Abstract
Hepatocyte growth factor (HGF) is an angiogenic, cardioprotective factor important for tissue and vascular repair. High levels of HGF are associated with chronic inflammatory diseases, such as coronary artery disease (CAD) and periodontitis, and are suggested as a marker of the ongoing atherosclerotic event in patients with CAD. Periodontal disease is more prevalent among patients with CAD than among healthy people. Recent studies indicate a reduced biological activity of HGF in different chronic inflammatory conditions. Biologically active HGF has high affinity to heparan sulfate proteoglycan (HSPG) on cell-membrane and extracellular matrix. The aim of the study was to investigate the serum concentration and the biological activity of HGF with ELISA and surface plasmon resonance (SPR), respectively, before and at various time points after percutaneous coronary intervention (PCI) in patients with CAD, and to examine the relationship with periodontal condition. The periodontal status of the CAD patients was examined, and the presence of P. gingivalis in periodontal pockets was analyzed with PCR. The HGF concentration was significantly higher, at all time-points, in patients with CAD compared to the age-matched controls (P< 0.001), but was independent of periodontal status. The HGF concentration and the affinity to HSPG adversely fluctuated over time, and the biological activity increased one month after intervention in patients without periodontitis. We conclude that elevated concentration of HGF but with reduced biological activity might indicate a chronic inflammatory profile in patients with CAD and periodontitis.
Keywords: Hepatocyte growth factor, Coronary artery disease, Angiography, Periodontal disease, Porphyromonas gingivalis
Highlights
► We examine the concentration and the biological activity of HGF in patients with CAD, and the relation to periodontal condition. ► Higher serum HGF concentration before and up to one year after percutaneous coronary intervention. ► HGF concentration fluctuated adversely to the HGF biological activity. ► Slightly reduced HGF biological activity in groups with periodontitis and P. gingivalis.
1. Introduction
Hepatocyte growth factor (HGF), an angiogenic and regenerative factor with cytoprotective effects, has gained substantial attention for its cardioprotective involvement in coronary ischemia and infarction [1–4]. The HGF receptor, the proto-oncogene c-Met, is a transmembrane tyrosine kinase receptor expressed by almost all epithelial, endothelial, and erythroid progenitor cells [5]. In addition, a low-affinity binding site has been identified on heparan sulfate proteoglycan (HSPG) [6], a sulfated glycoprotein present on the cell surface in essentially all tissues and in the extracellular matrix [7]. HSPG binds and facilitates the cytokine–receptor interaction [8–10] as well as the conversion of promitogen HGF to the two-chain active form [6,11].
A number of studies have demonstrated elevated HGF levels in patients with various chronic diseases [12,13]; however, despite high concentrations, HGF may appear in a form with reduced biological activity [14]. Furthermore, administration of exogenous HGF is shown to prevent tissue fibrosis and dysfunction in chronic disease models [15–17]. High levels of HGF are also associated with coronary artery disease (CAD) [18,19], and it has been suggested that circulating levels of HGF may provide a useful marker of atherothrombosis in patients with established CAD, reflecting the extent or activity of atherosclerosis. In addition, HGF and c-Met are expressed in the atherosclerotic vessel wall and in plaque [4,20].
There is an emerging interest in the link between oral health and systemic diseases, such as CAD. Indeed, periodontal disease is more prevalent among patients with CAD than among healthy people [21,22]. Porphyromonas gingivalis (P. gingivalis) is an etiological agent strongly associated with periodontal disease [23] and correlates with numerous inflammatory disorders, such as cardiovascular and rheumatic disease. P. gingivalis has been detected in human atheromatous carotid plaques [24], and serum antibodies to P. gingivalis have been shown to predict myocardial infarction [25]. Furthermore, periodontal pockets with a high number of periodontally pathogenic bacteria are a risk factor for acute coronary syndrome [26]. Chronically inflamed periodontal pockets may serve as a reservoir for inflammatory stimuli, and by entering the circulation, oral bacteria and their components activate neutrophils and platelets, thereby inducing production of reactive oxygen species [27], and triggering the inflammatory process in the carotid vessels. P. gingivalis contributes to periodontal disease via virulence factors such as cysteine proteinases (gingipains), fimbriae, and LPS, and an infection may lead to chronic inflammation in which hyper-responsive neutrophils contribute to host-mediated tissue destruction. In periodontitis, HGF concentrations, in gingival crevicular fluid and in saliva, increase proportionally with the progression of periodontal disease [28], and P. gingivalis stimulates HGF synthesis in human gingival fibroblasts [29].
While ELISA is a reliable, sensitive method for protein detection, this method does not differentiate between biologically active and inactive HGF [14]. The binding of HGF to HSPG has previously been shown to be important for the biological activity of HGF and for the induction of cellular responses [30,31]. Surface plasmon resonance (SPR) is an optical technique that can determine the affinity of a protein for several ligands or epitopes [32,33]. SPR-based assessment of the binding profile of HGF to HSPG may rapidly and sensitively distinguish HGF variants with different biological activities. Appropriate for clinical studies, this method can be used for evaluation of the quality of endogenous HGF [30].
The aim of the present study was to investigate the concentration and the biological activity of HGF with ELISA and SPR, respectively, in patients with confirmed CAD, and to examine the relationship with periodontal disease and the presence of P. gingivalis in periodontal pockets.
2. Material and methods
2.1. Study subjects and protocol
Thirty six (mean age 59.9 years; range 41–73 years; 26 men and 10 women) out of a total of 90 patients who underwent coronary angiography between February 2003 and 2007 due to chest pain were randomly selected and enrolled in this study (Table 1). Exclusion was based on the following criteria: age above 75 years, ongoing cortisone medication, ongoing treated infection, or malignity. Peripheral venous blood was drawn into heparin-containing (20 U/mL) vacutainer tubes from the patients before, as well as 24 h, 1 month, 6 and 12 months after angiography and percutaneous intervention (PCI). The blood samples were centrifuged at 3000g for 10 min; the serum was collected and frozen at −70 °C within 45 min of collection. All patients were treated with acetyl salicylic acid (75 mg) once daily before and after the procedures, and they were also given a loading dose of 300 mg clopidogrel approximately 12 h prior to PCI. Patients with unstable angina (n=4) were given acetylsalicylic acid, clopidogrel, and low molecular heparin until the time of the revascularization. Clopidogrel was also given for 3 months after the PCI.
Table 1.
Baseline data. Serum hepatocyte growth factor (HGF) concentration (ng/mL) and binding affinity to heparan sulfate proteoglycan (HSPG) (response units; RU) in patients with coronary artery disease (CAD) and in healthy controls. The patients are classified into periodontal conditions (gingivitis, moderate periodontitis, or severe periodontitis) and negative or positive for Porphyromonas gingivalis (P. gingivalis) in periodontal pockets. The data are presented as mean±SD, and P<0.05 is considered statistically significant.
|
Patients |
Controls | |||||||
|---|---|---|---|---|---|---|---|---|
|
Gingivitis |
Moderate periodontitis |
Severe periodontitis |
||||||
|
P. gingivalis |
P. gingivalis |
P. gingivalis |
||||||
| Neg | Pos | Neg | Pos | Neg | Pos | p-Valuesa | ||
| Age (mean ±SD) | ||||||||
| Subgroups | 57.0±5.0 | 59.0±8.6 | 57.8±8.4 | 64.0±6.5 | 62.8±6.3 | 60.7±6.9 | – | 0.240b |
| Groups | 57.8±6.7 | 60.5±8.5 | 61.7±6.8 | – | 0.475 | |||
| Total | 59.9±7.4 | 59.3±7.3 | 0.608 | |||||
| Sex (M/F (total)) | ||||||||
| Subgroups | 8/0 (8) | 3/1 (4) | 5/4 (9) | 1/4 (5) | 4/1 (5) | 5/0 (5) | – | – |
| Groups | 11/1 (12) | 6/8 (14) | 9/1 (10) | – | – | |||
| Total | 26/10 (36) | 41/15 (56) | 01.00 | |||||
| HGF baseline (mean ±SD) | ||||||||
| Subgroups | 1.14±0.47 | 1.17±0.24 | 1.12±0.79 | 1.10±0.47 | 1.14±0.40 | 1.06±0.16 | – | 0.881b |
| Groups | 1.15±0.40 | 1.11±0.44 | 1.07±0.17 | – | 0.270 | |||
| Total | 1.11±0.37 | 0.78±0.29 | <0.001 | |||||
| HSPG baseline (mean ±SD) | ||||||||
| Subgroups | 16.2±21.1 | 11.0±14.9 | 14.8±13.3 | 8.2±6.8 | 14.2±15.7 | 9.8±4.9 | – | 0.702b |
| Groups | 15.0±19.0 | 12.4±12.3 | 11.3±12.4 | – | 0.sta | |||
| Total | 13.0±14.6 | 14.5±15.8 | 0.400 | |||||
Fisher's exact test for sex and Mann–Whitney's U-test and Kruskal–Wallis ANOVA by ranks for continuous variables.
Differences between positive and negative P. gingivalis (Mann–Whitney's U-test).
A control group matched for age and gender comprised of 56 subjects randomly drawn from eligible participants in the population based on “Life Conditions, Stress and Health-study” [34]. Participants with reported coronary heart disease or angina pectoris were excluded prior to matching. Peripheral venous blood was drawn at one occasion and handled in the same way as described earlier.
The study protocol was approved by the Regional Ethical Review Board in Linköping, Sweden, and all participants provided written, informed consent.
2.2. Periodontal examination
Three months after each subject had undergone intervention, a dental examination was performed. Anamnesis, clinical periodontal examination, and a panoramic radiograph were recorded for each subject. Periodontal conditions were scored according to Lindhe and Nyman [35]. The number of remaining teeth (excluding third molars) was recorded, and plaque scores (PlI%) were calculated based on the presence or absence of visible plaque at the gingival margin on four surfaces (buccal, lingual, mesial, and distal) of each tooth. Periodontal pockets were recorded on each tooth surface with a depth exceeding 4 mm using a manual periodontal probe (HuFriedy PCP 11). Based on clinical and radiographic findings, all subjects were classified into one of the three groups according to the severity of periodontal disease, criteria modified from Hugoson and Jordan [36]: gingivitis: normal alveolar bone height, and >12 bleeding gingival units in the molar–premolar regions; moderate periodontitis: alveolar bone loss around the majority of the teeth not exceeding 1/3 of normal bone height; severe periodontitis: alveolar bone loss around the majority of the teeth exceeding 1/3 of normal bone height and presence of angular bony defects and/or furcation defects.
In patients with preserved teeth, subgingival microbial samples were collected from the four deepest periodontal pockets in the mouth. Supragingival plaque was removed, the root surface was dried, and a sterile endodontic paper point was inserted into the bottom of the pocket for 20 s. The pooled samples from each patient were stored in a sterile tube and transported to the laboratory for analysis of the prevalence of P. gingivalis.
2.3. PCR detection of P. gingivalis
The presence of P. gingivalis in the subgingival samples was analyzed by PCR [37]. Briefly, DNA was isolated using the Jetquick tissue DNA spin kit (Genomed, Löhne, Germany) according to the manufacturer's instructions. Five microliters of the extracted template was added to puRe Taq Ready-To-Go PCR Beads (Amersham Biosciences, Uppsala, Sweden) containing 200 μM of each dNTP, 2.5 U puReTaq DNA polymerase, 10 mM Tris–HCl [pH 9.0 at room temperature], 50 mM KCl, and 1.5 mM MgCl2 together with 0.4 μM of each primer (upstream primer: 5′-AGG CAG CTT GCC ATA CTG CG-3′ and downstream primer: 5′-ATC GTT AGC AAC TAC CAG TGT-3′; MWG Biotech AG, Ebersberg, Germany). P. gingivalis DNA (ATCC 33277D) was used as positive control. The template was amplified (Mastercycler®: Eppendorf, Hamburg, Germany) with an initial denaturation step at 95 °C for 10 min, followed by 36 cycles of denaturation at 95 °C for 30 s, annealing at 60 °C for one minute, and extension at 72 °C for one minute. The amplified product was stored at 4 °C for at most one hour before being separated by gel electrophoresis in 1.2% E-Gel® Agarose Gels (Invitrogen, Carlsbad, CA, USA) containing ethidium bromide, together with the Jetway 1000/100 bp ladder (Genomed, Löhne, Germany). The amplified product of 404 bp was visualized by UV light.
2.4. ELISA measurement of serum HGF concentration
The HGF concentration in serum collected before and at 24 h, 1 month, 6 months, and 12 months after coronary angiography with PCI was determined using an ELISA kit (Quantikine Human HGF immunoassay, minimum detectable limit: 0.04 ng/mL; R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions. The measurements of the samples from the different groups and time points were performed in duplicate at 450 nm using an ELISA reader (Expert 96; Asys Hitech GmbH, Eugendorf, Austria), and calibrated using the recombinant human HGF reference samples and standards that were provided in the ELISA kit.
2.5. Serum HGF activity
The biological activity of HGF was analyzed by Surface Plasmon Resonance (SPR) measuring binding affinity to HSPG (Sigma-Aldrich, St. Louis, MO, USA) as previously described [30]. Briefly, SPR measurements and ligand immobilization procedures were conducted at 760 nm in a fully automatic Biacore 2000 instrument (GE-Healthcare GmbH, Uppsala, Sweden) equipped with four flow cells; the flow cell temperature was 25 °C in all experiments. HBS-EP buffer (0.01 M HEPES [pH 7.4], 0.15 M NaCl, 3 mM EDTA, 0.005% surfactant P20) (GE-Healthcare GmbH) was used as a running buffer. The ligand (HSPG) was coupled to carboxymethylated dextran CM5 chips (GE Healthcare GmbH) by conventional carbodiimide chemistry using 200 mM N-ethyl-N0-(3-diethylaminopropyl) carbodiimide and 50 mM N-hydroxysuccinimide. The activation time was 7 min, followed by a 7 min ligand injection. Deactivation of the remaining active esters was performed by a 7 min injection of ethanolamine/hydrochloride [pH 8.5]. A flow rate of 5 μL/min was used during immobilization and measurement procedures. HSPG (≥400 μg/mL protein and 400 μg/mL glycosaminoglycan) was diluted in a ratio of 1:10 in 10 mM acetate buffer [pH 4.5] below the isoelectric point of the protein, thus enhancing the electrostatic interactions between the dextran matrix and the ligand. The contact time was 7 min, which resulted in immobilization levels around 15,000 response units (RU).
Serum samples were diluted in a ratio of 1:20 in PBS [pH 7.4] (Apoteket AB, Umeå, Sweden). An equal mixture of 1 M NaCl and 10 mM glycine [pH 2] followed by one injection of borate [pH 8.5] was used as a regeneration buffer. A positive and a negative control were included at the beginning and at the end of each run to confirm the reliability of the surfaces.
2.6. Statistical analysis
The data were normally distributed after logarithmation and analyzed by repeated measures of ANOVA followed by Neuman–Keuls Post Hoc test, using Statistica Software and/or Graph Pad Prism. P-values below 0.05 were considered statistically significant and results are expressed as mean±SD.
3. Results
The periodontal status was distributed as following: gingivitis n=12, moderate periodontitis n=14, severe periodontitis n=10. 14 (39%) of the patients were positive for P. gingivalis and 22 (61%) negative (Table 1). Periodontal conditions are shown in Table 2.
Table 2.
Periodontal conditions of the patients with CAD. Periodontal parameters in the group of 36 heart patients with PCI; mean number of remaining teeth, % of surfaces with visible plaque at the gingival margin (PI), % of surfaces with bleeding at the gingival margin (GI), % of surfaces with bleeding on probing (BOP), number of probing pocket depths (PPD).
| Variable | Mean | SD | Range (Max–Min) |
|---|---|---|---|
| Number of teeth | 24.6 | 2.0 | 7 (28–21) |
| Plaque (%) | 44.2 | 17.8 | 44 (66–22) |
| GI (%) | 45.4 | 21.0 | 58 (69–11) |
| BOP (%) | 14.8 | 14.0 | 43 (43–0) |
| 4–6 mm PPD | 14.0 | 12.3 | 39 (40−1) |
| >6 mm PPD | 1.56 | 3.25 | 9 (9–0) |
Prior to the PCI intervention, the mean HGF concentration in the patient group was significantly higher (P<0.001) than in the age-matched healthy control group, and the HGF concentration did not significantly differ between sampling times (Table 1). Additionally, there were no differences in HGF levels between the groups with different periodontal status (Fig. 1A), neither between groups negative or positive for P. gingivalis in periodontal pockets (Fig. 1C) (Table 1).
Fig. 1.
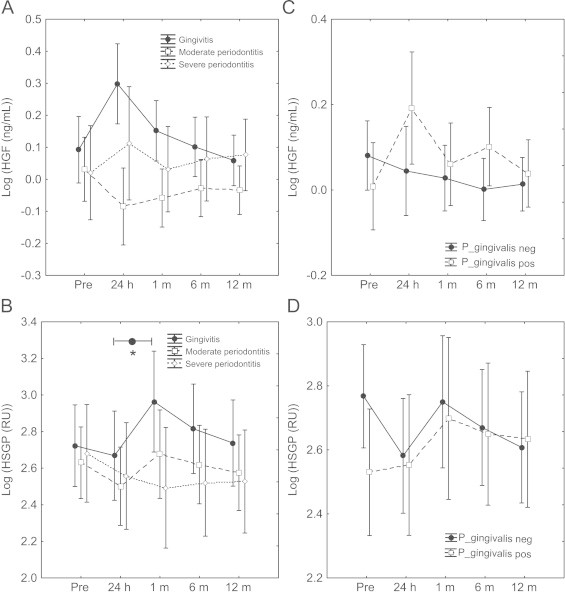
Serum concentration and biological activity of hepatocyte growth factor in patients with CAD. The concentration and the biological activity (binding to heparan sulfate proteoglycan; HSPG) of hepatocyte growth factor (HGF) in serum taken before (pre), 24 h, 1 month, 6 and 12 months after angiography and percutaneous coronary intervention (PCI) from patients with coronary artery disease (CAD) and with gingivitis, moderate periodontitis, or severe periodontitis (A, B). Patients were also grouped into negative or positive for Porphyromonas gingivalis (P. gingivalis) in dental pockets (C, D). The concentration of HGF (ng/mL) was measured with ELISA (A, C), and the binding affinity to HSPG was measured with surface plasmon resonance (SPR) and data are expressed as response units (RU) (B, D). Repeated measures of ANOVA was used for statistical analysis and data are presented as mean±SD. ⁎P<0.05.
The HGF serum concentration fluctuated adversely to the binding affinity to HSPG (which indicates the biological activity of HGF); the mean HGF concentration increased 24 h after the PCI intervention in patients without periodontitis (patients with gingivitis), while the binding affinity to HSPG at the same time decreased. After one month the HGF concentration showed the lowest level, while the affinity to HSPG reached the highest peak at the same time (P<0.05; binding affinity to HSPG 24 h vs. one month in patients with gingivitis) (Fig. 1).
4. Discussion
As observed in previous studies of chronic inflammatory diseases [13], we demonstrate in this study significantly higher serum concentration of HGF in patients with CAD, compared to an age/sex-matched control group. However, the binding affinity to HSPG, reflecting the biological activity of HGF, increased in the patients with CAD without periodontitis (but with gingivitis), one month after PCI, but decreased to the same level as prior to intervention after 12 months. Thus, the HGF serum concentration fluctuated adversely to the biological activity of HGF, i.e. when the concentration of HGF increased the biological activity of HGF decreased and vice versa. This phenomenon indicates that in chronic inflammatory conditions the binding affinity to HSPG is decreased, possibly through proteolytic cleavage and thereby inactivation of HGF. This leads to disabled interaction with the receptor [8–10,38,39], causing additional elevated circulatory levels of HGF [40]. According to these data, the PCI in the CAD patients in our study may temporarily open the occluded vessel and improve the blood flow, but have no effects on the inflammatory process of atherosclerosis in the vessel wall. These observations support the role of chronic inflammation in the pathophysiology of CAD, and may have an impact on future strategies in diagnosis, evaluation, and treatment of CAD.
Indeed, several previous studies strengthen the notion of the importance of endogenous biologically active HGF in the regeneration, and healing of injuries, during chronic inflammatory diseases, such as in the cardiovascular system [9,19,41,42], where endogenous HGF may be present in a reduced biological active form. The HGF concentration, which is elevated during chronic inflammation, has been used as a marker of the chronic status and as a monitor to predict the response to therapy [28]. In previous studies, we used an in vitro SPR system to analyze the binding affinity to HSPG, in comparison to the motogenic activity of HGF measured in a model of cell injury, to establish a method for evaluation of the biological activity of HGF [30]. We found that the biological activity of HGF was decreased in ulcer secretion from patients with chronic ulcers, who responded well to administration of biologically active HGF [19]. Whether HGF is a potent therapeutic tool for the treatment of chronic damage induced by untreated chronic infections requires further investigations.
The present study has limitations that should be considered in future prospective investigations. The study material includes not more than 36 patients that were sub grouped in either three periodontal status, or in P. gingivalis positive or negative groups. Another limitation of the study is that it was not possible to perform periodontal examinations on the healthy controls, since the samples were already collected and included previously as a part of another study. However, it is plausible to presume that the periodontal status in this control group more or less varied, and consequently this suggests that the differences between patients and a periodontal healthy control may be even more pronounced than shown in this study.
5. Conclusion
In summary, we conclude that patients with CAD have a systemic HGF profile reflecting a chronic inflammatory condition with a high concentration, but low biological activity of HGF. The biological activity of HGF is temporarily elevated after dilation of the stenotic artery, however, only in patients without periodontitis. Consequently, administration of biologically active HGF may be an important research focus of future strategies in the treatment of patients with CAD.
References
- 1.Aoki M., Morishita R., Taniyama Y., Kaneda Y., Ogihara T. Therapeutic angiogenesis induced by hepatocyte growth factor: potential gene therapy for ischemic diseases. Journal of Atherosclerosis and Thrombosis. 2000;7:71–76. doi: 10.5551/jat1994.7.71. [DOI] [PubMed] [Google Scholar]
- 2.Nakamura T., Mizuno S., Matsumoto K., Sawa Y., Matsuda H. Myocardial protection from ischemia/reperfusion injury by endogenous and exogenous HGF. Journal of Clinical Investigation. 2000;106:1511–1519. doi: 10.1172/JCI10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Belle E., Witzenbichler B., Chen D., Silver M., Chang L., Schwall R. Potentiated angiogenic effect of scatter factor/hepatocyte growth factor via induction of vascular endothelial growth factor: the case for paracrine amplification of angiogenesis. Circulation. 1998;97:381–390. doi: 10.1161/01.cir.97.4.381. [DOI] [PubMed] [Google Scholar]
- 4.McKinnon H., Gherardi E., Reidy M., Bowyer D. Hepatocyte growth factor/scatter factor and MET are involved in arterial repair and atherogenesis. American Journal of Pathology. 2006;168:340–348. doi: 10.2353/ajpath.2006.050379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura T., Sakai K., Matsumoto K. Hepatocyte growth factor twenty years on: much more than a growth factor. Journal of Gastroenterology and Hepatology. 2011;26(Suppl. 1):188–202. doi: 10.1111/j.1440-1746.2010.06549.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu K.X., Kato Y., Narukawa M., Kim D.C., Hanano M., Higuchi O. Importance of the liver in plasma clearance of hepatocyte growth factors in rats. American Journal of Physiology. 1992;263:G642–G649. doi: 10.1152/ajpgi.1992.263.5.G642. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura T., Teramoto H., Ichihara A. Purification and characterization of a growth factor from rat platelets for mature parenchymal hepatocytes in primary cultures. Proceedings of the National Academy of Sciences of United States of America. 1986;83:6489–6493. doi: 10.1073/pnas.83.17.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karihaloo A., Kale S., Rosenblum N.D., Cantley L.G. Hepatocyte growth factor-mediated renal epithelial branching morphogenesis is regulated by glypican-4 expression. Molecular and Cellular Biology. 2004;24:8745–8752. doi: 10.1128/MCB.24.19.8745-8752.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin J.S., Day R.M., Breckenridge D., Atabey N., Taylor W.G., Stahl S.J. Dissociation of heparan sulfate and receptor binding domains of hepatocyte growth factor reveals that heparan sulfate-c-met interaction facilitates signaling. Journal of Biological Chemistry. 2001;276:32977–32983. doi: 10.1074/jbc.M105486200. [DOI] [PubMed] [Google Scholar]
- 10.Sakata H., Stahl S.J., Taylor W.G., Rosenberg J.M., Sakaguchi K., Wingfield P.T. Heparin binding and oligomerization of hepatocyte growth factor/scatter factor isoforms. Heparan sulfate glycosaminoglycan requirement for Met binding and signaling. Journal of Biological Chemistry. 1997;272:9457–9463. doi: 10.1074/jbc.272.14.9457. [DOI] [PubMed] [Google Scholar]
- 11.Miyazawa K., Shimomura T., Kitamura A., Kondo J., Morimoto Y., Kitamura N. Molecular cloning and sequence analysis of the cDNA for a human serine protease reponsible for activation of hepatocyte growth factor. Structural similarity of the protease precursor to blood coagulation factor XII. Journal of Biological Chemistry. 1993;268:10024–10028. [PubMed] [Google Scholar]
- 12.Nayeri F., Olsson H., Peterson C., Sundqvist T. Hepatocyte growth factor; expression, concentration and biological activity in chronic leg ulcers. Journal of Dermatological Science. 2005;37:75–85. doi: 10.1016/j.jdermsci.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Funakoshi H., Nakamura T. Hepatocyte growth factor: from diagnosis to clinical applications. Clinica Chimica Acta. 2003;327:1–23. doi: 10.1016/s0009-8981(02)00302-9. [DOI] [PubMed] [Google Scholar]
- 14.Nayeri F., Holmgren-Pettersson K., Perskvist N., Forsberg P., Peterson C., Sundqvist T. An in vitro model for assessment of the biological activity of hepatocyte growth factor. Growth Factors. 2007;25:33–40. doi: 10.1080/08977190600997200. [DOI] [PubMed] [Google Scholar]
- 15.Yaekashiwa M., Nakayama S., Ohnuma K., Sakai T., Abe T., Satoh K. Simultaneous or delayed administration of hepatocyte growth factor equally represses the fibrotic changes in murine lung injury induced by bleomycin. A morphologic study. American Journal of Respiratory and Critical Care Medicine. 1997;156:1937–1944. doi: 10.1164/ajrccm.156.6.9611057. [DOI] [PubMed] [Google Scholar]
- 16.Ueki T., Kaneda Y., Tsutsui H., Nakanishi K., Sawa Y., Morishita R. Hepatocyte growth factor gene therapy of liver cirrhosis in rats. Nature Medicine. 1999;5:226–230. doi: 10.1038/5593. [DOI] [PubMed] [Google Scholar]
- 17.Mizuno S., Kurosawa T., Matsumoto K., Mizuno-Horikawa Y., Okamoto M., Nakamura T. Hepatocyte growth factor prevents renal fibrosis and dysfunction in a mouse model of chronic renal disease. Journal of Clinical Investigation. 1998;101:1827–1834. doi: 10.1172/JCI1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimura M., Ushiyama M., Ohtsuka K., Nishida M., Inoue N., Matsumuro A. Serum hepatocyte growth factor as a possible indicator of vascular lesions. Journal of Clinical Endocrinology and Metabolism. 1999;84:2475–2480. doi: 10.1210/jcem.84.7.5814. [DOI] [PubMed] [Google Scholar]
- 19.Susen S., Sautière K., Mouquet F., Cuilleret F., Chmaït A., McFadden E.P. Serum hepatocyte growth factor levels predict long-term clinical outcome after percutaneous coronary revascularization. European Heart Journal. 2005;26:2387–2395. doi: 10.1093/eurheartj/ehi436. [DOI] [PubMed] [Google Scholar]
- 20.Liu Y., Wilkinson F.L., Kirton J.P., Jeziorska M., Iizasa H., Sai Y. Hepatocyte growth factor and c-Met expression in pericytes: implications for atherosclerotic plaque development. Journal of Pathology. 2007;212:12–19. doi: 10.1002/path.2155. [DOI] [PubMed] [Google Scholar]
- 21.Starkhammar Johansson C., Richter A., Lundström A., Thorstensson H., Ravald N. Periodontal conditions in patients with coronary heart disease: a case-control study. Journal of Clinical Periodontology. 2008;35:199–205. doi: 10.1111/j.1600-051X.2007.01185.x. [DOI] [PubMed] [Google Scholar]
- 22.Bahekar A.A., Singh S., Saha S., Molnar J., Arora R. The prevalence and incidence of coronary heart disease is significantly increased in periodontitis: a meta-analysis. American Heart Journal. 2007;154:830–837. doi: 10.1016/j.ahj.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 23.Petersen P.E., Ogawa H. Strengthening the prevention of periodontal disease: the WHO approach. Journal of Periodontology. 2005;76:2187–2193. doi: 10.1902/jop.2005.76.12.2187. [DOI] [PubMed] [Google Scholar]
- 24.Haraszthy V.I., Zambon J.J., Trevisan M., Zeid M., Genco R.J. Identification of periodontal pathogens in atheromatous plaques. Journal of Periodontology. 2000;71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 25.Pussinen P.J., Alfthan G., Tuomilehto J., Asikainen S., Jousilahti P. High serum antibody levels to Porphyromonas gingivalis predict myocardial infarction. European Journal of Cardiovascular Prevention and Rehabilitation. 2004;11:408–411. doi: 10.1097/01.hjr.0000129745.38217.39. [DOI] [PubMed] [Google Scholar]
- 26.Renvert S., Pettersson T., Ohlsson O., Persson G.R. Bacterial profile and burden of periodontal infection in subjects with a diagnosis of acute coronary syndrome. Journal of Periodontology. 2006;77:1110–1119. doi: 10.1902/jop.2006.050336. [DOI] [PubMed] [Google Scholar]
- 27.Goulet V., Britigan B., Nakayama K., Grenier D. Cleavage of human transferrin by Porphyromonas gingivalis gingipains promotes growth and formation of hydroxyl radicals. Infection and Immunity. 2004;72:4351–4356. doi: 10.1128/IAI.72.8.4351-4356.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagaraja C., Pradeep A.R. Hepatocyte growth factor levels in gingival crevicular fluid in health, disease, and after treatment. Journal of Periodontology. 2007;78:742–747. doi: 10.1902/jop.2007.060249. [DOI] [PubMed] [Google Scholar]
- 29.Sugiyama A., Ogawa T., Daikuhara Y., Takada H. Enhancement of hepatocyte growth factor (scatter factor) production by human gingival fibroblasts in culture stimulated with Porphyromonas gingivalis fimbriae. Journal of Medical Microbiology. 2000;49:319–325. doi: 10.1099/0022-1317-49-4-319. [DOI] [PubMed] [Google Scholar]
- 30.Nayeri F., Nayeri T., Aili D., Brudin L., Liedberg B. Clinical impact of real-time evaluation of the biological activity and degradation of hepatocyte growth factor. Growth Factors. 2008;26:163–171. doi: 10.1080/08977190802128083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nayeri F., Strömberg T., Larsson M., Brudin L., Söderström C., Forsberg P. Hepatocyte growth factor may accelerate healing in chronic leg ulcers: a pilot study. Journal of Dermatological Treatment. 2002;13:81–86. doi: 10.1080/095466302317584449. [DOI] [PubMed] [Google Scholar]
- 32.Liedberg B., Nylander C., Lundström I. Biosensing with surface plasmon resonance—how it all started. Biosensors and Bioelectronics. 1995;10:i–ix. doi: 10.1016/0956-5663(95)96965-2. [DOI] [PubMed] [Google Scholar]
- 33.Nayeri F., Aili D., Nayeri T., Xu J., Almer S., Lundström I. Hepatocyte growth factor (HGF) in fecal samples: rapid detection by surface plasmon resonance. BMC Gastroenterology. 2005;5:13. doi: 10.1186/1471-230X-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garvin P., Nilsson L., Carstensen J., Jonasson L., Kristenson M. Circulating matrix metalloproteinase-9 is associated with cardiovascular risk factors in a middle-aged normal population. PLoS One. 2008;3:e1774. doi: 10.1371/journal.pone.0001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindhe J., Nyman S. The effect of plaque control and surgical pocket elimination on the establishment and maintenance of periodontal health. A longitudinal study of periodontal therapy in cases of advanced disease. Journal of Clinical Periodontology. 1975;2:67–79. doi: 10.1111/j.1600-051x.1975.tb01727.x. [DOI] [PubMed] [Google Scholar]
- 36.Hugoson A., Jordan T. Frequency distribution of individuals aged 20–70 years according to severity of periodontal disease. Community Dentistry and Oral Epidemiology. 1982;10:187–192. doi: 10.1111/j.1600-0528.1982.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 37.Slots J., Ashimoto A., Flynn M.J., Li G., Chen C. Detection of putative periodontal pathogens in subgingival specimens by 16S ribosomal DNA amplification with the polymerase chain reaction. Clinical Infectious Diseases. 1995;20(Suppl. 2):S304–S307. doi: 10.1093/clinids/20.supplement_2.s304. [DOI] [PubMed] [Google Scholar]
- 38.Rescan P.Y., Loréal O., Hassell J.R., Yamada Y., Guillouzo A., Clément B. Distribution and origin of the basement membrane component perlecan in rat liver and primary hepatocyte culture. American Journal of Pathology. 1993;142:199–208. [PMC free article] [PubMed] [Google Scholar]
- 39.Schlessinger J., Lax I., Lemmon M. Regulation of growth factor activation by proteoglycans: what is the role of the low affinity receptors? Cell. 1995;83:357–360. doi: 10.1016/0092-8674(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 40.Liu K.X., Kato Y., Kato M., Kaku T.I., Nakamura T., Sugiyama Y. Existence of two nonlinear elimination mechanisms for hepatocyte growth factor in rats. American Journal of Physiology. 1997;273:E891–E897. doi: 10.1152/ajpendo.1997.273.5.E891. [DOI] [PubMed] [Google Scholar]
- 41.Wang N., Tong G., Yang J., Zhou Z., Pan H., Huo Y. Effect of hepatocyte growth-promoting factors on myocardial ischemia during exercise in patients with severe coronary artery disease. International Heart Journal. 2009;50:291–299. doi: 10.1536/ihj.50.291. [DOI] [PubMed] [Google Scholar]
- 42.Urbanek K., Rota M., Cascapera S., Bearzi C., Nascimbene A., De Angelis A. Cardiac stem cells possess growth factor–receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circulation Research. 2005;97:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]


