Highlights
► The youngest case of endometrial carcinoma in the English literature ► Endometrial cancer is diagnosed in approximately 13–19% of women with Cowden Syndrome. ► Screening guidelines should follow that of Lynch Syndrome.
Keywords: Endometrial cancer, Cowden Syndrome, Phosphatase and tensin (PTEN) homolog gene, PTEN hamartoma tumor syndrome, Genetic mutation
Introduction
Cowden Syndrome (CS) is a rare, autosomal dominant disorder affecting approximately 1 in every 200,000 people. CS involves a germline mutation in the phosphatase and tensin (PTEN) homolog gene, which encodes a tumor suppressor antagonizing the phosphatidylinositol 3-kinase (PI3K/AKT) and mitogen-activated protein kinase (MAPK) pathway (Hobert and Eng, 2009). CS is associated with the development of hamartomas, as well as an increased lifetime risk of breast (25–50%), thyroid (10%), renal (13–34%), colorectal (16%) and endometrial carcinomas (13–19%); forming the prototype of the PTEN hamartoma tumor syndrome (PHTS) (Gammon et al., 2009). Physical manifestations may include macrocephaly, trichilemmomas and papillomatous papules.
Case report
A 15-year-old virginal female complained to her primary care physician of excessive vaginal bleeding. She reported 2–3 cycles per month with menses lasting 5–7 days. Menarche was at 13 years of age and had previously been normal. On examination, a cervical mass was seen but poorly evaluated at this time due to intolerance of exam. Papanicolaou test revealed atypical squamous cells of undetermined significance. She was examined under anesthesia, and a 3 cm polyp at the cervical os was found and removed with ring forceps. Final pathology was grade 1 endometrial carcinoma arising from endometrial/cervical polyp (Fig. 1). The patient was then referred to our gynecologic oncology practice.
Fig. 1.
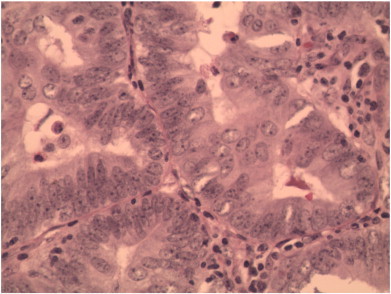
Endocervical polyp demonstrating the crowded branching gland component of the polyp with cytologic atypia (400 ×).
The patient's past medical history was notable for both motor and cognitive developmental delays. On physical exam, the patient was noted to have a head circumference of 63 cm (> 97th percentile), two thyroid masses measuring 3 and 6 cm and involving both lobes of the gland, and a right upper medial quadrant breast mass measuring approximately 2 cm. Sexual development was Tanner stage IV for both breast and pubic hair. The patient's father had a similar appearance of macrocephaly, as well as developmental delay, skin nodules suggestive of papillomatous papules, a remote history of thyroid goiter and a recent non-cancerous pancreatic tumor.
The patient was referred to St. Jude Children's Research Hospital for ongoing care. The diagnosis of CS was suspected clinically, and comprehensive PTEN mutational analysis identified a heterozygous truncating mutation in the PTEN gene (c. 467 470dupGGGA). Given the patient's young age, a conservative approach was appropriate. Computed tomography scan of the chest, abdomen, and pelvis was negative for metastatic disease. The patient then underwent hysteroscopy with dilation and curettage. Hysteroscopy revealed multiple polyps in the endometrium. Curettage and evacuation of polyps with polyp forceps were performed. Pathology revealed endometrioid adenocarcinoma, grade 1.
The patient and family were extensively counseled on treatment options by both pediatric oncology at St. Jude and gynecologic oncology. Options consisted of fertility preserving therapy with hormonal agents versus definitive therapy consisting of hysterectomy. The patient and family elected for definitive therapy, and the patient underwent a total abdominal hysterectomy with pelvic and paraaortic lymphadenectomy. Intraoperatively, bilateral ovarian biopsies were obtained. Pathology showed atypical cells in the right ovary; thus, a right oopherectomy was performed with preservation of the left ovary. Final pathology revealed stage IA endometrial adenocarcinoma, FIGO grade 1, invasive into the superficial 1/3 of the myometrium. No lymphatic/vascular invasion was identified. The right ovary demonstrated normal ovarian parenchyma on final pathology, with no malignancy; pelvic and paraaortic lymph nodes were negative for malignancy, and abdominal washings were negative for neoplastic cells. The patient's postoperative recovery was uneventful.
Two months after surgery, the patient underwent near total thyroidectomy and received radioactive iodine for residual thyroid ablation (32.5 mCi I-131). Biopsy of the breast mass showed focally fibrotic mammary gland tissue suggestive of, but not diagnostic of, lobular neoplasia. The patient has now been in remission for 3 years from both thyroid and endometrial cancer at the time of this submission.
Comment
PTEN tumor suppressor gene somatic mutations have been reported in approximately 40% of endometrial adenocarcinomas and constitute the most frequent genetic lesion (Mutter et al., 2000). Furthermore, persons with CS have a 13 to 19% lifetime risk of endometrial cancer (Riegert-Johnson et al., 2010). Despite these findings, the National Comprehensive Cancer Network (NCCN) guidelines do not endorse any endometrial cancer screening aside from patient education and awareness of symptoms of endometrial cancer.
Conversely, cancer screening in regard to breast and thyroid malignancies is well defined and recommended for CS patients beginning at 18 years of age or 5 years before the family's earliest age of cancer diagnosis (Hobert and Eng, 2009; Gammon et al., 2009).
This case illustrates the importance of recognizing both the features of CS and the clinical manifestations of its associated cancers, which may present during adolescence. Due to the inherent risk of endometrial cancer and the possibility of premenopausal development of endometrial cancer, adopting the guidelines for Lynch Syndrome should be considered; use of annual endometrial biopsies beginning at age 30 to 35 or 5 years before the earliest family diagnosis of endometrial cancer (Renkonen-Sinisalo et al., 2007; Auranen and Joutsiniemi, 2011). Postmenopausal recommendations may include annual ultrasonographic examination with biopsy of suspicious areas due to the improved sensitivity of detecting uterine pathology in this population (Smith-Bindman et al., 1998).
Another important recommendation is that of prophylactic hysterectomy. In patients with germ-line mutations associated with Lynch Syndrome, prophylactic hysterectomy and bilateral salpingo-oophorectomy prevented 100% of new uterine or ovarian malignancies when compared with controls who did not undergo prophylactic surgery (Schmeler et al., 2006).
With our experience, there seems to be no minimal age for performing endometrial biopsy if abnormal bleeding is found in patients suspected of CS. Collaboration with a genetic specialist is essential in diagnosing and managing CS. Awareness of the clinical phenotype (macrocephaly, trichilemmomas, and/or papillomatous papules) combined with a thorough physical examination are important in alerting physicians to a possible diagnosis of CS. For patients confirmed to have CS, counseling about the risks of breast, thyroid, and endometrial malignancy is important, as is routine screening for breast and thyroid cancer.
Conflict of interest statement
Each of the authors contributed to critical aspects of the case and production of this manuscript. On behalf of the authors, there are no conflicts of interest to report.
References
- Auranen A., Joutsiniemi T. A systematic review of gynecological cancer surveillance in women belonging to hereditary nonpolyposis colorectal cancer (Lynch syndrome) families. Acta Obstet. Gynecol. Scand. 2011;90(5):437–444. doi: 10.1111/j.1600-0412.2011.01091.x. [DOI] [PubMed] [Google Scholar]
- Gammon A. Hamartomatous polyposis syndromes. Best Pract. Res. Clin. Gastroenterol. 2009;23(2):219–231. doi: 10.1016/j.bpg.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert J.A., Eng C. PTEN hamartoma tumor syndrome: an overview. Genet. Med. 2009;11(10):687–694. doi: 10.1097/GIM.0b013e3181ac9aea. [DOI] [PubMed] [Google Scholar]
- Mutter G.L. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J. Natl. Cancer Inst. 2000;92(11):924–930. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- Renkonen-Sinisalo L. Surveillance for endometrial cancer in hereditary nonpolyposis colorectal cancer syndrome. Int. J. Cancer. 2007;120(4):821–824. doi: 10.1002/ijc.22446. [DOI] [PubMed] [Google Scholar]
- Riegert-Johnson D.L. Cancer and Lhermitte–Duclos disease are common in Cowden syndrome patients. Hered. Cancer Clin. Pract. 2010;8(1):6. doi: 10.1186/1897-4287-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeler K.M. Prophylactic surgery to reduce the risk of gynecologic cancers in the Lynch syndrome. N. Engl. J. Med. 2006;354(3):261–269. doi: 10.1056/NEJMoa052627. [DOI] [PubMed] [Google Scholar]
- Smith-Bindman R. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA. 1998;280(17):1510–1517. doi: 10.1001/jama.280.17.1510. [DOI] [PubMed] [Google Scholar]


