Abstract
The aim of this study was to determine the effect of HNSCC tumour treatment on systemic Th1 and Th2 cytokine levels and investigate correlations with clinicopathological parameters. IL2, IL4, IL5, IL6, IL8, IL10, IL13, GMCSF, IFNγ and TNFα were measured in the serum of 101 newly-presenting HNSCC patients (9 oral cavity, 27 oropharynx, 57 laryngopharynx, 1 sinonasal, 1 parotid and 6 unknown), prior to and following treatment, using a Quantibody® array based multiplex sandwich ELISA (Raybiotech). Data were analysed with respect to T stage, nodal status, age and sex of the patient as well as time between collection of pre- and post-treatment serum. A significant decrease in the levels of the Th2 cytokines IL4, IL5, IL6 and IL10 and the Th1 cytokines IL2 and IL8 was observed between the pre- and post-treatment serum samples. IL13 and TNFα were significantly higher in early stage (T1/T2) tumours compared with late stage (T3/T4) and this trend was maintained for nodal involvement. IL4 was higher in node positive patients compared with node negative, whereas the converse was true for IL2; IL4 was also higher in younger patients compared with the older age group. These results suggest that removal of HNSCC tumours from patients results in reduced circulating Th2 cytokines without a concurrent increase in Th1 cytokines, indicative of a partial rebalance of the Th1/Th2 system following treatment. Furthermore the cytokine profile may be influenced by the size and nodal involvement of the tumour.
Keywords: Head and neck cancer, Th1/Th2, Treatment, Multiplex sandwich ELISA
1. Introduction
Head and neck cancer is the sixth most common solid tumour in the western world accounting for approximately 5% of all cancer incidences globally. Head and neck encompasses a number of distinct subsites and thus should not be considered a single disease entity. About 90% of head and neck tumours arise from cells of the squamous epithelium lining the oral cavity, larynx, nasopharynx, oropharynx and hypopharynx, forming the sub-group of head and neck squamous cell carcinomas (HNSCC).
The incidence of head and neck cancers is more frequent in males compared with females and although recent reports indicate a better prognosis for patients with human papillomavirus (HPV) positive oropharyngeal tumour [1], those patients with HPV negative tumours still have a 5 year survival rate of less than 50%, despite advances in surgical, chemo- and radiotherapeutic treatment strategies.
Immune suppression in cancer patients is well recognised, particularly in patients with HNSCC [2,3], where suppression can include changes in the levels of immunoregulatory cytokines [4,5] and the balance of key immune cells including natural killer cells, dendritic cells, cytotoxic T cells, T-regulatory cells and T helper cells [6–8].
T helper cells play a key role in controlling the immune response and can be subdivided into T-helper 1 (Th1) and T-helper 2 (Th2)-like cells, defined by the cytokine repertoire they produce and subsequent responses. Th1-like cells are principally involved in promoting cell-mediated immunity, and generally are considered as the host's main anti-cancer mechanism, whereas Th2-like cells stimulate an antibody-mediated response, principally targeting extracellular pathogens [9]. The Th1 and Th2 responses are normally reciprocally balanced but a shift towards a Th2-like response has been observed in cancer patients, including those with HNSCC, by measuring serum cytokines [10–12].
Previous reports have investigated cytokines in serum from HNSCC patients with a view to identifying biomarkers or prognostic indicators [13,14]. The current study has employed a multiplex cytokine array to establish the levels of ten different cytokines, involved in the control of the Th1/Th2 response, simultaneously in a large cohort (n=101) of newly-presenting HNSCC patients both pre- and post-treatment. This has established the effect of removing the tumour, a possible cytokine source, on the systemic levels of these cytokines. The large cohort of patients has also enabled sub-site specific differences to be determined. The results provide a better understanding of the regulatory pathways involved in tumour immune evasion, which is essential for the development of future anti-tumour therapies.
2. Materials and methods
2.1. Patients
Subsequent to ethical approval (South Humber local research ethics committee; LREC-05/Q1105/55) patients with newly-presenting HNSCC were recruited (n=101). Exclusion criteria included previous diagnosis and treatment for any other form of cancer, active autoimmune or co-existing infectious disease and previous radio- or chemotherapy. Tumour samples included 9 oral cavities (anterior tongue, floor of mouth, palate, lip), 27 oropharynx (tongue base, tonsil), 57 laryngopharynx (larynx, hypopharynx), 1 sinonasal, 1 parotid and 6 unknown sub-sites (Table 1).
Table 1.
Clinicopathological features of HNSCC patients.
| Tumour site | n | Age |
Sex |
T stage |
Node status |
|||
|---|---|---|---|---|---|---|---|---|
| Median (range) | F | M | T1/T2 | T3/T4 | N0 | N+ | ||
| Laryngopharynx | 57 | 65 (41–92) | 5 | 52 | 25 | 32 | 34 | 23 |
| Oropharynx | 27 | 57 (30–67) | 4 | 23 | 16 | 10a | 5 | 22 |
| Oral cavity | 9 | 59 (53–69) | 3 | 6 | 6 | 3 | 3 | 6 |
| Naso/sinonasal | 1 | 57 | 0 | 1 | 0 | 1 | 1 | 0 |
| Parotid | 1 | 85 | 0 | 1 | 1 | 0 | 1 | 0 |
| Unknown | 6 | b | 1b | 3 | c | b | 4 | |
| Total | 101 | – | 13 | 86 | 48 | 46 | 44 | 55 |
One oropharynx was of unknown T stage.
Two unknown primary tumours were of unknown age, sex and nodal status.
All of the unknown primary tumours were of unknown T stage.
2.2. Serum separation
Following written informed consent venous blood was collected into two 7 ml serum separator vacutainers (SSTTM II, BD Biosciences, Oxford, UK), both prior to and after allocated treatment (between 0.5 and 16 months post-surgery, radio- and/or chemotherapy). The blood was clotted for 30 min at 4 °C before centrifugation (400g for 10 min) and the resulting upper layer of serum was aliquoted and stored at −80 °C prior to cytokine determination.
2.3. Serum cytokine determination
Serum from 101 paired pre- and post-treatment samples stored at −80 °C were thawed and used in the Quantibody® Human Th1/Th2 Array 1 (Raybiotech Inc, Tebu-bio, Cambs, UK) as directed by the manufacturer. Briefly, the kit consisted of a glass slide with 16 wells, spotted in quadruplicate with capture antibodies directed against 10 human cytokines (IL2, IL4, IL5, IL6, IL8, IL10, IL13, GMCSF, IFNγ and TNFα). Following air drying, each well was incubated with serial dilutions of the provided cytokine standard (IL2, IL4, IL5, IFNγ, TNFα, 2–1600 pg/ml; IL6, IL8, IL10, IL13, GMCSF, 1–800 pg/ml) or sample, overnight at 4 °C. Cytokines were evaluated in pre- and post-treatment serum samples on the same array, on the same day, to minimise intra-sample variation. Following stringent washes with supplied buffers, detection antibody was added to each well for 1 h at room temperature and Cy3-equivalent dye-conjugated streptavidin was added for another hour at room temperature to detect bound cytokine. Excess fluorophore was washed off and the slide dried by centrifugation (150g for 3 min).
The signal intensity for each spot was determined using an Axon GenePix laser scanner equipped with Cy3 wavelength detection (555 nm excitation, 565 nm emission) and cytokine concentrations were determined using Q analyser software v8.10.4. The software automatically removed outliers and generated standard curves; both linear and log regression curves were generated and the curve, which gave the best regression line (r2 closest to 1), was used to determine sample concentrations.
2.4. Statistical analysis
The results were analysed using SPSS version 18 (SPSS Inc, Chicago, USA). Cytokine levels and detection were analysed as continuous and dichotomous variables, respectively. Missing data were excluded from each analysis and non-parametric tests were used as appropriate where the data were not normally distributed.
Differences between pre- and post-treatment serum cytokine levels or detectability were determined using the Wilcoxon's signed rank sum test for related samples and the Fisher's exact test, respectively. Associations between cytokine levels or detectability and tumour sub-site or time between pre- and post-treatment sample collection were determined using the Kruskal–Wallis one way ANOVA for unrelated samples and the Fisher's exact test, respectively. Relationships between cytokine levels or detectability with T stage (early T1/T2; late T3/T4), nodal status (N0, N+), sex or age were determined using the Mann Whitney U test for unrelated samples and the Fisher's exact test, respectively. The laryngopharynx group was the only one with a sufficient number to investigate separately for relationships with clinicopathological parameters.
3. Results
3.1. Patient demographics
Newly-presenting patients with HNSCC (n=101) were recruited in the study between August 2008 and November 2010. The most abundant subtypes of HNSCC tumour were the laryngopharynx group (n=57) and the oropharynx tumours (n=27; Table 1). For all tumour sub-sites there was a greater incidence in males. There was a relatively even distribution of both early (T1/T2) and late (T3/T4) stage tumours at all sub-sites, however, there was significantly less nodal involvement in the laryngopharynx group compared with other tumour sites (p=0.001). The median age of the whole group of HNSCC patients was 62 (range 30–92), with the laryngopharynx group containing significantly more patients over the age of 60 (p=0.004), data not shown.
3.2. Effect of treatment on cytokine expression
Of the ten cytokines investigated IL2, IL5, IL8, IL13 and TNFα were detected in less than 50% of the pre- and post-treatment serum samples; IL4 was also detected in less than 50% of the post-treatment samples. There were no significant differences in the number of samples having detectable levels for any of the cytokines between the pre and post-treatment samples. In contrast although the median levels of IL2, IL4, IL5, IL6, IL8 and IL10 were zero, the Wilcoxon signed rank test showed significantly higher cytokine levels in pre-treatment as compared with post-treatment samples (Table 2).
Table 2.
Median concentrations of cytokines in serum from HNSCC patients pre- and post-treatment.
| Cytokine |
Pre-treatment |
Post-treatment |
Pvalue | ||
|---|---|---|---|---|---|
| nDetectable/ntotal | Median (IQR) (pg/ml) | nDetectable/ntotal | Median (IQR) (pg/ml) | ||
| IL2 | 44/101 | 0 (0–7.1) | 37/101 | 0 (0–4.2) | 0.02a |
| IL4 | 52/101 | 1.8 (0–7.4) | 41/101 | 0 (0–6.3) | 0.04a |
| IL5 | 48/101 | 0 (0–11.1) | 42/101 | 0 (0–6.3) | 0.03a |
| IL6 | 72/101 | 2.1 (0–6.6) | 65/101 | 1.9 (0–3.5) | 0.01a |
| IL8 | 37/101 | 0 (0–0.7) | 31/101 | 0 (0–0.3) | 0.02a |
| IL10 | 95/101 | 15.6 (8.4–40.9) | 92/101 | 15 (5.9–30.9) | 0.001a |
| IL13 | 46/101 | 0 (0–3.4) | 45/101 | 0 (0–2.6) | 0.26 |
| GM-CSF | 64/101 | 1.7 (0–7.5) | 61/101 | 1.5 (0–4.7) | 0.07 |
| IFNγ | 82/96b | 33.5 (10.1–77.9) | 80/96b | 28.8 (8.6–123.7) | 0.59 |
| TNFα | 43/91b | 0 (0–8.0) | 44/91b | 0 (0–6.0) | 0.90 |
IQR=Interquartile range.
Significant P value from Wilcoxon signed rank sum test for related samples.
Serum samples where levels of IFNγ (n=5), and TNFα (n=10) were unable to be determined due to the quality of the standard curve.
3.3. Relationship of cytokine expression with patient clinicopathology and demographics
Following grouping of tumour samples into 3 sub-sites: oral cavity, oropharynx and laryngopharynx, excluding the sinonasal, parotid and the 6 samples with unknown sub-site from analysis, no significant differences were determined in the levels of any of the cytokines between the sub-groups. The only significant difference observed was that pre-treatment IL5 was significantly less detectable in laryngopharynx compared with oral cavity or oropharynx (Fig. 1).
Fig. 1.
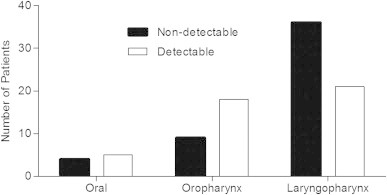
Bar chart showing the number of patients with non-detectable/detectable levels of IL5 in pre-treatment serum samples in relation to the subsite of the tumour. *p<0.05 Fishers exact test.
Of the tumour cohort one oropharynx and 6 samples of unknown primary site had an undefined T stage. The remaining 94 samples were grouped into early (n=48) or late (n=46) stage tumours and IL13 and TNFα had both significantly higher levels and were more detectable in both pre- and post-treatment serum from the early stage tumour group compared with the late stage group, with the difference being most apparent for TNFα (Table 3). The higher levels observed for IL2 and IL5 in both pre- and post-treatment serum samples from patients with early stage tumours compared with the late stage samples also approached significance and IL5 was significantly more detectable in early stage tumours but only in the pre-treatment samples (Table 3). Analysing laryngopharynx samples on their own maintained the significance for IL13 and TNFα and the difference for IL2 level with T stage became significant (data not shown).
Table 3.
Median concentrations pg/ml (IQR) of cytokines in serum from HNSCC patients both pre- and post-treatment in relation to T stage of the tumour.
| Cytokine | nDetectable/ntotal | Early stage (T1/2) | nDetectable/ntotal | Late stage (T3/4) | Pvalue |
|---|---|---|---|---|---|
| IL2 pre | 26/48 | 2.1 (0–13.7) | 16/46 | 0 (0–5.7) | 0.06 |
| IL2 post | 22/48 | 0 (0–8.3) | 14/46 | 0 (0–1.9) | 0.06 |
| IL4 pre | 25/48 | 2.0 (0–6.8) | 23/46 | 0.8 (0–6.5) | 0.88 |
| IL4 post | 18/48 | 0 (0–3.1) | 19/46 | 0 (0–6.6) | 0.71 |
| IL5 pre | 28/48b | 4.4 (0–17.0) | 17/46b | 0 (0–7.9) | 0.07 |
| IL5 post | 24/48 | 0.3 (0–7.5) | 14/46 | 0 (0–4.9) | 0.06 |
| IL6 pre | 35/48 | 2.7 (0–6.9) | 31/46 | 1.8 (0–6.5) | 0.55 |
| IL6 post | 32/48 | 1.9 (0–6.6) | 27/46 | 1.9 (0–3.1) | 0.26 |
| IL8 pre | 17/48 | 0 (0–0.7) | 18/46 | 0 (0–0.9) | 0.86 |
| IL8 post | 16/48 | 0 (0–1.0) | 15/46 | 0 (0–0.3) | 0.60 |
| IL10 pre | 47/48 | 16.4 (9.6–37.4) | 42/46 | 15.3 (6.2–50.1) | 0.58 |
| IL10 post | 45/48 | 17 (7.0–32.7) | 41/46 | 12.7 (5.1–30.1) | 0.43 |
| IL13 pre | 30/48b | 1.1 (0–6.2) | 14/46b | 0 (0–0.8) | 0.002a |
| IL13 post | 27/48b | 0.6 (0–5.6) | 15/46b | 0 (0–0.6) | 0.006a |
| GM-CSF pre | 34/48 | 2.2 (0–7.7) | 26/46 | 0.9 (0–7.6) | 0.39 |
| GMCSF post | 31/48 | 1.8 (0–4.1) | 27/46 | 1.6 (0–5.4) | 0.87 |
| IFNγ pre | 40/47c | 35 (10.5–77.7) | 36/42c | 28.2 (10.6–96.3) | 0.98 |
| IFNγ post | 42/47c | 39.9 (13.7–109.5) | 32/42c | 21.5 (3.2–144.8) | 0.47 |
| TNFα pre | 27/45bc | 2.5 (0–13.3) | 13/39c,b | 0 (0–2.4) | 0.009a |
| TNFα post | 27/45b,c | 3.5 (0–12.6) | 12/39c,b | 0 (0–2.7) | 0.003a |
Significant P value from Mann–Whitney U test for unrelated samples.
Significant difference in detectability p<0.05 Fisher's exact test.
Serum samples where levels of IFNγ, and TNFα were unable to be determined due to the quality of the standard curve.
The results of the cytokine levels relating to T stage were mirrored to a certain extent when they were considered in relation to the nodal status of the patient, in that levels of both IL13 and TNFα in serum from patients both pre- and post-treatment were higher in node negative compared with node positive patients (Table 4; 2 patients were of unknown nodal status). However, although the difference in levels for the IL13 pre-treatment samples and the TNFα post-treatment samples approached significance only the IL13 levels in the post-treatment samples were significantly higher in node negative patients. Levels of IL2 were also significantly higher in the pre-treatment serum from node negative patients compared with those in serum from node positive patients whereas the converse was true for IL4 in pre-treatment serum. The results for the levels of the cytokines in relation to nodal status of the tumour were also reflected in the detectability of these cytokines (Table 4). When the laryngopharynx group was considered separately the parameters mentioned above, which approached significance in relation to nodal status, became significant, as did the higher level of IL5 in both the pre- and post-treatment serum samples of the node negative group compared with the node positive group (p=0.04 and 0.03; data not shown).
Table 4.
Median concentrations pg/ml (IQR) of cytokines in serum from HNSCC patients both pre- and post-treatment in relation to nodal status of the patient.
| Cytokine | nDetectable/ntotal | N0 | nDetectable/ntotal | N+ | Pvalue |
|---|---|---|---|---|---|
| IL2 pre | 25/44b | 2.9 (0–13.7) | 19/55b | 0 (0–5.5) | 0.03a |
| IL2 post | 19/44 | 0 (0–6.5) | 18/55 | 0 (0–2.0) | 0.16 |
| IL4 pre | 17/44b | 0 (0–3.3) | 33/55b | 2.7 (0–10.9) | 0.04a |
| IL4 post | 14/44 | 0 (0–2.0) | 25/55 | 0 (0–8.8) | 0.11 |
| IL5 pre | 22/44 | 0.8 (0–30.3) | 26/55 | 0 (0–8.8) | 0.37 |
| IL5 post | 20/44 | 0 (0–9.1) | 21/55 | 0 (0–5.4) | 0.23 |
| IL6 pre | 30/44 | 1.9 (0–9.4) | 40/55 | 2.6 (0–5.1) | 0.89 |
| IL6 post | 24/44 | 1.4 (0–3.2) | 39/55 | 2.1 (0–3.7) | 0.25 |
| IL8 pre | 16/44 | 0 (0–0.7) | 19/55 | 0 (0–0.7) | 0.84 |
| IL8 post | 10/44 | 0 (0–0) | 21/55 | 0 (0–0.5) | 0.15 |
| IL10 pre | 40/44 | 11.3 (7.1–31.3) | 53/55 | 18.4 (9.6–41.5) | 0.12 |
| IL10 post | 40/44 | 12.4 (4.7–27.3) | 50/55 | 17.1 (7.1–32.0) | 0.20 |
| IL13 pre | 25/44 | 0.8 (0–5.6) | 21/55 | 0 (0–1.9) | 0.06 |
| IL13 post | 25/44b | 0.8 (0–5.6) | 19/55b | 0 (0–0.7) | 0.005a |
| GM-CSF pre | 25/44 | 1.4 (0–6.8) | 37/55 | 2.1 (0–8.1) | 0.52 |
| GMCSF post | 26/44 | 1.4 (0–4.3) | 34/55 | 1.8 (0–4.9) | 0.86 |
| IFNγ pre | 36/43c | 28.5 (4.4–66.4) | 44/51c | 37.8 (12.2–79.8) | 0.46 |
| IFNγ post | 38/43c | 28.4 (8.4–93.8) | 40/51c | 26.4 (8.9–153.5) | 0.78 |
| TNFα pre | 22/41c | 2.4 (0–12.7) | 20/48c | 0 (0–4.5) | 0.13 |
| TNFα post | 22/41c | 3.4 (0–12.6) | 20/48c | 0 (0–4.8) | 0.08 |
Significant P value from Mann–Whitney U test for unrelated samples.
Significant difference in detectability p<0.05 Fisher's exact test.
Serum samples where levels of IFNγ, and TNFα were unable to be determined due to the quality of the standard curve.
Significantly more patients presenting HNSCC were male (n=86) compared with female (n=13; unknown n=2), however the only differences observed in the levels of cytokines with respect to the sex of the patient were that IL2, IL5 and IL13 were all detected at significantly higher levels in females compared with males (p=0.03, 0.01 and 0.01, respectively) but there was no significant difference in the numbers of samples having detectable levels of cytokines in relation to gender (data not shown).
Samples were divided into two age groups: those from patients ≤60 (n=46) and those from patients >60 (n=53; unknown n=2), and it was determined that levels of IL4 in both pre- and post-treatment serum samples were significantly higher in the younger patients compared with the older group as were the levels of IL8 in the post-treatment serum samples (p=0.03, 0.01 and 0.02). There were also significantly more samples with detectable levels of IL4 in the post-treatment samples from the younger patient group (data not shown).
3.4. Effect of time of post-treatment sample collection on serum cytokines
The post-treatment collection sample was taken after patients had completed all their treatment (surgery, radio- and chemotherapy), however the time between pre- and post-treatment samples varied between 0.5 and 16 months. The samples were therefore divided into three groups: 0.5–3 months (n=23), 4–6 months (n=51) and 7–16 months (n=27) between collections. However, apart from the fact that the IL2 level was higher and more detectable in the 4–6 months group and the IL8 level was higher and more detectable in the 0.5–3 months group, the time of collection of the post-treatment sample had no significant influence on the cytokine level or detectability.
4. Discussion
The problem with many studies of head and neck cancer to date is that they have used mixed cohorts of patients, which are often relatively small and may have received prior treatment. The current study describes a large cohort of newly-presenting HNSCC patients from which data for individual subgroups has been obtained and is, to our knowledge, the largest study of multiplex cytokine analysis in HNSCC patients pre- and post-treatment to date. The advantage of the Quantibody® array over conventional ELISA methodology is that, the level of multiple cytokines can be detected simultaneously in a small volume of serum, saving time and generating a picture of cytokine interactions.
Some systemic cytokines in cancer patients may arise from the tumour itself, skewing the immune response towards Th2 promoting evasion of host anti-tumour mechanisms [15–18]. The current study supports this since the levels of the Th2 cytokines IL4, IL5, IL6 and IL10 were all found to decrease significantly following tumour excision. However, the tumour may influence peripheral blood mononuclear cells from HNSCC patients, which can have elevated Th2 cytokines and suppressed Th1 cytokines compared with those from controls shifting to Th1 post-operatively [19,10].
In contrast to Jebreel et al. who found a decrease in Th1 (IL12) and an increase in Th2 (IL10) cytokines in HNSCC patients (n=57) compared with controls (n=40) [5], the decrease in Th2 cytokines observed in the current study was not accompanied by an increase in the levels of the Th1 cytokines, in fact IL2 and IL8 also significantly decreased following treatment. This agrees with Lathers et al. who found that HNSCC patients (n=101) had increased levels of the Th2 cytokines IL4, IL6 and IL10 compared with healthy controls (n=40), but that the Th1 cytokines IL2 and GMCSF were also increased [17]. Hoffmann et al. also found elevated levels of the pro-inflammatory cytokines IL6 and IL8 in HNSCC patients (n=20) compared with healthy controls (n=20) [20] and Günaydin et al. found that IL10 was significantly more detectable in patients with laryngeal carcinoma (n=50) than controls (n=15) [21], however, neither Hoffman nor Lathers looked at treatment or sub-sites effects.
Sub-site discrimination is important because the survival and recurrence rates of patients with tumours from distinct sub-sites of the head and neck differ, although the reasons are controversial [22]—it may depend on a combination of the anatomical location, i.e. being more visible (oral cavity) or causing functional impairment (larynx), leading to earlier diagnosis or molecular differences [23]. The current study was unable to identify differences in cytokine levels between sub-sites, the only difference observed was the lower frequency of detection of IL5 in the laryngopharynx patients compared with the other sub-sites, the significance of which is unknown. This is in contrast to earlier studies, which showed that patients with tumours of the oral cavity had less detectable IL10 [5,18]. The current results would suggest the physical location of the tumour rather than the Th1/Th2 balance influences the prognosis of HNSCC patients [24].
Both IL13 and TNFα were higher in pre- and post-treatment serum from patients with early stage tumours compared with that from late stage patients and concurrently higher, although not always significantly so, in patients with node negative tumours compared with node positive tumours, suggesting their association with smaller more localised tumours, particularly in the laryngopharynx group. This is consistent with the role of TNFα as a Th1-like cytokine having anti-tumour effects targeting tumour vasculature. However, TNFα is also involved in cachexia in cancer patients [25] and has been found associated with advanced stage breast tumours with nodal involvement but this difference could be due to site variations [26]. IL13 is a Th2 cytokine negatively influencing anti-tumour immunity and can be an autocrine growth factor for some cancer cells [27,28], therefore may have been expected to be elevated in patients with late stage, more aggressive, HNSCC tumours. IL13 however is less likely to be involved in peripheral immune evasion as it cannot act directly on T cells unlike IL4 [27], which was detected at higher levels in the pre-treatment serum from patients with node positive tumours where it may be involved in immune evasion allowing the tumour to spread. In contrast the Th1 cytokine IL2 was higher in the pre-treatment serum of patients with node negative tumours where it may promote anti-tumour responses limiting the spread of the tumour. Sparano et al. also found that the Th2 cytokine IL10 was greater in HNSCC patients (n=58) with nodal involvement and T3/T4 stage tumours whereas the Th1 cytokine IL12 was greater in patients without nodal involvement and with T1/T2 stage tumours [4]. Günaydin et al. also found that IL10 was more detectable in patients with advanced stage laryngeal tumours with nodal involvement, however out of 50 patients investigated, there were only 10 with detectable IL10 levels [21].
Younger patients with HNSCC tend to have a poorer prognosis than their older counterparts [29] and in the current study it was found that the Th2 cytokine IL4 was higher in both the pre- and post-treatment serum from younger patients, which fits with the theory that the Th2 cytokines have a negative impact on the immune environment influencing patient prognosis.
The post-treatment samples in the current study were collected when the patients had completed all of their treatments, however, due to differences in the regimen this varied between 0.5 and 16 months after the pre-treatment sample, but this had little influence on the cytokine levels.
In conclusion, treatment of HNSCC reduces the levels of certain Th2-like cytokines however, an inverse Th1 gain is not observed and some cytokines are associated with the size and nodal involvement of the tumour. In addition to those in the periphery, cytokines in the microenvironment of the tumour should also be considered and are currently being investigated by our group along with other immunological parameters to gain a clearer picture of the overall immune responses occurring in these patients. Survival data are also being accumulated under the current ethical approval, to determine the relationship of these parameters with patient prognosis.
Acknowledgements
We would like to thank Mr Jose and other members of the head and neck surgical team in Hull for consenting the patients and for collection of serum samples and Dr Victoria Allgar for statistical advice. We gratefully acknowledge Yorkshire Cancer Research for financial support of this project.
References
- 1.Ang K.K., Harris J., Wheeler R. Human papillomavirus and survival of patients with oropharyngeal cancer. New England Journal of Medicine. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young M.R. Protective mechanisms of head and neck squamous cell carcinomas from immune assault. Head and Neck. 2006;28:462–470. doi: 10.1002/hed.20331. [DOI] [PubMed] [Google Scholar]
- 3.Whiteside T.L. The tumour microenvironment and its role in promoting tumour growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sparano A., Lathers DM, Achille N, Petruzzelli GJ, Young MR. Modulation of Th1 and Th2 cytokine profiles and their association with advanced head and neck squamous cell carcinoma. Otolaryngology—Head and Neck Surgery. 2004;131:573–576. doi: 10.1016/j.otohns.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Jebreel A., Mistry D., Loke D. Investigation of interleukin 10, 12 and 18 levels in patients with head and neck cancer. Journal of Laryngology and Otology. 2007;121:246–252. doi: 10.1017/S0022215106002428. [DOI] [PubMed] [Google Scholar]
- 6.Almand B., Resser J.R., Lindman B. Clinical significance of defective dendritic cell differentiation in cancer. Clinical Cancer Research. 2000;6:1755–1766. [PubMed] [Google Scholar]
- 7.Albers A.E., Strauss L., Liao T., Hoffman T.K., Kaufmann A.M. T cell-tumor interaction directs the development of immunotherapies in head and neck cancer. Clinical and Developmental Immunology. 2010 doi: 10.1155/2010/236378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schott A.K., Pries R., Wollenberg Permanent up-regulation of regulatory T-lymphocytes in patients with head and neck cancer. International Journal of Molecular Medicine. 2010;26:67–75. doi: 10.3892/ijmm_00000436. [DOI] [PubMed] [Google Scholar]
- 9.Becker Y. Molecular immunological approaches to biotherapy of human cancers-a review, hypothesis and implications. Anticancer Research. 2006;26(2A):1113–1134. [PubMed] [Google Scholar]
- 10.Melinceanu L., Sarafoleanu C., Lerescu L., Tucureanu C., Caraş I., Saˇlaˇgeanu A. Impact of smoking on the immunological profile of patients with laryngeal carcinoma. Journal of Medicine and Life. 2009;2:211–218. [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal A., Agarwal U., Verma S., Mohanty N.K., Saxena S. Serum Th1 and Th2 cytokine balance in patients of superficial transitional cell carcinoma of bladder pre- and post-intravesical combination immunotherapy. Immunopharmacology and Immunotoxicology. 2010;32:348–356. doi: 10.3109/08923970903300151. [DOI] [PubMed] [Google Scholar]
- 12.Nelson E.L., Wenzel L.B., Osann K. Stress, immunity and cervical cancer: biobehavioural outcomes of a randomized clinical trial. Clinical Cancer Research. 2008;14:2111–2118. doi: 10.1158/1078-0432.CCR-07-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen C., Duffy S., Teknos T. Nuclear factor-κB-related serum factors as longitudinal biomarkers of response and survival in advanced oropharyngeal carcinoma. Clinical Cancer Research. 2007;13:3182–3190. doi: 10.1158/1078-0432.CCR-06-3047. [DOI] [PubMed] [Google Scholar]
- 14.Bigbee Wl Grandis JR, Siegfried J.M. Mulitple cytokine and growth factor serum biomarkers predict therapeutic response and survival in advanced-stage head and neck cancer patients. Clinical Cancer Research. 2007;13:3107–3108. doi: 10.1158/1078-0432.CCR-07-0746. [DOI] [PubMed] [Google Scholar]
- 15.Ginos M.A., Page G.P., Michalowicz B.S. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Research. 2004;64:55–63. doi: 10.1158/0008-5472.can-03-2144. [DOI] [PubMed] [Google Scholar]
- 16.Mann E.A., Spiro J.D., Chen L.L., Kreutzer D.L. Cytokine expression by head and neck squamous cell carcinomas. American Journal of Surgery. 1992;164:567–573. doi: 10.1016/s0002-9610(05)80708-1. [DOI] [PubMed] [Google Scholar]
- 17.Lathers D.M.R., Achille N.J., Young M.R.I. Incomplete Th2 skewing of cytokines in plasma of patients with squamous cell carcinoma of the head and neck. Human Immunology. 2003;64:1160–1166. doi: 10.1016/j.humimm.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Alhamarneh O., Agada F., Madden L., Stafford N., Greenman J. Serum IL10 and circulating CD4(+) CD25(high) regulatory T cell numbers as predictors of clinical outcome and survival in patients with head and neck squamous cell carcinoma. Head and Neck. 2011;33:415–423. doi: 10.1002/hed.21464. [DOI] [PubMed] [Google Scholar]
- 19.Bose A., Cakraborty T., Chakraborty K., Pal S., Baral R. Dysregulation in immune functions is reflected in tumour cell cytotoxicity by peripheral blood mononuclear cells from head and neck squamous cell carcinoma patients. Cancer Immunology. 2008;12:8–10. [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann T.K., Sonkoly E., Homey B. Aberrant cytokine expression in serum of patients with adenoid cystic carcinoma and squamous cell carcinoma of the head and neck. Head and Neck. 2007;29:472–478. doi: 10.1002/hed.20533. [DOI] [PubMed] [Google Scholar]
- 21.Günaydin R.O., Kesikli S.A., Kansu E., Hoşal A.S. Identification of the peripheral blood levels of interleukin-12, interleukin-10, and transforming growth factor-β in patients with laryngeal squamous cell carcinoma. Head and Neck. 2011 doi: 10.1002/hed.21738. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Hoffman H.T., Karnell L.H., Funk G.F., Robinson R.A., Menck H.R. The National Cancer Data Base report on cancer of the head and neck. Archives of Otolaryngology—Head and Neck Surgery. 1998;124:951–962. doi: 10.1001/archotol.124.9.951. [DOI] [PubMed] [Google Scholar]
- 23.Freier K., Bosch F.X., Flechtenmacher C. Distinct site specific oncoprotein over expression in head and neck squamous cell carcinoma: a tissue microarray analysis. Anticancer Research. 2003;23(5A):3971–3977. [PubMed] [Google Scholar]
- 24.Weinberger P.M., Merkley M., Lee J.R. Use of combination proteomic analysis to demonstrate molecular similarity of head and neck squamous cell carcinoma arising from different subsites. Archives of Otolaryngology—Head and Neck Surgery. 2009;135:694–703. doi: 10.1001/archoto.2009.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Horssen R., Ten Hagan T.L., Eggermont A.M. TNF-α in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11:397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 26.Papadoppulou E., Tripsianis G., Anagnostopoulos K. Significance of serum tumor necrosis factor-alpha and its combination with HER-2 codon 655 polymorphism in the diagnosis and prognosis of breast cancer. International Journal of Biological Markers. 2010;25:126–135. doi: 10.1177/172460081002500302. [DOI] [PubMed] [Google Scholar]
- 27.Terabe M., Park J.M., Berzofsky J.A. Role of IL-13 in regulation of anti-tumor immunity and tumor growth. Cancer Immunology Immunotherapy. 2004;53:79–85. doi: 10.1007/s00262-003-0445-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawakami K., Kawakami M., Joshi B.H., Puri R.K. Interleukin-13 receptor-targeted cancer therapy in an immunodeficient animal model of human head and neck cancer. Cancer Research. 2001;61:6194–6200. [PubMed] [Google Scholar]
- 29.Park J.O., Sun Di, Cho K.J., Joo Y.H., Yoo H.J., Kim M.S. Clinical outcome of squamous cell carcinoma of the tongue in young patients: a stage-matched comparative analysis. Clinical and Experimental Otorhinolaryngology. 2010;3:161–165. doi: 10.3342/ceo.2010.3.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]


