Highlights
-
•
Intravenous leiomyosarcomatosis is a rare condition in which malignant myometrial tissue metastasizes to the heart.
-
•
We discuss the complicated diagnosis and treatment of a patient afflicted with intravenous leiomyosarcomatosis.
-
•
To ensure a favorable prognosis, therapy should comprise comprehensive and multi-disciplinary treatment.
Keywords: Intravenous leiomyosarcomatosis, Gynecologic oncology, Management
Introduction
Uterine leiomyosarcoma is an aggressive malignancy associated with an unfavorable clinical outcome (Coard and Fletcher, 2002). Intravenous leiomyosarcomatosis with cardiac extension is an extremely uncommon phenomenon wherein malignant smooth muscle cells of myometrial provenance metastasize to the heart (Ma et al., 2007). Since cardiac intravenous leiomyosarcomatosis was initially reported (Moorjani et al., 2005; Montemezzi, 1966), only scant cases have been documented in the literature (Coard and Fletcher, 2002; Ma et al., 2007; Moorjani et al., 2005).
Typically, intravenous leiomyosarcomatosis, similar to intravenous leiomyomatosis (IVL), is confined to the pelvis; but when intravenous leiomyosarcomatosis is exposed to cardiac venous blood flow, the inferior vena cava, or right-sided atrial, ventricular or pulmonary arterial blood flow, infiltration potentially occurs (Coard and Fletcher, 2002). In cases of intra-cardiac leiomyosarcomatosis, patients can present with a murmur, syncope or spontaneous cardiac death (Ma et al., 2007; McDonald et al., 2007; Ahmed et al., 2004). Unfortunately, since intravenous leiomyosarcomatosis is quite rare and the clinical index of suspicion is extensive, an expeditious diagnosis may be confounded.
Case report
A 43-year old female (gravida 3, para 1) presented to an outside hospital with pelvic pain and vaginal bleeding in December 2012, for which she was admitted to the emergency room; following a physical examination and CT scan of the pelvis, a 20 × 10 × 15 cm pelvic mass was identified. The lesion was compressing the rectum, bladder and left ureter, which caused severe, ipsilateral hydronephrosis. The patient's medical history was significant for a supracervical hysterectomy in 2008 to address uterine fibroids; her most recent Pap smear and pelvic exam, both of which were negative for malignancy, coincided with the aforesaid hysterectomy.
In January 2013, the patient underwent an exploratory laparotomy, biopsy and lysis of adhesions, which revealed a 10 × 8 cm necrotic, vaginal mass with a 5 × 5 cm pedicle at the vaginal apex. Subsequently, the mass and lysis of adhesions were biopsied. Abdominally, she had a 10 × 15 cm solid mass posterior to the bladder, normal-appearing right tube and ovary; there was a large amount of omental adhesions, which were lysed. The patient received 2 units of blood prior to the surgery and received an additional 2 L of blood intra-operatively; ultimately, she tolerated the procedure well.
Pathology of the pelvic mass revealed a hypercellular lesion without significant mitotic activity, atypia or necrosis, consistent with a cellular leiomyoma. The vaginal mass was composed predominantly of non-viable/necrotic tissue and only focal viable atypical tissue was available for evaluation (Fig. 1a); albeit suspicious for malignancy, given the scant nature of the atypical focus (Fig. 1b), a definitive diagnosis of malignancy could not be rendered at that time. Consequently, in lieu of a re-biopsy, the oncology team opted for complete removal of the disease.
Fig. 1.

a–b. Only focal, viable atypical spindle cells were identified, showing nuclear atypia and possible increased mitotic activity (H&E, 200 × magnification) (a). High power view of cellular spindle cell proliferation showing the lack of malignant features consistent with a cellular leiomyoma (H&E, 200 × magnification) (b).
The patient continued to suffer from intractable vaginal bleeding and thus, she underwent an abdominal aortogram, selective bilateral internal iliac arteriography and embolization of the pelvic mass in January 2013. Selective left hypogastric branch vessels demonstrated significant tumor vascularity; they were catheterized and then embolized, which effectuated a substantial reduction of tumor flow. Moreover, super selective injection of one branch demonstrated active arterial extravasation; this was successfully resolved following coil embolization.
A follow-up CT of the chest, abdomen and pelvis revealed a large thrombus in the inferior vena cava (IVC) and left iliac veins, which extended into the right atrium (Fig. 2); there was also evidence of enhancement, indicative of tumor thrombus or benign metastasizing leiomyomatosis. In consideration of the lesion's presence in the right atrium, cardiovascular surgery consultation was emergently recommended and an ensuing transthoracic echocardiogram was performed (Fig. 3).
Fig. 2.
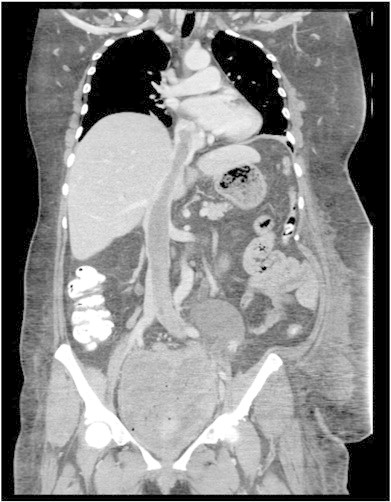
A coronal CT image of tumor thrombus extending from the pelvis through the inferior vena cava into the right atrium.
Fig. 3.
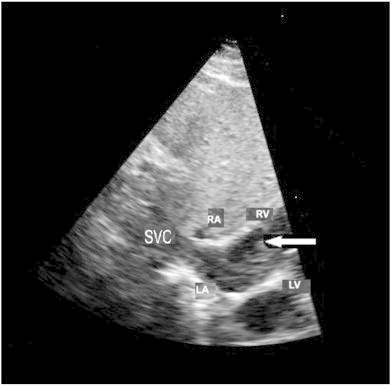
Transthoracic echocardiogram exhibiting a mass (arrow) extending from the superior vena cava (SVC) into the right atrium (RA); the left atrium (LA), right ventricle (RV) and left ventricle (LV) are also visualized.
Interestingly, the patient's medical history was negative for any cardiac or thoracic symptomatology. She also denied any specific knowledge of having a myocardial infarction, dysrhythmias, palpitations, or cardiac murmurs. Nonetheless, the patient reported increased dyspnea in the few months preceding her hospitalization; her sitting blood pressure was 132/92 mm Hg and pulse rate was 133 beats/min.
In February 2013, the patient was admitted to the emergency room due to asthenia, vertigo and a urinary tract infection; she also reported moderate bleeding. The patient was febrile at 102 °F; her hematocrit was 29% and creatinine was 1.2 mg/dL. The patient was administered antibiotic therapy and transfused with 2 units of packed red blood cells to ameliorate her symptoms.
Intra-cardiac leiomyosarcomatosis is an extremely complex and precarious condition and thus, the patient's management necessitated a multidisciplinary, formulated approach; this involved substantive pre-operative discussion among the cardiovascular, vascular, and gynecologic oncology surgeons. Since significant patient blood loss was anticipated, a comprehensive transfusion protocol was thereby instituted. Moreover, the operating room was reserved for the entire day to accommodate this multifaceted surgical procedure.
Initially, the patient underwent cardiovascular surgery, comprising a sternotomy via a combination of central and peripheral cannulation in the right common femoral vein, extending up to the common iliac artery. The heart was arrested; cardioplegia was administered at 30-minute intervals and hypothermic circulatory arrest was performed after the head was packed in ice. Simultaneously, a right atriotomy was performed with removal of the right ventricular and atrial components of the mass; there was no tumor identified in the ventricle and/or coronary sinus.
The tumor was removed en bloc approximately 2 cm into the IVC; however, the mass was friable and had to be removed in discrete sections (Fig. 4), indicative of a malignant process. A Foley catheter was used as a Fogarty arterial embolectomy catheter into the IVC, of which successive passes were negative for evidence of tumor or thrombus. The right atrium was closed with 5-0 Prolene suture in a running technique; the IVC was closed and cardiopulmonary bypass was reinstituted; the aortic crossclamp was removed, allowing for cardiac resuscitation.
The tumor was removed en bloc approximately 2 cm into the IVC; however, the mass was friable and had to be removed in discrete sections (Fig. 4), indicative of a malignant process. A Foley catheter was used as a Fogarty arterial embolectomy catheter into the IVC, of which successive passes were negative for evidence of tumor or thrombus. The right atrium was closed with 5-0 Prolene suture in a running technique; the IVC was closed and cardiopulmonary bypass was reinstituted; the aortic crossclamp was removed, allowing for cardiac resuscitation.
Vascular surgery consultation was then indicated; thereafter, a vena cava thrombectomy, right common iliac tumor thrombectomy and left common and external iliac tumor thrombectomy were planned. Total circulatory arrest was necessary to extract the tumor from the patient's ventricle and IVC. The neoplasm's removal from the iliac system occurred in 2 stages. During total circulatory arrest, the heart and vena cava were opened. The tumor was extracted; similar to the cardiovascular surgery, the mass was incohesive and thus, removed in distinct segments; estimated blood loss was approximately 4 L.
The final operative component encompassed gynecologic oncology surgery. Initially, the retroperitoneum on the right side was entered. Once the vagina was identified, the posterior region was divided, entered and the rectovaginal space was developed. An ovarian cyst was visualized and subsequently removed using blunt dissection; the bladder and ureters were both mobilized. Accordingly, the pelvic mass was completely mobilized.
Once the lesion was completely unencumbered, the neoplasm was extracted from the pelvis. Hemostasis was appreciated although there was large volume blood loss (10 L), necessitating a massive transfusion. The patient received 34 units of packed red blood cells, 30 units of cryoprecipitate, 8 units of fresh frozen plaza, 7 units of platelets and one dose of Recombinant Factor VIIa. When the procedure was concluded, she was taken to the Cardiovascular Intensive Care Unit in stable condition.
Despite the initial pre-operative pathology results (i.e., a cellular leiomyoma), additional evaluation of the patient's gynecologic and vascular specimens revealed a high grade leiomyosarcoma of uterine origin. At the conclusion of surgery, the patient did well; she did not suffer from any significant complications and fulfilled all appropriate recovery criteria prior to discharge, which occurred on postoperative day 18. Consequently, she began six cycles of gemcitabine (1000 mg/m2) and docetaxel (75 mg/m2) chemotherapy.
Discussion
Intravenous leiomyosarcomatosis potentially represents a malignant subtype of IVL (McDonald et al., 2007). This more aggressive condition is extremely uncommon and can portend inauspicious health consequences. In particular, when there is cardiac extension, the patient is at significant risk for a thrombotic and neoplastic induced pulmonary embolic event (McDonald et al., 2007; Ahmed et al., 2004; Burns et al., 1979).
One proposed theory regarding the pathogenesis of intravenous leiomyomatosis suggests that the intima of myometrial sinuses is invaded by leiomyomatosis cells of uterine myometrial provenance (Sizenfrey, 1911). Alternatively, with intravenous leiomyosarcomatosis, the malignancy consists of proliferating smooth muscle cells originating from the venous wall of the uterine or pelvic veins (Norris and Parmley, 1975). In the current study, the disease presumably migrated from the internal iliac veins, proximal to the uterus. Interestingly, our patient was initially treated via supracervical hysterectomy; one could conjecture that the residual cervical tissue contributed to the disease's ultimate efflorescence, since unanticipated malignancies have been reported in conjunction with this surgical procedure (Theben et al., 2013).
Successful therapy relies on advanced preparation comprising multi-disciplinary collaboration (Moorjani et al., 2005). Collectively, the surgical divisions developed a procedure to effectively manage the disease; this involved reserving the operative room for the entire day to accommodate the protracted surgical period and preparing a transfusion protocol in anticipation of the significant blood loss. When managing intravenous leiomyosarcomatosis, we elected to emphasize loco-regional treatment comprising surgery and adjuvant therapy (Coard and Fletcher, 2002; Ma et al., 2007; Moorjani et al., 2005); albeit speculative, anti-estrogens may also confer some clinical benefit (Theben et al., 2013). Furthermore, since this disease is associated with significant morbidity and patients have a propensity for developing recurrent disease, active patient counseling and surveillance are necessary.
The following is the supplementary data related to this article.
Fig. 4.
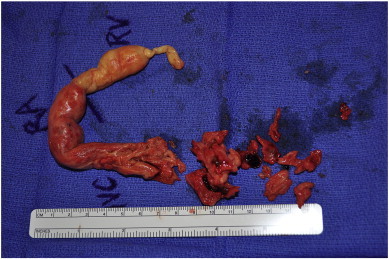
Gross specimen.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.gynor.2013.07.001.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
This study was supported by the Women's Cancer Research Foundation.
References
- Ahmed M., Zangos S., Bechstein W.O., Vogl T.J. Intravenous leiomyomatosis. Eur. Radiol. 2004;14:1316–1317. doi: 10.1007/s00330-003-2186-z. [DOI] [PubMed] [Google Scholar]
- Burns B., Curry R.H., Bell M.E. Morphologic features of prognostic significance in uterine smooth muscle tumors: a review of eighty-four cases. Am. J. Obstet. Gynecol. 1979;135:109–114. [PubMed] [Google Scholar]
- Coard K.C., Fletcher H.M. Leiomyosarcoma of the uterus with a florid intravascular component (“intravenous leiomyosarcomatosis”) Int. J. Gynecol. Pathol. 2002;21:182–185. doi: 10.1097/00004347-200204000-00012. [DOI] [PubMed] [Google Scholar]
- Ma L., Wu S., Zou Y., Li W., Gong L., Gerelle G., Ni Y. One-stage surgery of low-grade malignant intravenous uterine leiomyosarcomatosis with right atrium extension. Ann. Thorac. Surg. 2007;84:644–647. doi: 10.1016/j.athoracsur.2007.03.056. [DOI] [PubMed] [Google Scholar]
- McDonald D.K., Kalva S.P., Fan C.M., Vasilyev A. Leiomyosarcoma of the uterus with intravascular tumor extension and pulmonary tumor embolism. Cardiovasc. Intervent. Radiol. 2007;30:140–142. doi: 10.1007/s00270-006-0118-4. [DOI] [PubMed] [Google Scholar]
- Montemezzi L. Leiomyosarcoma with extensive venous and cardiac invasion of probable uterine origin. Arch De Vecchi Anat. Patol. 1966;48:37–56. [PubMed] [Google Scholar]
- Moorjani N., Kuo J., Ashley S., Hughes G. Intravenous uterine leiomyosarcomatosis with intracardial extension. J. Card. Surg. 2005;20:382–385. doi: 10.1111/j.1540-8191.2005.200476.x. [DOI] [PubMed] [Google Scholar]
- Norris H.J., Parmley T. Mesenchymal tumors of the uterus. V. Intravenous leiomyomatosis. A clinical and pathologic study of 14 cases. Cancer. 1975;36:2164–2178. doi: 10.1002/cncr.2820360935. [DOI] [PubMed] [Google Scholar]
- Sizenfrey A. Uber Venenmyome des Uterus mit Intravaskularem Wachstum. Z. Geburtsh. Gynaekol. 1911;68:1. [Google Scholar]
- Theben J.U., Schellong A.R., Altgassen C., Kelling K., Schneider S., Große-Drieling D. Unexpected malignancies after laparoscopic-assisted supracervical hysterectomies (LASH): an analysis of 1,584 LASH cases. Arch. Gynecol. Obstet. 2013;287:455–462. doi: 10.1007/s00404-012-2559-0. [DOI] [PubMed] [Google Scholar]


