs. eGFP). ttest: ***, P < 0.001. **, P < 0.005; *, P < 0.05.
To verify that expression of ΔN-Bcl-xL can trigger death of neurons, we co-expressed ΔN61-Bcl-xL with eGFP to mark transfected primary hippocampal neurons (DIV 18, Fig. 6b). At 1-2 days after transfection, neurons expressing eGFP exhibited little or no cell death. In contrast, neurons expressing ΔN61-Bcl-xL exhibited substantial cell death, relative to the eGFP control, as monitored by caspase-3-like activity (44% ± 2, n = 30 cells expressing ΔN61-Bcl-xL+eGFP; 8% ± 2, cells expressing eGFP alone, n = 16 from three independent experiments, P<0.05). In a separate experiment, ABT-737 markedly attenuated death of neurons expressing ΔN61-Bcl-xL as assessed by cell morphology (see Methods) and propidium iodide uptake (a marker of degenerating neurons/cells) by eGFP-positive neurons (Fig. 6c). In contrast, neurons expressing eGFP alone exhibited little or no cell death. Neurons expressing Bax exhibited less cell death relative to that of neurons expressing ΔN61-Bcl-xL, and the small fraction of cells exhibiting Bax-elicited cell death were not protected by ABT-737.
Knock-in of cleavage-resistant Bcl-xL protects mice from ischemic injury
To determine whether protease cleavage of endogenous Bcl-xL contributes to cell death in CA1 neurons following ischemia-reperfusion, we constructed knock-in mice in which Bcl-xL harbors mutations at both caspase cleavage sites, D61A and D76A, rendering Bcl-xL resistant to cleavage by caspases (Fig. 7c; Supplementary Fig. 7). The mice were viable and fertile and exhibited no gross morphological changes in brain anatomy. We subjected wild type and homozygous Bcl-xL cleavage-resistant mice to either sham surgery or transient global ischemia induced by bilateral common artery occlusion (BCCO), followed by reperfusion (see Methods). Sham-operated (control) wild type and knock-in mice did not differ in the number of neurons in the hippocampal CA1 (Fig. 7a,b) and showed no signs of degeneration, as assessed by FJ staining (Supplementary Fig 8). In wild type mice subjected to global ischemia, we observed significant cell death in the CA1, as assessed by neuronal counts of the hippocampal CA1 in toluidine blue-stained sections at 6 days after surgery, relative to sham wild type controls. In contrast to wild type mice, cleavage-resistant Bcl-xL knock-in mice were resistant to ischemia-induced neuronal death in the hippocampal CA1 (Fig. 7a,b). Furthermore, knock-in mice were rescued from ischemia-induced neuronal degeneration, as assessed by FJ-staining at 5 d after ischemia (Supplementary Fig 8). To determine if blocking cleavage of Bcl-xL attenuated mitochondrial channel activity, we isolated mitochondria from sham and post-ischemic knock-in mice and wild type littermates, and performed patch clamp recordings of whole mitochondria. Non-ischemic wild type and KI mice failed to show significant differences in large and intermediate conductance channel activity. In contrast, while ischemia elicited a significant increase in channel activity in wild type mitochondria compared to non-ischemic wild type controls, mitochondria isolated from ischemic knock-in animals had significantly less channel activity than that of ischemic wild type mice (Fig. 7d, e), suggesting that cleavage of endogenous Bcl-xL was required for the increase in mitochondrial channel activity after ischemia in mouse brain.
Figure 7. Bcl-xL cleavage-resistant mice are protected against ischemia-induced neuronal death.
(a) Toluidine blue-stained coronal brain sections at the level of the dorsal hippocampus at 6 d after in vivo ischemia from wild-type and homozygous Bcl-xL cleavage-resistant knockin mice. (b) Summary data of neuronal counts within the region of interest. Number of animals per treatment group is indicated on bars, 4 sections per animal. (c) Westerns probed with an anti-Bcl-xL antibody that detects both full-length and N-terminally truncated forms of Bcl-xL. Residual cleavage of Bcl-xL in slices of homozygous mice is presumably due to calpain-mediated proteolytic activity, which increases after ischemia (Yamashima et al., 1996). (d) Sample recordings from mitochondria isolated from the hippocampus of control (sham-operated) or ischemic wild-type and knock-in animals. Organelle-attached patches recorded at Vh = −100 mV. (e) Histograms showing closed, small, intermediate and large channel activity for recordings like those illustrated in d. Channel activity was classified as follows: closed, small: < 180 pS, intermediate: 180 to 760 pS, and large: > 760 pS., n = 5-14 10-s traces per condition. The number of mice was: 4 wild-type sham, 4 KI homozygous sham, 3 wild-type ischemic, 3 KI homozygous ischemic. For sham vs. ischemia: ttest *, P < 0.05; **, P < 0.01; for wild-type vs. knock-in: #, P < 0.05; ##, P < 0.01. Western blots illustrated in panel c have been cropped. Full length blot is presented in Supplementary Fig. 11.
To examine the effect of knock-in mice in a second model of neuronal death, we prepared hippocampal slice cultures from homozygous knock-in mice, heterozygous and wild type littermates and from wild type rats (DIV 9) and subjected slices to oxygen-glucose deprivation (OGD), a well-established in vitro model of global ischemia (45 min, followed by 2 days of reperfusion). Hippocampal slices from knock-in mice exhibited a marked reduction in OGD-induced neuronal death, compared with slices from heterozyous or wild type littermates, as assessed by propidium iodide uptake (Supplementary Fig. 9 a, b). OGD-induced formation of ΔN-Bcl-xL in hippocampal slices from wild type mice was increased compared to knock-in cleavage-resistant Bcl-xL mice (Fig. 7c). To examine the effect of ABT-737, OGD was performed in rat hippocampal slices. OGD induced cleavage of Bcl-xL to generate ΔN-Bcl-xL and elicited neuronal death (Supplementary Fig. 10 a, b). Application of ABT-737 (5 μM) to rat hippocampal slices attenuated OGD-induced neuronal death, assessed by propidium iodide uptake at 24-48 h after ischemia (Supplementary Fig. 10 b). Taken together, these data strongly suggest that cleavage of Bcl-xL contributes to the selective, delayed neurodegeneration associated with global ischemia and that truncated Bcl-xL contributes importantly to delayed ischemia-induced death of hippocampal neurons.
DISCUSSION
Transient global or forebrain ischemia arising as a consequence of cardiac arrest or open heart surgery elicits selective, delayed death of hippocampal CA1 neurons and cognitive deficits1-3, 33, 34. Appearance of large channel activity in mitochondrial membranes and release of cytochrome c are hallmarks of the early post-ischemic period. Proteolytic cleavage of the anti-apoptotic protein, Bcl-xL, to generate ΔN-Bcl-xL is associated with the formation of large-conductance mitochondrial channels, and with release of cytochrome c in postischemic neurons6, 21. Here we show that expression of ΔN-Bcl-xL in hippocampal neurons and mouse embryonic fibroblasts lacking Bax and Bak elicits cell death. We further show the unexpected finding that pretreatment of animals with the Bcl-2/Bcl-xL inhibitor ABT-737, which elicits apoptosis in a wide array of tumor cells10-13, when given before or after ischemia, markedly attenuates ischemia-induced cleavage of Bcl-xL, formation of large channel activity in the mitochondrial outer membrane and affords robust protection of CA1 neurons. Consistent with this, ischemia-induced mitochondrial channel activity and death of hippocampal neurons is attenuated in vivo and in organotypically cultured hippocampal slices from knock-in mice expressing a mutated form of Bcl-xL resistant to protease-dependent cleavage. These findings support a role for ΔN-Bcl-xL in the delayed cell death of hippocampal CA1 neurons and implicate ΔN-Bcl-xL as a putative target for therapeutic intervention in brain ischemic injury. Although not addressed by the present study, it remains to be seen whether ABT-737 administered many hours after the ischemic event would still afford protection. ABT-737 is up to now the most specific and selective small molecule inhibitor designed against Bcl-xL and thus provides important proof-of-principle for development of future therapeutic compounds. Other agents similar to ABT-737 have been designed that may cross the blood brain barrier, but their specificity and selectivity have not withstood the rigorous tests that have been applied to ABT-737. Nevertheless, these other drugs may have clinical relevance in brain ischemia paradigms in future studies.
ABT-737 protects hippocampal neurons from ischemia-induced cell death
A novel finding of the present study is that treatment of animals with ABT-737 prior to or after induction of global ischemia affords robust protection of hippocampal CA1 neurons in a clinically relevant model of global ischemia. These findings are somewhat unexpected in that ABT-737 is a BH3-mimetic which inhibits anti-apoptotic Bcl-2 family members by binding within the hydrophobic cleft typically occupied by pro-apoptotic, BH3-domain only proteins such as Bim and Bad10. ABT-737 exhibits high affinity for Bcl-xL, Bcl-2 and Bcl-w, with approximately 10-fold higher affinity for Bcl-xL than for Bcl-210. ABT-737 promotes apoptosis in lymphoma, multiple myeloma and small-cell lung carcinoma lines, as well as primary patient-derived cancer cells, thereby effectively suppressing tumors10-13. ABT-737 (ABT-263) is presently in clinical trials as an anti-tumorigenic agent that promotes regression of solid tumors10-13. In tumor cells, the mechanism by which ABT-737 elicits cell death is well delineated: ABT-737 sequesters Bcl-xL away from pro-apoptotic BH3-only proteins. When unleashed, these players initiate the oligomerization of Bax and Bak, which permeabilize the outer mitochondrial membrane8, 9. Nevertheless, given that ABT-737 can bind both anti-apoptotic full-length Bcl-xL, as well proapoptotic ΔN-Bcl-xL, it would be difficult to infer from findings in squid (our previous work) or rats (present study) what the actual impact of ABT-737 would be in humans in a clinical context.
Findings in the present study are consistent with a model whereby ABT-737 also binds both anti-apoptotic and pro-apoptotic ΔN-Bcl-xL, thereby aborting ischemia-induced neuronal death by preventing cleavage of full length Bcl-xL (as in Fig. 4a, b) and by blocking the channel activity and death-inducing effects of cleaved Bcl-xL. Consistent with this, expression of ΔN-Bcl-xL, but not Bax, elicited cell death in hippocampal neurons and ΔN-Bcl-xL also produced death in mouse embryonic fibroblasts lacking Bax and Bak. Whereas ABT-737 attenuated ΔN-Bcl-xL-elicited cell death in hippocampal neurons and in bax−/−/bak−/− double knockout MEFs (present study see Fig. 6a), ABT-737 had little or no effect on cell death in single knockout MEFs that contain Bax or Bak. Thus, Bax/Bak may also contribute to cell death observed in MEFs. However, in adult neurons, endogenous Bcl-xL abundance is still high at ages when Bax and Bak expression have declined23, and, although genetic ablation of Bax protects against cardiac ischemia35 and Bax inhibitors protect against brain ischemia in other models29, Bax inhibitors do not protect adult mice from ischemic injury induced by MCAO36 and endogenous and overexpressed Bax and Bak also can be profoundly protective in the brain depending on the developmental stage, death stimulus and brain subregion37, 38. Furthermore, we show that exogenously expressed Bax does not significantly increase death of cultured hippocampal neurons (see Fig. 6c), indicating that these neurons are not very sensitive to Bax. Nevertheless, cleaved Bcl-xL may stimulate both Bax/Bak-dependent and independent death.
Generation of cleaved Bcl-xL in ischemic injury
Bcl-xL can be cleaved by both calpain and caspases17, 39. Unlike calpain, which has less defined substrate cleavage sites, caspases cleave specifically after Asp residues, and we found that mutation of the two known caspase cleavage sites at Asp61 and Asp76 of endogenous Bcl-xL protects neurons significantly from ischemic injury. The specific caspases required to generate the pro-death ΔN-Bcl-xL fragment in neurons subjected to global ischemia in vivo or to oxygen glucose deprivation in organotypically-cultured hippocampal slices are not known. The responsible caspases could be activated by a pathway prior to mitochondrial involvement, or by mechanisms not involving mitochondrial permeabilization. These possibilities are consistent with the non-consensus caspase cleavage recognition sites in Bcl-xL. Caspase-cleaved Bcl-xL could also potentially lead to increased cleavage of full-length Bcl-xL if ΔN-Bcl-xL leads to mitochondrial permeabilization and subsequent amplification of caspase activity. Regardless of the specific caspases involved, these findings are consistent with previous observations that caspase inhibitors ameliorate global ischemia-induced neuronal death24, 40, 41.
ABT-737 inhibits ΔN-Bcl-xL induced channel activity
Another finding of the current study is that ABT-737 can inhibit functional activity associated with truncated, pro-apoptotic forms of Bcl-xL, leading to protection of postischemic neurons. First, ABT-737 inhibits the large channel activity in the outer mitochondrial membrane elicited by recombinant ΔN61- and ΔN76-Bcl-xL applied via the patch pipette. Second, ABT-737 attenuates ΔN-Bcl-xL-elicited cell death in hippocampal neurons and mouse embryonic fibroblasts lacking Bax and Bak. These findings provide a mechanism by which ABT-737 affords neuroprotection: namely, ABT-737 directly binds and inhibits ΔN-Bcl-xL and thereby prevents its ability to permeabilize the outer mitochondrial membrane and promote death in hippocampal neurons. Whereas these studies address inhibition of ΔN-Bcl-xL function by ABT-737, they do not establish exactly how ABT-737 inhibits channel activity. We envision at least three possible mechanisms by which ABT-737 might function. First, ABT-737 could act directly on ΔN-Bcl-xL. This is supported by the finding that ABT-737 can inhibit the effects of recombinant, as well as overexpressed ΔN-Bcl-xL. Second, ABT-737 might act directly on full-length Bcl-xL to render it less sensitive to proteolytic digestion, thereby blocking the cleavage of Bcl-xL to ΔN-Bcl-xL. Third, ABT-737 could act indirectly to reduce caspase cleavage of full-length Bcl-xL to ΔN-Bcl-xL in a feed-forward loop by suppressing the ability of ΔN-Bcl-xL to stimulate mitochondrial permeabilization leading to amplification of caspase activity. The latter two possibilities are consistent with our immunoblot analysis of processed Bcl-xL in ischemic brain.
In our study we identify a specific role for the cleavage of Bcl-xL in ischemia-induced neuronal death. We further show evidence for a ΔN-Bcl-xL-dependent activation of a Bax-like apoptotic pathway. However, these observations do not necessarily implicate apoptotic cell death as defined morphologically. Whereas global ischemia-induced neuronal death in the hippocampal CA1 has been attributed to apoptosis defined as caspase-dependent death42, analysis of the hippocampal CA1 by electron microscopy following global ischemic injury, and in other ischemia models reveals necrotic morphology26,43. In addition, we have recently reported that Bcl-xL increases mitochondrial energetic efficiency44, 45. Thus, loss of full-length Bcl-xL together with ΔN-Bcl-xL–induced mitochondrial damage could result in a plethora of cellular defects leading to a range of cell morphologies.
In summary, the present study extends our previous findings that ischemic insult triggers cleavage of full length Bcl-xL to generate its pro-apoptotic cleavage product ΔN-Bcl-xL in that we show that ΔN-Bcl-xL expressed in hippocampal neurons or in mouse embryonic fibroblasts lacking Bax and Bak elicits cell death. We further show the novel finding that ΔN-Bcl-xL is critical to neuronal death in a clinically relevant model of global ischemia. A key event is disruption of the functional integrity of the outer mitochondrial membrane, as evident by the increase in large channel activity in the early post-ischemic period. In addition, we show the novel finding that ABT-737, known to promote apoptosis and death of tumorigenic cells, affords robust protection of hippocampal neurons from global ischemia-induced neuronal death. Together these findings identify Bcl-xL as a putative therapeutic target for intervention in the neuronal injury and cognitive deficits associated with global ischemia.
Supplementary Material
Acknowledgements
We thank Adrianna Latuszek-Barrantes and Fabrizio Pontarelli for excellent technical assistance and Drs. John Hickman, Richard Kitsis and Leonard K. Kaczmarek for insightful scientific discussion and constructive review of the manuscript. We thank Casey Kinnally and Nika Danial for the gift of bax−/− bak−/− mouse embryonic fibroblasts and Institut de Recherches Servier, Croissy sur Seine, France for ABT-737. This work was supported by NIH NS045876 (E.A.J.), NS46742 (R.S.Z), and NS37402 (JMH), a McKnight Foundation Brain Disorders Award (RSZ), and a generous grant from the F.M. Kirby Foundation (RSZ).. R.S.Z. is the F.M. Kirby Professor of Neural Repair and Protection.
Appendix
METHODS
Global ischemia
Male Sprague Dawley rats (150–200 g; Charles River Laboratories, Wilmington, MA) were maintained in a temperature- and light-controlled environment and were treated in accordance with the principles and procedures of the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. All protocols were approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine. Animals were subjected to global ischemia by four-vessel occlusion (10 min)), followed by reperfusion, as described previously46. Briefly, on the day before surgery, rats were anesthetized with isoflurane (2% was decreased to 1% after the induction of anesthesia) in a mixture of N2/O2 (70:30) delivered by mask attached to a Vapomatic anesthetic vaporizer (CWE, Ardmore, PA). The vertebral arteries were permanently occluded by electrocauterization, the common carotid arteries were exposed through a ventral midline neck incision and isolated with 3-0 silk ligatures, and the wound was closed. Rats were fasted overnight and anesthetized the next day. The wound was reopened, and the carotid arteries were occluded with aneurysm clips (10 min). Although all forebrain areas experience oxygen and glucose deprivation during the brief ischemic insult, only selected neuronal populations degenerate and die in humans and in animals subjected experimentally to global ischemia42 Pyramidal neurons in the hippocampal CA1 are particularly vulnerable. Other neurons that may be damaged are hilar neurons of the dentate gyrus, pyramidal neurons in neocortical layers II, V and VI, and Purkinje neurons of the cerebellum. After removal of clips, the arteries were visually inspected to ensure adequate reflow. Anesthesia was discontinued immediately after initiation of occlusion. Body temperature was maintained at 37.5 ± 0.5°C with a rectal thermostat and heat lamp until the carotids were unclamped/at onset of reperfusion. Animals generally regained normal grooming and other social behaviors within 30 min of reperfusion. This observation suggests that brain (and body) temperature have returned to normal, but does not rule out the possibility that the drug induces mild cooling or prevents fever, either of which might afford an improved outcome. Animals that failed to recover within 30 min. were excluded from the study. Animals that failed to show complete loss of the righting reflex and dilation of the pupils from 2 min after occlusion was initiated until the end of occlusion, and the rare animals that subsequently exhibited obvious behavioral manifestations (abnormal vocalization when handled, generalized convulsions, hypoactivity) were also excluded from the study. Sham animals were subjected to the identical procedure, except that carotid arteries were not occluded.
Male and female wild type and homozygous Bcl-xL cleavage-resistant mice knock-in mice (aged 6 weeks) were subjected to sham surgery or transient global ischemia using the bilateral common artery occlusion (BCCO) model of global ischemia. In brief, the common carotid arteries were exposed through a ventral midline neck incision and isolated with 3-0 silk ligatures, and the arteries were occluded with aneurysm clips (15-30 min). Neuronal cell loss was assessed by histological examination of toluidine blue-stained brain sections at the level of dorsal hippocampus from animals killed 6 d after ischemia or sham operation, as previously described46. In brief, Animals were deeply anesthetized with pentobarbital (50 mg/kg, i.p.) and fixed by transcardiac perfusion with ice-cold 4% paraformaldehyde in 0.1 M PBS, pH 7.4. Brains were removed and immersed in fixative at 4°C overnight. Coronal sections (15 mm) were cut at the level of the dorsal hippocampus with a cryotome and stained with toluidine blue (0.05%). The number of surviving neurons in a 250 μm mediolateral segment of CA1 stratum pyramidale was counted under a light microscope at 40 x magnification. Fluoro-Jade–stained brains were also prepared as above. The numbers of Fluoro-Jade–positive cells per 500 μm length of the medial sector of the CA1 pyramidal cell layer were counted in 9–10 rats per treatment group (4 sections per rat).
Isolation of mitochondria
Mitochondria were isolated from control and experimental rat or mouse brains and purified by centrifugation over a discontinuous percoll gradient47, 48. In brief, rodents (n = 3–6 per group; tissue from three to six animals pooled for each assay) were killed by decapitation 1 h after ischemia, and hippocampi were dissected on ice and transferred to ice-cold isolation buffer (250 mM sucrose, 20 mM HEPES, pH 7.2, 1 mM EDTA, and 0.5% BSA). Tissue was minced, homogenized (seven times with a homogenizer; Wheaton, Millville, NJ), and centrifuged at 1300 xg to pellet nuclear material. The supernatant was centrifuged at high-speed (13,000 x g for 10 min at 4°C); the pellet containing the mitochondria was resuspended in isolation buffer and layered onto a percoll gradient ranging from 7.5 to 10%. After 30 min ultracentrifugation, at 32,500 rpm, 4°C, the mitochondrial and synaptosomal layers were removed and washed in isolation buffer by centrifugation at 13,000 x g. Mitochondria were frozen on dry ice and stored at −80°C until use. Previously frozen mitochondria were used for electrophysiology, and immunoblots.
Cytochrome c release from brain mitochondria
Mitochondria were isolated from the CA1 region of the hippocampus from control Sprague-Dawley rats, as described above. Isolated mitochondria (50 or 100 μg of protein) were incubated with recombinant Bcl-xL or ΔN-Bcl-xL protein (1 μM) in 50 μl of PBS for 60 min at 30°C. Reaction mixtures were centrifuged at 14,000 × g for 10 min, mitochondrial membrane pellets and the corresponding volume of supernatants were separated by SDS-PAGE on 4–12% Bis-Tris gels, and their respective cytochrome c contents were estimated by immunoblotting.
Electrophysiology
For patch-clamp recordings as described6, 21, mitochondria were placed in the internal patch solution (containing 120 mM KCl, 8 mM NaCl, 0.5 mM EGTA, 2 mM MgATP, and 10 mM HEPES, pH 7.3) Patch-clamp pipettes (80–100 MΩ) were filled with the same internal patch solution. The membrane potential was maintained at voltages ranging from −100 to 100 mV for periods of 12 s. Data were collected at 20 kHz and filtered at 500–1000 Hz. In recordings with recombinant ΔN-Bcl-xL (8 μg/ml), the protein was added to the internal patch pipette solution of PBS, and the bath perfusate was changed to PBS.
Primary cultures of hippocampal neurons
Primary cultures of hippocampal neurons were prepared from embryonic day 18 Sprague -Dawley rat brains and plated on poly-L-lysine–coated coverslips (12 mm) at low density (30,000 cells per dish), as described46. Neurons were transfected by the calcium phosphate method46. Cell death was assayed by the presence of caspase 3-like activity, propidium iodide staining, and/or morphological changes such as cell shrinkage or fragmentation.
Mouse embryonic fibroblasts cell culture
Murine embryonic fibroblasts (MEFs) were generated from bax−/− bak−/− embryos (a kind gift of Drs. Danial, Kinnally and Korsmeyer) as described 49. MEFs were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% of penicillin/streptomycin. The cells were plated at 15,000 per well in a 96-well plate and were transfected, after 24 hours, with 0.5 μg ΔN-Bcl-xL DNA construct, using Perfectin (Genlantis, USA), according to manufacturer’s protocol with and without 200nM ABT. The transfection mix minus DNA was added to the control wells. 18 h post-transfection 30 μl CellTiter 96® AQueous One (MTS) solution (Promega, USA) was added to each well. The rate of survival was measured by recording the absorbance at 490nm, using the Victor3 Multilabel Plate Reader (PerkinElmer, USA).
Generation of knockin caspase-resistant Bcl-xL mutant mice
We generated knockin mice expressing a mutationally altered form of Bcl-xL carrying two Asp to Ala point mutations in the loop domain rendering it resistant to cleavage by caspases. Heterozygous ES cells, where the neo allele corresponds to the mutant allele with caspase cleavage site mutations were screened by Southern and PCR. These cells were used for blastocyst injection. The Cre-LoxP system was used to delete the selection marker used for construction of the mice, leaving behind only the 34 bp loxP site in an intron. The genotypes of the final heterozygous or homozygous caspase-resistant knockin (KI) mice were confirmed by PCR methods.
Slice culture oxygen-glucose deprivation
Organotypic hippocampal slice cultures were prepared, as described 22, 50. In brief, hippocampi were removed from the brains of 7- to 9-d-old Wistar rats or from Bcl-xL knockin mice and placed in ice-cold HBSS supplemented with glucose (5 mg/ml) and sucrose (7 mg/ml). Transverse slices (400 μm) were cut with a McIlwain tissue chopper and transferred to humidified semiporous membranes (30 mm Millicell-CM tissue culture plate inserts; four slices per membrane), placed in six-well tissue culture plates containing 1.2 ml of culture medium (50% Eagle’s MEM, 25% heat-inactivated horse serum, 25% HBSS, 5 mg/ml glucose, and 1 mM glutamine), and maintained (37°C in 95% air/5% CO2) for 14 d in vitro. To induce oxygen glucose deprivation (OGD), slices were exposed to serum-free, glucose-free medium saturated with 95% N2/5% CO2 (45 min at 37°C) in an airtight anoxic chamber (Billups-Rothenberg, Del Mar, CA). After OGD, slices were returned to oxygenated, glucose-containing culture medium containing propidium iodide (5 μg/ml) (Molecular Probes, Eugene OR) under normoxic conditions until evaluation of neuronal injury. Cell death was assessed by measuring the uptake of this dye into dead/dying cell by quantifying the total fluorescence using ImageJ software.
Western Blot Analyses
For detection of the proteolytic fragment of Bcl-xL, protein samples (20μg) isolated from control and experimental hippocampal mitochondria were subjected to gel electrophoresis on a 4-12% SDS gradient gel and probed with chicken anti- Bcl-xL polyclonal antibody raised against the N terminal 16 amino acids of ΔN 61 Bcl-xL (Aves scientific) or a polyclonal antibody against Bcl-xL (Cell signaling, 54H6). As a positive control, 10 ng of recombinant human Bcl-xL C-terminal truncation (lacking the last 61 or 76 amino acids) was also subjected to gel electrophoresis 6. The following other antibodies were also used Bax (Santa Cruz, #7480), Bak (Invitrogen, #AHO0252), VDAC (Cell Signaling, #4866), cytochrome c (BD Biosciences, #556433), β-actin (Sigma, #A2228) cyclooxygenase IV (COX IV, Molecular Probes, #A21348)). Membranes were treated with ECL reagents (Amersham Life Science, Arlington Heights, IL) and apposed to XAR-5 x-ray film (Eastman Kodak). To quantify protein abundance, bands on Western blots were analyzed with a Scan Jet 4-C computing densitometer using NIH ImageJ software. Bands of samples from experimental animals were normalized to a loading control and expressed as a percentage of the corresponding control value. Protein standard curves were constructed to ensure that samples were in the linear range.
Statistical analysis
For comparisons involving 2 groups, paired or unpaired Student’s t-tests (2-tailed) were used. In figures, *=p<0.05, **=p<0.01, and ***=p<0.001 to denote significance level, unless otherwise specified in figure legend.
Footnotes
Author contributions:
Dimitry Ofengeim performed experiments, wrote and prepared the manuscript and made intellectual contributions. Yingbei Chen created the KI mouse. Takahiro Miyawaki performed experiments. Hongmei Li performed experiments. Silvio Sacchetti performed experiments. Richard J. Flannery performed experiments. Kambiz N. Alavian performed experiments and made intellectual contributions. Fabrizio Pontarelli performed experiments. Brian A. Roelofs assisted with KI mouse colony preparation. John A. Hickman provided intellectual contributions. J. Marie Hardwick designed experiments, wrote the manuscript and provided intellectual contributions. R. Suzanne Zukin designed experiments, wrote the manuscript and provided intellectual contributions. Elizabeth A. Jonas designed experiments, performed experiments, wrote the manuscript and provided intellectual contributions.
References
- 1.Moskowitz MA, Lo EH, Iadecola C. The science of stroke: Mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zukin RS, et al. Molecular and cellular mechanisms of ischemia-induced neuronal death. In: Mohr JP, Choi DW, Grotta JC, Weir B, Wolf PA, editors. Stroke: Pathophysiology, Diagnosis and Management. 2004. pp. 829–854. [Google Scholar]
- 3.Liou AK, Clark RS, Henshall DC, Yin XM, Chen J. To die or not to die for neurons in ischemia, traumatic brain injury and epilepsy: a review on the stress-activated signaling pathways and apoptotic pathways. Prog Neurobiol. 2003;69:103–142. doi: 10.1016/s0301-0082(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 4.Sugawara T, Fujimura M, Morita-Fujimura Y, Kawase M, Chan PH. Mitochondrial release of cytochrome c corresponds to the selective vulnerability of hippocampal CA1 neurons in rats after transient global cerebral ischemia. J Neurosci. 1999;19:RC39. doi: 10.1523/JNEUROSCI.19-22-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka H, et al. Ischemic preconditioning: neuronal survival in the face of caspase-3 activation. J Neurosci. 2004;24:2750–2759. doi: 10.1523/JNEUROSCI.5475-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miyawaki T, et al. Ischemic preconditioning blocks BAD translocation, Bcl-xL cleavage, and large channel activity in mitochondria of postischemic hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:4892–4897. doi: 10.1073/pnas.0800628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis. 2009;14:469–477. doi: 10.1007/s10495-008-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 9.Galonek HL, Hardwick JM. Upgrading the BCL-2 network. Nat Cell Biol. 2006;8:1317–1319. doi: 10.1038/ncb1206-1317. comment. [DOI] [PubMed] [Google Scholar]
- 10.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 11.Richardson A, Kaye SB. Pharmacological inhibition of the Bcl-2 family of apoptosis regulators as cancer therapy. Curr Mol Pharmacol. 2008;1:244–254. doi: 10.2174/1874467210801030244. [DOI] [PubMed] [Google Scholar]
- 12.Cragg MS, Harris C, Strasser A, Scott CL. Unleashing the power of inhibitors of oncogenic kinases through BH3 mimetics. Nat Rev Cancer. 2009;9:321–326. doi: 10.1038/nrc2615. [DOI] [PubMed] [Google Scholar]
- 13.Vogler M, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 14.Jonas EA, et al. Modulation of synaptic transmission by the BCL-2 family protein BCL-xL. J Neurosci. 2003;23:8423–8431. doi: 10.1523/JNEUROSCI.23-23-08423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickman JA, Hardwick JM, Kaczmarek LK, Jonas EA. Bcl-xL inhibitor ABT-737 reveals a dual role for Bcl-xL in synaptic transmission. J Neurophysiol. 2008;99:1515–1522. doi: 10.1152/jn.00598.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z, et al. Caspase-3 activation via mitochondria is required for long-term depression and AMPA receptor internalization. Cell. 2010;141:859–871. doi: 10.1016/j.cell.2010.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clem RJ, et al. Modulation of cell death by Bcl-XL through caspase interaction. Proc Natl Acad Sci U S A. 1998;95:554–559. doi: 10.1073/pnas.95.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng EH, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 19.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 20.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 21.Bonanni L, et al. Zinc-dependent multi-conductance channel activity in mitochondria isolated from ischemic brain. J Neurosci. 2006;26:6851–6862. doi: 10.1523/JNEUROSCI.5444-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calderone A, et al. Ischemic insults derepress the gene silencer REST in neurons destined to die. J Neurosci. 2003;23:2112–2121. doi: 10.1523/JNEUROSCI.23-06-02112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krajewska M, et al. Dynamics of expression of apoptosis-regulatory proteins Bid, Bcl-2, Bcl-X, Bax and Bak during development of murine nervous system. Cell Death Differ. 2002;9:145–157. doi: 10.1038/sj.cdd.4400934. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, et al. Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia. J Neurosci. 1998;18:4914–4928. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jover T, et al. Estrogen protects against global ischemia-induced neuronal death and prevents activation of apoptotic signaling cascades in the hippocampal CA1. J Neurosci. 2002;22:2115–2124. doi: 10.1523/JNEUROSCI.22-06-02115.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colbourne F, Sutherland GR, Auer RN. Electron microscopic evidence against apoptosis as the mechanism of neuronal death in global ischemia. J Neurosci. 1999;19:4200–4210. doi: 10.1523/JNEUROSCI.19-11-04200.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujita N, Nagahashi A, Nagashima K, Rokudai S, Tsuruo T. Acceleration of apoptotic cell death after the cleavage of Bcl-XL protein by caspase-3-like proteases. Oncogene. 1998;17:1295–1304. doi: 10.1038/sj.onc.1202065. [DOI] [PubMed] [Google Scholar]
- 28.Dejean LM, et al. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol Biol Cell. 2005;16:2424–2432. doi: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hetz C, et al. Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J Biol Chem. 2005;280:42960–42970. doi: 10.1074/jbc.M505843200. [DOI] [PubMed] [Google Scholar]
- 30.van Delft MF, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirsch DG, et al. Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem. 1999;274:21155–21161. doi: 10.1074/jbc.274.30.21155. [DOI] [PubMed] [Google Scholar]
- 32.Basanez G, et al. Pro-apoptotic cleavage products of Bcl-xL form cytochrome c-conducting pores in pure lipid membranes. J Biol Chem. 2001;276:31083–31091. doi: 10.1074/jbc.M103879200. [DOI] [PubMed] [Google Scholar]
- 33.Graham SH, Chen J. Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab. 2001;21:99–109. doi: 10.1097/00004647-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Okuno S, Saito A, Hayashi T, Chan PH. The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia. J Neurosci. 2004;24:7879–7887. doi: 10.1523/JNEUROSCI.1745-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hochhauser E, et al. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am J Physiol Heart Circ Physiol. 2003;284:H2351–2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, et al. Developmental shift of cyclophilin D contribution to hypoxic-ischemic brain injury. J Neurosci. 2009;29:2588–2596. doi: 10.1523/JNEUROSCI.5832-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis J, et al. Inhibition of virus-induced neuronal apoptosis by Bax. Nature Medicine. 1999;5:832–835. doi: 10.1038/10556. [DOI] [PubMed] [Google Scholar]
- 38.Fannjiang Y, et al. BAK alters neuronal excitability and can switch from anti-to pro-death function during postnatal development. Developmental Cell. 2003;4:575–585. doi: 10.1016/s1534-5807(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 39.Nakagawa T, Yuan J. Cross-talk between two cysteine protease families. Activation of caspase-12 by calpain in apoptosis. J Cell Biol. 2000;150:887–894. doi: 10.1083/jcb.150.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hara H, et al. Attenuation of transient focal cerebral ischemic injury in transgenic mice expressing a mutant ICE inhibitory protein. J Cereb Blood Flow Metab. 1997;17:370–375. doi: 10.1097/00004647-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 41.Cao G, et al. Cloning and characterization of rat caspase-9: implications for a role in mediating caspase-3 activation and hippocampal cell death after transient cerebral ischemia. J Cereb Blood Flow Metab. 2002;22:534–546. doi: 10.1097/00004647-200205000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Ofengeim D, Miyawaki T, Zukin RS. In: Molecular and Cellular Mechanisms of Ischemia-induced Neuronal Death in Stroke: Pathophysiology, Diagnosis and Management. Mohr JP, et al., editors. Churchill Livingstone Elsevier; Philadelphia: 2011. pp. 1–39. [Google Scholar]
- 43.Northington FJ, Chavez-Valdez R, Martin LJ. Neuronal cell death in neonatal hypoxia-ischemia. Ann Neurol. 2011;69:743–758. doi: 10.1002/ana.22419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alavian L, Collis, Bonanni, Zeng, Sacchetti, Lazrove, Nabili, Flaherty, Graham, Chen, Messerli, Mariggio, Rahner, McNay, Shore, Smith, Hardwick, Jonas Bcl-x(L) regulates metabolic efficiency of neurons through interaction with the mitochondrial F(1)F(O) ATP synthase. Nat Cell Biol. 2011;13:1224–1233. doi: 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen YB, Aon MA, Hsu YT, Soane L, Teng X, McCaffery JM, Cheng WC, Qi B, Li H, Alavian KN, Dayhoff-Brannigan M, Zou S, Pineda FJ, O’Rourke B, Ko YH, Pedersen PL, Kaczmarek LK, Jonas EA, Hardwick JM. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol. 2011;195:263–276. doi: 10.1083/jcb.201108059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyawaki T, et al. The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci. 2009;12:618–626. doi: 10.1038/nn.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- 48.Sims NR, Anderson MF. Isolation of mitochondria from rat brain using Percoll density gradient centrifugation. Nat. protoc. 2008;3:1228–1239. doi: 10.1038/nprot.2008.105. [DOI] [PubMed] [Google Scholar]
- 49.Wei MC, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. see comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Formisano L, et al. Ischemic insults promote epigenetic reprogramming of mu opioid receptor expression in hippocampal neurons. Proc Natl Acad Sci U S A. 2007;104:4170–4175. doi: 10.1073/pnas.0611704104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.