Abstract
Cholangiocarcinomas (CCAs) are hepatobiliary cancers with features of cholangiocyte differentiation; they can be classified anatomically as intrahepatic (iCCA), perihilar (pCCA), or distal CCA (dCCA). These subtypes differ not only in their anatomic location but in epidemiology, origin, etiology, pathogenesis, and treatment. The incidence and mortality of iCCA has been increasing over the past 3 decades, and only a low percentage of patients survive until 5 y after diagnosis. Geographic variations in the incidence of CCA are related to variations in risk factors. Changes in oncogene and inflammatory signaling pathways, as well as genetic and epigenetic alterations and chromosome aberrations, have been shown to contribute to development of CCA. Furthermore, CCAs are surrounded by a dense stroma that contains many cancer-associated fibroblasts, which promotes their progression. We have gained a better understanding of the imaging characteristics of iCCAs and have developed advanced cytologic techniques to detect pCCAs. Patients with iCCAs are usually treated surgically, whereas liver transplantation following neoadjuvant chemoradiation is an option for a subset of patients with pCCAs. We review recent developments in our understanding of the epidemiology, pathogenesis, of CCA, along with advances in classification, diagnosis and treatment.
Keywords: Cancer associated fibroblasts, distal cholangiocarcinoma, intrahepatic cholangiocarcinoma, molecular pathogenesis, perihilar cholangiocarcinoma, tumor microenvironment
Introduction
Cholangiocarcinoma is the most common biliary malignancy and the second most common hepatic malignancy after hepatocellular carcinoma (HCC).1 Cholangiocarcinomas (CCAs) are epithelial tumors with features of cholangiocyte differentiation. Intrahepatic cholangiocarcinomas (iCCAs) are located within the hepatic parenchyma. The second-order bile ducts serve as the point of separation between iCCAs and perihilar CCAs (pCCAs) or distal CCAs (dCCAs)—the cystic duct is the anatomical boundary between these latter two subtypes (Figure 1A).2 The Bismuth -Corlette classification stratifies perihilar tumors on the basis of biliary involvement. This classification has recently been extended to also take into account arterial and venous encasement.3 pCCA is the most common type of CCA. In a large series of patients with bile duct cancer, 8% had iCCA, 50% had pCCA, and 42% had distal CCA.4 CCA has a poor prognosis; patients have a median survival of 24 months after diagnosis. The only curative treatment option is surgery, for early-stage disease.5
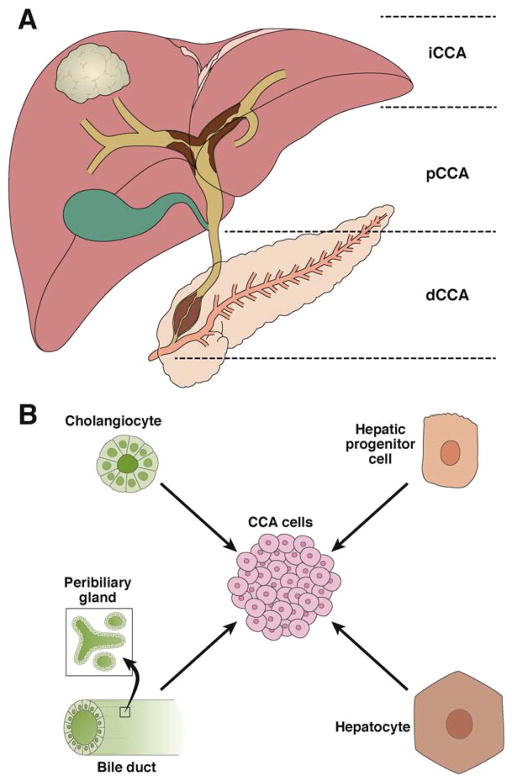
Figure 1. Anatomic localization of CCA and cells of origin in CCA.
(A)Anatomic localization of CCA. CCA is divided into 3 subtypes, based on anatomic location. Modified with permission from Razumilava et al.17 (B) Cells of origin in CCA. CCA, cholangiocarcinoma; dCCA, distal cholangiocarcinoma, iCCA, intrahepatic cholangiocarcinoma, pCCA, perihilar cholangiocarcinoma.
Epidemiology
Cholangiocarcinoma accounts for 3% of all gastrointestinal tumors. Over the past 3 decades, the overall incidence of CCA appears to have increased.6 The percentage of patients who survive 5 y after diagnosis has not increased during this time period, remaining at 10%.7, 8
In the United States, Hispanics and Asians have the highest incidence of CCA (2.8/100,000 and 3.3/100,000 respectively), whereas African Americans have the lowest (2.1/100,000). African Americans also have lower age-adjusted mortality compared with whites (1.4/100,000 vs. 1.7/100,000). Men have a slightly higher incidence of CCA and mortality from the cancer than women.7 With the exception of patients with primary sclerosing cholangitis (PSC), a diagnosis of CCA is uncommon before age 40 y.
Globally, hepatobiliary malignancies account for 13% of cancer-related deaths; 10%–20% of these are attributable to CCA. The mean age of diagnosis of CCA is 50 y. The global incidence of iCCA varies widely, from rates of 113/100,000 in Thailand to 0.1/100,000 in Australia.9, 10 Differences in the prevalence of genetic and other risk factors presumably account for this extensive variation.
Epidemiologic studies indicate that age-adjusted mortality for iCCA is increasing whereas mortality from pCCA and dCCA could be decreasing.9–14 A study of a WHO database reported a substantial global increase in iCCA mortality, with a decreasing trend in mortality from pCCA plus dCCA.15 Although this observed increase in the incidence of CCA over the past 30 y has been recorded as an increase in iCCA, it could result from misclassification of perihilar tumors as iCCAs.16 According to the US Surveillance, Epidemiology, and End Results database, the age-adjusted incidence rate for iCCA increased from 0.59/100,000 in 1990 to 0.91 in 2001. It subsequently decreased to 0.6/100,000 by 2007. Conversely, the incidence rate for pCCA plus dCCA remained around 0.8/100,000 until 2001 then gradually increased to 0.97 by 2007. Perihilar tumors were coded as iCCAs before 2001 and subsequently were coded as pCCAs after implementation of the third edition of the International Classification of Disease for Oncology (ICD-O-3). This update likely influenced the aforementioned changes in incidence rates of both CCA subtypes. Similar trends in the incidence of CCA subtypes were noted in the United Kingdom after the change to ICD -O-3 in 2008.6, 16
Risk Factors
There are several established risk factors for CCA, and most cases are sporadic.6, 8, 17 Geographic variations in incidence rates of CCA are related in part to variations in risk factors. For example, in Southeast Asia, which has one of the highest incidence rates of CCA, infection with the hepatobiliary flukes Opisthorchis viverrini and Clonorchis sinensis has been associated with development of CCA. Both parasites cause chronic inflammation and are considered carcinogens.8, 18 Hepatolithiasis is another risk factor for CCA (mainly iCCA) in Asian countries.8 Chronic biliary inflammation secondary to calculi has been proposed to increase the risk of malignancy. Moreover, infestation with hepatobiliary flukes has been shown to be more common in patients with hepatolithiasis.8, 19 The incidence and prevalence of CCA in patients with bile duct (choledochal) cysts are also higher in Asian than western countries.20, 21 Choledochal cystic diseases, including Caroli’s disease, are rare congenital abnormalities of the pancreatic and biliary ducts. Choledochal cysts can be intrahepatic or extrahepatic, and are diagnosed in patients at an average age of 32 y old.8, 17 Thorotrast, a previously used contrast agent that is now banned, was found to increase risk for CCA by 300-fold in a Japanese study.22
In the West, PSC is the most common predisposing condition for CCA. Among patients with PSC, the annual risk of development of CCA is 0.5%–1.5% with a lifetime prevalence of 5%–10%;17 CCA is diagnosed within 2 y of PSC in most of these patients. A number of potential risk factors for CCA in patients with PSC have been studied, including smoking and alcohol, though definitive data are lacking.8
Hepatitis B virus (HBV) or hepatitis C virus (HCV) infection and cirrhosis have been proposed as potential etiologies of iCCA.23–25 A recent meta-analysis of 11 studies found that cirrhosis, HBV, and HCV were major risk factors for iCCA, with odds ratios (ORs) of 22.92, 5.1, and 4.8, respectively.26 A case–control study from Korea found a significant association between HBV (OR 2.3), but not HCV, and CCA. Cirrhosis was also found to be a significant risk factor for CCA, with an OR of 13.6. HCV and cirrhosis were associated with iCCA in a US case–control study. Compared to controls, patients with iCCA had a higher prevalence of anti-HCV antibodies, with an OR of 7.9.24
CCA development has been associated with other risk factors, including inflammatory bowel disease independent of PSC, alcohol, smoking, fatty liver disease, diabetes, cholelithiasis, and choledocholithiasis.8, 27–29 Additional studies have associated variants of genes that regulate DNA repair, inflammation, and carcinogen metabolism with CCA development.8 Further studies are necessary to verify these potential associations.
Cells of Origin
iCCA is a histologically diverse hepatobiliary malignancy considered to develop from biliary epithelial cells or hepatic progenitor cells (Figure 1B). A recently proposed classification of iCCAs subdivided these tumors into the conventional, bile ductular, or intraductal neoplasm type, or rare variants (combined hepatocellular CCA, undifferentiated ICC, squamous/adenosquamous type). The conventional type include small duct or peripheral type and large duct or perihilar type.30 Neural cell adhesion molecule, a marker of hepatic progenitor cells (HPCs), has been detected in the bile ductular and combined hepatocellular-CCA types, so these might have originated from HPCs.30–32
Distal and pCCA have been proposed to arise from the biliary epithelium and peribiliary glands.33 Extrahepatic bile ducts and large intrahepatic bile ducts are lined by mucin-producing cuboidal cholangiocytes. A recent study demonstrated that mucin-producing iCCAs and hilar CCAs had gene expression and immunohistochemical profiles similar to those of the cylindrical, mucin-producing cholangiocytes that linehilar and intrahepatic large bile ducts.34
A model in which iCCAs arise from trans-differentiation and subsequent neoplastic conversion of normal hepatocytes into malignant cholangiocytes has been proposed. Fan et al. demonstrated in mice that overexpression of Notch1 and AKT resulted in development of invasive cystadenocarcinomas, via conversion of hepatocytes into cholangiocyte precursors of iCCA.35 Sekiya and Suzuki also showed that in mice, Notch-mediated conversion of hepatocytes into biliary cells leads to macronodular cirrhosis and iCCAs.36 Therefore, iCCAs may not have a single lineage, but instead derive from different cells of origin. In support of this theory, a recent study demonstrated that transformed hepatocytes, hepatoblasts, and HPCs can give rise to a broad spectrum of liver tumors, ranging from CCA to HCC.37 These studies indicate that multiple cell types, rather than only cholangiocytes, transform and develop into CCAs. Additional animal models of CCA and lineage tracing studies are necessary to help identify the cells of origin for CCA.
Inflammation
CCA frequently arises under conditions of inflammation, which is believed to contribute to pathogenesis. A variety of cytokines, growth factors, tyrosine kinases, and bile acids can contribute to the alterations in proliferation, apoptosis, senescence, and cell cycle regulation required for cholangiocarcinogenesis.5 Inflammatory cytokines activate inducible nitric oxide synthase, leading to excess nitric oxide with resultant single-stranded, double-stranded, and oxidative DNA lesions, as well as inhibition of DNA repair enzymes.38 IL6, an inflammatory mediator secreted by CCA and stromal inflammatory cells, can function in an autocrine or paracrine manner to promote cell survival and provide mitogenic signals.39, 40 Myeloid cell leukemia sequence 1 (MCL1) is an anti-apoptotic BCL2 family member that mediates tumor necrosis factor-related resistance to apoptosis-inducing ligand in CCAs.41 IL6 increases expression of MCL1 via constitutive activation of signal transducer and activator of transcription (STAT) signaling and protein kinase B (Akt).40, 42 MCL1 transcription is activated by IL6 via a p38 mitogen activated protein kinase (MAPK)-dependent pathway.43 IL6 binds to the gp130 receptor, leading to its subsequent dimerization and activation of the gp130-associated janus kinases (JAKs), including JAK1 and JAK2, which leads to STAT3 activation.44, 45 Epigenetic silencing of suppressor of cytokine signaling 3 (SOCS3) results in sustained IL6 signaling via STAT3.46 Inflammatory signaling pathways therefore appear to promote development of CCA by causing DNA damage and blocking the apoptosis normally induced by the DNA damage response. These cytokines also promote cell proliferation. The combination of DNA damage, evasion of apoptosis, and cell proliferation are all components of cell transformation.
Epidermal growth factor receptor (EGFR) signaling also contributes to cholangiocarcinogenesis and CCA progression. Activation of EGFR leads to activation of extracellular-signal regulated kinases (ERKs) 1 and 2 (also known as p44/42 MAPK). EGFR inhibitors decrease expression of cyclooxygenase-2 (COX2) by CCA cells.47 ERBB2 is another member of the EGFR family that contributes to CCA development. In mice, overexpression of ERBB2 led to formation of tumors along the biliary epithelium.48 Hepatocyte growth factor (hepapoietin A; scatter factor) (HGF) is a stromal paracrine mediator that regulates tumor invasiveness and metastasis.49–51 Activation of MET, the receptor for HGF, upregulates several signaling pathways, including those involving PI3K–AKT, STAT3, and MAPK.52 CCAs express higher levels of MET and HGF than non-tumor tissues.53, 54 MET overexpression was associated with activation of members of the EGFR family, particularly of HER2.54, 55
Cholestasis also contributes to development of CCA, and bile acids have important roles in this process, activating growth factors that mediate proliferation. Bile acids activate EGFR and increase expression of COX2 via a MAPK cascade.56 In addition to bile acids, COX2 overexpression is induced by oxysterols and iNOS.57 Oxysterols are overlooked in the pathogenesis of CCA.58 These oxidative degradation products of cholesterol are abundant in bile. They are endogenous ligands for the hedgehog signaling pathway59—a developmental pathway implicated in CCA progression.60
Genetics
A few studies have assessed the roles of genetic factors, such as chromosome aberrations or genetic and epigenetic alterations in tumor suppressor genes and oncogenes, in pathogenesis of human CCA. However, these studies have produced no definitive results, because they analyzed a limited number of genes in combined CCA specimens, without separate analyses of different subtypes.61 A comparative genomics hybridization analysis of 32 CCA samples from patients (7 iCCA, 13 pCCA, and 12 dCCA) showed that they all contained gains at 16q, 17p, 17q, 19p, and 19q, which included regions encoding ERBB2, MEK2, and platelet-derived growth factor β (PDGFβ).62 A meta-analysis of 5 studies that used comparative genomics hybridization to analyze 98 iCCAs found copy number losses at 1p, 4q, 8p, 9p, 17p, and 18q and gains at 1q, 5p, 7p, 8q, 17q, and 20q.61 In this meta-analysis, there was considerable variation among the 4 studies that were performed in Asia63–66 and the 1 study from Europe.67 This variation could have resulted from differences in ethnicity and etiological associations among the studies.
Whole-exome sequencing analyses of 8 liver fluke-related CCAs identified 206 somatic mutations in 187 genes.68 The frequency of these mutations was validated in an additional 46 liver fluke-related CCAs. Mutations were frequently detected in oncogenes and tumor suppressor genes such as those encoding TP53 (mutations in 44.4% of CCAs), KRAS (16.7%), and SMAD family member 4 (16.7%). Mutations were also found in MLL3 (14.8% of cases), RNF43 (9.3%), PEG3 (5.6%), and ROBO2 (9.3%). These genes are involved in deactivation of histone modifiers, activation of G-proteins, and loss of genomic stability.68 This study, performed in Asia, has been the only whole-exome sequence analysis of CCAs. Further whole-genome sequencing studies are needed to evaluate CCAs from Western patients.
A recent study comprising single nucleotide polymorphism array, gene expression profile, and mutation analyses of 149 iCCAs identified inflammation and proliferation classes of this tumor.45 Several copy number alterations were identified, including losses at 3p, 4q, 6q, 9pq, 13q, 14q, 8p, 17p, and 21q and gains at 1q and 7p.45 Features of the inflammation class included activation of inflammatory pathways, overexpression of cytokines, and activation of STAT3. The proliferation class was characterized by activation of oncogene signaling pathways involving RAS, MAPK, and MET. Activating mutations in KRAS have been frequently detected in CCAs.69–71 At least 2 studies have reported a higher incidence of activating mutations in KRAS in pCCAs compared to iCCAs.71, 72 In one cohort, the incidence of these mutations was 53% in pCCAs compared to 17% in iCCAs.71 In a transcriptome profile analysis of 104 CCAs and 59 matched non-tumor samples (controls), patients could be categorized based on overall survival time, early recurrence, and presence or absence of KRAS mutations; a detailed class comparison identified 4 subclasses of patients. Those with CCAs with altered expression of genes that regulate proteasome activity; with dysregulation of HER2; and with overexpression of EGFR, MET, and Ki67 had the worst outcomes.71
Inactivation of TP53, which regulates the cell cycle, is one of the most common genetic abnormalities in cancer cells and has also been detected during cholangiocarcinogenesis. A review of 10 studies, comprising 229 patients with CCA from Europe, Asia, and the US, reported TP53 mutations in 21% of CCAs.73 Mutations in other genes, including EGFR, NRAS, PI3K, and APC, have been less frequently described.44
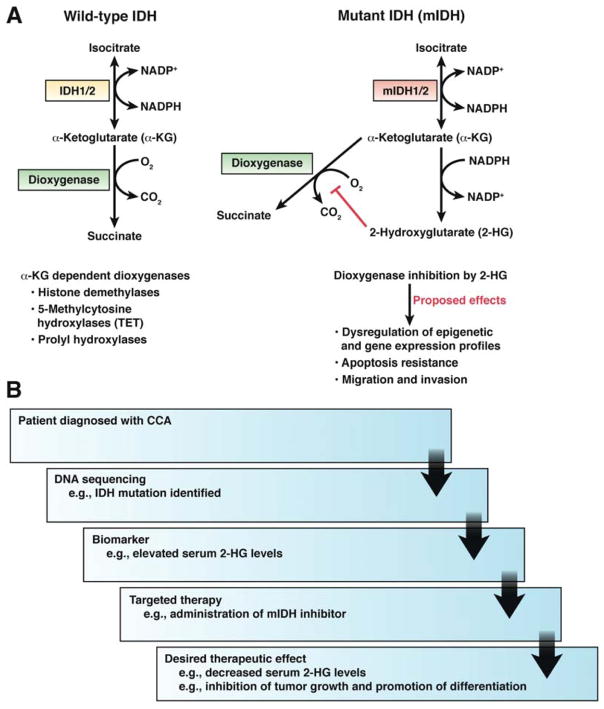
There has been growing interest in the effects of somatic mutations in genes encoding isocitrate dehydrogenases (IDH) 1 and 2. IDH1 and IDH2 mutations have been frequently detected in gliomas but rarely observed in other solid tumors. IDH mutations were detected in 22% of CCA specimens—more frequently in iCCAs (28%) than pCCA and dCCAs (7%).74 Recurrent mutations in IDH1 were observed in a subset of biliary tract tumors samples in a recent broad-based mutation profile analysis of gastrointestinal tumors.75 A subsequent analysis of 62 CCAs detected IDH1 mutations in only iCCAs.75 IDH1 and IDH2 mutations were significantly associated with increased levels of p53 and DNA hypermethylation.76 Epigenetic changes associated with IDH mutations likely mediate their oncogenic effects. The product of the enzymatic activity of mutant IDH1 and IDH2 is 2-hydroxyglutarate (Figure 2A). This metabolite might therefore serve as a biomarker for IDH1 and IDH2 mutations, and for a subset of patients that might be treated with IDH inhibitors77–79(Figure 2B).
Figure 2. IDH mutations.
(A) Function of wild-type and mutant IDH. Wild-type enzymes catalyze a reaction that converts isocitrate to α-ketoglutarate and reduction of NADP to NADPH. The mutant enzymes acquire a neomorphic activity that converts the normal metabolite α-KG to 2-HG and consumption rather than production of NADPH. 2-HG leads to inhibition of certain dioxygenases, which has been postulated to result in cancer promoting events. (B) Potential of personalized medicine for CCA, using mIDH inhibitors, as an example. α-KG, α-ketoglutarate; 2-HG, 2-hydroxyglutarate; IDH, isocitrate dehydrogenase; mIDH, mutant isocitrate dehydrogenase; NADPH, nicotinamide adenine dinucleotide phosphate.
A number of epigenetic alterations, such as promoter hypermethylation and microRNA dysregulation, have been associated with development of CCA. However, whole epigenome analysis has not been conducted and microRNA profiling is possible with only small numbers of tumor samples.80, 81 Promoter hypermethylation has been reported to silence tumor suppressor genes including CDKN2 (observed in 17%–83% of CCAs), SOCS3 (in 62%46), RASSF1A (in 31%–69%), and APC (in 27%–47%). 45, 61
Gene fusions, such as the BCR-ABL gene in chronic myeloid leukemia, are driver mutations in cancer which play a role in certain cancers.82 Fibroblast growth factor receptor (FGFR) fusions are active kinases. A recent study identified novel FGFR2 gene fusions in CCA.82 Cells with these FGFR fusions were susceptible to FGFR inhibitors, signifying that FGFR kinase inhibition may be a valid therapeutic strategy in CCA patients harboring these gene fusions.82
MicroRNAs (miRs) are non-coding RNAs that function in post-transcriptional regulation of gene expression. A cluster of 38 miRs was differentially expressed in 27 iCCA samples, compared with non-tumor tissues. miR21 is overexpressed in CCAs and could have oncogenic effects, partly by inhibiting programmed cell death 4 and tissue inhibitor of matrix metalloproteinase (MMP)3.83 miR21 was also found to regulate phosphatase and tensin homolog deleted on chromosome ten (PTEN)-dependent activation of PI3K signaling in CCAs, to affect chemosensitivity.84 miR200C prevents the epithelial–mesenchymal transition (EMT); changes in its level might be used as a prognostic factor.80 Further studies are needed to determine how alterations in miRs contribute to development of CCA, and how these changes might be used to determine patients’ prognoses.
Developmental Pathways
The Notch signaling pathway regulates embryonic development and proliferation of the biliary tree.85 Not surprisingly, therefore, Notch dysregulation has also been implicated in cholangiocarcinogenesis. Two recent studies in mice have demonstrated that Notch activation is required for conversion of normal adult hepatocytes to biliary cells that are precursors of iCCA.35, 36 Overexpression of intracellular domain of the Notch 1 receptor in liver cells of mice resulted in formation of iCCAs.86 In this model, an inhibitor of γ-secretase, an enzyme necessary for Notch signaling, suppressed tumor formation.
Another evolutionary conserved, developmental pathway is the Hedgehog signaling pathway. Hedgehog signaling is deregulated in many types of tumors, including CCAs. Inhibition of hedgehog signaling with cyclopamine impedes CCA cell migration, proliferation, and invasion.87, 88 Hedgehog signaling has also been implicated in survival signaling by myofibroblast-derived CCAs. PDGFβ protects CCA cells and promotes tumor survival in mice with CCAs, but cyclopamine reverses these effects.60
Wnt signaling is also required for intrahepatic bile duct development and proliferation.89 Wnt-inducible signaling pathway protein 1v (WISP1v) is overexpressed in stroma nests around CCAs, and levels of WISP1v are associated with reduced survival times of patients. WISP1v stimulated the invasive activity of CCA cell lines by activating MAPK1 andMAPK3.90
Tumor Microenvironment
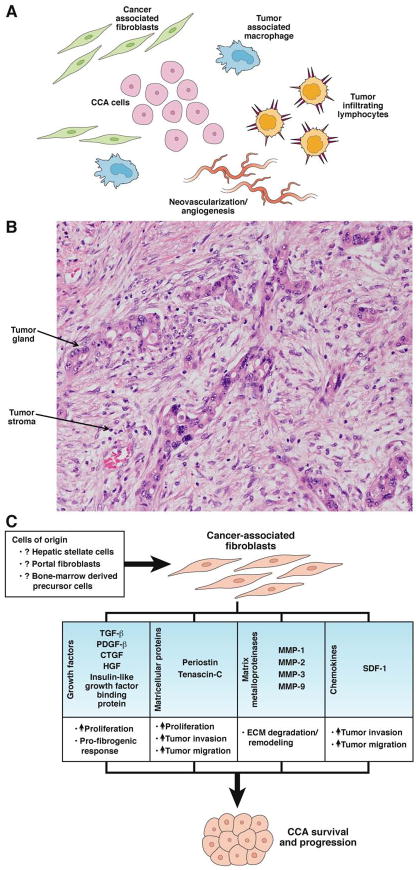
Carcinogenesis in CCA includes alterations in the stroma, recruitment of fibroblasts, remodeling of the extracellular matrix(ECM), changing patterns of immune cell migration, and promotion of angiogenesis (Figure 3A).91 iCCAs and pCCAs are characterized by a dense and reactive desmoplastic stroma (Figure 3B) that contains many α-smooth muscle actin (αSMA)–positive myofibroblasts, also known as cancer associated fibroblasts (CAFs). The tumor stroma surrounds the malignant ducts and glands and comprises most of the tumor mass.92, 93 The stroma promotes tumor progression, via reciprocal communication between the stromal cells and cancer cells.92
Figure 3. Microenvironment of cholangiocarcinoma.
(A) Components of the tumor microenvironment in CCA. (B) Micrograph of a stromal CCA. (C) Factors secreted by cancer-associated fibroblasts. CCA, cholangiocarcinoma; CTGF, connective tissue growth factor; ECM, extracellular matrix; HGF, hepatocyte growth factor; MMP, matrix metalloproteinase; PDGF-β, platelet-derived growth factor beta; SDF-1, stromal cell-derived factor 1; TGF-β, transforming growth factor beta.
The precise origin of CAFs is unclear, although several cell types, including hepatic stellate cells, portal fibroblasts, and bone-marrow derived precursor cells, have been proposed as candidates.92, 94–96 The EMT has also been proposed to produce CAFs.93 During tumorigenesis, the EMT is characterized by the presence of tumor cells that express mesenchymal markers such as vimentin, tenascin, fibronectin, and the zinc finger protein Snail.92 Immunohistochemical studies have demonstrated the expression of these markers by human CCA cell lines.97–99 In mice, xenograft tumors grown from EGFP-expressing human CCA cells were found to be surrounded and infiltrated by αSMA-expressing CAFs. Interestingly, EGFP was not co-expressed with αSMA, indicating that the EMT does not produce CAFs in CCAs.100 Based on combined evidence, αSMA-expressing CAFs appear to be a heterogeneous population of cells that originate from several cell lineages, but not from epithelial cancer cells.
CAFs produce factors that stimulate ECM production, leading to a fibrogenic response (Figure 3C).92 Factors produced by CAFs include transforming growth factor-β, PDGF isomers, connective tissue growth factor, and insulin-like growth factor binding proteins.92 PDGF-mediated interactions between CAFs and tumor cells have been observed, such as recruitment of CAFs by PDGFD secreted by CCA cells.60, 100, 101 PDGFD stimulates CAF migration via its receptor PDGFR, which is highly expressed on CAFs, and activation of small Rho GTPases and the JNK signaling pathway.100
Activated CAFs also secrete paracrine factors that promote initiation and progression of cancer. These include matricellular proteins, growth factors, chemokines, and ECM proteases. Periostin is a matricellular protein which is overexpressed by CAFs, compared to normal fibroblasts; its presence correlates with shorter survival times of patients. Knockdown of the periostin receptor, the alpha 5 subunit of integrin, with small interfering RNA reduced stimulation of tumor proliferation and invasion by periostin.102 The ECM that surrounds pancreatic tumors has also been shown to overexpress periostin, which promotes tumor invasiveness.103 Tenascin-C, another ECM protein produced by CAFs, also promotes tumor migration and invasiveness.92 In CCA cell lines, HGF promoted invasiveness and motility by inducing phosphorylation of Akt and ERK 1/2.104 Similarly, stromal cell derived factor-1, through activation of its receptor CXCR4, induced CCA cell invasion via ERK 1/2 and Akt.105, 106 This process was disrupted by the CXR4 inhibitor AMD3100.106
ECM degradation and remodeling is required for tumor progression. MMPs degrade and remodel the ECM during fibrogenesis and carcinogenesis. MMP1, MMP2, MMP3, and MMP9 are strongly expressed in CCAs and are associated with invasive tumors.107, 108 Fibroblast activation protein is a stromal protein; its high expression by CAFs has been associated with tumors with an aggressive phenotype.109
The exact mechanisms by which tumor and stroma communicate are not clear. However, the importance of the desmoplastic stroma in CCA progression indicates that it could be a new therapeutic target, perhaps via selective targeting of CAFs.110
Animal Models
Animal models are essential for development of new therapeutic strategies and diagnostic tools.111 Animal models of CCA (Table 1) include mice with xenograft tumors, 43, 112–119 mice with genetic changes that lead to CCA formation,86, 120–124 rats with orthotopic tumors125,126, and animals that develop CCAs following exposure to carcinogens.55, 127–129 Although these models offer an opportunity to bridge the chasm between in vitro findings and clinical applicability, they have limitations. The tumor microenvironment is an important feature in CCA development. It can sometimes be a challenge to study interactions between cancer cells and the stroma in mice with xenograft tumors, because the tumor is not growing in the same microenvironment as it does in humans. A model described by Sirica et al., in which rat CCA cells were injected into rat biliary trees, is unique in that the stroma and epithelial cells are derived from the same species.125 These animals allow for investigations of tumor–stroma interactions that more closely resemble those of patients. Although orthotopic tumor models do allow for study of the tumor microenvironment, they tend to be technically challenging and expensive. Animals with genetic alterations that lead to production of CCAs that resemble human tumors are needed.
Table 1.
Animal models of cholangiocarcinoma.
| Mice with xenograft tumors | ||
|---|---|---|
| Experimental Approach | Key Features | Study |
| Injection of 3 × 106 Mz-ChA-1 cells | Tumor development in 3 weeks | Fava et al. 112 |
| Injection of 5 × 106 Sk-ChA-1 cells +/− intratumoral tamoxifen injections | Significantly decreased CCA development with intratumoral tamoxifen injections | Pawar et al. 113 |
| Injection of 2 × 106 QBC939 cells +/− magnetic nanoparticle injections via tail vein | CCA tumor growth inhibition with magnetic nanoparticles | Tang et al. 114 |
| Injection of IL6 overexpressed Mz- ChA-1 stable cell line (Mz-ChA-IL6) vs. control vector Mz-ChA-1 cell line | Overexpression of IL6 increased growth of xenograft tumors | Meng et al. 43 |
| Injection of miR26a overexpressed CCLP1 cell line vs. scramble control CCLP1 cell line | Overexpression of miR26a increased growth of xenograft tumors | Zhang et al. 115 |
| Injection of miR494 overexpressed stable HuCCT1 cell line vs. control vector HuCCT1 cell line | Overexpression of miR494 increased growth of xenograft tumors | Olaru et al. 116 |
| Injection of stable QBC939 cell line transfected with Slug siRNA vs. control vector QBC939 cell line | Slug silencing suppressed growth of xenograft tumors | Zhang et al. 117 |
| Injection of CypA silenced stable M139 cell line vs. control vector M139 cell line | CypA silencing decreased growth of xenograft tumors | Obchoei et al. 118 |
| Injection of stable QBC939 cell line transfected with Beclin-1 siRNA vs. control vector QBC939 cell line | Beclin-1 silencing decreased growth of xenograft tumors | Hou et al. 119 |
| Genetically Engineered Mouse Models | ||
| Experimental Approach | Key Features | Study |
| Liver specific inactivation of SMAD4 and PTEN. | Tumor formation in all animals at 4–7 months of age | Xu et al. 120 |
| Chronic carbon tetrachloride exposure in TP53-deficient mice. | Development of tumors with dense peritumoral fibrosis and other histologic and genetic features of human iCCA. | Farazi et al. 121 |
| Liver-specific inactivation of macrophage stimulating factor 1 and 2 | Tumor development (HCC or CCA) in all mice by 6 months of age | Song et al. 122 |
| Liver-specific ablation of WW45, a homolog of Drosophila Salvador and adaptor for the Hippo kinase | Development of tumors with mixed histological features of HCC and CCA | Lee et al. 123 |
| Liver-specific activation of KRAS and deletion of TP53. | Development of stroma-rich tumors. Shortened time to tumor development and increased metastasis with the combination of KRAS activation and TP53 deletion | O’Dell et al. 124 |
| Overexpression of intracellular domain of Notch1 in livers of transgenic mice | Formation of tumors with features characteristic of iCCA | Zender et al. 86 |
| Orthotopic Rat Models | ||
| Experimental Approach | Key Features | Study |
| Inoculation of BDEneu cells into bile duct of isogenic rats | Rapid (21–26 d) development of cholangiocarcinoma characterized by biliary obstruction and gross peritoneal metastasis; origin of tumor stroma and tumor tissue from same species (rat) | Sirica et al. 125 |
| Three-dimensional organotypic culture model of CCA in rats | Stromal microenvironment, gene expression profile, and pathophysiological characteristics which mimic desmoplastic iCCA in vivo | Campell et al. 126 |
| Carcinogen-induced Models | ||
| Experimental Approach | Key Features | Study |
| Furan induced cholangiocarcinogenesis in rat liver. | Formation of mucin-producing CCA tumors; overexpression of C-NEU and MET | Radaeva et al. 55 |
| Chronic administration of thioacetamide in lean rats and rats with faulty leptin receptors | Increased development and growth of CCA tumors in lean rats treated with thioacetamide | Fava et al. 127 |
| Administration of diethylnitrosamine (DEN) +/− left and median bile duct ligation (LMBDL) to induce chronic cholestasis and CCA development | Increased CCA progression in mice with LMBDL given DEN compared to mice without LMBDL given DEN | Yang et al. 128 |
| Inoculation with Opisthorchis viverrini and administration of dimethynitrosaminein hamsters | Development of pus and tumor in liver starting at 20 weeks after O. viverrini infection/DEN administration; all hamsters in experimental group were dead by 28 weeks | Plengsuriyakarn et al. 129 |
BDEneu, highly malignant cholangiocarcinoma cell line; CCA, cholangiocarcinoma; CCLP1, cholangiocarcinoma cell line; C-NEU, rat homologue of human ERBB2; CypA, cyclophilin A; HCC, hepatocellular carcinoma; HuCCT1, cholangiocarcinoma cell line; IL6, interleukin-6; KRAS, Kirsten rat sarcoma viral oncogene homolog; MET, met proto-oncogene; M139, cholangiocarcinoma cell line; miR, microRNA; Mz-ChA-1, cholangiocarcinoma cell line; O. viverrini, Opisthorchis viverrini; PTEN, phosphatase and tensin homolog deleted on chromosome ten; QBC939, human hilar bile duct carcinoma cell line; Sk-ChA-1, cholangiocarcinoma cell line; siRNA, SMAD4, SMAD family member 4; small interfering RNA; TP53, tumor protein 53.
Diagnosis and Management
It can be a challenge to diagnose CCA because of its paucicellular nature, anatomic location, and silent clinical character. Diagnosis requires a high index of suspicion and a multidisciplinary approach that involves clinical, laboratory, endoscopic, and radiographic analyses.
iCCA
iCCA is divided into mass-forming, periductal infiltrating, and intraductal growth types.130 The clinical manifestations of iCCA include nonspecific symptoms such as abdominal pain, cachexia, malaise, fatigue, and night sweats.2 iCCA frequently presents as an intrahepatic mass lesion; imaging modalities including computed tomography (CT) and magnetic resonance imaging (MRI) aid in the diagnosis. The use of contrast enhancement improves the sensitivity of MRI for detection of iCCA, as these tumors typically have progressive uptake of contrast during the venous phase. HCCs, on the other hand, are characterized by rapid contrast uptake during the arterial phase, followed by a delayed venous washout phase.131 CT and MRI have similar utility in evaluation of tumor size and detection of satellite lesions. However, CT may be better for assessment of vascular encasement, identification of extrahepatic metastasis, and determination of resectability.17, 132
Serum levels of CA 19-9, a tumor biomarker, can aid in diagnosis, but this assay detects iCCA with only 62% sensitivity and 63% specificity.133 Moreover, increased levels of CA 19-9 are also observed in patients with benign diseases such as bacterial cholangitis or choledocholithiasis.5 Nonetheless, very high levels of CA 19-9 (≥ 1000 U/mL) have been associated with metastatic iCCA, so this assay might be used in disease staging rather than diagnosis.134 Mixed tumors are characterized by histologic and imaging features of HCC and iCCA. In these cases, immunohistochemical analysis for cytokeratins 7 and 19 can be useful—tumors positive for cytokeratins can be considered to be mixed hepatocellular-CCA.17, 135 A definitive diagnosis of iCCA requires liver biopsy analysis. According to the WHO classification criteria, iCCAs can be adenocarcinomas or mucinous carcinomas.2
The treatment of choice for iCCA is surgical resection. Patients should only undergo surgery if they have potentially resectable tumors and are appropriate surgical candidates. Following surgical resection, the median time of disease-free survival is 26 months; reported rates of recurrence are 60%–65%.136, 137 Approximately 60% of patients survive for 5 y after resection. Factors associated with recurrence and reduced survival time following resection include vascular invasion, lymph node metastasis, multiple tumors, and cirrhosis.4, 138 Nuclear expression of S100A4, a member of the S100 family of calcium-binding proteins, in neoplastic ducts were associated with metastasis and reduced time of survival after surgical resection in a subset of patients with CCA.139
Liver transplantation as a curative option for iCCA is highly controversial. iCCA was reported to recur in 70% of patients within 5 y of liver transplantation, and the median disease-free survival time was 8 months in a series of 14 patients with iCCA or mixed HCC-iCCA.135 Patients with very small iCCAs (< 2 cm) in the context of cirrhosis, however, do as well as patients undergoing liver transplantation for HCC. Locoregional therapy, including transarterial chemoembolization and radiofrequency ablation, has garnered interest as a therapeutic option for patients with unresectable iCCA. 140 The standard practice of care for advanced-stage iCCA is systemic chemotherapy with gemcitabine and cisplatin.141
pCCA
pCCAs can have exophytic or intraductal macroscopic growth patterns. The exophytic or mass-forming type can be of the nodular subtype or the periductal subtype (the most common subtype).142 There are also subtypes of intraductal patterns, including the intraductal growing type, mucin-producing type, papilloma type, and cystic type.17 Patients with pCCA can present with nonspecific symptoms including abdominal discomfort, cachexia, weight loss, and malaise. However, their presentation is typically consistent with biliary obstruction presenting with jaundice and less commonly cholangitis.17 Hypertrophy–atrophy complex, a phenomenon characterized by hypertrophy of the unaffected liver lobe and atrophy of affected lobe, presents as unilobar palpable prominence on physical examination.2 Laboratory analyses, including measurements of alkaline phosphatase and bilirubin levels, do not provide specific information, because they typically reflect concomitant cholestasis and cholangitis. For the same reason, serum levels of CA 19-9 are less specific in detecting pCCA than iCCA. IgG4 disease can present in a similar manner, so its presence should be excluded by evaluation for serum levels of IgG4.2
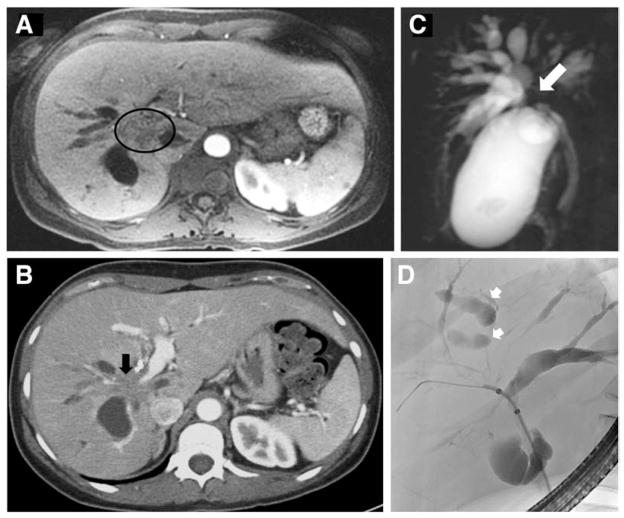
In addition to MRI and CT, magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiography (ERC), and endoscopic ultrasound (EUS) are used in the diagnosis of pCCA (Figure 4). Of these, MRI plus MRCP is the preferred imaging modality as it can assess resectability and tumor extent with an accuracy of up to 95%.2 EUS aids in evaluation for the presence of regional lymphadenopathy and omental metastasis, via fine-needle aspiration. However, fine-needle aspiration should not be performed on the primary tumor, because it can disseminate the tumor.143 ERC serves a diagnostic and therapeutic purpose—it is used to assess and sample the biliary tree via brush cytology and endoscopic biopsy, as well as dilatation and stent placement in cases of biliary obstruction.
Figure 4. Diagnostic modalities used for cholangiocarcinoma.
(A) MRI image of a pCCA mass (outlined in circle). (B) CT image of a pCCA mass with right portal vein encasement (indicated by black arrow). (C) MRCP image of common hepatic duct involvement by tumor (indicated by white arrow). (D) ERC image depicting excluded segmental ducts (white arrows) in a patient with a hilar biliary stricture extending into the right main hepatic duct. CT, computed tomography; ERC, endoscopic retrograde cholangiography; MRCP, magnetic resonance cholangiopancreatography; MRI, magnetic resonance imaging; pCCA, perihilar cholangiocarcinoma.
Fluorescence in situ hybridization (FISH) analysis increases the sensitivity of cytology in diagnosing pCCA.144 FISH can detect polysomy or amplification of at least 2 chromosomes, tetrasomy, and trisomy 7. Of these, polysomy in the presence of a dominant stricture is considered sufficient for diagnosis of pCCA, especially if the polysomy can be confirmed over time.145 Tetrasomy can be seen during the M phase of mitosis and should be interpreted with caution.5 Trisomy 7 is often observed with inflammation of the biliary tree. Detection of polysomy by FISH also been shown to predict the development of malignancies in patients with PSC with no mass and equivocal cytology. In a recent study, patients with PSC who had polysomy and levels of CA 19-9 greater than 129 U/ml all went on to develop cancer, mainly within 2 y (Figure 5).146
Figure 5.
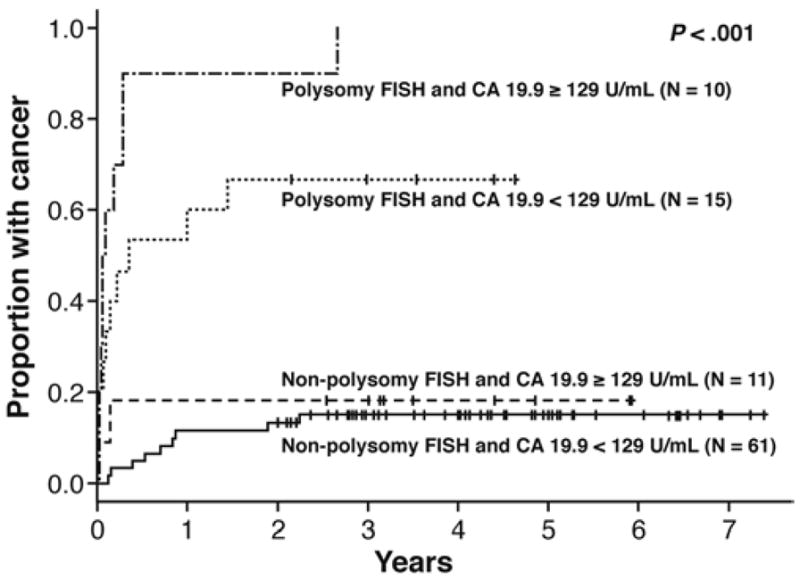
Time to diagnosis of cholangiocarcinoma based on FISH analysis and CA 19-9 levels. CA 19-9, carbohydrate antigen 19-9; FISH, fluorescence in situ hybridization.
Reused with permission from Wiley InterScience and Barr et al.146
The only curative options for pCCA are surgical resection and neoadjuvant chemoradiation followed by liver transplantation. The Bismuth-Corlette staging classification is based on the anatomic location of the CCA within the biliary tree and is meant to help guide decision making. Recently, this classification was expanded to take into account vascular encasement and parenchymal value of the potential remnant lobe.3 Surgical resection entails lobar hepatic and bile duct resection, regional lymphadenectomy, and Roux-en-Y hepaticojejunostomy. Potential contraindications to curative surgical resection include contralateral or bilateral vascular encasement and pCCA extension bilaterally to the level of the secondary biliary branches. The presence of regional lymphadenopathy does not necessarily preclude surgery.147 Occasionally, a tumor may be resectable but the remnant lobe has limited volume. In such cases, resectability can be achieved by preoperative relief of biliary obstruction and portal vein embolization of the affected lobe with resultant compensatory hyperplasia of the contralateral unaffected liver lobe.147 Rates of 5 y survival following surgical resection with negative margins range from 11% to 41%.147
With the advent of new liver transplantation protocols, neoadjuvant chemoradiation followed by transplantation has become an appealing option for patients selected carefully using stringent criteria (Table 2). Sixty-five percent of patients who were treated with neoadjuvant therapy followed by liver transplantation at 12 large-volume transplant centers survived for 5 y 148 Rigorous selection is imperative for successful outcomes. Eligibility criteria includes radial diameter of tumor less than 3 cm, absence of intrahepatic or extrahepatic metastasis, and in the case of patients without PSC, unresectability.149 Due to the presence of parenchymal liver disease, patients with PSC typically require liver transplantation rather than surgical resection.
Table 2.
Criteria for liver transplantation in pCCA.
Diagnosis of Cholangiocarcinoma
Tumor Size
Tumor confined to biliary tree
Unresectability
|
CA 19-9, carbohydrate antigen 19-9; CCA, cholangiocarcinoma; ERC, endoscopic retrograde cholangiography; FISH, fluorescence in situ hybridization; MRCP, magnetic resonance cholangiopancreatography; pCCA, perihilar cholangiocarcinoma; PSC, primary sclerosing cholangitis.
For patients who are not candidates for surgical resection or liver transplantation, systemic chemotherapy with gemcitabine and cisplatin is recommended. For patients with biliary obstruction, adequate drainage is essential to relieve cholestasis and increase tolerance to chemotherapy.17
dCCA
Intraductal papillary neoplasm and biliary intraepithelial neoplasia are the precursor lesions of dCCA.30 dCCA arises from the point of insertion of the cystic duct to the ampulla of vater and can therefore be difficult to distinguish from early pancreatic cancer.17 Analogous to pCCA, patients typically present with painless jaundice, and laboratory analysis is consistent with biliary obstruction. Although pCCA and dCCA are distinct with respect to their pathogenesis and treatment, most studies evaluating diagnostic modalities have grouped these as extrahepatic CCAs. Cross-sectional imaging, EUS, and ERC are therefore used in the same manner in diagnosis of dCCA as with pCCA. Diagnosis is made on the basis of presence of a dominant stricture and positive cytology and/or detection of polysomy by FISH.2 Surgical treatment of dCCA typically entails a Whipple procedure. Only 27% of patients survive for 5 y after surgical resection that attains negative margins.4 The role of neoadjuvant chemoradiation is limited. For patients who are not candidates for surgical resection, chemotherapy may be considered.17
Future Directions
Treatment options for CCA are limited and overall rates of survival are low. Earlier detection of CCA increases chances of having curative treatment options. However, despite recent advances in diagnosis, such as improved imaging and cytology techniques, including FISH, further work is necessary to overcome the challenge of diagnosing CCA at an earlier stage. CCA is still often diagnosed based on clinical criteria, such as a malignant-appearing bile duct stricture, increased serum levels of CA 19-9, appearance of a mass during MRI, normal serum levels of IgG4 level, etc.
There are significant geographic and ethnic variations in the incidence of CCA, so genetic factors are likely to contribute to its pathogenesis. Inflammatory and oncogenic signaling pathways are also involved in cholangiocarcinogenesis, and are potential therapeutic targets. Further studies are necessary to elucidate the role of genetic aberrations, particularly in regions encoding key components of signaling pathways. Additionally, the role of miRs as biomarkers remains to be fully elucidated. CCAs are heterogeneous; treatments are likely to be designed based on features of each individual tumor.150 Potential therapeutic targets could include the MET tyrosine receptor kinase, the PI3K–Akt–mTOR pathway, and IDH mutations. Molecular profiling of tumors, to identify their specific mutations, could make it possible to offer targeted therapiesin personalized treatments (Figure 2B).
Although cancer cells contain many genetic and functional aberrations, the tumor stroma appears to be more uniform and has strong potential as a target for new combination therapies. Further work is needed to highlight the dynamic reciprocal communication between tumor and stroma.
Acknowledgments
This work was supported by National Institutes of Health grants DK59427 (G.J.G.) and T32DK007198 (S.R.), and the Mayo Foundation.
The authors would like to thank Dr. Thomas Smyrk for kindly providing the stromal CCA photomicrograph and Ms. Courtney Hoover for outstanding secretarial support.
Abbreviations
- α-SMA
alpha-smooth muscle actin
- CA 19-9
carbohydrate antigen 19-9
- CAF
cancer associated fibroblast
- CCA
cholangiocarcinoma
- CT
computed tomography
- dCCA
distal cholangiocarcinoma
- ECM
extracellular matrix
- ERC
endoscopic retrograde cholangiography
- ERK
extracellular signal regulated kinase
- EUS
endoscopic ultrasound
- FGFR
fibroblast growth factor receptor
- FISH
fluorescence in situ hybridization
- HGF
hepatocyte growth factor
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HPC
hepatic progenitor cell
- IDH
isocitrate dehydrogenase
- IL6
interleukin-6
- miR
microRNA
- MCL1
myeloid cell leukemia sequence 1
- MMP
matrix metalloproteinase
- MRCP
magnetic resonance cholangiopancreatography
- MRI
magnetic resonance imaging
- mIDH
mutant IDH inhibitor
- OR
odds ratio
- pCCA
perihilar cholangiocarcinoma
- PDGF
platelet-derived growth factor
- PSC
primary sclerosing cholangitis
- PTEN
phosphatase and tensin homolog deleted on chromosome ten
- TP53
tumor protein 53
- WISP1v
Wnt-inducible signaling pathway protein 1v
Footnotes
The authors have nothing to disclose.
Authors‘ contributions: Dr. Sumera Rizvi contributed to the outline and drafting of the manuscript, critical revision of the manuscript, and important intellectual content; Dr. Gregory J. Gores contributed to the outline of the manuscript, critical revision of the manuscript for important intellectual content, and provided manuscript writing supervision.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Welzel TM, McGlynn KA, Hsing AW, O’Brien TR, Pfeiffer RM. Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 2006;98:873–5. doi: 10.1093/jnci/djj234. [DOI] [PubMed] [Google Scholar]
- 2.Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;8:512–22. doi: 10.1038/nrgastro.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deoliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus P, Clavien PA. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363–71. doi: 10.1002/hep.24227. [DOI] [PubMed] [Google Scholar]
- 4.DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, Choti MA, Yeo CJ, Schulick RD. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–62. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blechacz BG, GJ . Feldman: Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 9. Vol. 1. Saunders; 2010. Tumors of the Bile Ducts, Gallbladder, and Ampulla; pp. 1171–1176. [Google Scholar]
- 6.Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WM, Tait P, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Wasan H British Society of G. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61:1657–69. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 7.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–44. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 8.Tyson GL, El-Serag HB. Risk factors for cholangiocarcinoma. Hepatology. 2011;54:173–84. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaib Y, El-Serag HB. The epidemiology of cholangiocarcinoma. Semin Liver Dis. 2004;24:115–25. doi: 10.1055/s-2004-828889. [DOI] [PubMed] [Google Scholar]
- 10.Sripa B, Pairojkul C. Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 2008;24:349–56. doi: 10.1097/MOG.0b013e3282fbf9b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol. 2002;37:806–13. doi: 10.1016/s0168-8278(02)00297-0. [DOI] [PubMed] [Google Scholar]
- 12.Khan SA, Toledano MB, Taylor-Robinson SD. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB (Oxford) 2008;10:77–82. doi: 10.1080/13651820801992641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:1198–203. doi: 10.1158/1055-9965.EPI-05-0811. [DOI] [PubMed] [Google Scholar]
- 14.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–7. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 15.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, Toledano MB. Rising trends in cholangiocarcinoma: Is the ICD classification system misleading us? Journal of Hepatology. 2012;56:848–854. doi: 10.1016/j.jhep.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 17.Razumilava N, Gores GJ. Classification, diagnosis, and management of cholangiocarcinoma. Clin Gastroenterol Hepatol. 2013;11:13–21. e1. doi: 10.1016/j.cgh.2012.09.009. quiz e3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin HR, Oh JK, Lim MK, Shin A, Kong HJ, Jung KW, Won YJ, Park S, Park SJ, Hong ST. Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in Korea. J Korean Med Sci. 2010;25:1011–6. doi: 10.3346/jkms.2010.25.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang MH, Chen CH, Yen CM, Yang JC, Yang CC, Yeh YH, Chou DA, Yueh SK, Yang YY, Nien CK. Relation of hepatolithiasis to helminthic infestation. J Gastroenterol Hepatol. 2005;20:141–6. doi: 10.1111/j.1440-1746.2004.03523.x. [DOI] [PubMed] [Google Scholar]
- 20.Edil BH, Cameron JL, Reddy S, Lum Y, Lipsett PA, Nathan H, Pawlik TM, Choti MA, Wolfgang CL, Schulick RD. Choledochal cyst disease in children and adults: a 30-year single-institution experience. J Am Coll Surg. 2008;206:1000–5. doi: 10.1016/j.jamcollsurg.2007.12.045. discussion 1005–8. [DOI] [PubMed] [Google Scholar]
- 21.Mabrut JY, Bozio G, Hubert C, Gigot JF. Management of congenital bile duct cysts. Dig Surg. 2010;27:12–8. doi: 10.1159/000268109. [DOI] [PubMed] [Google Scholar]
- 22.Kato I, Kido C. Increased risk of death in thorotrast-exposed patients during the late follow-up period. Jpn J Cancer Res. 1987;78:1187–92. [PubMed] [Google Scholar]
- 23.Lee TY, Lee SS, Jung SW, Jeon SH, Yun SC, Oh HC, Kwon S, Lee SK, Seo DW, Kim MH, Suh DJ. Hepatitis B virus infection and intrahepatic cholangiocarcinoma in Korea: a case-control study. Am J Gastroenterol. 2008;103:1716–20. doi: 10.1111/j.1572-0241.2008.01796.x. [DOI] [PubMed] [Google Scholar]
- 24.Shaib YH, El-Serag HB, Nooka AK, Thomas M, Brown TD, Patt YZ, Hassan MM. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102:1016–21. doi: 10.1111/j.1572-0241.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 25.Sorensen HT, Friis S, Olsen JH, Thulstrup AM, Mellemkjaer L, Linet M, Trichopoulos D, Vilstrup H, Olsen J. Risk of liver and other types of cancer in patients with cirrhosis: a nationwide cohort study in Denmark. Hepatology. 1998;28:921–5. doi: 10.1002/hep.510280404. [DOI] [PubMed] [Google Scholar]
- 26.Palmer WC, Patel T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J Hepatol. 2012;57:69–76. doi: 10.1016/j.jhep.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620–6. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- 28.Welzel TM, Graubard BI, El-Serag HB, Shaib YH, Hsing AW, Davila JA, McGlynn KA. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221–8. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Welzel TM, Mellemkjaer L, Gloria G, Sakoda LC, Hsing AW, El Ghormli L, Olsen JH, McGlynn KA. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer. 2007;120:638–41. doi: 10.1002/ijc.22283. [DOI] [PubMed] [Google Scholar]
- 30.Nakanuma Y, Sato Y, Harada K, Sasaki M, Xu J, Ikeda H. Pathological classification of intrahepatic cholangiocarcinoma based on a new concept. World J Hepatol. 2010;2:419–27. doi: 10.4254/wjh.v2.i12.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komuta M, Spee B, Vander Borght S, De Vos R, Verslype C, Aerts R, Yano H, Suzuki T, Matsuda M, Fujii H, Desmet VJ, Kojiro M, Roskams T. Clinicopathological study on cholangiolocellular carcinoma suggesting hepatic progenitor cell origin. Hepatology. 2008;47:1544–56. doi: 10.1002/hep.22238. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchiya A, Kamimura H, Tamura Y, Takamura M, Yamagiwa S, Suda T, Nomoto M, Aoyagi Y. Hepatocellular carcinoma with progenitor cell features distinguishable by the hepatic stem/progenitor cell marker NCAM. Cancer Lett. 2011;309:95–103. doi: 10.1016/j.canlet.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 33.Cardinale V, Carpino G, Reid L, Gaudio E, Alvaro D. Multiple cells of origin in cholangiocarcinoma underlie biological, epidemiological and clinical heterogeneity. World J Gastrointest Oncol. 2012;4:94–102. doi: 10.4251/wjgo.v4.i5.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Komuta M, Govaere O, Vandecaveye V, Akiba J, Van Steenbergen W, Verslype C, Laleman W, Pirenne J, Aerts R, Yano H, Nevens F, Topal B, Roskams T. Histological diversity in cholangiocellular carcinoma reflects the different cholangiocyte phenotypes. Hepatology. 2012;55:1876–88. doi: 10.1002/hep.25595. [DOI] [PubMed] [Google Scholar]
- 35.Fan B, Malato Y, Calvisi DF, Naqvi S, Razumilava N, Ribback S, Gores GJ, Dombrowski F, Evert M, Chen X, Willenbring H. Cholangiocarcinomas can originate from hepatocytes in mice. J Clin Invest. 2012;122:2911–5. doi: 10.1172/JCI63212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekiya S, Suzuki A. Intrahepatic cholangiocarcinoma can arise from Notch-mediated conversion of hepatocytes. J Clin Invest. 2012;122:3914–8. doi: 10.1172/JCI63065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holczbauer A, Factor VM, Andersen JB, Marquardt JU, Kleiner DE, Raggi C, Kitade M, Seo D, Akita H, Durkin ME, Thorgeirsson SS. Modeling pathogenesis of primary liver cancer in lineage-specific mouse cell types. Gastroenterology. 2013;145:221–31. doi: 10.1053/j.gastro.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184–90. [PubMed] [Google Scholar]
- 39.Park J, Tadlock L, Gores GJ, Patel T. Inhibition of interleukin 6-mediated mitogen-activated protein kinase activation attenuates growth of a cholangiocarcinoma cell line. Hepatology. 1999;30:1128–33. doi: 10.1002/hep.510300522. [DOI] [PubMed] [Google Scholar]
- 40.Kobayashi S, Werneburg NW, Bronk SF, Kaufmann SH, Gores GJ. Interleukin-6 contributes to Mcl-1 up-regulation and TRAIL resistance via an Akt-signaling pathway in cholangiocarcinoma cells. Gastroenterology. 2005;128:2054–65. doi: 10.1053/j.gastro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 41.Taniai M, Grambihler A, Higuchi H, Werneburg N, Bronk SF, Farrugia DJ, Kaufmann SH, Gores GJ. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004;64:3517–24. doi: 10.1158/0008-5472.CAN-03-2770. [DOI] [PubMed] [Google Scholar]
- 42.Isomoto H, Kobayashi S, Werneburg NW, Bronk SF, Guicciardi ME, Frank DA, Gores GJ. Interleukin 6 upregulates myeloid cell leukemia-1 expression through a STAT3 pathway in cholangiocarcinoma cells. Hepatology. 2005;42:1329–38. doi: 10.1002/hep.20966. [DOI] [PubMed] [Google Scholar]
- 43.Meng F, Yamagiwa Y, Ueno Y, Patel T. Over-expression of interleukin-6 enhances cell survival and transformed cell growth in human malignant cholangiocytes. J Hepatol. 2006;44:1055–65. doi: 10.1016/j.jhep.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sia D, Tovar V, Moeini A, Llovet JM. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene. 2013 doi: 10.1038/onc.2012.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sia D, Hoshida Y, Villanueva A, Roayaie S, Ferrer J, Tabak B, Peix J, Sole M, Tovar V, Alsinet C, Cornella H, Klotzle B, Fan JB, Cotsoglou C, Thung SN, Fuster J, Waxman S, Garcia-Valdecasas JC, Bruix J, Schwartz ME, Beroukhim R, Mazzaferro V, Llovet JM. Integrative molecular analysis of intrahepatic cholangiocarcinoma reveals 2 classes that have different outcomes. Gastroenterology. 2013;144:829–40. doi: 10.1053/j.gastro.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isomoto H, Mott JL, Kobayashi S, Werneburg NW, Bronk SF, Haan S, Gores GJ. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology. 2007;132:384–96. doi: 10.1053/j.gastro.2006.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon JH, Gwak GY, Lee HS, Bronk SF, Werneburg NW, Gores GJ. Enhanced epidermal growth factor receptor activation in human cholangiocarcinoma cells. J Hepatol. 2004;41:808–14. doi: 10.1016/j.jhep.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Kiguchi K, Carbajal S, Chan K, Beltran L, Ruffino L, Shen J, Matsumoto T, Yoshimi N, DiGiovanni J. Constitutive expression of ErbB-2 in gallbladder epithelium results in development of adenocarcinoma. Cancer Res. 2001;61:6971–6. [PubMed] [Google Scholar]
- 49.Matsumoto K, Nakamura T. Hepatocyte growth factor and the Met system as a mediator of tumor-stromal interactions. Int J Cancer. 2006;119:477–83. doi: 10.1002/ijc.21808. [DOI] [PubMed] [Google Scholar]
- 50.Nishimura K, Kitamura M, Miura H, Nonomura N, Takada S, Takahara S, Matsumoto K, Nakamura T, Matsumiya K. Prostate stromal cell-derived hepatocyte growth factor induces invasion of prostate cancer cell line DU145 through tumor-stromal interaction. Prostate. 1999;41:145–53. doi: 10.1002/(sici)1097-0045(19991101)41:3<145::aid-pros1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura T, Matsumoto K, Kiritoshi A, Tano Y, Nakamura T. Induction of hepatocyte growth factor in fibroblasts by tumor-derived factors affects invasive growth of tumor cells: in vitro analysis of tumor-stromal interactions. Cancer Res. 1997;57:3305–13. [PubMed] [Google Scholar]
- 52.Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7:504–16. doi: 10.1038/nrd2530. [DOI] [PubMed] [Google Scholar]
- 53.Lai GH, Radaeva S, Nakamura T, Sirica AE. Unique epithelial cell production of hepatocyte growth factor/scatter factor by putative precancerous intestinal metaplasias and associated “intestinal-type” biliary cancer chemically induced in rat liver. Hepatology. 2000;31:1257–65. doi: 10.1053/jhep.2000.8108. [DOI] [PubMed] [Google Scholar]
- 54.Miyamoto M, Ojima H, Iwasaki M, Shimizu H, Kokubu A, Hiraoka N, Kosuge T, Yoshikawa D, Kono T, Furukawa H, Shibata T. Prognostic significance of overexpression of c-Met oncoprotein in cholangiocarcinoma. Br J Cancer. 2011;105:131–8. doi: 10.1038/bjc.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Radaeva S, Ferreira-Gonzalez A, Sirica AE. Overexpression of C-NEU and C-MET during rat liver cholangiocarcinogenesis: A link between biliary intestinal metaplasia and mucin-producing cholangiocarcinoma. Hepatology. 1999;29:1453–62. doi: 10.1002/hep.510290524. [DOI] [PubMed] [Google Scholar]
- 56.Yoon JH, Higuchi H, Werneburg NW, Kaufmann SH, Gores GJ. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology. 2002;122:985–93. doi: 10.1053/gast.2002.32410. [DOI] [PubMed] [Google Scholar]
- 57.Yoon JH, Canbay AE, Werneburg NW, Lee SP, Gores GJ. Oxysterols induce cyclooxygenase-2 expression in cholangiocytes: implications for biliary tract carcinogenesis. Hepatology. 2004;39:732–8. doi: 10.1002/hep.20125. [DOI] [PubMed] [Google Scholar]
- 58.Kuver R. Mechanisms of oxysterol-induced disease: insights from the biliary system. Clin Lipidol. 2012;7:537–548. doi: 10.2217/clp.12.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nachtergaele S, Mydock LK, Krishnan K, Rammohan J, Schlesinger PH, Covey DF, Rohatgi R. Oxysterols are allosteric activators of the oncoprotein Smoothened. Nat Chem Biol. 2012;8:211–20. doi: 10.1038/nchembio.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fingas CD, Bronk SF, Werneburg NW, Mott JL, Guicciardi ME, Cazanave SC, Mertens JC, Sirica AE, Gores GJ. Myofibroblast-derived PDGF-BB promotes Hedgehog survival signaling in cholangiocarcinoma cells. Hepatology. 2011;54:2076–88. doi: 10.1002/hep.24588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andersen JB, Thorgeirsson SS. Genetic profiling of intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol. 2012;28:266–72. doi: 10.1097/MOG.0b013e3283523c7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McKay SC, Unger K, Pericleous S, Stamp G, Thomas G, Hutchins RR, Spalding DR. Array comparative genomic hybridization identifies novel potential therapeutic targets in cholangiocarcinoma. HPB (Oxford) 2011;13:309–19. doi: 10.1111/j.1477-2574.2010.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koo SH, Ihm CH, Kwon KC, Park JW, Kim JM, Kong G. Genetic alterations in hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Cancer Genet Cytogenet. 2001;130:22–8. doi: 10.1016/s0165-4608(01)00460-5. [DOI] [PubMed] [Google Scholar]
- 64.Uhm KO, Park YN, Lee JY, Yoon DS, Park SH. Chromosomal imbalances in Korean intrahepatic cholangiocarcinoma by comparative genomic hybridization. Cancer Genet Cytogenet. 2005;157:37–41. doi: 10.1016/j.cancergencyto.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Lee JY, Park YN, Uhm KO, Park SY, Park SH. Genetic alterations in intrahepatic cholangiocarcinoma as revealed by degenerate oligonucleotide primed PCR-comparative genomic hybridization. J Korean Med Sci. 2004;19:682–7. doi: 10.3346/jkms.2004.19.5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong N, Li L, Tsang K, Lai PB, To KF, Johnson PJ. Frequent loss of chromosome 3p and hypermethylation of RASSF1A in cholangiocarcinoma. J Hepatol. 2002;37:633–9. doi: 10.1016/s0168-8278(02)00269-6. [DOI] [PubMed] [Google Scholar]
- 67.Homayounfar K, Gunawan B, Cameron S, Haller F, Baumhoer D, Uecker S, Sander B, Ramadori G, Lorf T, Fuzesi L. Pattern of chromosomal aberrations in primary liver cancers identified by comparative genomic hybridization. Hum Pathol. 2009;40:834–42. doi: 10.1016/j.humpath.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 68.Ong CK, Subimerb C, Pairojkul C, Wongkham S, Cutcutache I, Yu W, McPherson JR, Allen GE, Ng CC, Wong BH, Myint SS, Rajasegaran V, Heng HL, Gan A, Zang ZJ, Wu Y, Wu J, Lee MH, Huang D, Ong P, Chan-on W, Cao Y, Qian CN, Lim KH, Ooi A, Dykema K, Furge K, Kukongviriyapan V, Sripa B, Wongkham C, Yongvanit P, Futreal PA, Bhudhisawasdi V, Rozen S, Tan P, Teh BT. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nat Genet. 2012;44:690–3. doi: 10.1038/ng.2273. [DOI] [PubMed] [Google Scholar]
- 69.Xu RF, Sun JP, Zhang SR, Zhu GS, Li LB, Liao YL, Xie JM, Liao WJ. KRAS and PIK3CA but not BRAF genes are frequently mutated in Chinese cholangiocarcinoma patients. Biomed Pharmacother. 2011;65:22–6. doi: 10.1016/j.biopha.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 70.Ohashi K, Nakajima Y, Kanehiro H, Tsutsumi M, Taki J, Aomatsu Y, Yoshimura A, Ko S, Kin T, Yagura K, et al. Ki-ras mutations and p53 protein expressions in intrahepatic cholangiocarcinomas: relation to gross tumor morphology. Gastroenterology. 1995;109:1612–7. doi: 10.1016/0016-5085(95)90650-9. [DOI] [PubMed] [Google Scholar]
- 71.Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, Conner EA, Gillen MC, Roskams T, Roberts LR, Factor VM, Thorgeirsson SS. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–1031. e15. doi: 10.1053/j.gastro.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tada M, Omata M, Ohto M. High incidence of ras gene mutation in intrahepatic cholangiocarcinoma. Cancer. 1992;69:1115–8. doi: 10.1002/cncr.2820690509. [DOI] [PubMed] [Google Scholar]
- 73.Khan SA, Thomas HC, Toledano MB, Cox IJ, Taylor-Robinson SD. p53 Mutations in human cholangiocarcinoma: a review. Liver Int. 2005;25:704–16. doi: 10.1111/j.1478-3231.2005.01106.x. [DOI] [PubMed] [Google Scholar]
- 74.Kipp BR, Voss JS, Kerr SE, Barr Fritcher EG, Graham RP, Zhang L, Highsmith WE, Zhang J, Roberts LR, Gores GJ, Halling KC. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum Pathol. 2012;43:1552–8. doi: 10.1016/j.humpath.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 75.Borger DR, Tanabe KK, Fan KC, Lopez HU, Fantin VR, Straley KS, Schenkein DP, Hezel AF, Ancukiewicz M, Liebman HM, Kwak EL, Clark JW, Ryan DP, Deshpande V, Dias-Santagata D, Ellisen LW, Zhu AX, Iafrate AJ. Frequent mutation of isocitrate dehydrogenase (IDH)1 and IDH2 in cholangiocarcinoma identified through broad-based tumor genotyping. Oncologist. 2012;17:72–9. doi: 10.1634/theoncologist.2011-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang P, Dong Q, Zhang C, Kuan PF, Liu Y, Jeck WR, Andersen JB, Jiang W, Savich GL, Tan TX, Auman JT, Hoskins JM, Misher AD, Moser CD, Yourstone SM, Kim JW, Cibulskis K, Getz G, Hunt HV, Thorgeirsson SS, Roberts LR, Ye D, Guan KL, Xiong Y, Qin LX, Chiang DY. Mutations in isocitrate dehydrogenase 1 and 2 occur frequently in intrahepatic cholangiocarcinomas and share hypermethylation targets with glioblastomas. Oncogene. 2012 doi: 10.1038/onc.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reitman ZJ, Parsons DW, Yan H. IDH1 and IDH2: not your typical oncogenes. Cancer Cell. 2010;17:215–6. doi: 10.1016/j.ccr.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B, Komisopoulou E, Kunii K, Pedraza A, Schalm S, Silverman L, Miller A, Wang F, Yang H, Chen Y, Kernytsky A, Rosenblum MK, Liu W, Biller SA, Su SM, Brennan CW, Chan TA, Graeber TG, Yen KE, Mellinghoff IK. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science. 2013;340:626–30. doi: 10.1126/science.1236062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang F, Travins J, DeLaBarre B, Penard-Lacronique V, Schalm S, Hansen E, Straley K, Kernytsky A, Liu W, Gliser C, Yang H, Gross S, Artin E, Saada V, Mylonas E, Quivoron C, Popovici-Muller J, Saunders JO, Salituro FG, Yan S, Murray S, Wei W, Gao Y, Dang L, Dorsch M, Agresta S, Schenkein DP, Biller SA, Su SM, de Botton S, Yen KE. Targeted inhibition of mutant IDH2 in leukemia cells induces cellular differentiation. Science. 2013;340:622–6. doi: 10.1126/science.1234769. [DOI] [PubMed] [Google Scholar]
- 80.Oishi N, Kumar MR, Roessler S, Ji J, Forgues M, Budhu A, Zhao X, Andersen JB, Ye QH, Jia HL, Qin LX, Yamashita T, Woo HG, Kim YJ, Kaneko S, Tang ZY, Thorgeirsson SS, Wang XW. Transcriptomic profiling reveals hepatic stem-like gene signatures and interplay of miR-200c and epithelial-mesenchymal transition in intrahepatic cholangiocarcinoma. Hepatology. 2012;56:1792–803. doi: 10.1002/hep.25890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen L, Yan HX, Yang W, Hu L, Yu LX, Liu Q, Li L, Huang DD, Ding J, Shen F, Zhou WP, Wu MC, Wang HY. The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma. J Hepatol. 2009;50:358–69. doi: 10.1016/j.jhep.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 82.Wu YM, Su F, Kalyana-Sundaram S, Khazanov N, Ateeq B, Cao X, Lonigro RJ, Vats P, Wang R, Lin SF, Cheng AJ, Kunju LP, Siddiqui J, Tomlins SA, Wyngaard P, Sadis S, Roychowdhury S, Hussain MH, Feng FY, Zalupski MM, Talpaz M, Pienta KJ, Rhodes DR, Robinson DR, Chinnaiyan AM. Identification of targetable FGFR gene fusions in diverse cancers. Cancer discovery. 2013;3:636–47. doi: 10.1158/2159-8290.CD-13-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamanaka S, Olaru AV, An F, Luvsanjav D, Jin Z, Agarwal R, Tomuleasa C, Popescu I, Alexandrescu S, Dima S, Chivu-Economescu M, Montgomery EA, Torbenson M, Meltzer SJ, Selaru FM. MicroRNA-21 inhibits Serpini1, a gene with novel tumour suppressive effects in gastric cancer. Dig Liver Dis. 2012;44:589–96. doi: 10.1016/j.dld.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Meng F, Henson R, Lang M, Wehbe H, Maheshwari S, Mendell JT, Jiang J, Schmittgen TD, Patel T. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology. 2006;130:2113–29. doi: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 85.Hofmann JJ, Zovein AC, Koh H, Radtke F, Weinmaster G, Iruela-Arispe ML. Jagged1 in the portal vein mesenchyme regulates intrahepatic bile duct development: insights into Alagille syndrome. Development. 2010;137:4061–72. doi: 10.1242/dev.052118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zender S, Nickeleit I, Wuestefeld T, Sorensen I, Dauch D, Bozko P, El-Khatib M, Geffers R, Bektas H, Manns MP, Gossler A, Wilkens L, Plentz R, Zender L, Malek NP. A critical role for notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell. 2013;23:784–95. doi: 10.1016/j.ccr.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 87.Jinawath A, Akiyama Y, Sripa B, Yuasa Y. Dual blockade of the Hedgehog and ERK1/2 pathways coordinately decreases proliferation and survival of cholangiocarcinoma cells. J Cancer Res Clin Oncol. 2007;133:271–8. doi: 10.1007/s00432-006-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El Khatib M, Kalnytska A, Palagani V, Kossatz U, Manns MP, Malek NP, Wilkens L, Plentz RR. Inhibition of hedgehog signaling attenuates carcinogenesis in vitro and increases necrosis of cholangiocellular carcinoma. Hepatology. 2013;57:1035–45. doi: 10.1002/hep.26147. [DOI] [PubMed] [Google Scholar]
- 89.Sirica AE, Nathanson MH, Gores GJ, Larusso NF. Pathobiology of biliary epithelia and cholangiocarcinoma: proceedings of the Henry M and Lillian Stratton Basic Research Single-Topic Conference. Hepatology. 2008;48:2040–6. doi: 10.1002/hep.22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tanaka S, Sugimachi K, Kameyama T, Maehara S, Shirabe K, Shimada M, Wands JR, Maehara Y. Human WISP1v, a member of the CCN family, is associated with invasive cholangiocarcinoma. Hepatology. 2003;37:1122–9. doi: 10.1053/jhep.2003.50187. [DOI] [PubMed] [Google Scholar]
- 91.Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–54. doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 92.Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2012;9:44–54. doi: 10.1038/nrgastro.2011.222. [DOI] [PubMed] [Google Scholar]
- 93.Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 94.Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–44. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Okabe H, Beppu T, Hayashi H, Horino K, Masuda T, Komori H, Ishikawa S, Watanabe M, Takamori H, Iyama K, Baba H. Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16:2555–64. doi: 10.1245/s10434-009-0568-4. [DOI] [PubMed] [Google Scholar]
- 96.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, Baik GH, Shibata W, Diprete B, Betz KS, Friedman R, Varro A, Tycko B, Wang TC. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell. 2011;19:257–72. doi: 10.1016/j.ccr.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li T, Li D, Cheng L, Wu H, Gao Z, Liu Z, Jiang W, Gao YH, Tian F, Zhao L, Wang S. Epithelial-mesenchymal transition induced by hepatitis C virus core protein in cholangiocarcinoma. Ann Surg Oncol. 2010;17:1937–44. doi: 10.1245/s10434-010-0925-3. [DOI] [PubMed] [Google Scholar]
- 98.Sato Y, Harada K, Itatsu K, Ikeda H, Kakuda Y, Shimomura S, Shan Ren X, Yoneda N, Sasaki M, Nakanuma Y. Epithelial-mesenchymal transition induced by transforming growth factor-{beta}1/Snail activation aggravates invasive growth of cholangiocarcinoma. Am J Pathol. 2010;177:141–52. doi: 10.2353/ajpath.2010.090747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Korita PV, Wakai T, Ajioka Y, Inoue M, Takamura M, Shirai Y, Hatakeyama K. Aberrant expression of vimentin correlates with dedifferentiation and poor prognosis in patients with intrahepatic cholangiocarcinoma. Anticancer Res. 2010;30:2279–85. [PubMed] [Google Scholar]
- 100.Cadamuro M, Nardo G, Indraccolo S, Dall’olmo L, Sambado L, Moserle L, Franceschet I, Colledan M, Massani M, Stecca T, Bassi N, Morton S, Spirli C, Fiorotto R, Fabris L, Strazzabosco M. Platelet-derived growth factor-D and Rho GTPases regulate recruitment of cancer-associated fibroblasts in cholangiocarcinoma. Hepatology. 2013 doi: 10.1002/hep.26384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fingas CD, Mertens JC, Razumilava N, Bronk SF, Sirica AE, Gores GJ. Targeting PDGFR-beta in Cholangiocarcinoma. Liver Int. 2012;32:400–9. doi: 10.1111/j.1478-3231.2011.02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Utispan K, Thuwajit P, Abiko Y, Charngkaew K, Paupairoj A, Chau-in S, Thuwajit C. Gene expression profiling of cholangiocarcinoma-derived fibroblast reveals alterations related to tumor progression and indicates periostin as a poor prognostic marker. Mol Cancer. 2010;9:13. doi: 10.1186/1476-4598-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Baril P, Gangeswaran R, Mahon PC, Caulee K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T, Lemoine NR. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: role of the beta4 integrin and the PI3k pathway. Oncogene. 2007;26:2082–94. doi: 10.1038/sj.onc.1210009. [DOI] [PubMed] [Google Scholar]
- 104.Menakongka A, Suthiphongchai T. Involvement of PI3K and ERK1/2 pathways in hepatocyte growth factor-induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2010;16:713–22. doi: 10.3748/wjg.v16.i6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ohira S, Sasaki M, Harada K, Sato Y, Zen Y, Isse K, Kozaka K, Ishikawa A, Oda K, Nimura Y, Nakanuma Y. Possible regulation of migration of intrahepatic cholangiocarcinoma cells by interaction of CXCR4 expressed in carcinoma cells with tumor necrosis factor-alpha and stromal-derived factor-1 released in stroma. Am J Pathol. 2006;168:1155–68. doi: 10.2353/ajpath.2006.050204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leelawat K, Leelawat S, Narong S, Hongeng S. Roles of the MEK1/2 and AKT pathways in CXCL12/CXCR4 induced cholangiocarcinoma cell invasion. World J Gastroenterol. 2007;13:1561–8. doi: 10.3748/wjg.v13.i10.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Terada T, Okada Y, Nakanuma Y. Expression of immunoreactive matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases in human normal livers and primary liver tumors. Hepatology. 1996;23:1341–4. doi: 10.1053/jhep.1996.v23.pm0008675149. [DOI] [PubMed] [Google Scholar]
- 108.Prakobwong S, Yongvanit P, Hiraku Y, Pairojkul C, Sithithaworn P, Pinlaor P, Pinlaor S. Involvement of MMP-9 in peribiliary fibrosis and cholangiocarcinogenesis via Rac1-dependent DNA damage in a hamster model. Int J Cancer. 2010;127:2576–87. doi: 10.1002/ijc.25266. [DOI] [PubMed] [Google Scholar]
- 109.Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z, Hoffman JP, Weiner LM, Cheng JD. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37:154–8. doi: 10.1097/MPA.0b013e31816618ce. [DOI] [PubMed] [Google Scholar]
- 110.Mertens JC, Fingas CD, Christensen JD, Smoot RL, Bronk SF, Werneburg NW, Gustafson MP, Dietz AB, Roberts LR, Sirica AE, Gores GJ. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73:897–907. doi: 10.1158/0008-5472.CAN-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ko KS, Peng J, Yang H. Animal models of cholangiocarcinoma. Curr Opin Gastroenterol. 2013;29:312–8. doi: 10.1097/MOG.0b013e32835d6a3e. [DOI] [PubMed] [Google Scholar]
- 112.Fava G, Marucci L, Glaser S, Francis H, De Morrow S, Benedetti A, Alvaro D, Venter J, Meininger C, Patel T, Taffetani S, Marzioni M, Summers R, Reichenbach R, Alpini G. gamma-Aminobutyric acid inhibits cholangiocarcinoma growth by cyclic AMP-dependent regulation of the protein kinase A/extracellular signal-regulated kinase 1/2 pathway. Cancer Res. 2005;65:11437–46. doi: 10.1158/0008-5472.CAN-05-1470. [DOI] [PubMed] [Google Scholar]
- 113.Pawar P, Ma L, Byon CH, Liu H, Ahn EY, Jhala N, Arnoletti JP, McDonald JM, Chen Y. Molecular mechanisms of tamoxifen therapy for cholangiocarcinoma: role of calmodulin. Clin Cancer Res. 2009;15:1288–96. doi: 10.1158/1078-0432.CCR-08-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tang T, Zheng JW, Chen B, Li H, Li X, Xue KY, Ai X, Zou SQ. Effects of targeting magnetic drug nanoparticles on human cholangiocarcinoma xenografts in nude mice. Hepatobiliary Pancreat Dis Int. 2007;6:303–7. [PubMed] [Google Scholar]
- 115.Zhang J, Han C, Wu T. MicroRNA-26a promotes cholangiocarcinoma growth by activating beta-catenin. Gastroenterology. 2012;143:246–56. e8. doi: 10.1053/j.gastro.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Olaru AV, Ghiaur G, Yamanaka S, Luvsanjav D, An F, Popescu I, Alexandrescu S, Allen S, Pawlik TM, Torbenson M, Georgiades C, Roberts LR, Gores GJ, Ferguson-Smith A, Almeida MI, Calin GA, Mezey E, Selaru FM. MicroRNA down-regulated in human cholangiocarcinoma control cell cycle through multiple targets involved in the G1/S checkpoint. Hepatology. 2011;54:2089–98. doi: 10.1002/hep.24591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang K, Chen D, Wang X, Zhang S, Wang J, Gao Y, Yan B. RNA Interference Targeting Slug Increases Cholangiocarcinoma Cell Sensitivity to Cisplatin via Upregulating PUMA. Int J Mol Sci. 2011;12:385–400. doi: 10.3390/ijms12010385. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Obchoei S, Weakley SM, Wongkham S, Wongkham C, Sawanyawisuth K, Yao Q, Chen C. Cyclophilin A enhances cell proliferation and tumor growth of liver fluke-associated cholangiocarcinoma. Mol Cancer. 2011;10:102. doi: 10.1186/1476-4598-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hou YJ, Dong LW, Tan YX, Yang GZ, Pan YF, Li Z, Tang L, Wang M, Wang Q, Wang HY. Inhibition of active autophagy induces apoptosis and increases chemosensitivity in cholangiocarcinoma. Lab Invest. 2011;91:1146–57. doi: 10.1038/labinvest.2011.97. [DOI] [PubMed] [Google Scholar]
- 120.Xu X, Kobayashi S, Qiao W, Li C, Xiao C, Radaeva S, Stiles B, Wang RH, Ohara N, Yoshino T, LeRoith D, Torbenson MS, Gores GJ, Wu H, Gao B, Deng CX. Induction of intrahepatic cholangiocellular carcinoma by liver-specific disruption of Smad4 and Pten in mice. J Clin Invest. 2006;116:1843–52. doi: 10.1172/JCI27282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Farazi PA, Zeisberg M, Glickman J, Zhang Y, Kalluri R, DePinho RA. Chronic bile duct injury associated with fibrotic matrix microenvironment provokes cholangiocarcinoma in p53-deficient mice. Cancer Res. 2006;66:6622–7. doi: 10.1158/0008-5472.CAN-05-4609. [DOI] [PubMed] [Google Scholar]
- 122.Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM, Wang CY, Gao B, Jiang J, Yang Y. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010;107:1431–6. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM, Lim DS. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–53. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.O’Dell MR, Huang JL, Whitney-Miller CL, Deshpande V, Rothberg P, Grose V, Rossi RM, Zhu AX, Land H, Bardeesy N, Hezel AF. Kras(G12D) and p53 mutation cause primary intrahepatic cholangiocarcinoma. Cancer Res. 2012;72:1557–67. doi: 10.1158/0008-5472.CAN-11-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sirica AE, Zhang Z, Lai GH, Asano T, Shen XN, Ward DJ, Mahatme A, Dewitt JL. A novel “patient-like” model of cholangiocarcinoma progression based on bile duct inoculation of tumorigenic rat cholangiocyte cell lines. Hepatology. 2008;47:1178–90. doi: 10.1002/hep.22088. [DOI] [PubMed] [Google Scholar]
- 126.Campbell DJ, Dumur CI, Lamour NF, Dewitt JL, Sirica AE. Novel organotypic culture model of cholangiocarcinoma progression. Hepatol Res. 2012;42:1119–30. doi: 10.1111/j.1872-034X.2012.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fava G, Alpini G, Rychlicki C, Saccomanno S, DeMorrow S, Trozzi L, Candelaresi C, Venter J, Di Sario A, Marzioni M, Bearzi I, Glaser S, Alvaro D, Marucci L, Francis H, Svegliati-Baroni G, Benedetti A. Leptin enhances cholangiocarcinoma cell growth. Cancer Res. 2008;68:6752–61. doi: 10.1158/0008-5472.CAN-07-6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang H, Li TW, Peng J, Tang X, Ko KS, Xia M, Aller MA. A mouse model of cholestasis-associated cholangiocarcinoma and transcription factors involved in progression. Gastroenterology. 2011;141:378–88. 388 e1–4. doi: 10.1053/j.gastro.2011.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Plengsuriyakarn T, Eursitthichai V, Labbunruang N, Na-Bangchang K, Tesana S, Aumarm W, Pongpradit A, Viyanant V. Ultrasonography as a tool for monitoring the development and progression of cholangiocarcinoma in Opisthorchis viverrini/dimethylnitrosamine-induced hamsters. Asian Pac J Cancer Prev. 2012;13:87–90. doi: 10.7314/apjcp.2012.13.1.087. [DOI] [PubMed] [Google Scholar]
- 130.Yamasaki S. Intrahepatic cholangiocarcinoma: macroscopic type and stage classification. J Hepatobiliary Pancreat Surg. 2003;10:288–91. doi: 10.1007/s00534-002-0732-8. [DOI] [PubMed] [Google Scholar]
- 131.Rimola J, Forner A, Reig M, Vilana R, de Lope CR, Ayuso C, Bruix J. Cholangiocarcinoma in cirrhosis: absence of contrast washout in delayed phases by magnetic resonance imaging avoids misdiagnosis of hepatocellular carcinoma. Hepatology. 2009;50:791–8. doi: 10.1002/hep.23071. [DOI] [PubMed] [Google Scholar]
- 132.Vilgrain V. Staging cholangiocarcinoma by imaging studies. HPB (Oxford) 2008;10:106–9. doi: 10.1080/13651820801992617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–21. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Patel AH, Harnois DM, Klee GG, LaRusso NF, Gores GJ. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204–7. doi: 10.1111/j.1572-0241.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 135.Sapisochin G, Fidelman N, Roberts JP, Yao FY. Mixed hepatocellular cholangiocarcinoma and intrahepatic cholangiocarcinoma in patients undergoing transplantation for hepatocellular carcinoma. Liver Transpl. 2011;17:934–42. doi: 10.1002/lt.22307. [DOI] [PubMed] [Google Scholar]
- 136.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, D’Angelica M, DeMatteo RP, Fong Y, Schwartz L, Kemeny N, O’Reilly E, Abou-Alfa GK, Shimada H, Blumgart LH, Jarnagin WR. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 137.Choi SB, Kim KS, Choi JY, Park SW, Choi JS, Lee WJ, Chung JB. The prognosis and survival outcome of intrahepatic cholangiocarcinoma following surgical resection: association of lymph node metastasis and lymph node dissection with survival. Ann Surg Oncol. 2009;16:3048–56. doi: 10.1245/s10434-009-0631-1. [DOI] [PubMed] [Google Scholar]
- 138.Li YY, Li H, Lv P, Liu G, Li XR, Tian BN, Chen DJ. Prognostic value of cirrhosis for intrahepatic cholangiocarcinoma after surgical treatment. J Gastrointest Surg. 2011;15:608–13. doi: 10.1007/s11605-011-1419-8. [DOI] [PubMed] [Google Scholar]
- 139.Fabris L, Cadamuro M, Moserle L, Dziura J, Cong X, Sambado L, Nardo G, Sonzogni A, Colledan M, Furlanetto A, Bassi N, Massani M, Cillo U, Mescoli C, Indraccolo S, Rugge M, Okolicsanyi L, Strazzabosco M. Nuclear expression of S100A4 calcium-binding protein increases cholangiocarcinoma invasiveness and metastasization. Hepatology. 2011;54:890–9. doi: 10.1002/hep.24466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kuhlmann JB, Blum HE. Locoregional therapy for cholangiocarcinoma. Curr Opin Gastroenterol. 2013;29:324–8. doi: 10.1097/MOG.0b013e32835d9dea. [DOI] [PubMed] [Google Scholar]
- 141.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J, Investigators ABCT. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–81. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 142.Yamashita Y, Takahashi M, Kanazawa S, Charnsangavej C, Wallace S. Hilar cholangiocarcinoma. An evaluation of subtypes with CT and angiography. Acta Radiol. 1992;33:351–5. [PubMed] [Google Scholar]
- 143.Heimbach JK, Sanchez W, Rosen CB, Gores GJ. Trans-peritoneal fine needle aspiration biopsy of hilar cholangiocarcinoma is associated with disease dissemination. HPB (Oxford) 2011;13:356–60. doi: 10.1111/j.1477-2574.2011.00298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR, Barr Fritcher EG, Levy MJ, Gores GJ. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–72. doi: 10.1053/j.gastro.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Barr Fritcher EG, Kipp BR, Voss JS, Clayton AC, Lindor KD, Halling KC, Gores GJ. Primary sclerosing cholangitis patients with serial polysomy fluorescence in situ hybridization results are at increased risk of cholangiocarcinoma. Am J Gastroenterol. 2011;106:2023–8. doi: 10.1038/ajg.2011.272. [DOI] [PubMed] [Google Scholar]
- 146.Barr Fritcher EG, Voss JS, Jenkins SM, Lingineni RK, Clayton AC, Roberts LR, Halling KC, Talwalkar JA, Gores GJ, Kipp BR. Primary sclerosing cholangitis with equivocal cytology: Fluorescence in situ hybridization and serum CA 19-9 predict risk of malignancy. Cancer Cytopathol. 2013 doi: 10.1002/cncy.21331. [DOI] [PubMed] [Google Scholar]
- 147.Nagorney DM, Kendrick ML. Hepatic resection in the treatment of hilar cholangiocarcinoma. Adv Surg. 2006;40:159–71. doi: 10.1016/j.yasu.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 148.Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, Botha JF, Mezrich JD, Chapman WC, Schwartz JJ, Hong JC, Emond JC, Jeon H, Rosen CB, Gores GJ, Heimbach JK. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88–98. e3. doi: 10.1053/j.gastro.2012.04.008. quiz e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hong JC, Jones CM, Duffy JP, Petrowsky H, Farmer DG, French S, Finn R, Durazo FA, Saab S, Tong MJ, Hiatt JR, Busuttil RW. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg. 2011;146:683–9. doi: 10.1001/archsurg.2011.116. [DOI] [PubMed] [Google Scholar]
- 150.Geynisman DM, Catenacci DV. Toward personalized treatment of advanced biliary tract cancers. Discov Med. 2012;14:41–57. [PubMed] [Google Scholar]