Highlights
-
•
A second case of low-grade myofibroblastic sarcoma of the vulva.
-
•
Six-year follow-up with no evidence of disease after radical local excision.
-
•
Adequate margin resection is important.
Keywords: Low-grade myofibroblastic sarcoma, Sarcoma of the vulva
Introduction
Sarcomas arising in the vulva are rare and are often misdiagnosed clinically as Bartholin gland cysts (Curtin et al., 1995). Low-grade myofibroblastic sarcomas (LGMSs), although rare, typically occur in the head and neck region (Fisher, 2004); they have been reported in the vulva in only 1 case report (Roth et al., 2004).
Local recurrence of LGMS, an indolent tumor, occurs in 47% of patients 17 to 46 months after the initial excision (Meng et al., 2007). In the previously reported case, a 5 × 3 cm tumor was resected by wide local excision, but the follow-up time was only 14 months. Here, we report a second case of LGMS of the vulva, with long-term disease-free survival after radical local excision (RLE).
Case
A 24-year-old nulligravida presented with a nodule on her left labia minora. She had felt discomfort while sitting approximately 1 month earlier and had noticed the tumor; however, at the time of presentation, she did not feel any pain. Physical examination revealed a subcutaneous, movable, non-tender mass of 2 × 3 cm in diameter. The initial clinical diagnosis was a previous Bartholin's gland infection, and oral antibiotics were prescribed. Three months later, the patient felt pain, and oral antibiotics were prescribed a second time. However, the pain did not improve, and a mass of 2 cm in diameter was surgically resected. The surrounding hardened tissues were removed with curettage, but some hard tissue, few millimeters in thickness, remained (Fig. 1A). The obtained specimen revealed that the tumor was composed of slender spindle cells with eosinophilic cytoplasm, sharing both features of fibroblasts and smooth muscle cells (Fig. 2A–B). Immunohistochemistry showed that most spindle cells were immunoreactive for α-smooth muscle actin (α-SMA) and vimentin and had an MIB-1 index of 10% (Fig. 2C–D). In contrast, immunostaining for other markers, such as CD34, S-100, c-kit, HMB-45, anaplastic lymphoma kinase (ALK), or desmin, was negative.
Fig. 1.
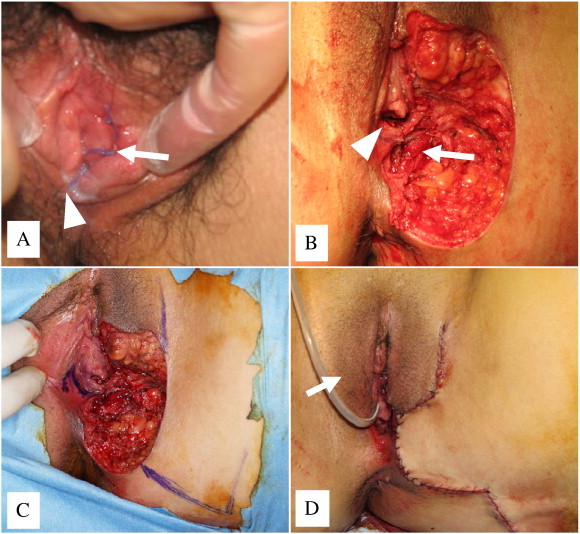
(A) After local excision of the primary surgery. Arrow indicates the left labia minora. Arrowhead indicates the introitus with hymen. (B) Defect after radical local excision. Arrowhead indicates the introitus with hymen. Arrow indicates the left levator ani muscle. (C) A combination of a labia major advancement flap, a rhomboid flap from inner thigh, and a mucosal flap from contralateral side of the vaginal vestibule was designed to cover the defect. (D) Reconstruction of the defect was completed by placement of a subcutaneous drain. Arrow indicates a Foley catheter passed through the urethra and into the bladder.
Fig. 2.
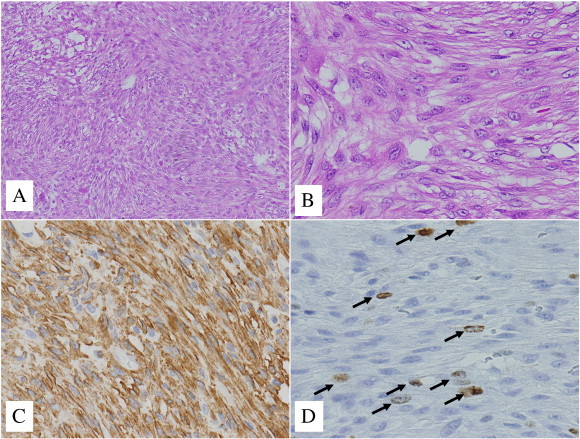
Immunohistochemistry of the tumor specimen. (A) The tumor comprised intersecting fascicles of spindle cells (hematoxylin and eosin [H&E], × 100). (B) Most tumor cells had eosinophilic cytoplasm with mild atypical nuclei (H&E, × 400). (C) The cytoplasm of the tumor cells were diffusely stained with antibody to α-smooth muscle actin (α-SMA) (× 400). (D) Arrows point to Ki-67 immunohistochemical reactivity, seen in 10% of the tumor cells (× 400).
Based on these findings, the patient was diagnosed with LGMS. A computed tomography scan of the abdomen and pelvis revealed no evidence of metastasis. The residual tumor could not be detected by palpation. RLE was performed to remove the surgical scar with a 2-cm margin, and the underlying fascia was resected. During surgery, free margins were confirmed by the examination of multiple frozen sections. The operation was completed using a combination of skin and mucosal flaps (Fig. 1B-D). The obtained specimen included residual tumor cells infiltrating into the surrounding tissue, although tumor-free margins, 2 cm in width and depth, were confirmed. The patient was followed-up without postoperative radiation or adjuvant therapy. She has been without evidence of recurrence for 72 months since RLE.
Discussion
Myofibroblasts, first described in granulation tissue 40 years ago, are mesenchymal spindle cells that share ultrastructural features of both fibroblasts and smooth-muscle cells. Occasionally, myofibroblasts undergo tumorigenic transformation and become malignant (Mentzel et al., 1998). A LGMS is a rare malignant tumor with myofibroblastic differentiation (Fisher, 2004) that is classified as a distinct entity according to the World Health Organization classification of soft tissue tumors. Histopathologically, LGMS is characterized by fusiform tumor cells that are arranged in complicated, sheet-like or storiform pattern and show diffuse, infiltrative growth (Mentzel et al., 1998). Immunohistochemically, tumor cells stain positive for at least one of the myogenic markers (α-SMA, desmin, and muscle-specific actin) (Meng et al., 2007; Mentzel et al., 1998; Montgomery et al., 2001).
The specimen in our case was composed of infiltrative spindle tumor cells with myofibroblastic differentiation and an MIB-1 index of 10%, indicative of low proliferation. The tumor cells were immunopositive for α-SMA and vimentin and negative for S-100, CD34, c-kit, and HMB-45. A diagnosis of inflammatory myofibroblastic tumor was ruled out due to the specimen's high cellularity, morphologically uniform appearance, and less infiltrative lymphocytes or plasma cells. Based on these findings, the specimen was diagnosed as LGMS. A recent study has shown that LGMS tumor cells were also negative for ALK (Qiu et al., 2008).
The clinical course of LGMS is relatively indolent (Meng et al., 2007). LGMS has infiltrative characteristics, and some cases have shown lesions with compression of the surrounding tissues (Montgomery et al., 2001). The recurrence rate in a large series was reported as 40% (24 out of 60 patients), and distant recurrence was rare (Meng et al., 2007; Mentzel et al., 1998; Montgomery et al., 2001; Qiu et al., 2008; Agaimy et al., 2008). Adjuvant chemotherapy or radiation therapy was conducted in some patients, but the therapeutic effect was unclear (Meng et al., 2007; Mentzel et al., 1998; Montgomery et al., 2001). In the previously reported case of LGMS of the vulva, the mass was easily shelled out from the subcutaneous tissue during the first surgery with a wide local excision, and the margins were confirmed negative pathologically (Roth et al., 2004). Aartsen et al. originally proposed the importance of adequate margin resection with local excision for the treatment of sarcoma of the vulva and suggested that the actual extent of the neoplasm was inevitably greater than what was initially apparent (Aartsen and Albus-Lutter, 1994). Although the width of the margins was not specifically defined, 2 cm has been considered sufficient by some authors (Aartsen and Albus-Lutter, 1994; Ulutin et al., 2003). In this case, the mass was slightly adhesive to the surrounding tissue, and en bloc resection was not completed during the primary surgery. Hence, RLE was conducted after diagnosis of LGMS.
Studies have reported that vulvar sarcoma mainly metastasizes via the hematogenous route, and groin dissection has been performed in few cases reported in the literature (Aartsen and Albus-Lutter, 1994; Ulutin et al., 2003). Aartsen EJ et al. performed groin dissection in 18 of 47 patients with vulvar sarcoma, but groin metastases were observed only in 5 patients (Aartsen and Albus-Lutter, 1994). Mentzel et al. reported 3 cases of recurrence among 18 LGMS cases: 2 patients developed local recurrence and 1 patient had multiple metastatic disease (Mentzel et al., 1998). Montgomery reviewed 13 patients, and of 7 patients with recurrence, only 1 showed distant metastasis to the lungs (Montgomery et al., 2001). Qiu et al. reported 3 cases of local recurrence, including 1 case of metastasis to the tongue, among 8 cases (Qiu et al., 2008). Thus, LGMS rarely shows lymphogenous metastasis and the benefit of therapeutic inguinal lymphadenectomy is minimal. Therefore, we did not perform inguinal lymphadenectomy in the current case.
Here, we report a rare case of LGMS of the vulva that was successfully treated with RLE and reconstruction. Because low-grade sarcoma or LGMS of the vulva is extremely rare, the definitive surgical procedure for infiltrative LGMS has not yet been determined. This case provides a useful reference to aid in the decision-making process for similar cases.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Conflict of interest statement
The authors have no financial conflicts of interest to disclose.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Curtin J.P., Saigo P., Slucher B., Venkatraman E.S., Mychalczak B., Hoskins W.J. Soft-tissue sarcoma of the vagina and vulva: a clinicopathologic study. Obstet. Gynecol. 1995;86:269–272. doi: 10.1016/0029-7844(95)00160-s. [DOI] [PubMed] [Google Scholar]
- Fisher C. Low-grade sarcomas with CD34-positive fibroblasts and low-grade myofibroblastic sarcomas. Ultrastruct. Pathol. 2004;28:291–305. doi: 10.1080/019131290882187. [DOI] [PubMed] [Google Scholar]
- Roth T.M., Fratkin J., Woodring T.C., McGehee R.P. Low-grade myofibroblastic sarcoma of the vulva. Gynecol. Oncol. 2004;92:361–364. doi: 10.1016/j.ygyno.2003.09.035. [DOI] [PubMed] [Google Scholar]
- Meng G.Z., Zhang H.Y., Bu H., Zhang X.L., Pang Z.G., Ke Q. Myofibroblastic sarcomas: a clinicopathological study of 20 cases. Chin. Med. J. (Engl.) 2007;120:363–369. [PubMed] [Google Scholar]
- Mentzel T., Dry S., Katenkamp D., Fletcher C.D. Low-grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. Am. J. Surg. Pathol. 1998;22:1228–1238. doi: 10.1097/00000478-199810000-00008. [DOI] [PubMed] [Google Scholar]
- Montgomery E., Goldblum J.R., Fisher C. Myofibrosarcoma: a clinicopathologic study. Am. J. Surg. Pathol. 2001;25:219–228. doi: 10.1097/00000478-200102000-00010. [DOI] [PubMed] [Google Scholar]
- Qiu X., Montgomery E., Sun B. Inflammatory myofibroblastic tumor and low-grade myofibroblastic sarcoma: a comparative study of clinicopathologic features and further observations on the immunohistochemical profile of myofibroblasts. Hum. Pathol. 2008;39:846–856. doi: 10.1016/j.humpath.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Agaimy A., Wünsch P.H., Schroeder J., Gaumann A., Dietmaier W., Hartmann A. Low-grade abdominopelvic sarcoma with myofibroblastic features (low-grade myofibroblastic sarcoma): clinicopathological, immunohistochemical, molecular genetic and ultrastructural study of two cases with literature review. J. Clin. Pathol. 2008;6:301–306. doi: 10.1136/jcp.2007.048561. [DOI] [PubMed] [Google Scholar]
- Aartsen E.J., Albus-Lutter C.E. Vulvar sarcoma: clinical implications. Eur. J. Obstet. Gynecol. Reprod. Biol. 1994;56:181–189. doi: 10.1016/0028-2243(94)90168-6. [DOI] [PubMed] [Google Scholar]
- Ulutin H.C., Zellars R.C., Frassica D. Soft tissue sarcoma of the vulva: a clinical study. Int. J. Gynecol. Cancer. 2003;13:528–531. doi: 10.1046/j.1525-1438.2003.13305.x. [DOI] [PubMed] [Google Scholar]


