Highlights
-
•
Usual-type VIN is an HPV-associated premalignant condition that can affect immunocompromised patients and may have multifocal, multicentric involvement.
-
•
Immunocompromised patients who may be poor surgical candidates, and in whom topical modalities may prove ineffective, treatment remains a challenge.
-
•
We present such a patient in whom sinecatechin was successfully used as an alternative to less desired and effective treatment options.
Keywords: Vulvar intraepithelial neoplasia, Carcinoma in situ, Immunocompromised, Sinecatechins
Introduction
Vulvar intraepithelial neoplasia (VIN) is a dysplastic premalignant condition that affects the vulvar squamous epithelium. It is classified into usual and differentiated types. If left untreated, VIN may progress to invasive cancer within 1–7 years (Jones et al., 2005). Although rare, VIN has become an increasingly common problem in younger women < 50 years of age with a reported 392% increase in incidence from 1994–1997 (Joura et al., 2000). Interestingly, it is not rare to diagnose invasive cancer at the time of surgery for VIN, with a reported incidence of up to 5% (ACOG, 2011). A similar trend has been observed in vulvar cancer patients with a striking increase in incidence noted in the 40–49 years age group (Joura et al., 2000). High risk human papillomavirus (HPV) infection, human immunodeficiency virus infection, smoking, and other pelvic neoplasia are some of the documented risk factors for development of VIN (ACOG, 2011). Recognized treatment modalities include partial superficial skinning vulvectomy, wide local excision, CO2 laser ablation, topical ointments (Imiquimod 5%), and 5-aminolevulinic acid-based photodynamic therapy (Hillemanns et al., 2006).
In 2006, Sinecatechins 15% ointment (Veregen®, PharmaDerm®), was the first botanical drug approved by the Food and Drug Administration for the treatment of external anogenital warts (U.S. Food and Drug Administration and Center for Drug Evaluation and Research, 2006). It is a formulation of a variety of catechins, a type of polyphenol with potent antioxidant activity. It has been identified for its immunostimulatory, antitumor, and antiproliferative properties (Stockfleth et al., 2008). Although Veregen® has an established role in complete clearance of anogenital warts; it has not been used for the treatment of VIN. We report a case of a woman with multifocal Usual-type VIN, warty type disease who was treated with Sinecatechins 15% ointment.
Case Report
A 45-year-old female with myelodysplastic syndrome, 10 months after stem cell transplant (CD4 count 143 cell/mcL), presented her primary care physician complaining of pruritic lesions on her vulva. She had no other pertinent risk factors. Physical exam revealed erythematous papules, plaques and ulcerated lesions on the vulva. An office biopsy was obtained which showed high grade VIN and squamous cell carcinoma in situ (CIS). The patient was then referred to the gynecologic oncology service at our institution for further workup and management. She was noted to have extensive multifocal and multicentric disease (Fig. 1). After performing further biopsies in the operating room, all biopsy specimens were then reviewed by our gynecologic pathologists and diagnosed as Usual-type VIN, warty subtype, with HPV changes and CIS. Initially, our recommendations included wide local excision of the lesions. However, due to its multifocal involvement and need for excision of the clitoris with extensive reconstruction, more conservative alternatives were considered. We prescribed topical Imiquimod 5% ointment, twice weekly. Patient was followed every two weeks in clinic and after 8 weeks of treatment, she failed to show any clinical response. In addition, due to extreme pain with application, patient was unable to tolerate the full course, despite use of narcotics and pudendal block.
Fig. 1.
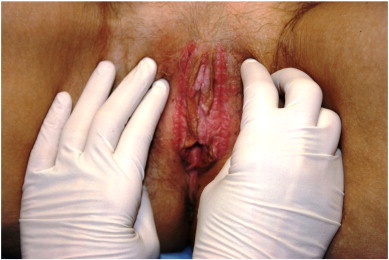
Mutiple lesions noted on the vulva of a 45 years old, immunocompromised patient, diagnosed with VIN3/CIS on biopsy.
Consequently, we prescribed Veregen® ointment, to be used three times a day for six weeks. Four weeks after start of therapy, visible improvement in the vulvar lesions was noted. The patient reported some pain and discomfort at the application sites, but stayed motivated to complete the treatment, as her lesions appeared to be resolving. At the end of 6 weeks, examination revealed complete resolution of all lesions, with mild scarring on the vulva (Fig. 2). Confirmatory biopsies of the scarred areas were taken and determined to be negative for dysplasia or carcinoma. She remains in remission after eleven follow up months and has resumed normal sexual function since then.
Fig. 2.
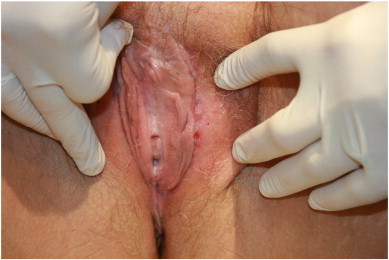
Healthy vulva, with resolution of all lesions after treatment with Sinecatechins ointment.
Discussion
Throughout the last decade, incidence of VIN in United States for women between ages 40–49 years has increased to a current rate of 2.9 per 100,000 women. This increase has been attributed to a number of factors, including changes in sexual behavior, smoking habits, HPV infection, and increased surveillance (Joura et al., 2000). There is a well-established association between high risk HPV genotypes and VIN. HPV 16 and 18 are associated with 70–80% of VIN lesions, and about 30–40% of invasive vulvar cancers (Hillemanns et al., 2006).
VIN1 is typically a self-limiting infection that does not require treatment. Conversely, higher grade lesions may require aggressive surgical management to prevent progression to invasive cancer. In women with multifocal and extensive disease, laser ablation is an alternative to excisional therapy, provided there is no coexistent invasive cancer.
Although surgical treatment provides tissue sampling to evaluate for invasive cancer, medical management achieves preservation of vulvar anatomy and function, particularly in younger women. Invasive disease must be excluded with colposcopy and biopsies prior to initiating medical treatment. It is also recommended to prevent extensive excisional and reconstructive surgery in women that are not good candidates for surgery due to coexisting medical comorbidities or extensive, multifocal vulvar lesions.
Medical options that include Imiquimod 5% applied three times weekly to affected areas for 12–20 weeks is an effective therapy for high grade VIN (Hillemanns et al., 2006). Side effects related to its use include erythema, erosions, and moderate vulvar pain. Imiquimod is a topical immune response modifier that acts by stimulating local cytokine production and cell mediated immunity, and thus has decreased effectiveness in immunocompromised patients. Other available options for conservative treatment include 5-Fluorouracil, topical 5-aminolevulinic acid based photodynamic therapy, and Cidofovir (antiviral agent) (ACOG, 2011).
Our patient had extensive multifocal disease involving her clitoris that would require extensive surgical excision and reconstruction, repeated biopsies showed absence of invasive cancer, and it failed treatment with Imiquimod 5%. It, thus, presented a few therapeutic dilemmas.
The failure of response to Imiquimod in our patient can be attributed to her low T cell count. We prescribed Sinecatechins as an alternative to surgical treatment and followed her closely to monitor side effects, response, and signs of recurrence.
Sinecatechins, or polyphenol E compound, is a defined green tea extract, available with trade name Veregen® (Sinecatechin 15% ointment) and is known to possess immunostimulatory, antiviral, antitumor and antiproliferative properties (Stockfleth et al., 2008). It has a well-established role in complete clearance of anogenital warts when used three times daily for up to 16 weeks. Three randomized, double blinded, placebo controlled trials have been published that prove the efficacy, safety and tolerability of Sinecatechins in treatment of genital and perianal warts (Stockfleth et al., 2008; Gross et al., 2007; Tatti et al., 2008). When compared to other patient applied drugs like Imiquimod 5% and 5-Fluorouracil, Sinecatechins was found superior in terms of clearance rates, cost, less recurrence rates and less patient discomfort (Tatti et al., 2008). Minor local reactions like erythema, burning pain, pruritus were noted in 10–20% of patients. These patients continued treatment due to noticeable improvement with use. The exact mechanism of action of Sinecatechins in wart regression is yet to be identified, but it has been shown to inhibit a broad range of enzymes and kinases involved in the generation of inflammatory mediators. Its wide array of biological activities shows great therapeutic potential in treatment of other HPV related tumors (Tyring, 2012).
To our knowledge, this is the first report of successful treatment of VIN/CIS with Sinecatechins 15%. Prospective studies are warranted to investigate its role in the treatment of VIN or other pelvic dysplastic diseases.
Conflict of interest statement
The authors have no conflict of interest to report.
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal upon request.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Natasha Gupta, Email: docnatasha3@gmail.com.
Ernesto Rodriguez, Email: rodrige1@mskcc.org.
Vaagn Andikyan, Email: andikyav@mskcc.org.
Stacy P. Salob, Email: stacypsg@aol.com.
Dennis Chi, Email: chid@mskcc.org.
References
- ACOG Committee Opinion No. 509 Management of vulvar intraepithelial neoplasia. Obstet. Gynecol. 2011;118(5):1192–1194. doi: 10.1097/AOG.0b013e31823b17c2. [DOI] [PubMed] [Google Scholar]
- Gross G. A randomized, double-blind, four-arm parallel-group, placebo-controlled Phase II/III study to investigate the clinical efficacy of two galenic formulations of Polyphenon E in the treatment of external genital warts. J. Eur. Acad. Dermatol. Venereol. 2007;21(10):1404–1412. doi: 10.1111/j.1468-3083.2007.02441.x. [DOI] [PubMed] [Google Scholar]
- Hillemanns P. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO(2) laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecol. Oncol. 2006;100(2):271–275. doi: 10.1016/j.ygyno.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Jones R.W., Rowan D.M., Stewart A.W. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstet. Gynecol. 2005;106(6):1319–1326. doi: 10.1097/01.AOG.0000187301.76283.7f. [DOI] [PubMed] [Google Scholar]
- Joura E.A. Trends in vulvar neoplasia. Increasing incidence of vulvar intraepithelial neoplasia and squamous cell carcinoma of the vulva in young women. J. Reprod. Med. 2000;45(8):613–615. [PubMed] [Google Scholar]
- Stockfleth E. Topical Polyphenon E in the treatment of external genital and perianal warts: a randomized controlled trial. Br. J. Dermatol. 2008;158(6):1329–1338. doi: 10.1111/j.1365-2133.2008.08520.x. [DOI] [PubMed] [Google Scholar]
- Tatti S. Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts: a randomized controlled trial. Obstet. Gynecol. 2008;111(6):1371–1379. doi: 10.1097/AOG.0b013e3181719b60. [DOI] [PubMed] [Google Scholar]
- Tyring S.K. Effect of Sinecatechins on HPV-Activated Cell Growth and Induction of Apoptosis. J. Clin. Aesthet. Dermatol. 2012;5(2) p. 34-41. [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration, Center for Drug Evaluation and Research . U.S. Food and Drug Administration; Washington (DC): 2006. Approval letter Veregen™ Ointment, 15%, NDA 021902. [Google Scholar]


