Abstract
Infectious bursal disease (IBD) is a highly contagious disease of chickens which leads to immunosuppression. In our previous study it was demonstrated that, possibly, CD4+ and CD8+ T cells may employ perforin and granzyme-A pathway for the clearance of IBDV-infected bursal cells. In this study, we evaluated the cytotoxic T cell responses involving two independently functioning but complementary mechanisms: Fas–Fas ligand and perforin–granzyme pathways in IBDV-infected chickens. As demonstrated previously, infection of chickens with IBDV was accompanied by influx of CD8+ T cells in the bursa and spleen. There was an upregulation in the gene expression of cytolytic molecules: Fas and Fas ligand (FasL), perforin (PFN) and granzyme-A (Gzm-A) in bursal and in the splenic tissues of IBDV inoculated chickens. Additionally, for the first time, we detected Fas, Fas ligand, Caspase-3 and PFN producing CD8+ T cells in the bursa and spleen of IBDV-infected chickens. The infiltration and activation of CD8+ T cells was substantiated by the detection of Th1 cytokine, IFN-γ. These data suggest that T cells may be involved in the clearance of virus from the target organ bursa and peripheral tissues such as spleen. The findings of these studies provide new insights into the pathogenesis of IBD and provide mechanistic evidence that the cytotoxic T cells may act through both Fas–FasL and perforin–granzyme pathways in mediating the clearance of virus-infected cells.
Abbreviations: Bursa of Fabricius, BF; Classical Infectious Bursal Disease Virus, cIBDV; Fas Ligand, FasL; Cytotoxic T Lymphocytes, CTLs; Granzyme, Gzm; Gamma Interferon, IFN-γ; Perforin, PFN; Post Inoculation Days, PIDs; Tumor Necrosis Factor, TNF; Quantitative RT-PCR, qRT-PCR
Keywords: Cytotoxic T cells, Fas–FasL, Granzyme, IBDV, Perforin, Virus clearance
1. Introduction
Poultry producers around the globe suffer significant economic losses inflicted by infectious bursal disease (IBD), which is a highly immunosuppressive viral disease of chickens. The IBD virus (IBDV) belongs to the family Birnaviridae and has a polyploid, bisegmented genome which enables the virus to reassort under field conditions [19]. The virus has predilection for lymphoid tissues especially the bursa of Fabricius (BF). IBDV antigens can also be detected in spleen, kidney, thymus and lungs [38], [39]. The BF becomes atrophic upon depletion of B cells during the acute phase of the disease which lasts for about 7–10 days [35].
T cells promptly infiltrate the bursa starting at an early stage of virus infection [42]. Colocalization of T cells with replicating virus suggested that T cells may be involved in the host defense. Although IBDV infection is controlled by antibody response, various studies have indicated T cell contribution in mediating protection against IBDV [29], [49].
Cytotoxic T cells exert antiviral functions via two principal mechanisms: a non cytolytic pathway through the secretion of antiviral cytokines such as gamma interferon (IFN-γ) and tumor necrosis factor alpha and a cytolytic pathway through the use of perforin–granzyme molecules or Fas and FasL interactions [7], [12], [13], [20], [30], [32]. Interactions between Fas on target infected cells and FasL on effector T cells lead to cytolysis via the activation of a death domain and a caspase apoptosis cascade [15], [22].
The Fas/FasL pathway uses a coordinated ligand which is able to lyse Fas receptor bearing-cells [18]. The Fas/FasL coordination transmits apoptotic signals from the surrounding milieu into the cell. Both Fas and FasL belong to the tumor necrosis factor (TNF) family and each contains a single transmembrane domain [11], [41]. The binding of FasL with Fas instigates receptor oligomerization, which engages Fas-associated death domain (FADD) [3]. The FADD binds procaspase-8 and allows activation of caspase-8 through self-cleavage [21]. Caspase-8 activates the effector caspases, which assign the cell to the controlled process of apoptosis [1]. Disruption of either the perforin or Fas–FasL cytolytic pathways adversely affected the control of several viral infections including, West Nile virus, lymphocytic choriomeningistis, mouse hepatitis, and Theiler's viruses [13], [24], [31], [37].
Previously we have shown the gene expression of PFN, Gzm-A and molecules involved in DNA repair and apoptosis and the presence of PFN producing CD4+ and CD8+ T cells in IBDV-infected bursa [27]. The goal of this study was to examine the activation of Fas–FasL pathway in the bursa and cytotoxic T responses in the spleen. Here we show the infiltration of CD8+ T cells and detection of Fas, FasL, caspase-3 and PFN positive cells and gene expression of Fas, FasL, PFN, Gzm-A, and IFN-γ genes in bursal and splenic tissues of IBDV infected chickens. These data indicate that activated T cells may be involved in antiviral immunity and mediation of virus clearance from the bursa and spleen of IBDV-infected chickens. The findings of this study will help in understanding the role of T cells in the pathogenesis of IBD and designing effective control strategies against this immunosuppressive viral disease of chickens.
2. Material and methods
The chicken experiment protocols (08-Ag-0029) were approved by the Animal Care and Use Committee of The Ohio State University.
2.1. Chickens and virus
Specific pathogen free (SPF) chicken eggs were incubated and hatched at The Ohio Agriculture Research and Development Center, The Ohio State University. The chickens were kept in a disease containment building that had rooms supplied with HEPA filter intake and exhaust air. At 3-weeks of age chickens were transferred to hard sided isolators supplied with HEPA filters for intake and exhaust air. The classical IBDV STC strain (cIBDV) [27] maintained in our laboratory was used to inoculate chickens.
2.2. Experimental design
Forty-eight 3 week-old chickens were allotted into 2 groups; 12 chickens were used as virus free controls and 36 chickens were inoculated intraocularly each with 104 embryo infectious dose50 (EID50)/200 μl of IBDV [27]. Twelve chickens were used as a virus free control group. At post inoculation days (PIDs) 3, 5 and 7, twelve chickens from the virus infected group and 4 chickens from the virus free group were euthanized; bursal and splenic tissues were collected. Bursa and spleen were examined for IBDV antigen presence and mononuclear cells were isolated and quantified for relative gene expression of Fas, FasL, PFN, Gzm-A, and IFN-γ by quantitative RT-PCR (qRT-PCR). In addition CD8+ T cells, Fas, FasL, caspase-3 and PFN positive cells were examined by immunohistochemistry.
2.3. Isolation of bursal and splenic mononuclear cells
Twelve individual bursa and spleens samples, collected at each PID were allotted into 4 pools of three bursa and spleens each from infected chickens. Bursa and spleens were also collected from 4 virus-free control chickens at each PID. Mononuclear cells were isolated from the bursal and splenic tissues as previously described [14], [26], [27]. The mononuclear cell suspensions were prepared from bursal and splenic tissues by density gradient centrifugation over Ficoll-Hypaque having a gradient density of 1.077 (GE Healthcare Bio-Sciences, Uppsala, Sweden), and washed twice in cold RPMI 1640 (Gibco, Carlsbad). The cell pellet was lysed by adding Trizol reagent (Invitrogen, Carlsbad, CA) and stored at −70 °C for RNA extraction.
2.4. RNA extraction and qRT-PCR
Total RNA from bursal and splenic mononuclear cells of IBDV-infected and virus free control chickens was extracted using the Trizol reagent (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. qRT-PCR was used to examine the gene expression of Fas, FasL, PFN, Gzm-A, and IFN-γ gene using previously published gene specific primers [5], [9], [27], [34]. qRT-PCR was performed using Power SYBER Green RNA—to-CT one step RT-PCR kit (Applied Bio System, Foster City, CA). Amplification and detection were performed in an automated 7500 Real time RT-PCR system (Applied Bio System, Foster city, CA) as described previously [14], [27].
Quantitation of the mRNA was determined by the comparative cycle threshold (CT) method as previously described [6], [14], [27].
2.5. Detection of CD8+ T cells, Fas/FasL, caspase-3 and PFN in IBDV-infected bursal and splenic tissues by immunohistochemistry
The IBDV antigen, CD8+ T cells, Fas, FasL, PFN and caspase-3 were detected in frozen sections of virus-free and IBDV-infected bursal and splenic tissues by immunohistochemistry as described previously [2], [27]. The primary and secondary antibodies used for the detection of IBDV antigen were R-63 anti-IBDV monoclonal antibody (ATCC, Manassas, VA, USA) and biotinylated goat anti-mouse IgG (H+L) 1:500 dilution (Vector laboratories, Burlingame, CA). The primary and secondary antibodies used for the detection of CD8+ T cells were: mouse anti-chicken CD8α at 1:200 dilutions (Southern Biotech, Birmingham, AL, USA) and biotinylated goat anti-mouse IgG (H+L) 1:500 dilution (Vector laboratories, Burlingame, CA). The primary and secondary antibodies used for the detection of Fas and FasL were: Mouse Anti-Human CD95 (Fas/Apo-1, clone DX-2) and Mouse Anti-Human Fas Ligand (Southern Biotech, Birmingham, AL, USA) at 1:100 and 1:200 dilutions respectively. The secondary antibody used for detection of Fas/FasL was biotinylated goat anti-mouse IgG (H+L) at 1:500 dilution (Vector laboratories, Burlingame, CA). After washing, the sections were incubated with ABC reagent (Vector laboratories, Burlingame, CA) for 30 min. The primary and secondary antibodies used for the detection of activated caspase-3 were: cleaved caspase-3 rabbit monoclonal antibody (1:200) (Cell Signaling Technologies, Inc., Danvers, MA, USA) and goat polyclonal anti rabbit IgG (H+L)–HRP at 1:1000 dilutions (Abcam Inc.; Cambridge, MA, USA). The primary and secondary antibodies used for the detection of PFN were: polyclonal rabbit anti-human PFN antibody (1:50) (BioVision Research Products, CA, USA) and goat polyclonal anti rabbit IgG (H+L)–HRP at 1:1000 dilutions (Abcam Inc.; Cambridge, MA, USA). The reaction was developed with 3-3′diaminobenzidine (DAB) substrate. The development of dark brown color indicates positive staining for CD8+ T cells, Fas/FasL, caspase-3 and PFN positives cells. The immunostained sections were evaluated using Olympus 1×70 microscope (Olympus Optical Co., Ltd., Tokyo, Japan). The number of CD8+ T cells, Fas/FasL, PFN and caspase-3 positive cells were counted as previously described [27], [28]. Briefly bursal and spleens sections from virus-free chickens and cIBDV-infected chickens were examined for the infiltration of CD8+ T cells and cytotoxic T cells mediators: Fas, FasL, Perforin and Caspase-3 by immunocytochemistry. Each immunostained section was examined at (20×) in blind manner given positive cells count based on T cell infiltration in tissues (1: 1–25%; 2: 26–50%; 3: 51–75%; 4: 76–100). The group means of the numbers of T cells and PFN-positive cells were determined per microscopic fields, at a magnification of 20× after counting 5 fields/bursa and or spleen/bird.
Additionally, Fas, PFN producing CD8+ T cells and viral antigen and caspase-3 combination were detected in the bursal and splenic tissues by double staining [2], [27]. In double staining, caspase-3, Fas and PFN staining was developed with DAB (brown color) and CD8+ T cells and viral antigen staining was developed by incubating the sections with commercial Vector-SG peroxidase substrate kit (gray blue color) (Vector laboratories, Burlingame, CA).
3. Results
3.1. Detection of IBDV genome and antigen
The IBDV infection was confirmed by gross bursal and splenic lesions at necropsy (PIDs 3, 5 and 7) and by detection of IBDV antigen at PID 5 in the bursal and splenic tissues (Fig. 1).
Fig. 1.
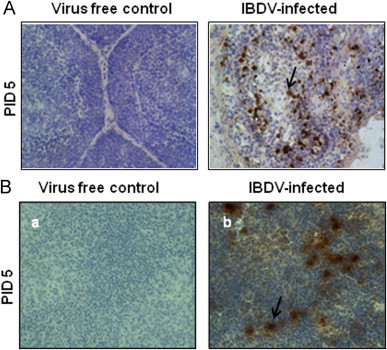
IBDV antigen detection by immunocytochemical staining in virus-infected bursa and spleen. SPF chickens were inoculated with 104EID50 of cIBDV; bursa and spleen tissues were collected at PIDs 3, 5 and 7. Bursal (A) and spleen (B) sections from virus-free chickens and cIBDV-infected chickens were examined (40×) for the presence of IBDV antigen by immunohistochemistry at PID 5.
3.2. Expression of PFN, Gzm-A, Fas, FasL and IFN-γ genes in bursa and spleen
Previously we have shown the expression of PFN and Gzm-A in IBDV infected bursa [27]. The relative gene expression of PFN, Gzm-A, Fas and FasL in bursal and splenic mononuclear cells was detected by qRT-PCR. Expression of PFN in splenic cells was downregulated early in the infection at PID 3 and upregulated at 5 and 7 PID (Fig. 2A). Consistent with PFN expression Gzm-A was also downregulated at PID 3 in splenic cells. It was at peak upregulated level at PID 5 and at lesser upregulated level at PID 7 in splenic cells (Fig. 2B). In contrast, Fas gene expression was at peak upregulation at PID 3, downregulated at PID 5 and upregulated at PID 7 in splenic cells (Fig. 2C). FasL gene expression was upregulated at all PIDs in splenic cells (Fig. 2D). The Fas/FasL gene expression was upregulated at all PIDs in bursal tissues of IBDV-infected chickens (Fig. 2E and F).
Fig. 2.
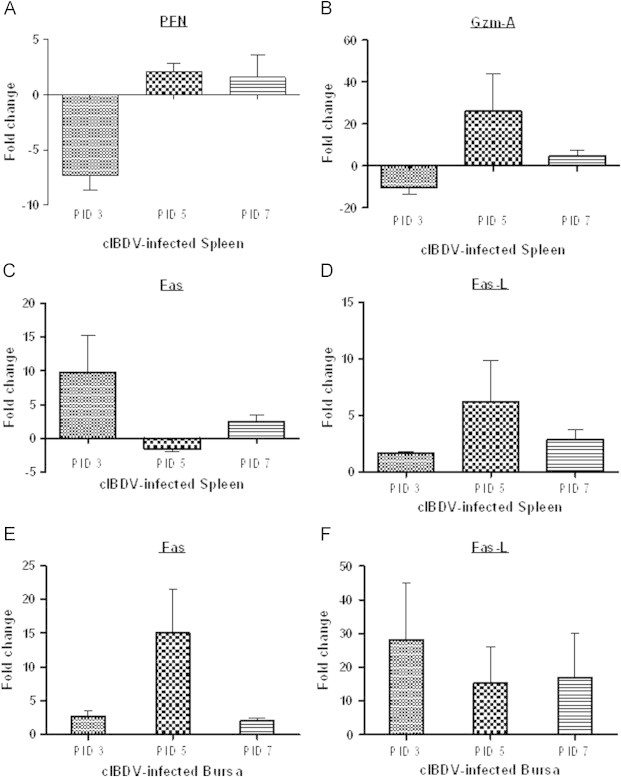
Relative gene expression of cytolytic molecules, PFN, Gzm-A, Fas and FasL mRNA in IBDV-infected spleen and bursa. At PIDs 3, 5 and 7, splenic and bursal mononuclear cells were isolated from cIBDV-infected and virus-free control chickens and examined for (A) PFN, (B) Gzm-A (C) Fas and (D) FasL gene expression by qRT-PCR in splenic cells and (E) and (F) in bursal mononuclear cells. Results are shown as transcription of the target gene relative to housekeeping gene 28S. The data are expressed as fold change expression in infected chickens over virus-free control. The values represent the mean±SEM of 4 pools of 3 spleens and bursa each at designated PID.
Activation of infiltrating T cells in bursa and spleen was examined by detecting the expression of IFN-γ in bursal and splenic cells by qRT-PCR. The IFN-γ expression in bursal cells was in accordance with our previous finding [27]. An increased relative expression of IFN-γ in IBDV-infected spleen cells was noted. The accumulation of IFN-γ mRNA was detectable at PID 3 (3.07±1.17 fold) which further increased at PID 5 to 7.51±2.86 fold. At PID 7, spleen cells from IBDV-inoculated chickens had approximately 9-fold (8.42±2.1 fold) higher levels of IFN-γ mRNA than spleen cells from control birds.
3.3. Detection of CD8+ T cells, Fas, FasL, PFN and caspase-3 in IBDV-infected bursal and splenic tissue sections by immunohistochemistry
The CD8+ T cells and PFN positive cells in the spleen (Figs. 3 and 4A) and Fas positive cells (Fig. 5A–B), FasL positive cells (Fig. 6A–B) and caspase-3 positive cells (Fig. 7A–B), were detected both in splenic and bursal tissues of IBDV-infected chickens by immunohistochemistry at PIDs 3, 5 and 7. The relative infiltration and accumulation of each cell type is shown in Table 1A and B. Caspase and both Fas/FasL infiltration was significantly different in cIBDV-infected chickens bursa as compared with control bursa. Peak infiltration was noted for FasL (3.33±0.61) and caspase-3 (2.7±0.23) at PID 5 whereas accumulation of Fas protein was slightly higher (2.93±0.64) at PID 3 (Table 1A). Similarly the Fas/FasL and caspase-3 infiltration was significantly higher in cIBDV-infected chickens splenic tissues at all PIDs. The Fas/FasL infiltration was at peak (3.7±0.11) and (3.46±0.5) at PID 5. Caspase 3 infiltration was also at peak (3.46±0.5) at PID 5.
Fig. 3.
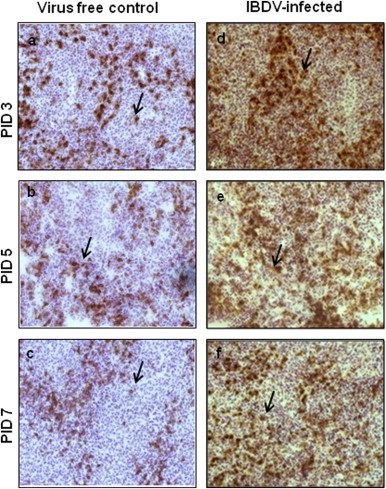
Infiltration of CD8+ T cells in the spleen. Three-week-old chickens were inoculated with IBDV and frozen sections of spleen tissues were prepared. At PIDs 3, 5 and 7 spleen sections from virus free chickens (a, b, and c) and IBDV-infected chickens (d, e, and f) were examined (40×) for the presence of CD8+ T cells by immunohistochemistry using anti-chicken CD8+ mAb. Brown color shown by arrow indicates the positive staining.
Fig. 4.
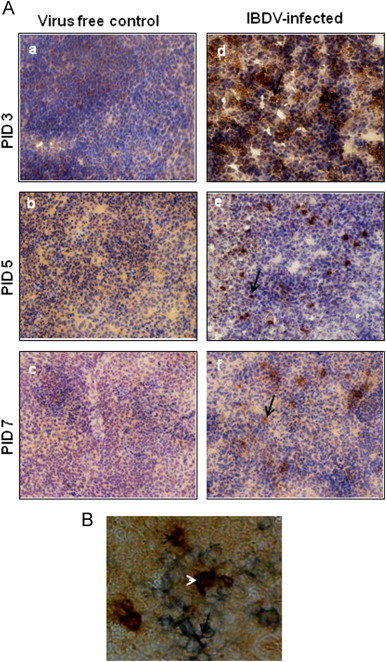
Detection of PFN producing CD8+ T cells in spleen. (A) Chickens were inoculated with IBDV and spleen tissues were collected at 3, 5 and 7 PID. Spleen sections from virus free chickens (a, b and c) and IBDV-infected chickens (d, e, and f) were examined (40×) for the presence of PFN by immunohistochemistry using anti-human PFN mAb. Development of brown color shown by arrow indicates positive staining for PFN. (B) Double staining for CD8+ cells, positive for PFN; blue staining shown by arrow represents CD8+ positive T cells and brown staining represented black arrow head shows PFN positive cells and white arrow head shows double positive cells respectively.
Fig. 5.
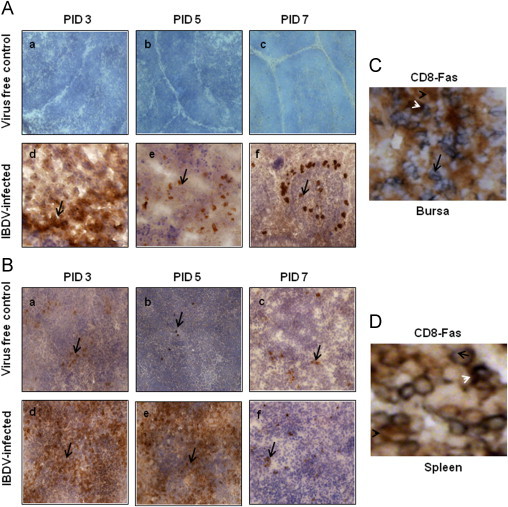
Detection of Fas positive cells in bursa and spleen (A, B). Chickens were inoculated with IBDV; bursa and spleen tissues were collected. At 3, 5 and 7 PID, bursa and spleen sections from virus free chickens (a, b and c) and IBDV-infected chickens (d, e, and f) were examined (40×) for the presence of Fas by immunohistochemistry using anti-human Fas mAb. Development of brown color shown by arrow indicates positive staining for Fas protein. Bursal and splenic tissue sections (C, D) were double stained by immunohistochemistry for the colocalization of CD8+ T cells and Fas positive cells. Blue staining shown by arrow represents CD8+ positive T cells and brown staining represented by black arrow head shows Fas positive cells, whereas white arrow head shows colocalization of CD8+ T cells and Fas positive cells respectively.
Fig. 6.
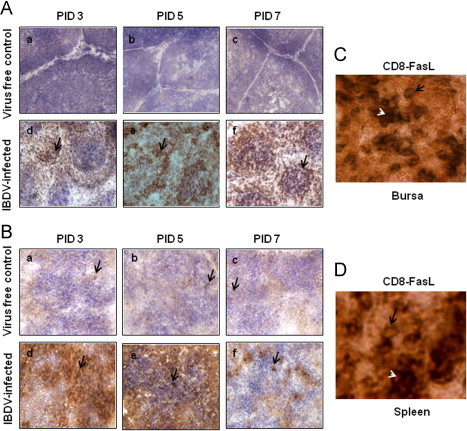
Detection of FasL in bursa and spleen (A-B). Chickens were inoculated with IBDV; bursa and spleen tissues were collected. At 3, 5 and 7 PID, bursa and spleen sections from virus free chickens (a, b and c) and IBDV-infected chickens (d, e, and f) were examined (40X) for the presence of FasL by immunohistochemistry using anti-human FasL mAb. Development of brown color shown by arrow indicates positive staining for FasL. Bursal and splenic tissue sections (C-D) were double stained by immunohistochemistry for the detection of CD8+ T cells and FasL double positive cells. Blue staining shown by arrow represents CD8+ positive T cells and blue/brown staining represented by white arrow head shows CD8+/FasL double positive cells.
Fig. 7.
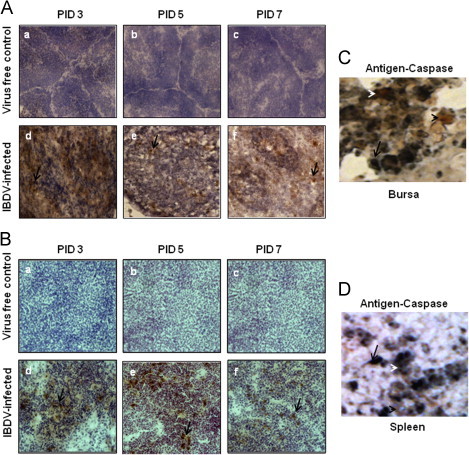
Detection of cleaved caspase-3 in bursa and spleen (A-B). Chickens were inoculated with IBDV; bursa and spleen tissues were collected. At 3, 5 and 7 PID, bursa and spleen sections from virus free chickens (a, b and c) and IBDV-infected chickens (d, e, and f) were examined (40X) for the presence of activated cleaved caspase-3 by immunohistochemistry using antihuman cleaved caspase-3mAb. Development of brown color shown by arrow indicates positive staining for cleaved caspase-3. Bursal and splenic tissue sections (C-D) were double stained by immunohistochemistry using cleaved caspase-3 and anti-IBDV R63 monoclonal antibodies. Blue staining shown by arrow represents IBDV antigen positive cells and brown staining represented by black arrow head shows caspase positive cells.
Table 1.
Detection and quantification of CD8+ T cells and cytotoxic T cells mediators: Fas, FasL, perforin and cleaved caspase-3 by immunohistochemistry in IBDV-infected chickens.
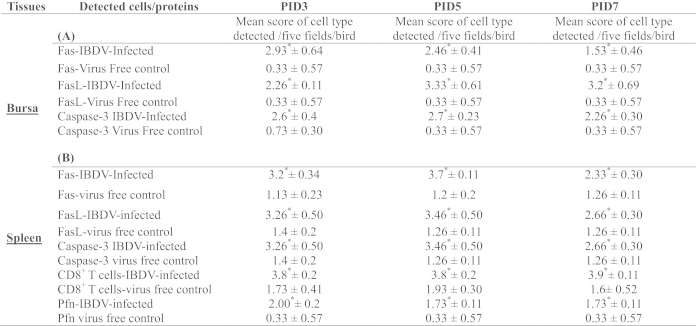 |
Foot notes. Three weeks old SPF chickens were inoculated with 104EID50 of cIBDV. Bursa and spleen tissues were collected at PIDs 3, 5 and 7. Bursal and spleens sections from virus-free chickens and cIBDV-infected chickens were examined for the detection of CD8+ T cells and cytotoxic T cells mediators: Fas, FasL, Perforin and cleaved caspase-3 by immunocytochemistry. Each immunostained section was examined (20×) and given positive cells count based on each cell type (Scores of positive cells/field: 1=1–25%; 2=26–50%; 3=51–75%; 4=76–100%). Student's t-test was used to detect significant differences between virus free control and IBDV-infected chickens. The values represent the mean ±SEM of 5 fields/bursa and/or spleen/chicken on designated PID (3, 5 and 7). * indicates statistically significant differences between virus free control and IBDV-infected groups (p<0.05).
Additionally we detected the colocalization of CD8+ T cells and Fas positive cells and caspase and IBDV antigen by double staining in IBDV-infected bursal and splenic tissues (Fig. 5C–D) and (Fig. 7C–D) respectively.
4. Discussion
The effective containment and clearance of virus-infected cells from invading pathogens require synchronized collaboration between effector lymphocytes [45]. There are two specific, cytotoxic mechanisms, that are functional in cytotoxic T lymphocytes (CTLs), one is based on cytolytic secretions of proteins (perforin and granzymes) and the other depends on cell surface ligand receptor (Fas/FasL) communications [18]. In a previous study we demonstrated that CD4+ and CD8+ T cells may be utilizing perforin and granzyme-A pathway to clear IBDV-infected bursal cells [27]. In this study, we report for the first time that, in addition to PFN and granzyme pathway, Fas/FasL pathway may also be activated in IBDV infected chickens. We for the first time, concurrently demonstrated the upregulated gene expression and detection of cytolytic molecules Fas, FasL, caspase-3, PFN and Gzm-A in the bursal and splenic tissues in IBDV-infected chickens. Extensive infiltration of CD8+ T cells in the bursal and splenic tissues of IBDV-infected chickens was also detected.
Cytotoxic T lymphocytes (CTLs) are the key players in the induction of immune response against virus-infected or transformed cells. The killing activity by cytotoxic cells of the target cells is achieved by releasing a membrane-disrupting protein known as perforin (PFN) and granzymes (Gzms) secretion by exocytosis which provoke apoptosis of the target cells [40], [45]. The granule-exocytosis pathway activates cell death through either caspase-dependent i.e. Fas/FasL or caspase-independent pathways i.e. PFN and Gzms pathway [33], [44], [45]. The Fas/FasL mechanism of cell cytotoxicity functions independent of PFN and requires the involvement of Fas ligand, which initiates the caspase-dependent pathway of apoptosis [23], [46].
To understand the mechanisms underlying T cell mediated virus clearance from the bursal and peripheral tissues of IBDV-infected chickens, we examined the two complimentary cytolytic pathways: caspase dependent Fas, FasL pathway and PFN and Gzm-A pathway in the bursal and splenic cells of IBDV-infected chickens. The FasL and cell death receptor Fas were upregulated both in bursal and splenic cells. We also detected the Fas, FasL and cleaved caspase-3 proteins in the bursal and splenic cells of IBDV infected-chickens. FasL is normally expressed and upregulated on activated T cells. The FasL binding to Fas have direct antiviral effects by inducing apoptosis of infected target cells [22]. Fas/FasL can activate the caspase family, especially caspase 3 and caspase 8, after combining with a homologous ligand and lead to apoptosis [17]. In our study, beside caspase gene expression caspase-3 accumulation was significantly higher in IBDV infected chickens, also indicating the Fas/FasL mediated clearance of IBDV infected cells. Fas/FasL mediated apoptosis/clearance of virus infected cells has been reported in several human viral infection including Dengue Virus, Influenza virus, HIV infection, and Herpes Simplex Virus [10], [17], [25], [48]. Similar to these viruses, it is likely that Fas/FasL mediated pathway is involved in the CD8+ T cells mediated clearance of IBDV-infected cells.
In our previous study, we demonstrated the PFN and Gzm-A gene expression in bursal tissues of IBDV infected chickens [27]. In this study, we detected higher expression of cytolytic proteins Gzm-A and PFN in splenic cells and PFN producing CD8+ T cells indicating the involvement of PFN and Gzms mediated cytotoxic mechanisms for the clearance of IBDV infected splenic cells which are in accordance with our previous findings. In PFN and Gzm-A pathway cytotoxic T cells kill virus-infected cells through the release of lytic proteins mainly PFN and Gzms that are secreted via exocytosis of pre-formed granules following recognition of infected targets [8], [16]. In several human viral infections including poliovirus infection, herpes simplex and West Nile virus infection, CTLs clear the virus infection via PFN mediated cytotoxic pathway [4], [36], [47].
The convergence of Fas/FasL mechanism and PFN and Gzm-A pathway for the induction of apoptosis has been reported in the clearance of lymphocytic choriomeningitis virus infection [18] influenza virus infection [43] and west Nile virus infection [37]. In choriomeningitis virus infection both perforin and FasL meditated cytotoxicity was required for the successful elimination of virus infected cells. Either perforin-deficient or FasL-deficient cytotoxic T-cells showed impaired lytic activity on target cells. The killing activity by CTLs was completely eliminated when both pathways were inactivated by using target cells from Fas-deficient mice and perforin free effector CTLs [18]. These findings strongly support our data and indicate that CD8+ T cells may be utilizing both perforin and FasL pathways for the elimination of IBDV-infected cells.
In summary, in this study, we demonstrated the gene expression of cytolytic molecules Fas, FasL, caspase-3 and PFN and the infiltration of CD8+ T cells in bursal and splenic tissues of IBDV infected chickens. Combining these data with our previous results [27], we conclude that CD8+ T cells may be using Fas/FasL and/or PFN-Gzm-A cytolytic pathways to clear IBDV virus in infected chickens. A more complete understanding of the effector mechanisms responsible for the T-cell clearance of IBDV infections may provide platform for the development of novel vaccines that stimulate a robust cell-mediated immune responses in addition to an antibody response.
Acknowledgments
Salaries and research support provided by state and federal funds appropriated to the Ohio Agriculture Research and Development Center, The Ohio State University. We are grateful to Dr. Juliette Hanson, Gregory Myers and Kingsly Berlin for their help in animal work.
Contributor Information
Mahesh Khatri, Email: khatri.14@osu.edu.
Yehia M. Saif, Email: saif.1@osu.edu.
References
- 1.Ashkenazi A., Dixit V.M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 2.Brewer L., LaRue R., Hering B., Brown C., Njenga M.K. Transplanting encephalomyocarditis virus-infected porcine islet cells reverses diabetes in recipient mice but also transmits the virus. Xenotransplantation. 2004;11:160–170. doi: 10.1046/j.1399-3089.2003.00102.x. [DOI] [PubMed] [Google Scholar]
- 3.Chinnaiyan A.M., O'Rourke K., Tewari M., Dixit V.M. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81:505–512. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 4.Dobbs M.E., Strasser J.E., Chu C.F., Chalk C., Milligan G.N. Clearance of herpes simplex virus type 2 by CD8+ T cells requires gamma interferon and either perforin- or Fas-mediated cytolytic mechanisms. Journal of Virology. 2005;79:14546–14554. doi: 10.1128/JVI.79.23.14546-14554.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eldaghayes I., Rothwell L., Williams A., Withers D., Balu S., Davison F. Infectious bursal disease virus: strains that differ in virulence differentially modulate the innate immune response to infection in the chicken bursa. Viral Immunology. 2006;19:83–91. doi: 10.1089/vim.2006.19.83. [DOI] [PubMed] [Google Scholar]
- 6.Giulietti A., Overbergh L., Valckx D., Decallonne B., Bouillon R., Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods. 2001;25:386–401. doi: 10.1006/meth.2001.1261. [DOI] [PubMed] [Google Scholar]
- 7.Harty J.T., Tvinnereim A.R., White D.W. CD8+ T cell effector mechanisms in resistance to infection. Annual Review of Immunology. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 8.Hersperger A.R., Pereyra F., Nason M., Demers K., Sheth P., Shin L.Y. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathogens. 2010;6:e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hghihghi H.R., Read L.R., Mohammadi H., Pei Y., Ursprung C., Nagy E. Characterization of host responses against a recombinant fowlpox virus-vectored vaccine expressing the hemagglutinin antigen of an avian influenza virus. Clinical and Vaccine Immunology. 2010;17:454–463. doi: 10.1128/CVI.00487-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ishikawa T., Yamada H., Oyamada A., Goshima F., Nishiyama Y., Yoshikai Y. Protective role of Fas–FasL signaling in lethal infection with herpes simplex virus type 2 in mice. Journal of Virology. 2009;83:11777–11783. doi: 10.1128/JVI.01006-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- 12.Kagi D., Ledermann B., Burki K., Seiler P., Odermatt B., Olsen K.J. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 13.Kagi D., Vignaux F., Ledermann B., Burki K., Depraetere V., Nagata S. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994;265:528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- 14.Khatri M., Palmquist J.M., Cha R.M., Sharma J.M. Infection and activation of bursal macrophages by virulent infectious bursal disease virus. Virus Research. 2005;113:44–50. doi: 10.1016/j.virusres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Krzyzowska M., Cymerys J., Winnicka A., Niemialtowski M. Involvement of Fas and FasL in Ectromelia virus-induced apoptosis in mouse brain. Virus Research. 2006;115:141–149. doi: 10.1016/j.virusres.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Kuerten S., Nowacki T.M., Kleen T.O., Asaad R.J., Lehmann P.V., Tary-Lehmann M. Dissociated production of perforin, granzyme B, and IFN-gamma by HIV-specific CD8(+) cells in HIV infection. AIDS Research and Human Retroviruses. 2008;24:62–71. doi: 10.1089/aid.2007.0125. [DOI] [PubMed] [Google Scholar]
- 17.Liao H., Xu J., Huang J. FasL/Fas pathway is involved in dengue virus induced apoptosis of the vascular endothelial cells. Journal of Medical Virology. 2010;82:1392–1399. doi: 10.1002/jmv.21815. [DOI] [PubMed] [Google Scholar]
- 18.Lowin B., Hahne M., Mattmann C., Tschopp J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature. 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 19.Luque D., Rivas G., Alfonso C., Carrascosa J.L., Rodriguez J.F., Caston J.R. Infectious bursal disease virus is an icosahedral polyploid dsRNA virus. Proceedings of the National Academy of Sciences USA. 2009;106:2148–2152. doi: 10.1073/pnas.0808498106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mullbacher A., Lobigs M., Hla R.T., Tran T., Stehle T., Simon M.M. Antigen-dependent release of IFN-gamma by cytotoxic T cells up-regulates Fas on target cells and facilitates exocytosis-independent specific target cell lysis. Journal of Immunology. 2002;169:145–150. doi: 10.4049/jimmunol.169.1.145. [DOI] [PubMed] [Google Scholar]
- 21.Muzio M., Chinnaiyan A.M., Kischkel F.C., O'Rourke K., Shevchenko A., Ni J. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 22.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 23.Nagata S., Golstein P. The Fas death factor. Science. 1995;267:1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- 24.Parra B., Lin M.T., Stohlman S.A., Bergmann C.C., Atkinson R., Hinton D.R. Contributions of Fas–Fas ligand interactions to the pathogenesis of mouse hepatitis virus in the central nervous system. Journal of Virology. 2000;74:2447–2450. doi: 10.1128/jvi.74.5.2447-2450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poonia B., Pauza C.D., Salvato M.S. Role of the Fas/FasL pathway in HIV or SIV disease. Retrovirology. 2009;6:91. doi: 10.1186/1742-4690-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rauf A., Khatri M., Murgia M.V., Jung K., Saif Y.M. Differential modulation of cytokine, chemokine and Toll like receptor expression in chickens infected with classical and variant infectious bursal disease virus. Veterinary Research. 2011;42:85. doi: 10.1186/1297-9716-42-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rauf A., Khatri M., Murgia M.V., Saif Y.M. Expression of perforin–granzyme pathway genes in the bursa of infectious bursal disease virus-infected chickens. Developmental and Comparative Immunology. 2011;35:620–627. doi: 10.1016/j.dci.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Rautenschlein S., Haase C. Differences in the immunopathogenesis of infectious bursal disease virus (IBDV) following in ovo and post-hatch vaccination of chickens. Veterinary Immunology and Immunopathology. 2005;106:139–150. doi: 10.1016/j.vetimm.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Rautenschlein S., Yeh H.Y., Sharma J.M. The role of T cells in protection by an inactivated infectious bursal disease virus vaccine. Veterinary Immunology and Immunopathology. 2002;89:159–167. doi: 10.1016/s0165-2427(02)00202-7. [DOI] [PubMed] [Google Scholar]
- 30.Regner M., Lobigs M., Blanden R.V., Mullbacher A. Effector cytolotic function but not IFN-gamma production in cytotoxic T cells triggered by virus-infected target cells in vitro. Scandinavian Journal of Immunology. 2001;54:366–374. doi: 10.1046/j.1365-3083.2001.00955.x. [DOI] [PubMed] [Google Scholar]
- 31.Rossi C.P., McAllister A., Tanguy M., Kagi D., Brahic M. Theiler's virus infection of perforin-deficient mice. Journal of Virology. 1998;72:4515–4519. doi: 10.1128/jvi.72.5.4515-4519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Russell J.H., Ley T.J. Lymphocyte-mediated cytotoxicity. Annual Review of Immunology. 2002;20:323–370. doi: 10.1146/annurev.immunol.20.100201.131730. [DOI] [PubMed] [Google Scholar]
- 33.Sarin A., Williams M.S., Alexander-Miller M.A., Berzofsky J.A., Zacharchuk C.M., Henkart P.A. Target cell lysis by CTL granule exocytosis is independent of ICE/Ced-3 family proteases. Immunity. 1997;6:209–215. doi: 10.1016/s1074-7613(00)80427-6. [DOI] [PubMed] [Google Scholar]
- 34.Sarson A.J., Abdul-Careem M.F., Read L.R., Brisbin J.T., Sharif S. Expression of cytotoxicity-associated genes in Marek's disease virus-infected chickens. Viral Immunology. 2008;21:267–272. doi: 10.1089/vim.2007.0094. [DOI] [PubMed] [Google Scholar]
- 35.Sharma J.M., Kim I.J., Rautenschlein S., Yeh H.Y. Infectious bursal disease virus of chickens: pathogenesis and immunosuppression. Developmental and Comparative Immunology. 2000;24:223–235. doi: 10.1016/s0145-305x(99)00074-9. [DOI] [PubMed] [Google Scholar]
- 36.Shrestha B., Diamond M.S. Role of CD8+ T cells in control of West Nile virus infection. Journal of Virology. 2004;78:8312–8321. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shrestha B., Diamond M.S. Fas ligand interactions contribute to CD8+ T-cell-mediated control of West Nile virus infection in the central nervous system. Journal of Virology. 2007;81:11749–11757. doi: 10.1128/JVI.01136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skeeles J.K., Lukert P.D., De Buysscher E.V., Fletcher O.J., Brown J. Infectious bursal disease viral infections. I. Complement and virus-neutralizing antibody response following infection of susceptible chickens. Avian Diseases. 1979;23:95–106. [PubMed] [Google Scholar]
- 39.Skeeles J.K., Lukert P.D., De Buysscher E.V., Fletcher O.J., Brown J. Infectious bursal disease viral infections. II. The relationship of age, complement levels, virus-neutralizing antibody, clotting, and lesions. Avian Diseases. 1979;23:107–117. [PubMed] [Google Scholar]
- 40.Smyth M.J., Trapani J.A. Granzymes: exogenous proteinases that induce target cell apoptosis. Immunology Today. 1995;16:202–206. doi: 10.1016/0167-5699(95)80122-7. [DOI] [PubMed] [Google Scholar]
- 41.Suda T., Takahashi T., Golstein P., Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993;75:1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- 42.Tanimura N., Sharma J.M. Appearance of T cells in the bursa of Fabricius and cecal tonsils during the acute phase of infectious bursal disease virus infection in chickens. Avian Diseases. 1997;41:638–645. [PubMed] [Google Scholar]
- 43.Topham D.J., Tripp R.A., Doherty P.C. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. Journal of Immunology. 1997;159:5197–5200. [PubMed] [Google Scholar]
- 44.Trapani J.A., Jans D.A., Jans P.J., Smyth M.J., Browne K.A., Sutton V.R. Efficient nuclear targeting of granzyme B and the nuclear consequences of apoptosis induced by granzyme B and perforin are caspase-dependent, but cell death is caspase-independent. Journal of Biological Chemistry. 1998;273:27934–27938. doi: 10.1074/jbc.273.43.27934. [DOI] [PubMed] [Google Scholar]
- 45.Trapani J.A., Smyth M.J. Functional significance of the perforin/granzyme cell death pathway. Nature Reviews Immunology. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 46.Van Parijs L., Abbas A.K. Role of Fas-mediated cell death in the regulation of immune responses. Current Opinion in Immunology. 1996;8:355–361. doi: 10.1016/s0952-7915(96)80125-7. [DOI] [PubMed] [Google Scholar]
- 47.Wahid R., Cannon M.J., Chow M. Virus-specific CD4+ and CD8+ cytotoxic T-cell responses and long-term T-cell memory in individuals vaccinated against polio. Journal of Virology. 2005;79:5988–5995. doi: 10.1128/JVI.79.10.5988-5995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wolf A.I., Mozdzanowska K., Quinn W J 3rd, Metzgar M., Williams K.L., Caton A.J. Protective antiviral antibody responses in a mouse model of influenza virus infection require TACI. Journal of Clinical Investigation. 2011;121:3954–3964. doi: 10.1172/JCI57362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeh H.Y., Rautenschlein S., Sharma J.M. Protective immunity against infectious bursal disease virus in chickens in the absence of virus-specific antibodies. Veterinary Immunology and Immunopathology. 2002;89:149–158. doi: 10.1016/s0165-2427(02)00206-4. [DOI] [PubMed] [Google Scholar]


