Key Points
Ezh2 is specifically required to induce effector cells producing IFN-γ and expansion of T cells late upon alloantigen activation.
Genetic inactivation of Ezh2 function reduces GVHD but preserves antitumor effects in mice after allogeneic BMT.
Abstract
Posttranscriptional modification of histones by methylation plays an important role in regulating Ag-driven T-cell responses. We have recently drawn correlations between allogeneic T-cell responses and the histone methyltransferase Ezh2, which catalyzes histone H3 lysine 27 trimethylation. The functional relevance of Ezh2 in T-cell alloimmunity remains unclear. Here, we identify a central role of Ezh2 in regulating allogeneic T-cell proliferation, differentiation, and function. Conditional loss of Ezh2 in donor T cells inhibited graft-versus-host disease (GVHD) in mice after allogeneic bone marrow (BM) transplantation. Although Ezh2-deficient T cells were initially activated to proliferate upon alloantigenic priming, their ability to undergo continual proliferation and expansion was defective during late stages of GVHD induction. This effect of Ezh2 ablation was largely independent of the proapoptotic molecule Bim. Unexpectedly, as a gene silencer, Ezh2 was required to promote the expression of transcription factors Tbx21 and Stat4. Loss of Ezh2 in T cells specifically impaired their differentiation into interferon (IFN)-γ–producing effector cells. However, Ezh2 ablation retained antileukemia activity in alloreactive T cells, leading to improved overall survival of the recipients. Our findings justify investigation of modulating Ezh2 as a therapeutic strategy for the treatment of GVHD and other T cell–mediated inflammatory disorders.
Introduction
Allogeneic bone marrow transplantation (BMT) remains limited by morbidity and mortality related to GVHD.1-5 GVHD is caused by donor T cells that recognize and react to histocompatibility differences between the donor and host.1-5 It occurs in 3 sequential characteristic phases: priming, induction, and effector.1,3-5 During the priming phase, host antigen-presenting cells (APCs) elicit initial activation and proliferation of donor T cells.1,3-6 During the induction phase, activated donor T cells undergo robust proliferation and differentiation into effector cells.1,2,6,7 The effector phase is characterized by migration and infiltration of alloreactive effector cells into GVHD target organs, causing host tissue injury.1,2,5-7
Histone methylation, which is catalyzed by histone methyltransferases (HMTs), has been correlated with the expression of genes associated with proliferation, differentiation, and survival of Ag-activated T cells.8,9 We have recently demonstrated that inhibition of histone methylation using 3-Deazaneplanocin A (DZNep) arrested ongoing GVHD through induction of apoptosis in alloreactive T cells.10 DZNep reduced multiple histone methylation marks, including trimethylation of histone H3 at lysine 4 (H3K4me3), H3K27me3, H3K36me3, and H4K20me3,10-12 each of which is catalyzed by a specific HMT.9,13 However, the key HMT that regulates allogeneic T-cell responses remains unclear.
Ezh2 is an HMT that catalyzes H3K27me3 and acts primarily as a gene silencer.14 Ezh2 controls cell proliferation, differentiation, and function in numerous contexts.14 Ezh2 can influence proliferation and differentiation of hematopoietic stem cells,15,16 B lymphopoiesis,17 and thymopoiesis.16 Naïve T cells expressed low levels of Ezh2 but rapidly upregulated Ezh2 upon TCR ligation and alloantigen stimulation.10,16,18 However, whether Ezh2 plays important roles in regulating T-cell immune responses in vivo has not been previously determined. In the present study, we used conditional inactivation models to isolate the stage-specific alterations in allogeneic T-cell responses engendered by Ezh2 ablation and its effect on GVHD and graft-versus-leukemia (GVL) activity.
Materials and methods
Our data regarding experimental mice, antibodies (Abs), flow cytometry analysis, cell lines, cell preparation, culture of T cells, cytotoxicity assay, BMT and induction of GVHD, induction of GVL, in vivo bioluminescence imaging, enzyme-linked immunosorbent assay (ELISA), real-time reverse transcriptase polymerase chain reaction (RT-PCR), and chromatin immunoprecipitation assay (ChIP) are detailed in supplemental files, available on the Blood Web site. Experimental protocols were approved by the University of Michigan’s Committee on Use and Care of Animals.
Statistical analysis
Survival in different groups was compared by using the log-rank test. Comparison of 2 means was analyzed by using the 2-sided 2-sample Student t test.
Results
Conditional loss of Ezh2 in donor T cells inhibits acute GVHD
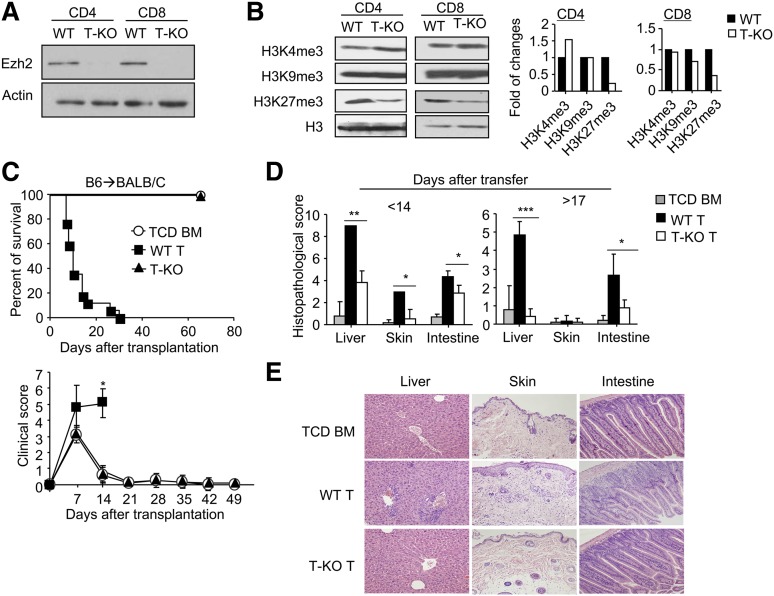
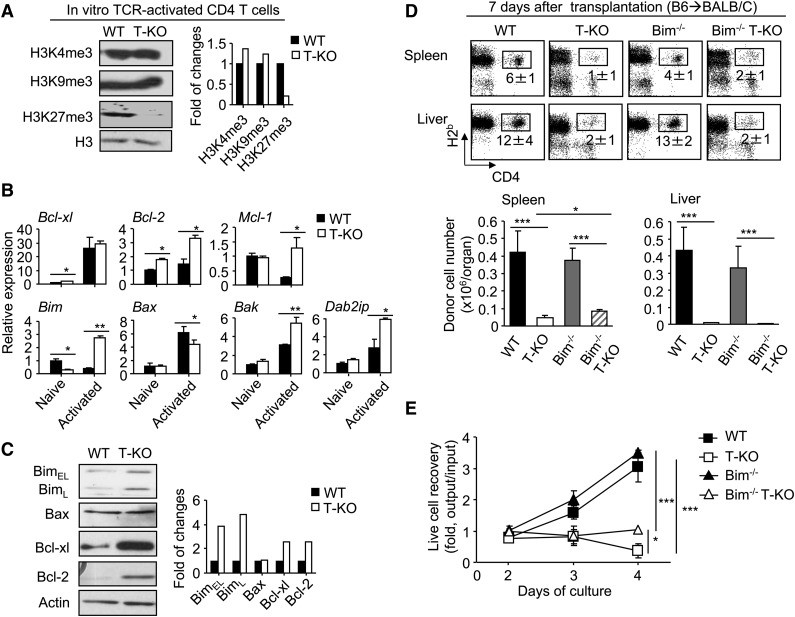
To inactivate the enzyme activity of Ezh2 in mature T cells, we bred mice with floxed alleles of Ezh2 (Ezh2fl/fl)17 to B6 mice expressing Cre recombinase under control of the CD4 promoter to generate T cell–specific Ezh2 conditional knockout B6 mice (named T-KO). In agreement with previous observations,16 the absence of Ezh2 had no significant effect on the percentage and number of double-negative (DN), double-positive (DP), and CD4+ and CD8+ single positive (SP) thymocytes (supplemental Figure 1A). Likewise, normal absolute numbers and phenotype (eg, CD25, CD44, CD69, CD62L) of T cells were found in the spleens and lymph nodes of T-KO and wild-type (WT) mice (supplemental Figure 1B-C). Western blot confirmed the deletion of Ezh2 (Figure 1A) and reduction of H3K27me3 in T-KO T cells (Figure 1B).
Figure 1.
Donor T cells lacking Ezh2 fail to mediate GVHD. (A-B) CD4+ and CD8+ T cells were isolated from the spleens and lymph nodes of WT and T-KO B6 mice, and the cell lysates were prepared for analysis of Ezh2 expression (A) and histone methylation marks (B). Data are representative of 2 independent experiments. (C-D) Lethally irradiated (8 Gy) BALB/C recipients (H2d) were transplanted with B6 (H2b) TCD BM (5 × 106) (○, n = 5) or TCD BM + CD44lo T cells (ie, 1 × 106 CD4+ + 1 × 106 CD8+ T cells) that were derived from WT (▪, n = 14) or T-KO (▲, n = 10) B6 mice. (C) Recipient survival and clinical signs were monitored over time (P < .001, ▪ vs ▲). (D) Histologic GVHD score. Tissues were collected at 14 days and later than 17 days after transplantation. (E). Images were obtained with an Olympus BX41 microscope (10/0.3 NA lens, 200× magnification, digital DP70 camera). *P < .05, **P < .01, ***P < .001. Error bars indicate mean ± SD.
We then examined the impact of Ezh2 ablation in allogeneic T cells using the major histocompatibility (MHC)-mismatched B6 anti-BALB/C mouse GVHD model. Lethally irradiated BALB/C mice were transplanted with T cell–depleted (TCD) bone marrow (BM) from B6 mice with or without WT or T-KO T cells. As expected, WT T-cell recipients died of GVHD. In contrast, T-KO T-cell recipients did not develop clinical signs of severe GVHD and all survived (Figure 1C). Histologic examination showed a significant reduction of inflammation in the intestine, skin, and liver of T-KO T-cell recipients (Figure 1D-E). In addition, compared with TCD BM recipients, T-KO T-cell recipients showed complete donor BM engraftment in the BM, spleen, thymus, and peripheral blood (supplemental Figure 2), suggesting that T-KO T cells do not impair hematopoietic niche and thymic stromal cells, which are also the GVHD targets.19,20 Thus, inactivation of Ezh2 in donor T cells prevents lethal GVHD.
Ezh2 plays a differentiation stage-specific role in alloantigen-driven T cells
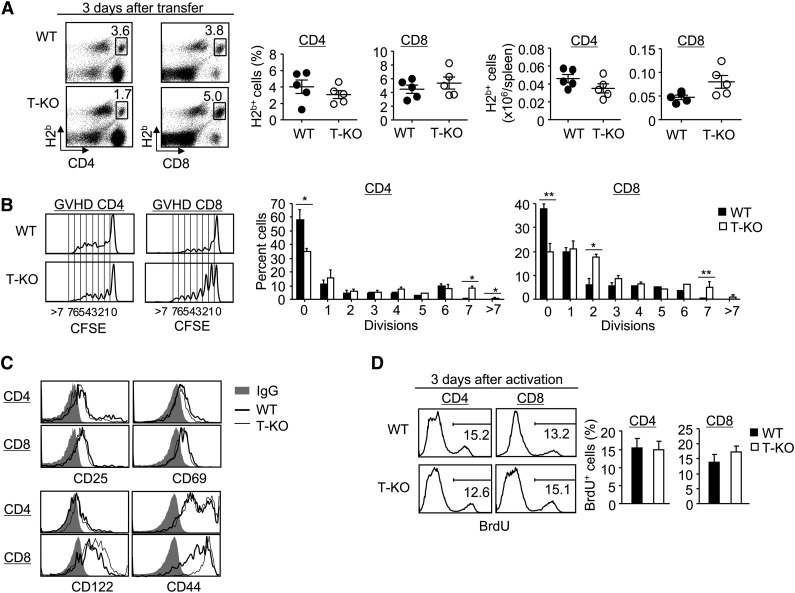
To understand the mechanism by which Ezh2-deficient T cells failed to induce GVHD, we first determined whether loss of Ezh2 impaired activation, engraftment, and/or proliferation of donor T cells during the GVHD priming phase. By 3 days after transplantation, there was no significant difference in the numbers of donor-derived T cells in the spleen in BALB/C recipients of T-KO T cells compared with WT T cells, with modestly increased numbers of donor CD8+ T cells (Figure 2A). When carboxyfluoroscein diacetate succinimidyl ester (CFSE) was used to track cell division, T-KO T cells had slightly higher percentages of dividing cells than WT T cells (Figure 2B). Furthermore, both T-KO and WT T cells expressed high levels of activation markers (eg, CD25, CD44, CD69, CD122) (Figure 2C). To assess proliferation of T-KO T cells in response to alloantigens, we assessed the BrdU incorporation by donor T cells 3 days after in vitro stimulation with allogeneic dendritic cells (DCs). There was no difference in BrdU+ percentage between activated WT and T-KO T cells (Figure 2D). We further examined the effect of Ezh2 deficiency on TCR signaling in T cells and showed normal activation of AKT and ERK signaling intermediates in T-KO T cells (supplemental Figure 3). These results suggest successful activation and proliferation of T-KO T cells during the priming phase.
Figure 2.
Ezh2 deficiency does not affect the initial activation and proliferation of donor T cells during alloantigen-priming phase. (A-C) Donor CD44lo T cells (ie, CD4+ + CD8+ T cells) derived from WT or T-KO B6 mice were labeled with CFSE and transplanted with TCD BM into lethally irradiated BALB/C recipients. Three days later, donor T cells were recovered from these BALB/C recipients for analysis. (A) Dot plots show the fraction of donor-derived H2b+ CD4+ or CD8+ T cells, and graphs show the percentage and absolute number of donor T cells. (B) Histograms show the CFSE dilution in donor T cells activated in allogeneic recipients. Graphs show the percentage of donor-derived T cells at each division. (C) Cells were collected for flow cytometry analysis. Histograms show the expression of CD25, CD69, CD122, and CD44 in donor-derived T cells. (D) CD44lo T cells derived from WT or T-KO B6 mice were stimulated by BALB/C BM-derived DCs. Three days later, cells were pulsed with BrdU for 2 hours to measure the incorporation of BrdU. Histograms and graphs show the percentage of T cells with BrdU incorporation. *P < .05, **P < .01. Error bars indicate mean ± SD. Data are representative of 2 independent experiments.
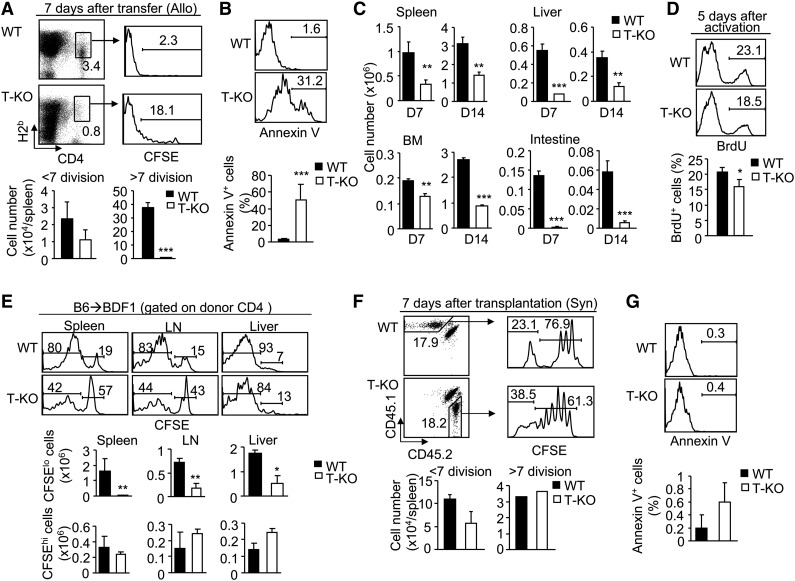
To evaluate whether Ezh2 deficiency affected alloreactive T cells at later stages, we tracked donor-derived T cells in the spleen of BALB/C recipients during the GVHD induction phase. By day 7 posttransplantation, the number of rapidly dividing T-KO CD4+ T cells (ie, >7 rounds of cell division) was markedly reduced (Figure 3A). T-KO CD4+ T cells showed increased apoptosis compared with WT cells (Figure 3B). As a result, there was an overall reduction in the total number of donor CD4+ T cells in the spleen, liver, BM, and intestine of BALB/C recipients at day 7 and 14 after transplantation (Figure 3C). BrdU incorporation assay showed the reduced proliferation of T-KO CD4+ T cells 5 days after allogeneic DC stimulation (Figure 3D). Similar results were observed for T-KO CD8+ T cells (supplemental Figure 4A-D).
Figure 3.
Loss of Ezh2 impairs the survival and expansion of alloantigen-activated T cells during the later stages of GVHD induction. Donor CD44lo T cells derived from B6/SJL WT (H2b) or B6 T-KO (H2b) mice were labeled with CFSE and transplanted with TCD BM into irradiated BALB/C recipients (H2d) (A-C). Seven days later, donor T cells were recovered for analysis. (A) Dot plots show the fraction of donor-derived CD4+ T cells, and histograms show their CFSE dilution. Graphs show the number of donor T cells. (B) Histograms and graphs show Annexin V+ CD4+ T cells. (C) Graphs show the number of donor-derived CD4+ T cells in different organs. (D) CD44lo T cells (CD4+ T cells) derived from WT or T-KO B6 mice were stimulated by BALB/C BM-derived DCs. Five days later, cells were collected for BrdU assay. (E) B6 (H2b) WT or T-KO T cells (CD4+ + CD8+ T cells) were transplanted into nonirradiated BDF1 (H2b/d) mice. Seven days later, cells were recovered for analysis. Histograms and graphs show the CFSE dilution and number of donor T cells. (F-G) A homeostatic proliferation assay was performed by transferring B6/SJL WT T cells (CD45.1) and B6 T-KO T cells (CD45.2) into lethally irradiated syngeneic B6xB6/SJL (CD45.1/CD45.2) recipients. (F) Dot plots show the fraction of donor-derived CD4+ T cells, and histograms show their CFSE dilution. (G) Histograms and graphs show Annexin V+ in donor CD8+ T cells. Data shown are representative of 2 independent experiments, each with 3 to 5 mice per group. *P < .05, **P < .01, ***P < .001. Error bars indicate mean ± SD.
In GVHD models, alloantigen-specific T-cell proliferation occurs parallel to homeostatic proliferation in irradiated hosts.21,22 To assess the effect of Ezh2 deficiency on alloantigen-driven T cells, we transferred B6 WT and T-KO T cells (H2b) labeled with CFSE into un-irradiated B6xDBA (BDF1) mice (H2b/d). Dilution of CFSE can be tracked to distinguish alloantigen-activated T cells (CFSElo) from alloantigen unresponsive T cells (CFSEhi).21 Loss of Ezh2 led to an overall reduction of donor CFSElo T cells rather than CFSEhi T cells (Figure 3E; supplemental Figure 4E). To test whether Ezh2 inhibition affected homeostatic proliferation, we transferred CFSE-labeled B6/SJL WT T cells (CD45.1) or B6 T-KO T cells (CD45.2) into irradiated syngeneic B6/SJLxB6 mice (CD45.1/CD45.2) to induce homeostatic proliferation.22 Seven days posttransplantation, there was no significant decrease in proliferation of CD4+ T cells in these syngeneic recipients (Figure 3F-G), or even an increase in the number of CD8+ T cells (supplemental Figure 4F-G). Thus, Ezh2 is essential for continual proliferation and expansion of alloantigen-reactive T cells later during GVHD induction but dispensable for T-cell homeostatic proliferation.
Ezh2 regulates alloreactive T cells in a cell-autonomous manner
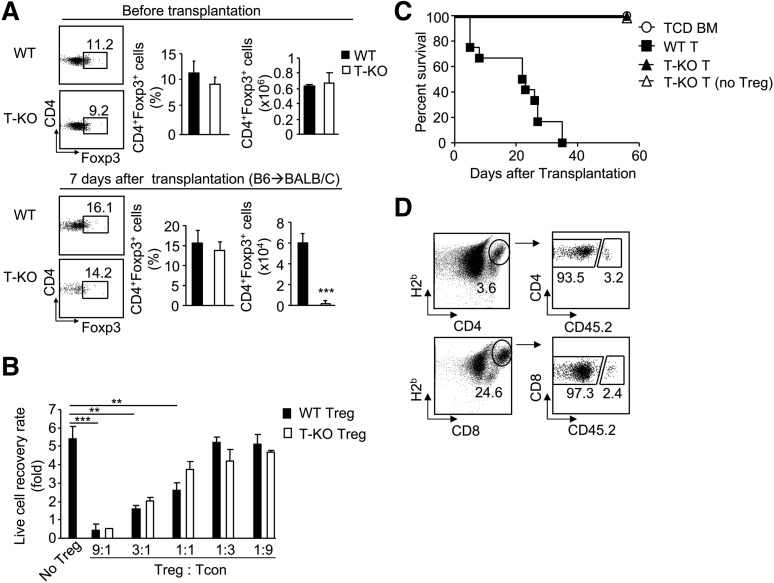
T regulatory cells (Tregs) play important roles in repressing allogeneic T-cell responses.23 We tested whether the absence of Ezh2 affected the number and function of Treg. The frequency of FoxP3+ cells among total CD4+ T cells and the total number of CD4+FoxP3+ Treg cells were the same in WT and T-KO mice (Figure 4A). Seven days after transplantation, the total numbers of T-KO Treg cells were markedly reduced compared with WT cells (Figure 4A). To evaluate the function of Ezh2-deficient Tregs, we first examined the effect of CD25-positive T-KO Tregs on proliferation of T-cell receptor (TCR)-activated CD4 T cells depleted of Tregs in cultures. Both WT and T-KO Tregs similarly reduced the expansion of TCR-activated CD4 T cells (Figure 4B). We also found that transfer of T-KO T cells depleted of Tregs did not rescue their inability to induce GVHD (Figure 4C), ruling out the possibility of enhanced regulatory function in T-KO cells.
Figure 4.
Ezh2 regulates the survival and expansion of alloantigen-activated T cells in a cell-autonomous manner. (A) Donor T cells were isolated from the spleen of WT and T-KO mice (before transplantation) and of allogeneic BMT BALB/C recipients 7 days after transplantation (after transplantation) to measure their expression of Foxp3. (B) CD4+CD25+ Tregs were isolated from WT and T-KO B6 mice and added to the wells of a 96-well plate containing CD25– CD4+ T cells derived from B6/SJL mice (CD45.1) at ratios as indicated. Seventy-two hours later, the recovery rate of proliferating CD45.1+ CD4+ T cells was analyzed. (C) B6 WT T cells, T-KO T cells, or T-KO T cells depleted of CD4+CD25+ Tregs were transplanted together with TCD BM into irradiated BALB/C recipients to induce GVHD. The survival was monitored over time. ○, n = 5; ▪, n = 5; ▲ n = 5; △ n = 10. (D) WT T cells derived from B6/SJL mice (CD45.1+) and T-KO T cells derived from B6 mice (CD45.2+) were mixed at a ratio of 1:1 and transplanted with TCD BM into lethally irradiated BALB/C recipients. Seven days later, cells were recovered for flow cytometry analysis. Dot plots show the fraction of donor WT and T-KO T cells. **P < .01, ***P < .001. Data shown in (A), (B), and (D) are from 3 to 5 mice per group. Data are representative of 2 independent experiments. Error bars indicate mean ± SD.
To examine the cell-intrinsic requirement for Ezh2 in T cells, we co-transferred B6 T-KO T cells (CD45.2) together with WT B6/SJL T cells (CD45.1) into irradiated BALB/C mice. Compared with WT T cells, there were approximately 30- to 40-fold fewer T-KO T cells in these allogeneic recipients at day 7 after transplantation, suggesting impaired proliferation of T-KO cells (Figure 4D). These data demonstrate that Ezh2 is intrinsically required to regulate the survival and expansion of alloantigen-activated T cells.
Deletion of Bim minimally rescues alloantigen-activated T cells lacking Ezh2
Having observed an increase of apoptosis in T-KO cells (Figure 3B and supplemental Figure 4B), we tested whether the altered expression of apoptosis genes accounted for impaired expansion of activated T-KO T cells. TCR-activated T-KO CD4+ T cells showed a reduction of H3K27me3 and upregulation of Dab2ip gene (which is an Ezh2-repressed gene24) (Figure 5A), confirming the inhibition of Ezh2 function. These activated T-KO CD4+ T cells expressed higher levels of the proapoptotic Bim mRNA and protein than did activated WT CD4+ T cells (Figure 5B-C). Similar results were found in T-KO CD8+ T cells (supplemental Figure 5A-B). ChIP assays confirmed that Ezh2 and its catalyzed H3K27me3 marked the promoter region of the Bim gene in T cells (supplemental Figure 5C-D). Thus, Ezh2 represses Bim transcription in activated T cells.
Figure 5.
Inactivation of Bim minimally rescues the expansion of alloantigen-activated Ezh2-deficient T cells in vivo. (A-C) Naïve CD4+ T cells from WT or T-KO B6 mice were stimulated with CD3/CD28 Abs. Three days later, total RNA and protein lysates were prepared. Western blot analysis shows the amount of histone methylation marks (A). The graph shows the gene expression analyzed by RT-PCR (B). Western blot analysis shows the protein levels (C). Data are representative of 2 independent experiments. (D) Donor T cells (CD4+ + CD8+ T cells) derived from WT, Ezh2-T-KO, Bim−/−, or Bim−/− EZH2-T-KO mice were transplanted with TCD BM into lethally irradiated BALB/C recipients. Seven days later, cells were recovered from these recipients, counted, and analyzed using flow cytometry. Data are representative of 2 independent experiments, each with 3 to 4 recipients per group. Dot plots show the fraction of donor-derived CD4+ T cells. Graphs show the absolute number of donor CD4+ cells. (E) WT, Ezh2-T-KO, Bim−/−, or Bim−/− Ezh2-T-KO B6 mouse-derived T cells were cultured with BALB/C BM-derived DCs. Graphs show the recovery rate of live cells. *P < .05, **P < .001, ***P < .001. Error bars indicate mean ± SD. Data are representative of 2 independent experiments.
Bim is known to initiate apoptosis by neutralizing or modifying Bcl-2– and Bcl-2–like prosurvival proteins.25 In WT and T-KO CD4+ and CD8+ T cells, expression of Bcl-xl and Bcl-2 proteins increased upon stimulation (Figure 5C; supplemental Figure 5B). To test whether the elevated ratio of Bim vs Bcl-2 and Bcl-xl might be responsible for increased apoptosis of activated T-KO T cells, we bred T-KO B6 mice to Bim−/− B6 mice and generated Bim-deficient T-KO B6 mice (named Bim−/−.T-KO mice). T cells from WT, T-KO, Bim−/−, or Bim−/−.T-KO B6 mice were transferred into irradiated BALB/C mice. We reasoned that if loss of Bim rescued T-KO T cells in vivo, it would suggest that Ezh2 repression of Bim was important for promoting the survival of alloreactive T cells. There was a modestly increased number of Bim−/−.T-KO CD4+ T cells in the spleen of these recipients compared with T-KO T cells 7 days after transplantation (Figure 5D). However, Bim deletion had no effect on the expansion of T-KO CD4+ T cells in the liver (Figure 5D). Upon in vitro stimulation with allogeneic DCs, we validated that Bim deletion minimally rescued alloantigen-activated T-KO CD4+ T cells (Figure 5E). Similar results were observed for alloantigen-activated Bim−/−.T-KO CD8+ T cells (supplemental Figure 6). Thus, it appears that increased Bim expression in activated T-KO T cells only partially, if at all, accounts for their impaired expansion. This effect of Ezh2 deletion on GVHD T cells differs from that of DZNep treatment.10
The anti-apoptotic molecule Mcl-1 protects activated T cells through antagonizing the proapoptotic role of Bax and Bak, which is independent of Bim-mediated apoptosis.26 Compared with activated WT T cells, T-KO T cells expressed approximately fivefold more Mcl-1, reduced expression of Bax, and moderately increased Bak (Figure 5B). It seems unlikely that increased apoptosis in activated T-KO T cells could be explained by the presence of high levels of Mcl-1, although additional experiments are needed to formally address it.
Ezh2-deficient T cells are specifically impaired in their induction of IFN-γ–producing effector cells
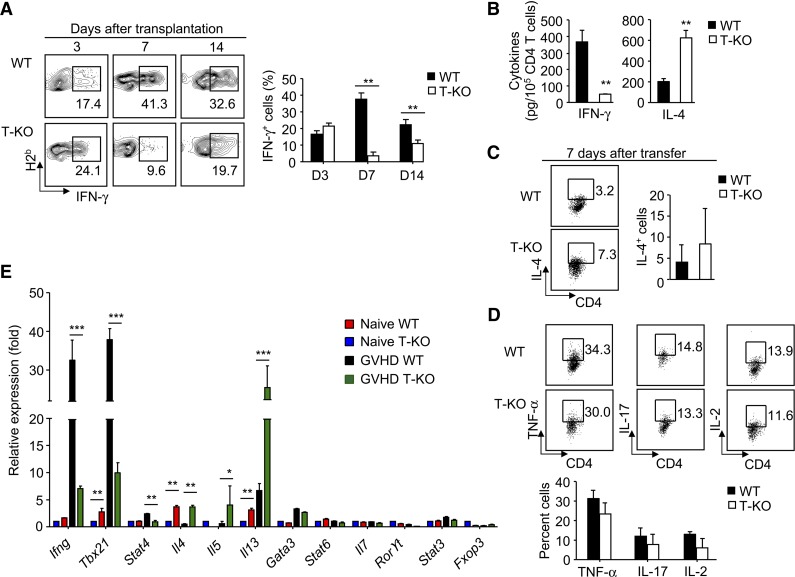
As a gene silencer, Ezh2 has been thought to repress T-cell production of interferon-γ (IFN-γ), interleukin-4 (IL-4), and IL-17.27,28 Previous studies demonstrate that IFN-γ–producing alloreactive T help (Th)1 effector cells cause damage to the liver and gastrointestinal tract but prevents lung injury mainly induced by IL-4–producing Th2 effector cells.29,30 To investigate the impact of Ezh2 on effector T-cell differentiation, we recovered donor T cells from BALB/C mice receiving B6 mouse–derived WT and T-KO T cells at various time points after transplantation. There was no significant difference in percentage of IFN-γ–producing CD4+ T cells between WT and T-KO T-cell recipients 3 days after transplantation (Figure 6A), consistent with a successful activation of T-KO T cells (Figure 2). In contrast, 7 and 14 days after transplantation, T-KO CD4+ T cells showed markedly reduced frequency of IFN-γ+ effector cells than WT CD4+ T cells (Figure 6A). ELISA confirmed the decreased secretion of IFN-γ by these T-KO CD4+ T cells (Figure 6B). Reduction of T-KO IFN-γ+CD4+ T cells also occurred in other GVHD target organs (supplemental Figure 7), ruling out the possibility that altered tissue distribution might contribute to the reduction of IFN-γ+ T cells. Similar results were observed for alloantigen-activated T-KO CD8+ T cells (supplemental Figure 8).
Figure 6.
Ezh2-deficient T cells are specifically impaired for their differentiation into effector cells producing IFN-γ Donor T cells (CD4+ + CD8+ T cells) derived from WT or T-KO B6 mice were transplanted with B6 TCD BM into lethally irradiated BALB/C recipients. At indicated time points after transplantation, the cells were collected, counted, and analyzed using flow cytometry. (A) The percentage of donor T cells producing IFN-γ. (B) Graphs show cytokine production assessed by ELISA. (C) The fraction of donor T cells producing IL-4. (D) The percentage of donor T cells producing TNF-α, IL-17, and IL-2. (E) RT-PCR analysis of gene expression in donor CD4+ T cells isolated from the spleens of BMT recipients 7 days after transplantation. **P < .01. Error bars indicate mean ± SD. Data are representative of 2 independent experiments with 3 to 5 mice per group.
Loss of Ezh2 led to increased overall production of IL-4 in alloreactive T cells (Figure 6B) without polarizing into a clearly distinguishable Th2 population 7 days after transfer (Figure 6C). Furthermore, compared with WT CD4+ T cells, alloantigen-stimulated T-KO CD4+ T cells produced equal levels of TNF-α, IL-17, and IL-2 (Figure 6D). Likewise, both WT and T-KO CD4+ and CD8+ T cells expressed similar percentages of cytolytic effector molecules (FasL, TRAIL, and GzmB) and the chemokine receptor CXCR3 (supplemental Figure 9A), and had similar killing activity against A20 leukemic cells (supplemental Figure 9B). Thus in addition to regulating the production of sufficient numbers of alloreactive T cells needed to mediate GVHD, Ezh2 is also specifically required to promote IFN-γ production in effector cells.
Lineage-specific transcription programs are responsible for inducing different subsets of effector CD4+ T cells.31 Stat4 and T-bet (encoded by Tbx21) promote Th1 cell differentiation, whereas Stat6 and Gata3 drive Th2 cell development.31 To understand the mechanism by which Ezh2 affected the development of Th1 cells during GVH reaction, we purified donor CD4+ T cells from BALB/C mice receiving WT and T-KO T cells 7 days after transplantation. Real-time RT-PCR revealed that although loss of Ezh2 led to decreased expression of Ifng, Tbx21, and Stat4, it caused upregulation of Th2-cell cytokine transcripts Il4, Il5, and Il13 without changing Stat6 and Gata3 (Figure 6E). Th17 cell differentiation involves activation of Stat3 and RORγt.31 Loss of Ezh2 did not alter the expression of Il17 and Rorγt transcripts (Figure 6E). Thus, Ezh2 promotes Th1-cell development through a mechanism of stimulating expression of Ifng, Tbx21, and Stat4. The underlying mechanism has yet to be determined.
Ezh2 ablation prevents GVHD against minor histocompatibility antigens (miHAs)
To assess whether Ezh2 deficiency prevented T cell–mediated GVHD directed against miHAs, we used the MHC-identical but miHA-mismatched B6 anti-BALB.B mouse model32 Our previous studies33 and others34 have shown that miHA H60–specific CD8+ effector cells (H60+CD8+) possess a potent ability to mediate GVHD. WT T cells caused lethal GVHD, with approximately 80% dying from the disease (supplemental Figure 10A). In contrast, 90% of T-KO T cell recipients survived (supplemental Figure 10A). The overall expansion of donor T-KO CD8+ and CD4+ T cells was markedly reduced in these BALB.B recipients compared with that of WT T cells (supplemental Figure 10B). Furthermore, Ezh2 deficiency drastically decreased donor H60+CD8+ T cells (supplemental Figure 10C). Thus, loss of Ezh2 prevents GVHD directed against miHAs.
Preservation of antileukemic effects in donor T cells in the absence of Ezh2
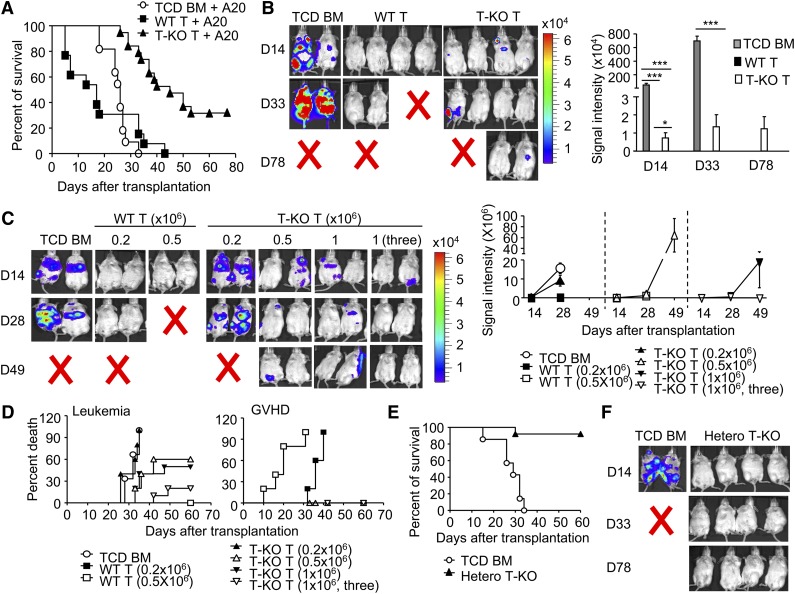
Donor lymphocyte infusion has been effective in treating hematologic malignant diseases after transplantation.3,4 To determine whether donor T cells lacking Ezh2 retained the beneficial GVL, we challenged these BALB/C recipients with A20 leukemia/lymphoma cells at the day of allogeneic BMT. We tracked luciferase-expressing A20 cells using bioluminescence imaging. Mice transplanted with TCD BM and challenged with A20 cells developed leukemia and died of the disease within 36 days (Figure 7A-B). No leukemia was found in mice receiving WT T cells, suggesting potent antileukemia effects. However, these WT T-cell recipients died of GVHD (Figure 7A-B). In contrast, transfer of T-KO T cells significantly reduced the growth of leukemic cells, with 30% surviving without leukemia and GVHD within the 80-day observation period (Figure 7A-B).
Figure 7.
Inhibition of Ezh2 preserves GVL effects. (A-B) Irradiated BALB/C recipients were transplanted with B6 TCD BM, with or without WT or T-KO T cells (1 × 106 CD4+ + 1 × 106 CD8+ T cells), and challenged with A20 cells (1 × 106/mouse). (A) The survival (pooled from at least 3 experiments) was monitored over time. ○, n = 11; ▪, n = 13; ▲, n = 19. P < .001, ○ versus ▲. (B) The graph shows the total body luminescence intensity. (C-D) Irradiated BALB/C recipients were transplanted with donor B6 TCD BM, with or without indicated doses of WT or T-KO T-KO cells and challenged with A20 cells (1 × 106/mouse) at the day of BMT. In one group, the recipient mice received T-KO (1 × 106) at days 0, 5, and 10 after transplantation, respectively. (C) The graph shows the total body luminescence intensity. (D) The survival was monitored. The death of GVHD or GVL was determined by necropsy. ○, n = 5; ▪, n = 5; □, n = 5; ▲, n = 5; △, n = 5; ▼, n = 5; ▽, n = 10. (Leukemia death: P < .05, ○ vs △, and ○ vs ▼; P < .01, ○ vs ▽. GVHD death: P < .001, ▪ vs ▲, ▪ vs △, ▪ vs ▼, ▪ vs ▽). (E-F) Lethally irradiated BALB/C recipients were transplanted with donor B6 TCD BM, with (▲, n = 10) or without (○, n = 7) T cells (0.2 × 106) isolated from hetero T-KO mice, and challenged with A20 cells. (E) The survival was monitored over time. P < .001, ○ vs ▲. (F) In vivo bioluminescence analysis of leukemia growth. Error bars indicate mean ± SD. *P < .05, ***P < .001.
To better define GVL effects of T-KO T cells, we titrated donor T cells and evaluated their ability to control leukemia growth. Transfer of 0.2 × 106 WT T cells was sufficient to eliminate leukemic cells but caused lethal GVHD (Figure 7C-D). In contrast, as many as 0.5 × 106 T-KO T cells are necessary for significantly reducing the leukemia growth and prolonging the mean survival time of these transplant recipients (Figure 7C-D). This suggests that T-KO T cells retain significant antileukemic activity but are less potent than WT T cells.
Because both T-KO and WT T cells had similar cytotoxicity against A20 leukemic cells (supplemental Figure 9B), we reasoned that increased apoptosis of alloreactive T-KO T cells could limit their GVL. To test it, we used 2 clinically relevant strategies to assess the antileukemia effect of Ezh2 inhibition in T cells. We first examined whether repetitive infusion of T-KO T cells could improve GVL effects. Compared with one injection of T-KO T cells (1 × 106), 3 injections of T-KO T cells (1 × 106, 3) at a schedule of once every 5 days after allo-BMT completely eradicated the leukemia by 28 days after infusion, with about 80% of mice surviving without GVHD and leukemia (Figure 7C-D).
We then examined whether partially reducing Ezh2 activity in donor T cells could augment GVL effects. We titrated the number of donor T cells derived from conditional Ezh2 heterozygous knockout B6 mice (named hetero T-KO), in which only one of 2 Ezh2 alleles was deleted in T cells. In contrast to WT T-cell recipients, mice receiving 0.2 × 106 hetero T-KO cells were protected against GVHD lethality and morbidity (supplemental Figure 11A). Increasing the dose of hetero T-KO T cells to 1.0 × 106 caused GVHD similar to that of WT T cells (supplemental Figure 11A). These data suggest that the capacity of Ezh2-haploinsufficient donor T cells to mediate GVHD is markedly reduced. Importantly, infusion of 0.2 × 106 hetero T-KO cells was sufficient to eliminate leukemic cells in transplant recipients (Figure 7E-F). Like T-KO T cells, hetero T-KO T cells showed impaired expansion and proliferation in cultures 5 days after stimulation (supplemental Figure 11B). These findings indicate that either repetitive infusion of T-KO T cells or partial inhibition of Ezh2 can efficiently prevent the malignant relapse without causing GVHD after BMT. These findings are important for future designing of Ezh2 inhibitor-based GVHD therapy.
Discussion
Identifying the molecular pathways important for allogeneic T-cell responses is crucial for modulating the balance between the beneficial and harmful effects of allogeneic BMT. Our findings reveal unpredicted but essential roles for Ezh2 in regulating allogeneic T-cell proliferation, differentiation, and expansion. Deletion of Ezh2 reduced the ability of donor T cells to mediate GVHD but retained GVL in mouse models of allogeneic BMT. Ezh2 deficiency in donor T cells did not affect their initial activation and proliferation responses to alloantigens during the priming phase but reduced their capacity to undergo continual proliferation and expansion during later stages of GVHD induction. In addition, Ezh2-deficient T cells were specifically impaired in their induction of IFN-γ–producing effector cells. Thus modulation of Ezh2 may lead to novel approaches to control GVHD while preserving antileukemia activity.
Emerging evidence indicates that targeting epigenetic pathways can effectively prevent GVHD. For example, pharmacologic inhibition of DNA methylation, which mediates global silencing of gene expression,35 prevents acute GVHD in mice without losing GVL effects through a mechanism of modulating Tregs.36,37 Pharmacologic inhibition of histone deacetylases, which relaxes chromatin structure and facilitates gene transcription,35 reduces GVHD.38-40 Treatment with the histone deacetylase inhibitor suberoylanilide hydroxamic acid decreases the production of inflammatory cytokines and enhances the regulatory function of APCs.38-40 For these reasons, it is of extreme importance to identify specific chromatin modifying enzyme(s) that regulate allogeneic T-cell responses. Identification of the selective effect of Ezh2 on allogeneic T cells suggests that pharmacologic modulating Ezh2 should be investigated as a novel therapeutic strategy after allogeneic BMT.
Intriguingly, we observed both similarity and discrepancy in modulation of alloreactive T cells between genetic inactivation of Ezh2 and DZNep inhibition of histone methylation. Similar to Ezh2 deficiency, DZNep treatment reduced cellular Ezh2, decreased H3K27me3, activated Bim, and caused apoptosis in alloreactive T cells.10 However, although Bim was clearly important for DZNep treatment–reduced GVHD,10 deletion of Bim in Ezh2-deficient T cells only partially rescued their survival and expansion. Two possibilities may explain this discrepancy. First, there is a fundamental difference in modulating histone methylation and gene expression between conditionally deleting Ezh2 and DZNep treatment. Ezh2 deficiency in alloreactive T cells led to their selective reduction of H3K27me3. In contrast, DZNep treatment caused a reduction of multiple histone methylation pathways (eg, H3K4me3, H3K27me3, H3K36me3, H4K20me3).10,11 Because each of these histone methylation pathways may have distinct roles in T-cell immune responses,9 it is likely that the reduction of Ezh2 function in activated T cells only represents one subset of multiple effects by DZNep treatment. Second, conditional knockout results in complete and permanent inactivation of Ezh2. In contrast, DZNep treatment may cause transient and reversible impact on Ezh2 and other HMTs. For example, the in vivo elimination half-life of DZNep was about 12.8 minutes,41 and some studies suggested that the expression of genes in DZNep-treated cells could return to the original level within 24 hours after removal of the inhibitor.11 Thus we propose that investigating the effect of modulating Ezh2 using specific inhibitors should consider the impact of dose, duration, and time of the inhibitor and its potential off-target effects.
Dissociation of beneficial GVL effects from harmful GVHD is important to harness donor T-cell responses to allogeneic BMT but has proven to be a difficult task.1,3,4 One major reason is that cytotoxic mechanisms mediating GVHD are also important for GVL.7 Recent studies suggest that the threshold of allogeneic T-cell responses mediating GVHD differs from that required for eliminating leukemic cells.42,43 For example, Notch inhibition results in dramatic reduction of multiple inflammatory cytokines and cytotoxic molecules in alloreactive T cells.42,44 As a result, inhibition of Notch signaling reduces GVHD but preserves potent GVL.42,45 Other studies also suggest that inhibition of PKC-θ, which is critical for expansion of alloreactive T cells, reduces GVHD and preserves GVL.43 These observations support the idea that elimination of leukemic cells requires either less alloreactivity or fewer alloreactive effector T cells than induction of lethal GVHD.46 Our findings provide a unique approach to regulate this threshold of GVH reaction because inhibition of Ezh2 reduced GVHD while preserving GVL after allogeneic BMT.
Our observations identify Ezh2 as a new central regulator of allogeneic T-cell responses. Ezh2 is not required for the initial activation and proliferation of alloantigen-activated T cells early during the priming phase. Ezh2 is also dispensable for T cells undergoing homeostatic survival and proliferation in response to irradiation-induced lymphopenia. In contrast, Ezh2 is essential for continual proliferation, effector differentiation, and expansion of alloantigen-responding T cells late during GVHD induction. Thus our findings will be important for designing novel and clinically relevant strategies for GVHD therapies using Ezh2-specific inhibitors, which have been most recently discovered by other groups for cancer treatment in patients.47-50 Because either repetitive infusion of Ezh2-deficient T cells or partial inactivation of Ezh2 function could reduce GVHD but preserve GVL, we suggest that pharmacologic strategies are highly likely to be effective at improving the efficacy of allogeneic BMT. In addition, although our observations are made in mouse GVHD models, they may extend to other types of Ag-driven T-cell responses. For instance, Ezh2 may control autoimmune diseases and rejection of allogeneic grafts. From a therapeutic perspective, we suggest that modulation of Ezh2 may lead to new strategies to treat other inflammatory disorders in broad context.
Supplementary Material
Acknowledgments
We thank Elizabeth Hexner (Department of Medicine, University of Pennsylvania) for the thoughtful discussion and critical reading of the manuscript.
This work was supported by a Damon Runyon-Rachleff Innovation Award (Y.Z.), the American Cancer Society (Y.Z.), the Department of Defense (Y.Z.), and National Institutes of Health, National Cancer Institute grant CA172106-01(Y.Z.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.H. and Y.Z. conceived and designed the project; S.H., F.X., Y.L., Q.T., K.M., P.E.L., R.-S.M., I.M., and Y.Z. performed experiments and analyzed the data; P.R., A.M.C., S.M., and P.D.K. designed experiments and analyzed data; P.D.K. edited the manuscript; and S.H. and Y.Z. wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yi Zhang, Department of Internal Medicine, University of Michigan, Ann Arbor, MI 48109-5942; e-mail: yizha@med.umich.edu.
References
- 1.Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12(6):443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373(9674):1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112(12):4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 4.Wu CJ, Ritz J. Induction of tumor immunity following allogeneic stem cell transplantation. Adv Immunol. 2006;90:133–173. doi: 10.1016/S0065-2776(06)90004-2. [DOI] [PubMed] [Google Scholar]
- 5.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98(12):3192–3204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 6.Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7(5):340–352. doi: 10.1038/nri2000. [DOI] [PubMed] [Google Scholar]
- 7.van den Brink MR, Burakoff SJ. Cytolytic pathways in haematopoietic stem-cell transplantation. Nat Rev Immunol. 2002;2(4):273–281. doi: 10.1038/nri775. [DOI] [PubMed] [Google Scholar]
- 8.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9(2):91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 9.He S, Tong Q, Bishop DK, Zhang Y. Histone methyltransferase and histone methylation in inflammatory T-cell responses. Immunotherapy. 2013;5(9):989–1004. doi: 10.2217/imt.13.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He S, Wang J, Kato K, et al. Inhibition of histone methylation arrests ongoing graft-versus-host disease in mice by selectively inducing apoptosis of alloreactive effector T cells. Blood. 2012;119(5):1274–1282. doi: 10.1182/blood-2011-06-364422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miranda TB, Cortez CC, Yoo CB, et al. DZNep is a global histone methylation inhibitor that reactivates developmental genes not silenced by DNA methylation. Mol Cancer Ther. 2009;8(6):1579–1588. doi: 10.1158/1535-7163.MCT-09-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan J, Yang X, Zhuang L, et al. Pharmacologic disruption of Polycomb-repressive complex 2-mediated gene repression selectively induces apoptosis in cancer cells. Genes Dev. 2007;21(9):1050–1063. doi: 10.1101/gad.1524107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badeaux AI, Shi Y. Emerging roles for chromatin as a signal integration and storage platform. Nat Rev Mol Cell Biol. 2013;14(4):211–224. doi: 10.1038/nrm3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469(7330):343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamminga LM, Bystrykh LV, de Boer A, et al. The Polycomb group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood. 2006;107(5):2170–2179. doi: 10.1182/blood-2005-09-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Su IH, Dobenecker MW, Dickinson E, et al. Polycomb group protein ezh2 controls actin polymerization and cell signaling. Cell. 2005;121(3):425–436. doi: 10.1016/j.cell.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 17.Su IH, Basavaraj A, Krutchinsky AN, et al. Ezh2 controls B cell development through histone H3 methylation and Igh rearrangement. Nat Immunol. 2003;4(2):124–131. doi: 10.1038/ni876. [DOI] [PubMed] [Google Scholar]
- 18.Kato K, Cui S, Kuick R, et al. Identification of stem cell transcriptional programs normally expressed in embryonic and neural stem cells in alloreactive CD8+ T cells mediating graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(6):751–771. doi: 10.1016/j.bbmt.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shono Y, Ueha S, Wang Y, et al. Bone marrow graft-versus-host disease: early destruction of hematopoietic niche after MHC-mismatched hematopoietic stem cell transplantation. Blood. 2010;115(26):5401–5411. doi: 10.1182/blood-2009-11-253559. [DOI] [PubMed] [Google Scholar]
- 20.Na IK, Lu SX, Yim NL, et al. The cytolytic molecules Fas ligand and TRAIL are required for murine thymic graft-versus-host disease. J Clin Invest. 2010;120(1):343–356. doi: 10.1172/JCI39395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166(2):973–981. doi: 10.4049/jimmunol.166.2.973. [DOI] [PubMed] [Google Scholar]
- 22.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2(8):547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 23.Edinger M, Hoffmann P, Ermann J, et al. CD4+CD25+ regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9(9):1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal P, Raghavan A, Nandiwada SL, et al. Gene regulation and chromatin remodeling by IL-12 and type I IFN in programming for CD8 T cell effector function and memory. J Immunol. 2009;183(3):1695–1704. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouillet P, O’Reilly LA. CD95, BIM and T cell homeostasis. Nat Rev Immunol. 2009;9(7):514–519. doi: 10.1038/nri2570. [DOI] [PubMed] [Google Scholar]
- 26.Tripathi P, Koss B, Opferman JT, Hildeman DA. Mcl-1 antagonizes Bax/Bak to promote effector CD4(+) and CD8(+) T-cell responses. Cell Death Differ. 2013;20(8):998–1007. doi: 10.1038/cdd.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacob E, Hod-Dvorai R, Schif-Zuck S, Avni O. Unconventional association of the polycomb group proteins with cytokine genes in differentiated T helper cells. J Biol Chem. 2008;283(19):13471–13481. doi: 10.1074/jbc.M709886200. [DOI] [PubMed] [Google Scholar]
- 28.Koyanagi M, Baguet A, Martens J, Margueron R, Jenuwein T, Bix M. EZH2 and histone 3 trimethyl lysine 27 associated with Il4 and Il13 gene silencing in Th1 cells. J Biol Chem. 2005;280(36):31470–31477. doi: 10.1074/jbc.M504766200. [DOI] [PubMed] [Google Scholar]
- 29.Burman AC, Banovic T, Kuns RD, et al. IFNgamma differentially controls the development of idiopathic pneumonia syndrome and GVHD of the gastrointestinal tract. Blood. 2007;110(3):1064–1072. doi: 10.1182/blood-2006-12-063982. [DOI] [PubMed] [Google Scholar]
- 30.Yi T, Chen Y, Wang L, et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114(14):3101–3112. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berger M, Wettstein PJ, Korngold R. T cell subsets involved in lethal graft-versus-host disease directed to immunodominant minor histocompatibility antigens. Transplantation. 1994;57(7):1095–1102. [PubMed] [Google Scholar]
- 33.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Alloreactive memory T cells are responsible for the persistence of graft-versus-host disease. J Immunol. 2005;174(5):3051–3058. doi: 10.4049/jimmunol.174.5.3051. [DOI] [PubMed] [Google Scholar]
- 34.Choi EY, Christianson GJ, Yoshimura Y, et al. Real-time T-cell profiling identifies H60 as a major minor histocompatibility antigen in murine graft-versus-host disease. Blood. 2002;100(13):4259–4265. doi: 10.1182/blood-2002-05-1299. [DOI] [PubMed] [Google Scholar]
- 35.De Carvalho DD, Sharma S, You JS, et al. DNA methylation screening identifies driver epigenetic events of cancer cell survival. Cancer Cell. 2012;21(5):655–667. doi: 10.1016/j.ccr.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi J, Ritchey J, Prior JL, et al. In vivo administration of hypomethylating agents mitigate graft-versus-host disease without sacrificing graft-versus-leukemia. Blood. 2010;116(1):129–139. doi: 10.1182/blood-2009-12-257253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sánchez-Abarca LI, Gutierrez-Cosio S, Santamaría C, et al. Immunomodulatory effect of 5-azacytidine (5-azaC): potential role in the transplantation setting. Blood. 2010;115(1):107–121. doi: 10.1182/blood-2009-03-210393. [DOI] [PubMed] [Google Scholar]
- 38.Reddy P, Maeda Y, Hotary K, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci USA. 2004;101(11):3921–3926. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi S, Reddy P. HDAC inhibition and graft versus host disease. Mol Med. 2011;17(5-6):404–416. doi: 10.2119/molmed.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reddy P, Sun Y, Toubai T, et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118(7):2562–2573. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coulombe RA, Jr, Sharma RP, Huggins JW. Pharmacokinetics of the antiviral agent 3-deazaneplanocin A. Eur J Drug Metab Pharmacokinet. 1995;20(3):197–202. doi: 10.1007/BF03189670. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Sandy AR, Wang J, et al. Notch signaling is a critical regulator of allogeneic CD4+ T-cell responses mediating graft-versus-host disease. Blood. 2011;117(1):299–308. doi: 10.1182/blood-2010-03-271940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valenzuela JO, Iclozan C, Hossain MS, et al. PKCtheta is required for alloreactivity and GVHD but not for immune responses toward leukemia and infection in mice. J Clin Invest. 2009;119(12):3774–3786. doi: 10.1172/JCI39692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mochizuki K, Xie F, He S, et al. Delta-like ligand 4 identifies a previously uncharacterized population of inflammatory dendritic cells that plays important roles in eliciting allogeneic T cell responses in mice. J Immunol. 2013;190(7):3772–3782. doi: 10.4049/jimmunol.1202820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tran IT, Sandy AR, Carulli AJ, et al. Blockade of individual Notch ligands and receptors controls graft-versus-host disease. J Clin Invest. 2013;123(4):1590–1604. doi: 10.1172/JCI65477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chao N. Control of GVHD: it’s in our DNA! Blood. 2012;119(5):1102–1103. doi: 10.1182/blood-2011-12-395905. [DOI] [PubMed] [Google Scholar]
- 47.Konze KD, Ma A, Li F, et al. An Orally Bioavailable Chemical Probe of the Lysine Methyltransferases EZH2 and EZH1. ACS Chem Biol. 2013 doi: 10.1021/cb400133j. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCabe MT, Ott HM, Ganji G, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492(7427):108–112. doi: 10.1038/nature11606. [DOI] [PubMed] [Google Scholar]
- 49.Knutson SK, Wigle TJ, Warholic NM, et al. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat Chem Biol. 2012;8(11):890–896. doi: 10.1038/nchembio.1084. [DOI] [PubMed] [Google Scholar]
- 50.Qi W, Chan H, Teng L, et al. Selective inhibition of Ezh2 by a small molecule inhibitor blocks tumor cells proliferation. Proc Natl Acad Sci USA. 2012;109(52):21360–21365. doi: 10.1073/pnas.1210371110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.