Abstract
Background and Aims
Our first objective was to evaluate the immune response to the adjuvanted 2009 A/H1N1 pandemic (pH1N1) vaccine in inflammatory bowel disease (IBD) patients treated with anti-TNF-α alone or combined with immunosuppressants (IS). Second and third aims were the safety of pH1N1 vaccine and the effects on IBD clinical activity.
Methods
36 patients with Crohn's disease (CD) and 26 with ulcerative colitis (UC) and thirty-one healthy control subjects (HC) were enrolled. 47 patients were on anti TNF-α maintenance monotherapy and 15 on anti TNF-α combined with IS. Sera were collected at baseline (T0) and 4 weeks after the vaccination (T1) for antibody determination by hemagglutination inhibition (HAI Disease activity was monitored at T0 and T1.
Results
Seroprotective titers (≥ 1: 40) in patients were comparable to HC. Seroconvertion rate (≥ 4 fold increase in HAI titer) was lower than HC in IBD patients (p=0,009), either on anti TNF-α monotherapy (p=0,034) or combined with IS (p=0,011). Geometric mean titer (GMT) of antibodies at T1 was significantly lower in patients on combined therapy versus those on monotherapy (p=0,0017) and versus HC (p=0,011). The factor increase of GMT at T1 versus T0 was significantly lower in IBD patients versus HC (p=0,042), and in those on combined immunosuppression, both versus monotherapy (p=0,0048) and HC (p=0,0015). None of the patients experienced a disease flare.
Conclusion
Our study has shown a suboptimal response to pH1N1 vaccine in IBD patients on therapy with anti TNF-α and IS compared to those on anti-TNF-α monotherapy and HC.
Keywords: Inflammatory bowel disease, H1N1 influenza vaccine, anti TNF-α agents, immunosuppression
Introduction
Patients with Crohn's disease (CD) and ulcerative colitis (UC) have subtle alterations in immunity. Genetic mutations associated with changes in innate immunity (including NOD2 and IL23R) have been found in inflammatory bowel disease (IBD) patients.[1-4] IBD patients are often placed on long term anti-inflammatory and immunosuppressive therapies, including aminosalicylates, corticosteroids, 6-mercaptopurine, azathioprine, methotrexate, and tumor necrosis factor (TNF)-α inhibitors. Although a majority of IBD patients do not develop serious infections, reports of life-threatening infections (e.g. varicella, tuberculosis) have been published, primarily in patients receiving immunosuppressive therapy.[5-8]
In April 2009, the US Centers for Disease Control and Prevention (CDC) identified the first two cases of a novel swine origin (H1N1) virus. The lack of immunity to this virus and ease of its human-human transmission resulted in widespread outbreaks in different countries, prompting the World Health Organization to declare an ongoing world pandemic of novel influenza H1N1 on June 11, 2009.[9] The clinical manifestations of 2009 H1N1 influenza were similar to seasonal influenza.[10] The risk factors for severe infection due to H1N1 influenza were also similar to seasonal influenza and include different chronic underlying medical conditions such as heart, lung, renal and liver disease, cancer, immunosuppression, as well as pregnancy.[11,12] The most effective way to prevent influenza and its complications is vaccination. Vaccination against influenza with inactivated vaccines is recommended for inflammatory bowel disease (IBD) patients according to published guidelines both in the US [13] and Europe.[14] In 2009 the outbreak of pandemic swine influenza of a new H1N1 strain lead to a strong recommendation for vaccination of at risk categories which comprise chronic inflammatory diseases and immunosuppressed patients.[15] However, vaccine studies in patients with chronic illnesses suggest there are varying degrees of serologic response to vaccination. Few studies about the influenza vaccine were conducted in IBD patients and they have shown some conflicting results. Two studies were conducted in pediatric IBD populations. One study [16] demonstrated that children with IBD and particularly those receiving both infliximab and immunomodulators had a lower response to some influenza strains in the vaccine compared to HC. At variance, Lu et al.[17] in a study on 146 children and young adults with IBD found that influenza vaccination produced a high prevalence of seroprotection in these subjects, particularly against A strains. No differences were detected among non-immunosuppressed and immunosuppressed patients. A few studies conducted in adult IBD patients found that the immune response to vaccinations in IBD patients may depend on the type of immunosuppressive therapy they receive, in particular anti TNF-α therapy.[18,19,20] Two of these studies[18,19] evaluated the response to H1N1 vaccination in IBD patients.
In recent years the issue of a higher efficacy of a combination of immunosuppressors and anti TNF-α, both in CD [21] and in UC [22] has been greatly debated; furthermore the potential higher efficacy needs to be balanced with the possible higher risks of opportunistic infections and lymphoproliferative diseases. The possible effect of combination therapy in IBD also on vaccine response needs to be better investigated.
The aims of our study were first to evaluate the immune response to the pH1N1 vaccine in patients with IBD treated with anti-TNF-α alone or combined with immunosuppressive therapy, and to compare them with healthy control subjects; then to analyze the safety and efficacy of influenza A/H1N1 vaccine in IBD patients and lastly to determine the effects of pH1N1 vaccine on IBD clinical activity.
Methods
Subjects
This prospective observational study was conducted at the outpatients IBD clinic of the Catholic University Sacro Cuore in Rome, Italy, from November 2009 to January 2010. The protocol was approved by our hospital's ethics committee, and written informed consent was obtained from all participants before enrollment. We enrolled 62 consecutive IBD patients on maintenance treatment at our IBD outpatient clinic with anti TNF-α agents (Infliximab, Adalimumab, Certolizumab pegol) for at least 4 months (median 16, range 4-78). Mean age of patients was 40 years (range 18-75), while mean age of HC was 28 (range 26-55). 26 patients were affected by Ulcerative Colitis (UC) and 36 by Crohn's disease (CD). Patients were divided into two subgroups: those on anti TNF-α monotherapy (N=47) and those on anti TNF-α combined with immunosuppressants (IS) or corticosteroids (N=15). Combined therapy was as follows: azathioprine/6MP (2-2,5 mg/Kg) in ten patients, methotrexate (10-15 mg/week) in four patients and methyl-prednisolone (20 mg/day) in one patient. All IS were at stable dose from at least 4 months, while patients on monotherapy were off IS therapy from at least 4 months. We also enrolled 31 healthy controls (HC) among hospital personnel, during the pandemic vaccine campaign, on a voluntary basis. All patients and controls lived in the same geografic area (central Italy).
Study protocol
At baseline (T0), a physical examination was performed on all patients, and blood specimens were obtained to measure clinical and laboratory parameters of IBD activity. We assayed erythrocyte sedimentation rate (ESR), C reactive protein (CRP), lymphocyte count and percentage and then we stored serum samples at −80°C for later assay of IgG antibodies to A/California/7/2009 (H1N1) by hemagglutination inhibition (HAI). Disease location and behaviour were described according to the Montreal classification,[23] and data were collected from patients' charts and interviews, relative to the duration of disease and to the duration of anti TNF-α therapy. We evaluated the disease activity in patients with ulcerative colitis (UC) by partial Mayo score (pMayo),[24] while in patients with Crohn's disease by Harvey Bradshaw index (HBI).[25] All the patients and controls were vaccinated with the MF59-adjuvanted vaccine A/California/7/2009 (H1N1), Focetria®, Novartis, the only p2009 influenza vaccine available in Italy. Each 0.5 ml dose of vaccine has the following composition: hemagglutinin (HA) and neuraminidase (NA) antigens from the influenza virus strain recommended by WHO/EU for the pandemic ≥ 7.5 μg HA (propagated in eggs) – employed strain NYMC X-181; adjuvant MF59C.1 composed of: squalene 9.75 mg, polysorbate 80 1.175 mg, sorbitan trioleate 1.175 mg; other ingredients: sodium chloride, potassium chloride, potassium dihydrogen phosphate, disodium phosphate dehydrate, magnesium chloride hexahydrate, calcium chloride dehydrate, Thiomersal (included only in multi-dose vials), sodium citrate, citric acid, water for injections.[26]
The subjects were contacted by phone at 72 hours after immunization to document the occurrence of adverse events.
At T1, 4-6 weeks after the vaccination, a physical examination was performed on all patients, HBI or pMayo was calculated, as appropriate, and blood specimens were obtained to measure ESR, CRP, lymphocyte count and percentage and to obtain serum samples to store at -80°C for later assay by HAI. Data were recorded also concerning the occurrence of previous flu vaccination in the last 3 years (2006, 2007, 2008), and the concomitant 2009 seasonal flu vaccination.
Blood samples were obtained at T0 and T1 also from HC and stored in the same conditions.
Outcomes
The primary outcome of the study was the immune response to H1N1 vaccine in HC and patients with IBD as measured by pre immunization and post immunization hemagglutination inhibition (HAI) titer. The secondary outcome was the safety of the H1N1 vaccine. The tertiary outcome was the effect of H1N1 vaccine on IBD clinical activity.
Hemagglutination inhibition (HAI) assay
HAI was performed simultaneously on all study samples according to previously described method.[27] Briefly, the sera were pretreated with receptor destroying enzyme (RDE II, Seiken Co Ltd) for 20 h at 37°C then heated at 56°C for 60 min, 25 μl of serial dilutions of sera were incubated with 4 HA units of whole vaccine for 1h at RT and 50 μl of 1,25% suspension of human RBC type O were added and after 2 h at RT titers were determined. HAI results were reported as follows: seroprotection rate (% subjects with T1 titer ≥ 1:40), seroconvertion rate (% subjects having ≥ 4 fold increase in titer), Geometric mean titer (GMT) = antilog (Σ log reciprocal HAI titer)/number of subjects and the Factor increase of GMT between T1 and T0, as described. [28] We considered as the cut off level for each measure of immunogenicity those indicated for adults by the European Committee for Propetary Medicinal Products (CPMP) for the assessment of influenza vaccines, i.e. 70% seroprotection, 40% seroconversion and >2,5 factor increase of GMT.[29] In particular, for a virus with the potential to cause pandemics, all three criteria should be fulfilled.[30]
Statistical analysis
We calculated the sample size based on the study of Mamula et al [16], who detected for H1N1 vaccine a difference in response ranging from 34 to 12% between IBD patients on anti TNF-α and/or IS therapy and HC,. With α value of 0.05 and a power of 80% the minimal required sample to detect a difference of 25% was 29. We enrolled a higher number of patients to allow subgroup comparison. Data analysis was undertaken with the use of MedCalc® software, version 9.2.1.0. We used a two-sides Fisher's exact test to compare proportions between vaccine groups. All p values reported are two-sided, with no adjustment for multiple testing; values of 0,05 or less were considered to indicate statistical significance. For immunogenicity analyses, the geometric mean antibody titers at each time point were used. Geometric mean titers and 95% confidence intervals were computed by taking the exponent (log10) of the mean and of the lower and upper limits of the 95% confidence intervals of the log10 transformed titers. Normality was checked by D'Agostino-Pearson test. The group comparisons were performed by Mann Whitney test, Wilcoxon test and t test for paired and unpaired samples (two-tailed) and statistical correlations were tested by Spearman's rho and regression analysis, as appropriate.
Results
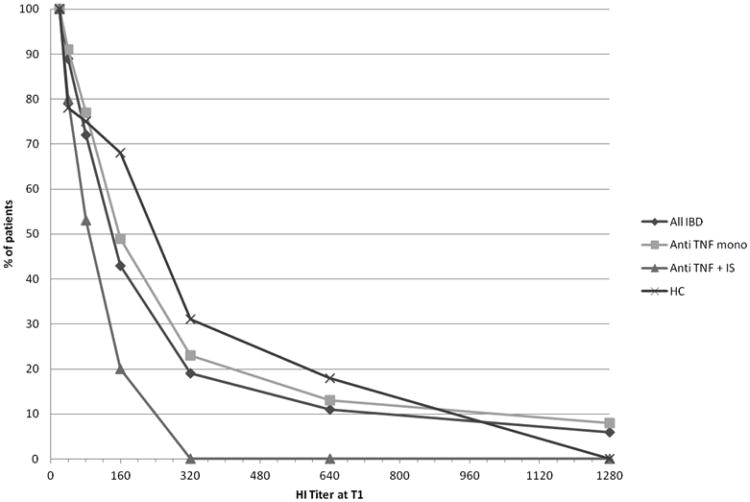
The demographic and clinical characteristics of IBD patients are shown in Tables I, II and III. The HAI response of patients and controls to the A/H1N1 influenza vaccination is shown in Table IV. Pre-vaccination HAI titer ≥ 1:40 were comparable between IBD patients an controls (54% vs 64%, p=not significant by χ2test). IBD patients on anti TNF-α therapy showed rates of seroprotection at T1 comparable to those of HC. The seroconversion rate (≥ 4 fold increase in titer at T1) was lower than HC in the whole population of patients (p=0,009) and in the group on anti TNF-α therapy, either alone (p=0,034) or combined with IS (p=0,011). The GMT at T1 was significantly lower in patients on anti TNF-α combined with IS compared both with HC and with patients on monotherapy (p=0,011 and p= 0,017, respectively). The reverse cumulative distribution curves of HAI titers at T1 are shown in Fig. 1, showing the markedly reduced titers of patients on anti TNF-α combined with IS. The factor increase of GMT at T1 was significantly lower in the IBD patients on anti TNF-α therapy versus the HC (p=0,042), and was lower in the group of patients on combined therapy versus those on anti TNF-α monotherapy (p= 0,0048) and versus the HC (p=0,0015). In the patients on combined therapy the seroconversion rate and the factor increase of GMT did not reach the EMEA requirements [30] for vaccine immunogenicity in a pandemic setting, but those on TNF-a monotherapy did (Table IV).
Table I.
Demographic and clinical characteristic of the studied subjects.
| N | Age* | Gender (M/F) | Disease duration (years)* | Previous flu vaccinanation 2006-2008 N (%) | Concomitant 2009 seasonal flu vaccination N (%) | |
|---|---|---|---|---|---|---|
| All IBD | 62 | 40 (18-75) | 28/34 | 7,5 (1-32) | 23 (37) | 20 (32) |
| Crohn's disease | 36 | 38 (18-69) | 22/14 | 7,5 (1-32) | 12 (33) | 13 (36) |
| Ulcerative colitis | 26 | 44 (29-75) | 6/20 | 7,5 (2-21) | 11 (42) | 7 (27) |
| Anti TNF monotherapy | 47 | 40 (18-69) | 24/23 | 8 (1-32) | 17 (36) | 17 (36) |
| Anti TNF and IS | 15 | 47 (20-75) | 11/4 | 7 (1-21) | 6 (40) | 3 (20) |
| HC | 31 | 31,5 (20-55) | 10/21 | na | 9 (29) |
Median (range)
Table II. Montreal Classification.
| A1 | A2 | A3 | L1 | L2 | L3 | B1 | B2 | B3 | p | E2 | E3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crohn's disease | 5 | 22 | 9 | 13 | 4 | 19 | 14 | 10 | 12 | 9 | ||
| Ulcerative Colitis | 10 | 16 |
Table III. Type of anti TNF-α treatment of the IBD patients (number of patients).
| Crohn's disease | Ulcerative Colitis | Total | |
|---|---|---|---|
| Anti TNF monotherapy | 27 | 20 | 47 |
| Infliximab | 14 | 19 | 33 |
| Adalimumab | 12 | 1 | 13 |
| Certolizumab pegol | 1 | 0 | 1 |
| Anti TNF and IS | 9 | 6 | 15 |
| Infliximab | 4 | 4 | 8 |
| Adalimumab | 5 | 2 | 7 |
Table IV. Response to the influenza A/H1N1 vaccination in IBD patients on anti TNF-α therapy.
| IBD on anti TNF-α | Anti TNF-α monotherapy | Anti TNF-α + IS | HC | EMEA Requirements* | |
|---|---|---|---|---|---|
| Seroprotection n/total (%) | 55/62 (88) | 43/47 (91) | 12/15 (80) | 25/31 (81) | 70 % |
| Seroconversion n/total (%) | 28/62 (45)Δ | 23/47 (49)∞ | 5/15 (33)& | 23/31 (74) | 40 % |
| GMT at T0 (95% CI) | 34,58 (30,43-39,31) | 35 (30,12-40,73) | 33,25 (25,38-43,56) | 29,25 (25,71-33,26) | |
| GMT at T1 (95% CI) | 102,30 (77,76-134,60) | 122,7 (88,43-170,23) | 57,9 (38,54-87)‡@ | 139,91 (90,8-215,63) | |
| Factor Increase of GMT (95% CI) | 2,96 (2,25-3,87)¤ | 3,5 (2,52-4,86) | 1,74 (1,21-2,5)§◆ | 4,68 (3,25-7,05) | 2,5 |
IS=immunosuppressant
HC=healthy controls
GMT= geometric mean titer
Fisher 's test:
p= 0,009 vs HC
p=0,034 vs HC
p=0,011 vs HC
t test:
p=0,011 vs HC
p= 0,042 vs HC
p=0,0015 vs HC
p=0,017 vs anti TNF mono
p=0,0048 vs anti TNF mono
Fig.1.
Response to influenza A/H1N1 vaccine in patients with IBD on anti TNF-α. Reverse cumulative distribution curves of HI titers at T1 of patients and HC.
No correlation was found between seroconversion rate, GMT and factor increase with age, gender, duration of disease and treatment, with the type of anti TNF-α, disease activity, Montreal classification, and flu vaccination in the last 3 years. The GMT at T1 of the IBD patients was significantly correlated with previous or concomitant vaccination for 2009 seasonal influenza (p = 0,036) and with GMT at T0 (p=0,027). Multiple regression analysis of GMT at T1 revealed the following independent variables: combined therapy (p=0,036) and the previous vaccination for 2009 seasonal influenza (p=0,017). After exclusion of the subjects vaccinated for seasonal flu the GMT at T1 was 94 for patients on anti TNF-α monotherapy and 53 for those on combined therapy (p=0.054) and the factor increase was 4,5 vs. 1,9, respectively (p=0.0068).
As far as the safety was concerned, none of the studied patients complained of injection site symptoms after the vaccination; 2 patients presented headache, one of them on combined therapy, 2 patients presented malaise and 1 patient presented shivering. None of them reported a flare of the IBD at the T1 visit. No difference was found among the mean HBI and p Mayo scores at T1 versus T0 (Table V).
Table V. Activity scores and laboratory parameters of IBD patients before and after A/H1N1 influenza vaccination mean values (95% CI).
| n. | T0 | T1 | p values (paired t test) | |
|---|---|---|---|---|
| Harvey Bradshaw Index | 36 | 1,64 (0,73 - 2,56) | 1,86 (0,93 - 2,79) | 0,15 |
| Partial Mayo score | 26 | 2,61 (1,58 - 3,65) | 3(2,01-3,98) | 0,18 |
| ESR (mm) | 62 | 19,67 (15,7-23,63) | 20,08 (15,88-24,29) | 0,73 |
| CRP (mg/l) | 62 | 5,05 (2,5 – 7,57) | 5,2 (2,25 – 8,16) | 0,83 |
| Peripheral Blood Lymphocyte count (cells/μl) | 62 | 2153 (1954 – 2351) | 2083 (1865 – 2301) | 0,42 |
| Peripheral Blood Lymphocyte % | 62 | 32,21 (29,66 -34,76) | 31,19 (28,38 – 34) | 0,37 |
Discussion
Our study has shown that our population of IBD patients on anti TNF-α therapy has reached the minimum seroprotective titer of 1:40 at HAI in proportions comparable to HC during the pandemic 2009 A/H1N1 vaccine campaign. However when the response is analyzed in a quantitative way significant differences are detectable: the percentage of patients achieving seroconversion is lower than HC and, furthermore, seroconversion rate is lower than HC in the patients on anti TNF-α therapy combined with immunosuppressors. In these patients on combined therapy, in particular, the GMT after the vaccination is significantly lower than in patients on anti TNF-α monotherapy. A limitation of our study is the limited number of enrolled patients and the lack of an IBD control group without IS.
In general, vaccine studies in IBD are very difficult to compare. Authors have collected patients affected by different IBD pathologies, at different stages of clinical activity. The effect of age, which is well known to affect immune response to vaccination,[27] has not been always taken in account. Also the effect of immunosuppressive treatments is often difficult to interpret: some studies compared anti TNF-α to other IS or to combined IS therapy, or to non IS drugs, like mesalamine. Furthermore, not all studies have included HC and they have not employed the same measures of the immune response to influenza vaccines. A diversity in immunization status before vaccination could happen in different years and in distinct populations and different vaccination schedules were employed in some studies.
The first studies on influenza vaccine in IBD were conducted in pediatric populations vaccinated with trivalent inactivated vaccine (TIV). In these studies[16] it was shown that children with IBD had a lower seroconversion rate compared to HC and that patients receiving both infliximab and immunomolulators were seroconverting less than HC. Another study on pediatric IBD patients receiving 2 doses of TIV, one month apart,[17] showed a high prevalence of seroconversion in IBD patients regardless of treatment. The very few studies conducted in adult IBD patients confirmed that the immune response to vaccinations in IBD patients may depend on the type of immunosuppressive therapy they receive, together with anti TNF-α therapy. Gelinck [19] studied 112 autoimmune patients treated with TNF-α inhibitors and showed that the percentage of subjects who had a seroprotective titer was high, but post-vaccination titers were significantly lower in subjects treated with TNF-α inhibitors as compared to HC. The A/H1N1 pandemic vaccine campaign represented a good chance to explore the response to a monovalent vaccine for a “relatively” new virus. Two recent studies were conducted during the 2009 pandemic vaccine campaign. Molnar et al.[20] studied 24 adult IBD patients (15 on IS, 7 on biologicals and 2 on combined therapy) by a different method (microneutralization assay). The authors showed seroprotective titers in every patient independently from the type of medication. The study, although limited by small number and lack of pre-vaccination measures, indicated, similarly to our study, that vaccination is able to induce seroprotection in the majority of IBD patients. At variance, a recently published study[18] described the results of a prospective study on 105 adult IBD patients who received a single dose of non adjuvanted A/H1N1 2009 pandemic vaccine. 28 patients were non immunosuppressed and 77 immunosuppressed. This study has shown a low rate of seroprotection, particulary among patients on IS. However, no HC were included and the overall proportion of seroprotection was 50%, with no differences among immunosuppressed and non immunosuppressed patients. In this population, at variance with ours, the pre-vaccination seroprotection rate was low.
The immune response to vaccines in IBD can be discussed in the frame of vaccine response in other immune mediated inflammatory diseases (IMID).[31] Several studies have shown that adult patients with rheumatoid arthritis (RA), treated with anti-TNFα, have similar antibody titer levels to influenza vaccine compared to controls.[32,33] Other studies in patients treated with IS or anti TNF-α have indicated some reduction in the response, however a protective immune response after influenza vaccination was achieved in the majority of patients.[34-36] The only randomized controlled trial (RCT) available in this field[37] was conducted in RA patients who were treated with adalimumab or placebo and underwent influenza and pneumococcal vaccination, showing no effect of biological therapy on vaccination.
The safety of vaccines has been a concern both for IBD patients and their treating physicians, and this may be one of the reasons for the poor compliance to influenza vaccination that has been reported in these patients.[38] The possibility that immune mediated diseases could flare after a vaccination cannot theoretically be excluded and, indeed, a case report has been published.[39] However a recent observational study[40] was conducted on 575 IBD patients during the pandemic vaccine campaign, to evaluate the safety of both adjuvanted and not adjuvanted vaccines, containing different virus antigen doses. The authors observed at four weeks after the vaccination no reactivation of the disease in the large majority of patients (96,7% CD and 95,6% UC), and they found that the increase of disease activity was not related to the type of vaccine. Our study confirmed those findings and also showed the lack of change of laboratory indices of inflammatory activity such as CRP and ESR, as well as of lymphocyte count and percentages.
Our study has shown a suboptimal response to A/H1N1 2009 vaccine in IBD patients on combined therapy with anti TNF-α and IS, although the seroprotective 1:40 titer was reached by the majority of patients. Similar results to ours where there was a normal response among non immunosuppressed IBD patients and an impaired response in IBD patients on combined immunosuppression were also described for pneumococcal polysaccharide vaccination.[41] The real meaning of these findings in terms of reduced protection from infection is unknown, however, considering the EMEA requirements for influenza vaccine in a pandemic setting, the response of IBD patients on combined therapy can be considered at risk to be insufficient. So our results clearly indicate quantitatively reduced response to influenza vaccine in IBD patients on combined immunosuppression. Different strategies to further overcome this reduced response can be explored. In at-risk groups, post-vaccination testing could be proposed. A double injection schedule with a recall one month after the first injection could also be used. Given our results the use of adjuvanted vaccine in IBD patients could be explored in appropriately designed studies. The efficacy and safety of these vaccine strategies in IBD patients on immunosuppressive therapy need to be ascertained by appropriately designed double blind randomized controlled studies, addressing different patient categories and including control groups.
Acknowledgments
Funding: This research received no specific funding
Footnotes
Competing Interests: There are no competing interests
Contributorship: GL,GA,DF,BBB: conception and design, analysis and interpretation of data, drafting the article, revising it critically for important intellectual content and final approval of the version to be published; MR,MM,CF,GM: data collection; AA,AP,IDV,GLR: analysis and interpretation of data revising the article for important intellectual content and final approval of the version to be published
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 2.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, Achkar JP, Brant SR, Bayless TM, Kirschner BS, Hanauer SB, Nuñez G, Cho JH. A frameshift mutation in NOD2 assoiated with susceptibily to Crohn's disease. Nature. 411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 3.Baldassano RN, Bradfield JP, Monos DS, Kim CE, Glessner JT, Casalunovo T, Frackelton EC, Otieno FG, Kanterakis S, Shaner JL, Smith RM, Eckert AW, Robinson LJ, Onyiah CC, Abrams DJ, Chiavacci RM, Skraban R, Devoto M, Grant SF, Hakonarson H. Association of variants of the interleuckin-23 receptor gene with susceptibility to pediatric Crohn's disease. Clin Gastroenterol Hepatol. 2007;5:972–976. doi: 10.1016/j.cgh.2007.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, Dassopoulos T, Bitton A, Yang H, Targan S, Datta LW, Kistner EO, Schumm LP, Lee AT, Gregersen PK, Barmada MM, Rotter JI, Nicolae DL, Cho JH. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rau R, Fitzhugh CD, Baird K, Cortez KJ, Li L, Fischer SH, Cowen EW, Balow JE, Walsh TJ, Cohen JI, Wayne AS. Triad of severe abdominal pain, inappropriate antidiuretic hormone secretion, and disseminated varicella-zoster virus infection preceding cutaneous manifestations after hematopoietic stem cell transplantation:untily of PCR for early recognition and therapy. Pediatr Infect Dis J. 2008;27:265–8. doi: 10.1097/INF.0b013e31815cb239. [DOI] [PubMed] [Google Scholar]
- 6.Deutsh DE, Olson AD, Kraker S, Dickinson CJ. Overwhelming varicella pneumonia in a patient with Crohn's disease treated with 6-mercaptopurine. J Pediatr Gastroenterol Nutr. 1995;20:351–3. doi: 10.1097/00005176-199504000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Korelitz BI, Fuller SR, Warman JI, Goldberg MD. Shingles during the course of treatment with 6 mercaptopurine for inflammatory bowel disease. Am J Gastroenterol. 1999;94:424–6. doi: 10.1111/j.1572-0241.1999.871_w.x. [DOI] [PubMed] [Google Scholar]
- 8.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N Engl J Med. 2001;345:1098–104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 9.Fraser C, Donnelly CA, Cauchemez S, Van Kerkhove MD, Hollingsworth TD, Griffin J, Baggaley RF, Jenkins HE, Lyons EJ, Jombart T, Hinsley WR, Grassly NC, Balloux F, Ghani AC, Ferguson NM, Rambaut A, Pybus OG, Lopez-Gatell H, Alpuche-Aranda CM, Chapela IB, Zavala EP, Guevara DM, Checchi F, Garcia E, Hugonnet S, Roth C. WHO Rapid Pandemic Assessment Collaboration.. Pandemic potential of a strain of influenza A (H1N1) virus in humans. Science. 2009 Jun 19;324(5934):1557–61. doi: 10.1126/science.1176062. Epub 2009 May 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowood FS, Jaiun S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 11.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289(2):179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 12.Kelly H, Grant K, Williams S, Smith D. H1N1 swine origin influenza infection in the United States and Europe in 2009 may be similar to H1N1 seasonal influenza infection in two Australian states in 2007 and 2008. Influenza Other Respi Viruses. 2009;3(4):183–8. doi: 10.1111/j.1750-2659.2009.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sands BE, Cuffari C, Katz J, Kugathasan S, Onken J, Vitek C, Orenstein W. Guidelines for immunizations in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004 Sep;10(5):677–92. doi: 10.1097/00054725-200409000-00028. [DOI] [PubMed] [Google Scholar]
- 14.Rahier JF, Ben-Horin S, Chowers Y, Conlon C, De Munter P, D'Haens G, Domènech E, Eliakim R, Eser A, Frater J, Gassull M, Giladi M, Kaser A, Lémann M, Moreels T, Moschen A, Pollok R, Reinisch W, Schunter M, Stange EF, Tilg H, Van Assche G, Viget N, Vucelic B, Walsh A, Weiss G, Yazdanpanah Y, Zabana Y, Travis SP, Colombel JF the European Crohn's and Colitis Organisation (ECCO) European evidence-based Consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009 Jun;3(2):47–91. doi: 10.1016/j.crohns.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Rahier JF, Yazdanpanah Y, Viget N, Viget N, Travis S, Colombel JF. Review article: influenza A (H1N1) virus in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2010 Jan;31(1):5–10. doi: 10.1111/j.1365-2036.2009.04161.x. [DOI] [PubMed] [Google Scholar]
- 16.Mamula P, Markowitz JE, Piccoli DA, Klimov A, Cohen L, Baldassano RN. Immune response to influenza vaccine in pediatric patients with inflammatory bowel disease. Cliln Gastroenterol Hepatol. 2007;5:851–856. doi: 10.1016/j.cgh.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y, Jacobson DL, Ashworth LA, Grand RJ, Meyer AL, McNeal MM, Gregas MC, Burchett SK, Bousvaros A. Immune response to influenza vaccine in children with inflammatory bowel disease. Am J Gastroenterol. 2009 Feb;104(2):444–53. doi: 10.1038/ajg.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cullen G, Bader C, Korzenik JR, Sands BE. Serological response to the 2009 H1N1 influenza vaccination in patients with inflammatory bowel disease. Gut. 2011 Jul 13; doi: 10.1136/gutjnl-2011-300256. [DOI] [PubMed] [Google Scholar]
- 19.Gelinck LB, van der Bijl AE, Beyer WE, Visser LG, Huizinga TW, van Hogezand RA, Rimmelzwaan GF, Kroon FP. The effect of anti- tumor necrosis factor alpha treatment on the antibody response top influenza vaccination. Ann Rheum Dis. 2008;67:713–716. doi: 10.1136/ard.2007.077552. [DOI] [PubMed] [Google Scholar]
- 20.Molnár T, Farkas K, Jankovics I, Melles M, Nagy F, Szepes Z, Wittmann T. Appropriate response to influenza A (H1N1) virus vaccination in patients with inflammatory bowel disease on maintenance immunomodulator and/or biological therapy. Am J Gastroenterol. 2011 Feb;106(2):370–2. doi: 10.1038/ajg.2010.395. [DOI] [PubMed] [Google Scholar]
- 21.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, Tang KL, van der Woude CJ, Rutgeerts P SONIC Study Group. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med. 2010 Apr 15;362(15):1383–95. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 22.Panaccione R, Ghosh S, Middleton S. Infliximab, azathioprine, or infliximab + azathioprine for treatment of moderate to severe ulcerative colitis: The UC SUCCESS trial. Journal of Crohn's and Colitis. 2011 Feb;5(1):S8. [Google Scholar]
- 23.Satsangi J, Silverberg MS, Vermeire S, Colombel JFl. The Montreal classification of IBD: controversies, consensus, and implications. Gut. 2006;55:749–753. doi: 10.1136/gut.2005.082909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis JD, Chuai S, Nessel L, Lichtenstein GR, Aberra FN, Ellenberg JH. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. 2008 Dec;14(12):1660–6. doi: 10.1002/ibd.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harvey RF, Bradshaw JM. A simple index of Crohn_s disease activity. Lancet. 1980;1:514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 26.Focetria® prescribing information. Novartis Vaccines & Diagnostics. Siena; Italy: 2009. [Google Scholar]
- 27.Frasca D, Diaz A, Romero M, Phillips M, Mendez NV, Landin AM, Blomberg BB. Unique biomarkers for B cell function predict the serum response to pandemic H1N1 influenza vaccine International Immunology. Int Immunol. 2012 Mar;24(3):175–82. doi: 10.1093/intimm/dxr123. Epub 2012 Jan 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McElhaney JE, Meneilly GS, Lechelt KE, Beattie BL, Bleackley RC. Antibody response to wholevirus and split-virus influenza vaccines in successful ageing. Vaccine. 1993;11(10):1055–60. doi: 10.1016/0264-410x(93)90133-i. [DOI] [PubMed] [Google Scholar]
- 29.EMEA Committee for Human Medicinal products Note for Guidance on Harmonization Of Requirements for Influenza Vaccines, CPMP/BWP/214/96
- 30.EMEA Committee for Human Medicinal products Guideline on Influenza Vaccines prepared from Viruses with the Potential to cause a Pandemic and intended for Use outside of the Core Dossier Context EMEA/CHMP/VWP/263499/2006)
- 31.Rahier JF, Moutschen M, Van Gompel A, Van Ranst M, Louis E, Segaert S, Masson P, De Keyser F. Vaccinations in patients with immune-mediated inflammatory diseases. Rheumatology (Oxford) 2010 Oct;49(10):1815–27. doi: 10.1093/rheumatology/keq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chalmes A, Scheifele D, Ptterson C, Williams D, Weber J, Shuckett R, Teufel A. Immunization of patients with rheumatoid arthritis against influenza: a study of vaccine safety and immunogenicity. J Rheumatol. 1994;21:1203–1206. [PubMed] [Google Scholar]
- 33.Kubota T, Nii T, Nanki T, Kohsaka H, Harigai M, Komano Y, Sugihara T, Nonomura Y, Hirose W, Nagasaka K, Sakurai T, Miyasaka N. Anti-tumor necrosis factor therapy does not diminish the immune response to influenza vaccine in Japanese patients with rheumatoid arthritis. Mod Rheumatol. 2007;17:531–533. doi: 10.1007/s10165-007-0632-5. [DOI] [PubMed] [Google Scholar]
- 34.Fomin I, Caspi D, Levy V, Varsano N, Shalev Y, Paran D, Levartovsky D, Litinsky I, Kaufman I, Wigler I, Mendelson E, Elkayam O. Vaccination against influenza in rheumatoid arthritis: the effect of disease modifying drugs, including TNF alpha blockers. Ann Rheum Dis. 2006 Feb;65(2):191–4. doi: 10.1136/ard.2005.036434. Epub 2005 Jul 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapetanovic MC, Saxne T, Nilsson JA, Geborek P. Influenza vaccination as model for testing immune modulation induced by anti-TNF and methotrexate therapy in rheumatoid arthritis patients. Rheumatology (Oxford) 2007 Apr;46(4):608–11. doi: 10.1093/rheumatology/kel366. Epub 2006 Nov 18. [DOI] [PubMed] [Google Scholar]
- 36.Aikawa NE, Campos LM, Silva CA, Carvalho JF, Saad CG, Trudes G, Duarte A, Miraglia JL, Timenetsky Mdo C, Viana VS, França IL, Bonfa E, Pereira RM. Glucocorticoid: Major Factor for Reduced Immunogenicity of 2009 Influenza A (H1N1) Vaccine in Patients with Juvenile Autoimmune Rheumatic Disease. J Rheumatol. 2012 Jan;39(1):167–73. doi: 10.3899/jrheum.110721. Epub 2011 Nov 15. [DOI] [PubMed] [Google Scholar]
- 37.Kaine JL, Kivitz AJ, Birbara C, Luo AY. Immune responses following administration of influenza and pneumococcal vaccines to patients with rheumatoid arthritis receiving adalimumab. J Rheumatol. 2007 Feb;34(2):272–9. [PubMed] [Google Scholar]
- 38.German IBD Study Group. Adherence to the H1N1 vaccination recommendation in patients with Crohn's disease or ulcerative colitis. Dtsch Med Wochenschr. 2011 May;136(18):939–43. doi: 10.1055/s-0031-1275829. [DOI] [PubMed] [Google Scholar]
- 39.Lisotti A, Roda G, Brillanti S, Roda E. Reactivation of Crohn's disease after pandemic aH1N1 and seasonal flu vaccinations. Dig Liver Dis. 2010 Dec;42(12)(10):909. doi: 10.1016/j.dld.2010.03.014. Epub 2010 Apr 24. [DOI] [PubMed] [Google Scholar]
- 40.Rahier JF, Papay P, Salleron J, Sebastian S, Marzo M, Peyrin-Biroulet L, Garcia-Sanchez V, Fries W, van Asseldonk DP, Farkas K, de Boer NK, Sipponen T, Ellul P, Louis E, Peake ST, Kopylov U, Maul J, Makhoul B, Fiorino G, Yazdanpanah Y, Chaparro M European Crohn's and Colitis Organisation (ECCO) H1N1 vaccines in a large observational cohort of patients with inflammatory bowel disease treated with immunomodulators and biological therapy. Gut. 2011 Apr;60(4):56–62. doi: 10.1136/gut.2010.233981. Epub 2011 Jan 26. [DOI] [PubMed] [Google Scholar]
- 41.Melmed GY, Agarwal N, Frenck RW, Ippoliti AF, Ibanez P, Papadakis KA, Simpson P, Barolet-Garcia C, Ward J, Targan SR, Vasiliauskas EA. Immunosuppression impairs response to pneumococcal polysaccharide vaccination in patients with inflammatory bowel disease. Am J Gastroenterol. 2010 Jan;105(1):148–54. doi: 10.1038/ajg.2009.523. Epub 2009 Sep 15. [DOI] [PubMed] [Google Scholar]