Highlights
► We present a rare case of a PYY-positive primary strumal carcinoid tumor of the ovary during pregnancy. ► MRI was useful for the preoperative diagnosis, and the prenatal course was uneventful after the operation. ► Colitis due to severe constipation caused by PYY, which is an inhibitor of intestinal mobility, might induce serum CEA elevation.
Keywords: Ovarian strumal carcinoid, Peptide YY, Ovarian tumor during pregnancy, Severe constipation, CEA elevation
Introduction
Although the incidence of ovarian malignancy during pregnancy is extremely low (approximately 1 per 20,000 deliveries), the possibility of a borderline tumor or cancer must be considered when adnexal masses are detected during a prenatal ultrasound (Cunningham et al., 2005a). Moreover, it is generally much more difficult to diagnose and manage adnexal masses during pregnancy than in non-pregnant women because of the restriction of radiological examinations.
Carcinoid tumors are neuroendocrine tumors arising from the embryologic primitive gut derivation and typically derive from the intestine and the bronchopulmonary system. They represent an unusual and complex disease spectrum with protean clinical manifestations. Primary ovarian carcinoid tumors are of germ cell origin and account for 1% of all carcinoid tumors and less than 0.1% of ovarian malignancies. Of the primary ovarian carcinoid tumors, the insular type is the most commonly observed in Western countries. This type is of midgut derivation and associated with classical or typical carcinoid syndrome caused by serotonin and its precursors and derivatives secreted from the tumors. In contrast, trabecular and strumal carcinoid tumors are primarily reported in Japan. They are of foregut or hindgut origin and are related to severe constipation induced by the production of peptide YY (PYY), an inhibitor of intestinal mobility (Talerman and Vang, 2011; Motoyama et al., 1992; Matsuda et al., 2002). Primary ovarian carcinoids present various findings on preoperative images. Therefore, the preoperative diagnosis of these tumors may be difficult, even in non-pregnant women.
Herein, we report a case of a primary ovarian strumal carcinoid tumor with small mucinous components accompanied by severe constipation and carcinoembryonic antigen (CEA) elevation during pregnancy.
Case
A 24-year-old Japanese woman gradually began to suffer from severe constipation since she had undergone an appendectomy for acute appendicitis at the age of 12. Although she passed a bowel movement once every 2 to 4 weeks despite using laxative agents, she had never had a physical examination for 12 years. Her menarche occurred at the age of 12, and periods were regular at 30-day intervals. The patient initially presented to a primary obstetrician because of amenorrhea and was diagnosed with pregnancy at 8 weeks of gestation. Transvaginal sonography also revealed a hypoechoic solid mass that is 10 cm in diameter in the cul-de-sac. The patient was referred to a general hospital. Magnetic resonance imaging (MRI) showed a solid mass, 10 × 8 cm in size, with low intensity on both T2- and T1-weighted images with small and high intensity portions on T1-weighted images behind the uterus (Fig. 1). Metastatic ovarian tumors were considered in the differential diagnosis because of this characteristic MRI finding and serum CEA elevation (14.9 ng/ml, the cutoff values were < 3.5 ng/ml). Upper gastrointestinal endoscopy for the exclusion of gastric cancer subsequently revealed no abnormalities. The patient was then referred to Kumamoto University Hospital because of a pelvic mass and a high serum CEA level at 10 weeks of gestation.
Fig. 1.
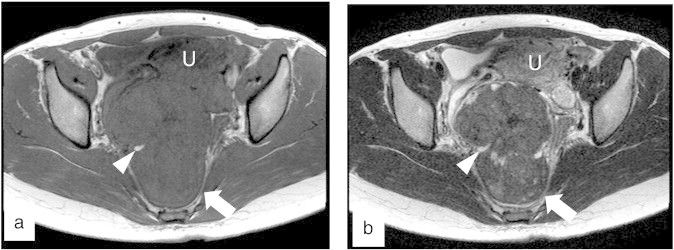
MRI findings of the tumor. (a) An axial T1-weighted image showed the high signal intensity area without fat suppression in the tumor of low signal intensity (arrow head). (b) An axial T2-weighted image showed an area of high signal intensity that was consistent with the high signal intensity area on the T1-weighted image (arrow head). The arrows indicate the pelvic mass. U: uterus.
A pelvic examination revealed an irregular and elastic hard mass, the size of a newborn's head, behind the uterus. Lower intestinal tract endoscopy showed non-specific colitis and dolichocolon. After the patient was prompted to defecate by the administration of multiple laxative drugs over the normal dose range, the CEA level decreased (7.9 ng/ml). We suspected an ovarian tumor with benign or low malignant potential. As the mass would undergo torsion or be an obstruction to delivery, the patient underwent a laparotomy at 13 weeks of gestation.
The tumor arose from the right ovary with a small amount of straw-colored ascites. A cytologic examination revealed no malignant cells in the ascites. A right salpingo-oophorectomy was performed. Macroscopically, the tumor was rigid with a reddish smooth surface. It was 8 × 10 × 12 cm and weighed 350 g. Cut sections revealed a yellowish-white solid mass with a few cystic areas containing mucus (Fig. 2). A pathological examination confirmed the diagnosis of a strumal carcinoid tumor with mucinous cystadenoma (Fig. 3a, b). The neuroendocrine tumor cells showed immunoreactivity with chromogranin A, synaptophysin, CD56 and PYY (Fig. 3c). No immunoreactivity was observed with CEA. Because mitosis and necrosis were not observed and the Mib-1 labeling index was less than 2% by immunohistochemistry, we decided that the patient could continue the pregnancy under careful observation. A postoperative bowel movement was observed within 48 h, and thereafter the patient had a bowel movement every day without the use of laxative drugs following tumor removal. The CEA level had fallen under the limit within 3 weeks (1.6 ng/ml). Her prenatal course was uneventful. The patient delivered a 2960 g female newborn by cesarean section at 38 weeks of gestation because of cephalopelvic disproportion. Since contrast-enhanced whole-body computed tomography (CT) scanning showed no abnormalities 2 months after delivery, she was diagnosed as stage Ia. The patient is in good condition without any evidence of tumor recurrence 11 months after her operation.
Fig. 2.
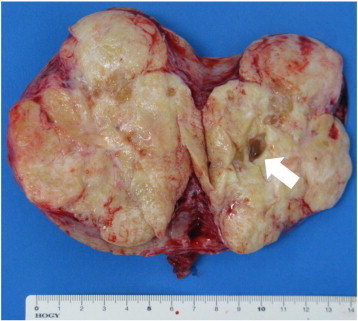
The cut section of the tumor. The cut section revealed a yellowish-white solid mass with a few cystic areas containing mucus (arrow).
Fig. 3.
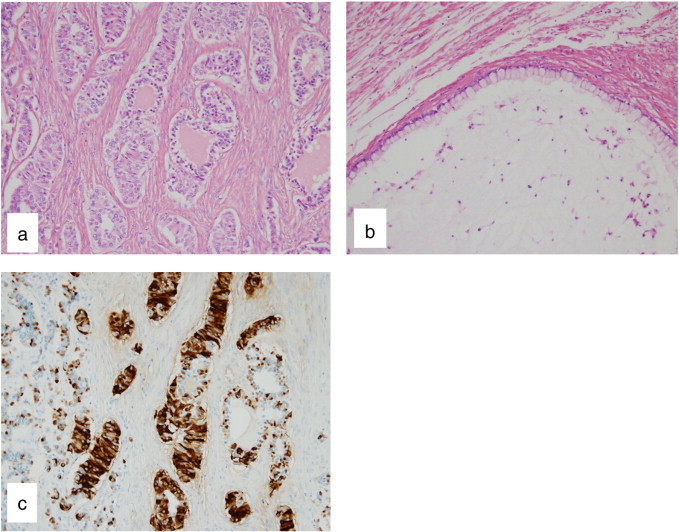
Pathological and immunohistochemical findings of the tumor. (a) The solid parts of the tumor showed that thyroid follicles containing colloid merge with ribbons of neoplastic cells in dense fibrous tissue stroma. Hematoxylin–eosin staining. Original magnification 200 ×. (b) The cystic components in the tumor were composed of single-layered mucinous epithelial cells. Hematoxylin–eosin staining. Original magnification 200 ×. (c) Carcinoid tumor cells showed positive staining for PYY. Methyl green counter-staining. Original magnification 200 ×.
Discussion
Solid tumors of the ovary must be differentially diagnosed from malignant epithelial tumors, germ cell tumors, sex cord stromal tumors and metastatic tumors. The preoperative diagnosis of an ovarian solid mass, particularly during pregnancy, is more difficult because possible examinations are restricted and blood examinations, including tumor markers and hormone tests, are variable during pregnancy (Cunningham et al., 2005b). The treatment course of a pregnant woman should also be determined deliberately by the exclusion of a malignant tumor.
Although preoperative serum levels of CEA, CA125 or CA19-9 can be elevated in a non-pregnant case of PYY-positive ovarian carcinoid tumor, it is difficult to interpret their elevation during pregnancy. Among them, CEA is not influenced by pregnancy (Cheli et al., 1999). In the present case, the patient's serum CEA level was high at the first visit. The level was decreased by approximately half before the tumor removal due to the reduced constipation following the administration of laxative agents. Furthermore, CEA was not detected in the tumor cells by immunostaining. We speculate that the high serum CEA level may have been induced by the existence of nonspecific colitis due to static stool for approximately 1 month because an elevated circulating CEA level can be observed in a patient with inflammatory bowel disease (Loewenstein and Zamcheck, 1978). The elevation of serum tumor markers must be evaluated as not only direct products from a tumor but also a reflector of other clinical manifestations.
Primary ovarian carcinoids histologically present a variety of patterns, including insular, trabecular, strumal and mucinous. A mixed type has also been reported, which is composed of any combination of the pure types. Primary ovarian carcinoids also arise in association with mature cystic teratomas or mucinous tumors; therefore, a preoperative diagnosis is extremely difficult (Talerman and Vang, 2011). As such, most have been diagnosed based on postoperative pathology findings. Small parts of the solid tumor in this case showed high signal intensity on both T1- and T2-weighted images without fat suppression, which generally indicates the existence of mucus. The histopathological findings of the tumor subsequently showed a strumal carcinoid with a mucinous component. This finding may contribute to the preoperative differential diagnosis from other solid tumors of the ovary.
A PYY-positive ovarian carcinoid accompanied by severe constipation was first reported in Japan (Motoyama et al., 1992). Although similar cases were thereafter reported in Japan and other Asian countries, no cases have been reported during pregnancy. PYY is the gastrointestinal (GI) hormone released from the endocrine cells of the GI mucosa in response to oral nutrient ingestion. PYY physiologically inhibits many GI functions, including gastric acid secretion, gastric emptying, small bowel and colonic chloride secretion, mouth to cecum transit time, pancreatic exocrine secretion and pancreatic insulin secretion. PYY also inhibits jejunal and colonic motilities. PYY circulates in two active forms: PYY (1–36) and PYY (3–36). PYY (1–36) increases appetite and stimulates weight gain, and PYY (3–36), the truncated form of PYY, has effect opposite to PYY (1–36) (Ballantyne, 2006). The structure of PYY localized in the tumor could not be analyzed; however, its major function was estimated to inhibit intestinal mobility. In basic research, the chronic administration of PYY to pregnant mice or the transgenic overexpression of PYY to pregnant mice has been shown to induce a neural tube defect in embryos (Yuzuriha et al., 2007). Therefore, the fetus must be carefully observed in cases that occur during pregnancy. In the present case, no adverse effects on her offspring have been observed until the last follow-up. According to previous reports, the incidence of PYY-positive ovarian carcinoid must differ between Western countries and Asian countries including Japan. The patient's race will also be useful information for diagnosis. Almost all primary trabecular and strumal carcinoid tumors occur in women with stage I disease and have an excellent outcome (Lee et al., 2003). Nevertheless, a case of multiple metastases with a higher mitotic rate and focal necrosis has been reported (Kurabayashi et al., 2010). The presence of mitoses or necroses in the tumor will be useful for predicting a poor prognosis. In view of her pathological findings, she will have a good prognosis, and she is scheduled to be followed up by physical examinations, tumor markers, ultrasounds, and CT scans. A redevelopment of severe constipation may be a predictive symptom during a follow-up period because a case of liver metastasis with reappearance of severe constipation of 10 months after surgery was reported (Matsuda et al., 2002).
In conclusion, it is necessary to know the clinical characteristics of primary ovarian strumal carcinoid tumors to ensure their appropriate diagnosis and management.
Conflict of interest statement
The authors of this manuscript declare that there are no conflicts of interest.
Acknowledgment
The authors would like to thank Dr. Ken-ichi Iyama (Department of Surgical Pathology, University Hospital, Kumamoto University) for technical assistance. This study was partially supported by a Grant-in-Aid for Scientific Research (C) ( 24592521) from the Japan Society for the Promotion of Science (K. Motohara).
References
- Cunningham F.G., Leveno K.J., Bloom S.L., Hauth J.C., Gilstrap L.C., III, Wenstrom K.D. Abnormalities of the reproductive tract. In: Rouse D., Rainey B., Spong C., Wendel G.D., editors. Williams Obstetrics. 22nd ed. McGraw-Hill; New York: 2005. pp. 949–970. [Google Scholar]
- Talerman A., Vang R. Germ cell tumors of the ovary. In: Kurman R.J., Ellenson L.H., Ronnett B.M., editors. Blaustein's Pathology of the Female Genital Tract. 6th ed. Springer; New York: 2011. pp. 847–907. [Google Scholar]
- Motoyama T., Katayama Y., Watanabe H., Okazaki E., Shibuya H. Functioning ovarian carcinoids induce severe constipation. Cancer. 1992;70:513–518. doi: 10.1002/1097-0142(19920715)70:2<513::aid-cncr2820700223>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Matsuda K., Maehama T., Kanazawa K. Strumal carcinoid tumor of the ovary: a case exhibiting severe constipation associated with PYY. Gynecol. Oncol. 2002;87:143–145. doi: 10.1006/gyno.2002.6785. [DOI] [PubMed] [Google Scholar]
- Cunningham F.G., Leveno K.J., Bloom S.L., Hauth J.C., Gilstrap L.C., III, Wenstrom K.D. Neoplastic diseases. In: Rouse D., Rainey B., Spong C., Wendel G.D., editors. Williams Obstetrics. 22nd ed. McGraw-Hill; New York: 2005. pp. 1257–1273. [Google Scholar]
- Cheli C.D., Morris D.L., Neaman I.E., Dai J., Allard W.J., Yeung K.K. Measurement of four tumor marker antigens in the sera of pregnant women. J. Clin. Lab. Anal. 1999;13:35–39. doi: 10.1002/(SICI)1098-2825(1999)13:1<35::AID-JCLA7>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein M.S., Zamcheck N. Carcinoembryonic antigen (CEA) levels in benign gastrointestinal disease states. Cancer. 1978;42:1412–1418. doi: 10.1002/1097-0142(197809)42:3+<1412::aid-cncr2820420805>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Ballantyne G.H. Peptide YY(1–36) and peptide YY(3–36): part I. Distribution, release and actions. Obes. Surg. 2006;16:651–658. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- Yuzuriha H., Inui A., Asakawa A., Ueno N., Kasuga M., Meguid M.M., Miyazaki J., Ninomiya M., Herzog H., Fujimiya M. Gastrointestinal hormones (anorexigenic peptide YY and orexigenic ghrelin) influence neural tube development. FASEB J. 2007;21:2108–2112. doi: 10.1096/fj.06-7621com. [DOI] [PubMed] [Google Scholar]
- Lee K.R., Tavassoli F.A., Prat J., Dietel M., Gersell D.J., Karseladze A.I., Hauptmann S., Rutgers J. Tumours of the ovary and peritoneum. In: Tavassoli F.A., Devilee P., editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. IARC Press; Lyon: 2003. pp. 113–202. [Google Scholar]
- Kurabayashi T., Minamikawa T., Nishijima S., Tsuneki I., Tamura M., Yanase T., Hashidate H., Shibuya H., Motoyama T. Primary strumal carcinoid tumor of the ovary with multiple bone and breast metastases. J. Obstet. Gynaecol. Res. 2010;36:567–571. doi: 10.1111/j.1447-0756.2010.01231.x. [DOI] [PubMed] [Google Scholar]


