Highlights
-
•
Primary malignant melanoma of cervix is an extremely rare neoplasm and regardless stage and treatment, prognosis is extremely poor.
-
•
The only available primary treatment that improves prognosis is radical surgical excision of the tumor with wide clear margins.
Keywords: Primary malignant melanoma, Cervix, Mucosal melanoma, Cancer
Introduction
Malignant melanomas involving mucosal membranes, mucosal melanomas, account for less than 0.03% of all newly diagnosed cancers and only 2% of those are discovered in female genital tract (Patrick et al., 2007). Primary malignant melanoma of the uterine cervix is a very rare entity with only 81 cases reported in the literature since 1889 and their prognosis is poor, regardless of the stage at the time of diagnosis (Pusceddu et al., 2011).
We present the case of a FIGO stage IIa1 primary cervical melanoma which has been successfully treated surgically and has an ongoing survival and disease free period of 40 months. This is an exceptionally long survival given the locally bulky disease and could be explained by the absence of occult distant metastasis and good local control offered by the radical surgical excision.
Case report
A 63-year-old patient, para-3 presented with a four-month increased vaginal foul-smelly discharge and occasional slight bleeding. She was generally fit and well, on medication for hypothyroidism, depression and atopic dermatitis. Her maternal grandmother died from breast cancer. She had normal smear tests and normal mammogram. She had been on Hormone Replacement Therapy (HRT) for 5 years from the age of 50 to 55 for menopausal symptoms. Her Body Mass Index (BMI) was 28.
Speculum examination revealed a 4 cm polypoid, fleshy, pale tumor based on the cervix with early involvement of the anterior vaginal fornix. A biopsy was taken and the histopathology showed a poorly differentiated malignant tumor (Fig. 1). Immunohistochemistry showed strong positivity for S100 (Fig. 2), vimentin, melanin-A, MITF and patchy positivity for HM45 (Fig. 3). Epithelial and neuroendocrine markers were negative, establishing the diagnosis of a malignant melanoma.
Fig. 1.
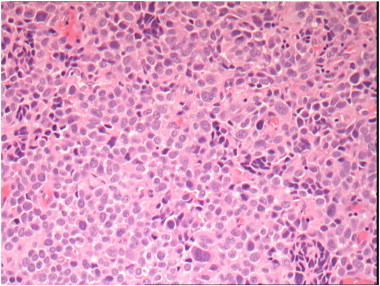
Immunohistochemistry (IHC) images H&E at × 200 magnification.
Fig. 2.
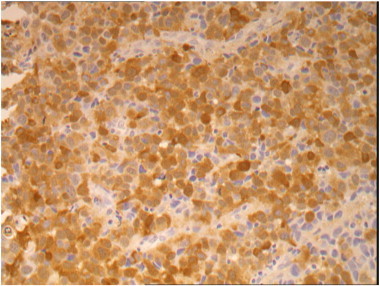
Immunohistochemistry (IHC) images for S100 at × 200 magnification.
Fig. 3.
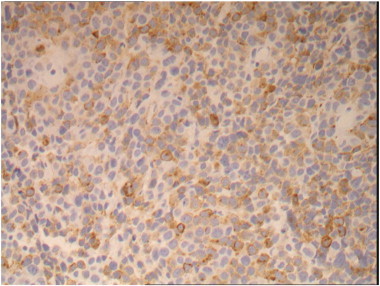
Immunohistochemistry (IHC) images for HMB45 at × 200 magnification.
Patient underwent an extensive search for melanotic lesion of skin or other sites, which was negative. There was no evidence of extra-cervical disease on a CT scan of chest, abdomen and pelvis. On MRI imaging, a lesion occupying the cervix and extending both anteriorly (31 × 11 × 12 mm) and posteriorly (13 × 16 × 14 mm) of the external os was identified. There was possible involvement of vaginal fornices but no evidence of parametrial involvement (Supplementary Fig. 4). Additionally a 12 mm equivocal right external iliac lymph node was noted.
Supplementary Fig. 4.
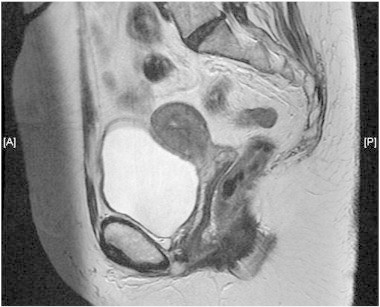
Pre-operative MRI showing the tumor, affecting both anterior and posterior cervical lips, with possible early involvement of the vaginal fornices. Clear fat plains where observed between the tumor and bladder anteriorly and rectosigmoid posteriorly.
Patient underwent an extensive search for melanotic lesion of skin or other sites, which was negative. There was no evidence of extra-cervical disease on a CT scan of chest, abdomen and pelvis. On MRI imaging, a lesion occupying the cervix and extending both anteriorly (31 × 11 × 12 mm) and posteriorly (13 × 16 × 14 mm) of the external os was identified. There was possible involvement of vaginal fornices but no evidence of parametrial involvement (Supplementary Fig. 4). Additionally a 12 mm equivocal right external iliac lymph node was noted.
Patient underwent a Type III, Radical Abdominal Hysterectomy with bilateral salpingo-oophorectomy, bilateral pelvic lymph node dissection and removal of the upper vagina with a cuff of 45 × 35 mm (Supplementary Figs. 5 and 6). Final histopathological report verified the presence of a cervical malignant melanoma measuring in total 33 × 22 × 35 mm, obscuring the external os and extending into the endocervical canal. The tumor composed of solid sheets of malignant epithelioid cells, in keeping with the biopsy. There was non-brisk lymphocytic response and the mitotic count was up to 16 per 10 high-powered fields. There were no ulcerations, features of regression, microsatellite lesions, lymphovascular or perineural space invasion. The tumor was confined within the cervix and all pelvic lymph nodes removed were free of neoplasia. Histopathologically, this was a FIGO stage IIa1 and Clark's Level IV. Following surgery, no adjuvant therapy was commenced as the tumor had been resected with a 12.1 mm clear peripheral microscopic cuff margin and 7.1 mm free parametrial soft tissue margin. Patient has been on a regular 3 monthly clinical follow-up with pelvic examination and skin inspection. She is disease-free and active in her daily life, 40 months following her diagnosis. There were no additional symptoms reported that needed investigating.
Supplementary Fig. 5.
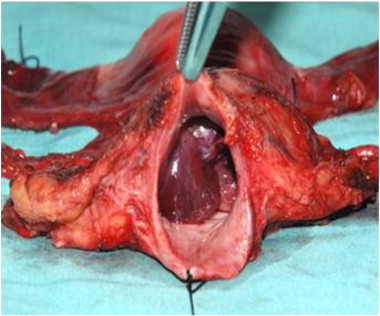
Postoperative radical hysterectomy specimen showing the cervical tumor through adequate vaginal cuff.
Supplementary Fig. 6.
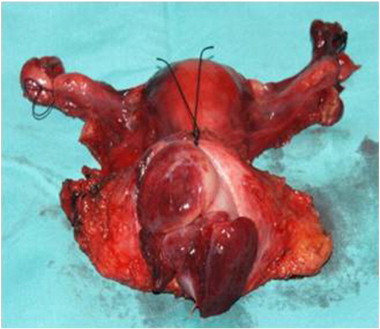
Postoperative radical hysterectomy specimen showing the cervical tumor through adequate vaginal cuff (vagina wall opened).
Patient underwent a Type III, Radical Abdominal Hysterectomy with bilateral salpingo-oophorectomy, bilateral pelvic lymph node dissection and removal of the upper vagina with a cuff of 45 × 35 mm (Supplementary Figs. 5 and 6). Final histopathological report verified the presence of a cervical malignant melanoma measuring in total 33 × 22 × 35 mm, obscuring the external os and extending into the endocervical canal. The tumor composed of solid sheets of malignant epithelioid cells, in keeping with the biopsy. There was non-brisk lymphocytic response and the mitotic count was up to 16 per 10 high-powered fields. There were no ulcerations, features of regression, microsatellite lesions, lymphovascular or perineural space invasion. The tumor was confined within the cervix and all pelvic lymph nodes removed were free of neoplasia. Histopathologically, this was a FIGO stage IIa1 and Clark's Level IV. Following surgery, no adjuvant therapy was commenced as the tumor had been resected with a 12.1 mm clear peripheral microscopic cuff margin and 7.1 mm free parametrial soft tissue margin. Patient has been on a regular 3 monthly clinical follow-up with pelvic examination and skin inspection. She is disease-free and active in her daily life, 40 months following her diagnosis. There were no additional symptoms reported that needed investigating.
Discussion
Mucosal Melanomas (MM) occur more frequent in older ages and postmenopausal women. Almost 73% of cases involved women aged over 60 years old. The incidence of genital tract mucosal melanomas has been estimated at 1.6 cases per 1 million females with only less than 2% accoutering for cervix MM (McLaughin et al., 2005).
The etiology of cervical MM is unknown. It was only in 1959 when basal melanocytes were identified in cervical biopsies by Cid S.J. Melanocyte migration from neural crest or melanocytic differentiation from the endocervical epithelium are two theories for the presence of those melanocytes on the cervix (Zamiati et al., 2001). Risk factors such as HPV 16, radiotherapy or estrogen hormonal influence has been proposed as per case reports in the literature. Furthermore, hypothetic mechanisms have been suggested, but none can be identified as specific, hence primary cervical MM is a very rare entity and conclusions can not be safely made (Pusceddu et al., 2011).
MM is more likely to present as vaginal discharge, dyspareunia and/or bleeding either post coital or more frequently post menopausal. Macroscopically, it appears as a polypoid or exophytic mass of the cervix. Tumor size may vary and pigmentation can be generally dark, black, blue or reddish and in occasions of amelanotic tumors, pale or white (Deshpande and Munshi, 2001). Diagnosis is made by immunostaining as MMs are frequently positive for S100, vimentin, melanin A, MART1 and HMB45. Protein S100 is considered more sensitive, and protein HMB45 a rather more specific stain to set the diagnosis of MM especially when the two markers are combined (Deshpande and Munshi, 2001). The criteria for diagnosis of a primary cervical MM include: presence of melanin in the normal cervical epithelium, absence of melanoma in other body areas, evidence of junctional changes in the cervix, and metastases according to the pattern of cervical cancer (Pusceddu et al., 2011).
Diagnosis is made by obtaining biopsy of those suspicious, polypoid cervical tumors at presentation with the above-mentioned symptoms, or incidentally after speculum assessment in routine gynecological examination. Early diagnosis in Papanicolaou smear test, colposcopy, or liquid-based cytology has also been reported (Deshpande and Munshi, 2001; Jin et al., 2007). This emphasizes the need of specialist cytopathologist reviewing cervical smears and biopsies, to keep in mind this infrequent pathology and apply the appropriate staining in cases that melanin pigmentated cells, high grade squamous intraepithelial lesions or poorly differentiated cells are encountered (Jin et al., 2007).
Although staging of primary cutaneous melanomas is based on the thickness of the primary lesion, primary cervical MM is staged accordingly to the FIGO system for cervical cancer; as this correlates better with survival (Pusceddu et al., 2011).
Treatment of choice for MM is surgical with the excision of the tumor in wide clear margins (Pusceddu et al., 2011; Piura, 2008). This approach stands also for other MM like anorectal, which is equally rare and better studied in the literature (Nilsson and Ragnarsson-Olding, 2010). Although there is no consensus for a standardized surgical approach in cervical MM, radical hysterectomy sometimes coupled with regional lymphadenectomy and/or vaginectomy are generally performed (Mousavi et al., 2006).
MM is a known radio-resistant tumor. Therefore, radiotherapy has been reserved for: a) palliative treatment of recurrences, b) in advance disease, c) unsatisfactory surgical margins, d) parametrial invasion, e) residual tumors, or f) pelvic lymph node involvement. Treatment outcomes are variable (Mousavi et al., 2006). At present, the same chemotherapy protocols used for skin melanoma are applied for cases of advanced or recurrent cervical MM (Piura, 2008). Although objective response to treatment was noticed, survival was not significantly improved. Immunotherapy with high dose Interleukin-2 has shown promising results in a small percentage of cases with metastatic disease (Bhatia et al., 2009).
Cervical MM is highly aggressive as both local recurrence and wide spread metastases usually occur within a short span of a few months to two years from initial diagnosis (Zamiati et al., 2001). Relapse is more likely to be local (vagina, vulva or alongside suture line) rather than distant, stressing the value of clinical examination as part of routine follow-up. Regardless of stage and treatment, prognosis of cervical MM is consequently extremely poor, as 87.5% of patients, reported in literature, died within 36 months of diagnosis (22.9 months overall mean survival). This is despite the fact that almost 41% of cases have been diagnosed at FIGO stage I disease. Globally, the 5-year survival is 18.8% for stage I, 11.1% for stage II and 0% for stages III–IV (Pusceddu et al., 2011). There is no consistency into the Cervical MM treatment approach, probably due to its rarity, so safe conclusions can't be made. Studies on other MM, like anorectal where larger numbers are available, have showed that resection status and tumor stage are independent prognostic variables and provided clear resection margins; there is a significantly better overall 5-year survival. (Nilsson and Ragnarsson-Olding, 2010) Due to MM radio resistance, Gynae Oncologists should consider and discuss with patient extend of surgical resection.
Conclusion
Primary Cervical MM prognosis is poor and literature review suggests that the only available primary treatment is surgical excision of the tumor in wide clear margins. Our case reinforces this statement. Although the size of the primary tumor, clear resection margins, negative lymph node status and absence of lymphovascular space invasion, led to a better disease free survival. We suggest that these rare tumors should be discussed by a specialist multidisciplinary team and surgical excision with curative intent should be the primary approach. Adjuvant chemotherapy and radiotherapy, with or without surgical excision to control local symptoms, are used as palliative treatment in advance stages and disease recurrences with variable outcomes.
It is very crucial to diagnose MM of the cervix and treat at an early stage. Routine pelvic examination and cervical inspection should be a part of clinical assessment especially for patients presenting with vaginal bleeding or discharge.
The following are the supplementary data related to this article.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.gynor.2013.04.004.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgment
The authors are grateful to Dr Michael Scott and Dr Paula Hyder (Consultant Histopathologists — University Hospitals of South Manchester, Manchester, UK) who provided histology images and description.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Bhatia S., Tykodi S.S., Thompson J.A. Treatment of metastatic melanoma: an overview. Oncology. 2009;23(6):488–496. (May) [PMC free article] [PubMed] [Google Scholar]
- Deshpande A.H., Munshi M.M. Primary malignant melanoma of the uterine cervix: report of a case diagnosed by cervical scrape cytology and review of the literature. Diagn. Cytopathol. 2001;25:108–111. doi: 10.1002/dc.2014. [DOI] [PubMed] [Google Scholar]
- Jin B., Goldsmith A., Budev H., Al-Abbadi M. Primary melanoma of the uterine cervix after supracervical hysterectomy. A case report. Acta Cytol. 2007;51(1):86–88. doi: 10.1159/000325690. (Jan–Feb) [DOI] [PubMed] [Google Scholar]
- McLaughin C.C., Wu X.C., Jemal A., Martin H.J., Roche L.M., Chen V.W. Incidence of noncutaneous melanomas in the U.S. Cancer. 2005;103:1000–1007. doi: 10.1002/cncr.20866. [DOI] [PubMed] [Google Scholar]
- Mousavi A.S., Fakor F., Nazari Z., Ghaemmaghami F., Hashemi F.A., Jamali M. Primary malignant melanoma of the uterine cervix: case report and review of the literature. J. Low Genet. Tract Dis. 2006;10(4):258–263. doi: 10.1097/01.lgt.0000229564.11741.4e. (Oct) [DOI] [PubMed] [Google Scholar]
- Nilsson P.J., Ragnarsson-Olding B.K. Importance of clear resection margins in anorectal malignant melanoma. Br. J. Surg. 2010;97(1):98–103. doi: 10.1002/bjs.6784. (Jan) [DOI] [PubMed] [Google Scholar]
- Patrick R.J., Fenske N.A., Messina J.L. Primary mucosal melanoma. J. Am. Acad. Dermatol. 2007;56(5):828–834. doi: 10.1016/j.jaad.2006.06.017. (May) [DOI] [PubMed] [Google Scholar]
- Piura B. Management of primary melanoma of the female urogenital tract. Lancet Oncol. 2008;9(10):973–981. doi: 10.1016/S1470-2045(08)70254-7. (Oct) [DOI] [PubMed] [Google Scholar]
- Pusceddu S., Bajetta E., Carcangiu M.L., Formisano B., Ducceschi M., Buzzoni R. A literature overview of primary cervical malignant melanoma: an exceedingly rare cancer. Crit. Rev. Oncol. Hematol. 2011;81(2):103–206. doi: 10.1016/j.critrevonc.2011.03.008. (Apr 22) [DOI] [PubMed] [Google Scholar]
- Zamiati S., Sahraoui S., Jabri L., Louahlia S., Sqalli S., Kahlain A. Primary malignant melanoma of the cervix uteri: apropos of 1 case with review of the literature. Gynecol. Obstet. Fertil. 2001;29(5):381–385. doi: 10.1016/s1297-9589(01)00148-5. (May) [DOI] [PubMed] [Google Scholar]


