Abstract
The requirement for new strategies for synthesizing five-membered carbocycles has driven an expansion in the study of the Nazarov cyclization. This renewed interest in the reaction has led to the discovery of several interesting new methods for generating the pentadienyl cation intermediate central to the cyclization. Methods reviewed include carbon-heteroatom ionization, functionalization of a double bond, nucleophilic addition, or electrocyclic ring opening. Additional variations employ unconventional substrates to produce novel pentacycles, such as the iso- and imino-Nazarov. Herein, we provide an overview of these unconventional, yet highly useful versions of the Nazarov cyclization.
Keywords: Nazarov, electrocyclization, carbocation, cyclopentane, synthesis
Introduction
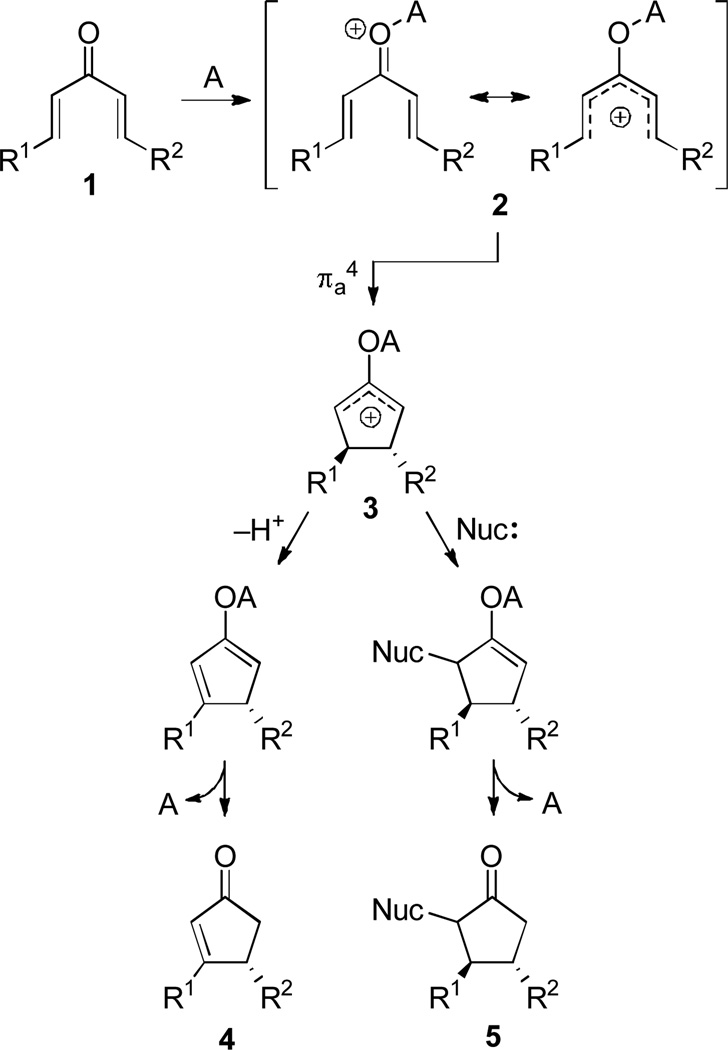
Since its initial discovery over 60 years ago,[1] the Nazarov cyclization has evolved to become a primary tool for the of cyclopentenones. The classical version (Scheme 1) is triggered by the Lewis- or Brønsted-acid activation of a divinyl ketone (1), which forms a pentadienyl cation (2). Electrocyclization produces an oxyallyl cation (3) which may eliminate or undergo trapping by a nucleophile to yield the product cyclopentenone (4 and 5). The characteristic diastereospecificity[2] of the cyclization is a consequence of orbital symmetry rules, which dictate an antarafacial overlap of the two cyclization termini in the transition state, leading to stereospecific conrotatory cyclization.
Scheme 1.
The Prototypical Nazarov Cyclization.
These powerful attributes have stimulated the extensive development and expansion of the reaction.[2,3] In recent years, this expansion has included the use of unconventional substrates or transformations to generate the requisite pentadienyl cation. In this microreview, we provide an overview of select examples of these methods.
We have organized them according to two defining features: 1) the reaction that generates the pentadienyl cation, and 2) the structure of the pentadienyl cation. There are several approaches which may fall under more than one classification, and in these instances we have used our discretion.
1. Formation of Pentadienyl Cations Through Ionization of a Carbon-Heteroatom Bond
Ionizing the C–O bond of an alcohol or ether is an effective way to initiate a Nazarov cyclization when the resulting carbocation is capable of conjugating with two double bonds, thus providing a straightforward route to cyclopentenes and cyclopentadienes.
Alcohol Ionization
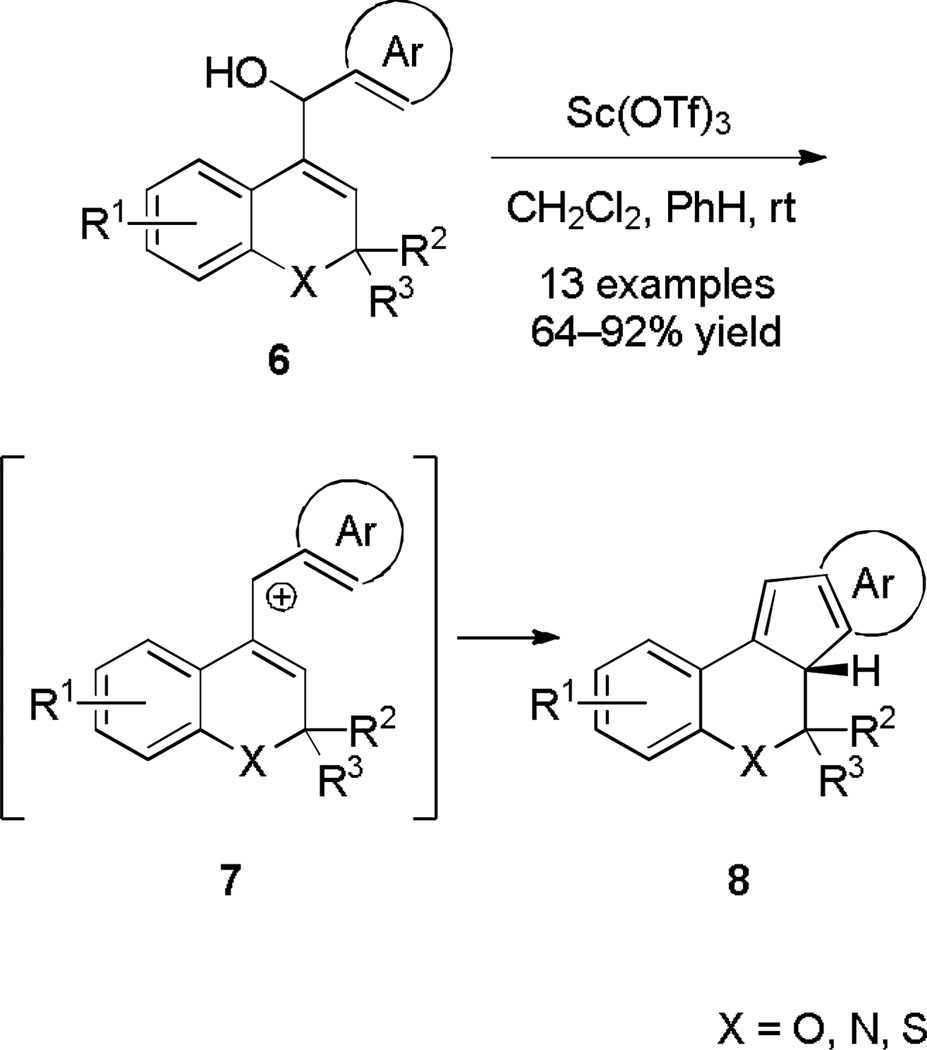
An increasingly common method for constructing such 5-membered carbocycles by means of the Nazarov cyclization is through the ionization of a divinyl carbinol substrate.[4] This is illustrated by the Sc(OTf)3-catalyzed ionization of alkenyl aryl carbinols (6) to form [6,6,5,6] and [6,6,5,5] heterocyclic ring systems (8, Scheme 2).[5] The cyclization efficacy was highest when the participating aromatic ring was electron-rich, with yields ranging from good-to-excellent. 2-Thienyl, 3-furyl, or 3-benzo[b]thienyl aryl groups resulted in decomposition.
Scheme 2.
Nazarov Cyclization Triggered by Ionization of Alkenyl Aryl Carbinols.
Ether Ionization
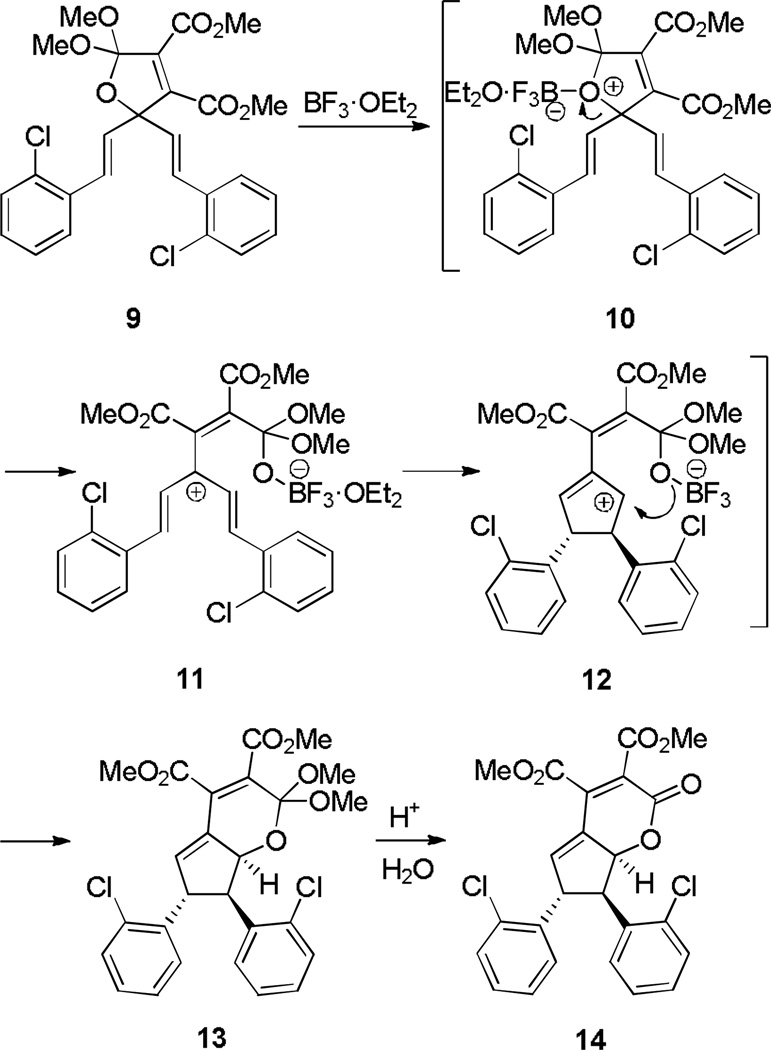
Ionization of an ether has also found application in several contexts. One that exemplifies the potent synthetic potential of this approach is demonstrated in Scheme 3. After C–O bond cleavage of dihydrofuran 9 by BF3·OEt2, a Nazarov cyclization interrupted by intramolecular alkoxide trapping occurs to form [6,5] ring system 13 which is hydrolyzed during workup to furnish lactone 14.[6] A related ether ionization–intramolecular trapping method uses an allene as one of the π components.[7]
Scheme 3.
Nazarov Cyclization Triggered by Ether Ionization Followed by Trapping.
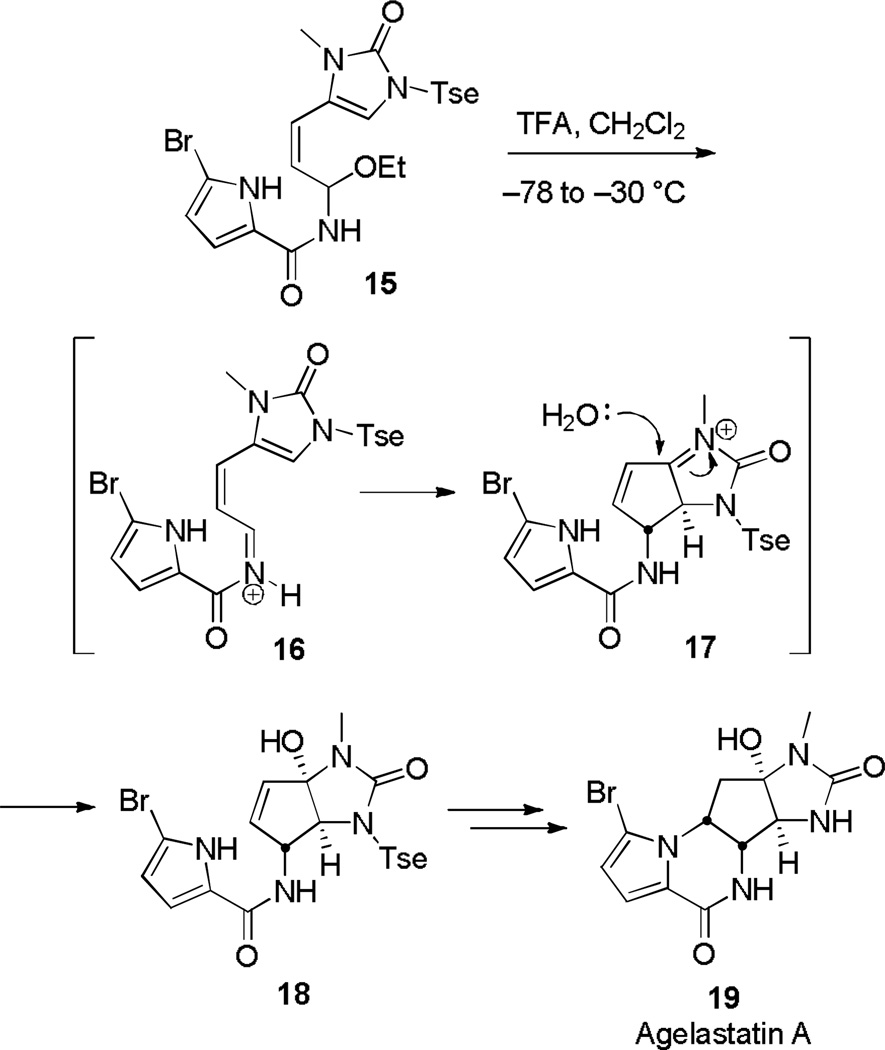
Quite recently, Romo has employed such a tactic to construct the C ring of Agelastatin A (Scheme 4).[8] Treatment of 15 with trifluoroacetic acid at low temperature yielded 18, presumably through removal of ethoxide in 15 to form pentadienyl cation 16 and subsequent water trapping of the stabilized allylic cation 17. The cyclization was notably complete within 5 minutes.
Scheme 4.
Nazarov Cyclization in the Synthesis of Agelastatin A.
2. Formation of Pentadienyl Cations Through Activation of a C=C Bond
Pentadienyl cation generation is also possible through activation of a conjugated or cross-conjugated triene with a Lewis or Brønsted acid.
Protonation of Alkoxytrienes
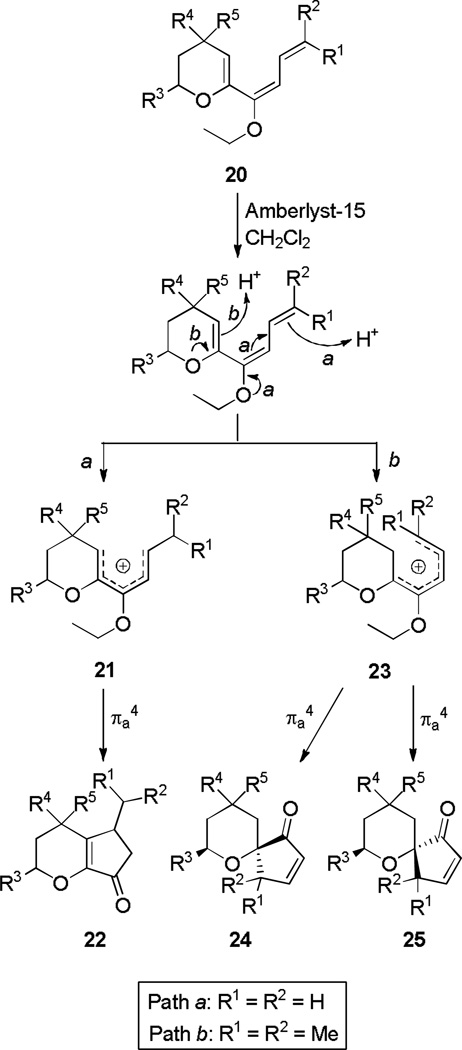
Alkoxytrienes can be excellent divinyl ketone surrogates. They undergo facile Nazarov cyclization immediately following protonation, under conditions which are too mild for the analogous divinyl ketone substrates to cyclize.[9] The erudite study of this reaction by Occhiato and Prandi has established a framework for several aspects of the reaction including torquoselectivity, size of the heterocyclic ring component, and regioselectivity. The latter parameter was found to be controlled primarily by substitution at the exocyclic triene terminus (Scheme 5): when R1 and/or R2 are unsubstituted, fused [6,5] ring system 22 was obtained (Path a), while Me or Et mono- or di-substitution allowed competition with spirocyclic [6,5] products 24 and 25 (Path b).
Scheme 5.
Regioselectivity in the Nazarov Cyclization of Conjugated Alkoxytrienes.
3. Formation of Pentadienyl Cations Through Activation of an Allene
The reactivity of the allene double bond, driven in part by the relief of allenic strain, has been harnessed to effect Nazarov cyclizations prompted by exposure to various electrophilic species such as oxidants, transition metal complexes, and Brønsted acids.[10]
Oxidation-Initiated Nazarov Cyclization
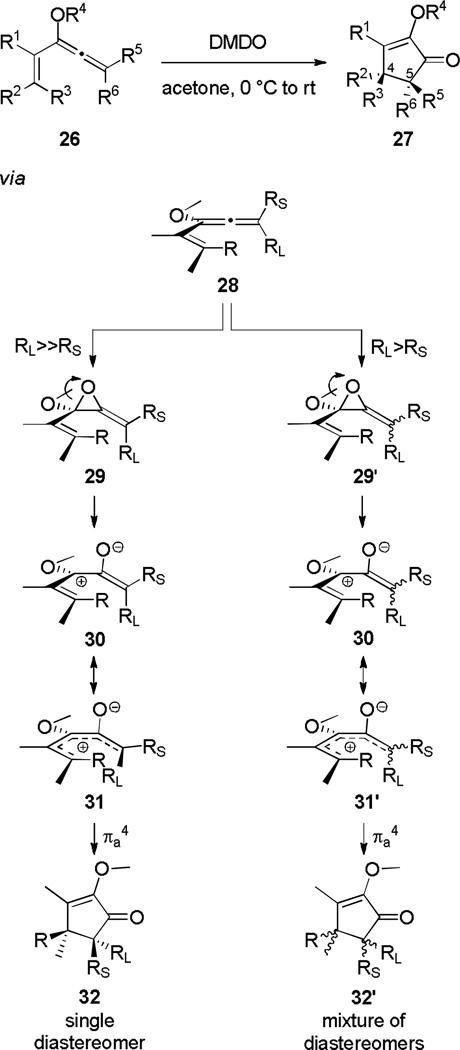
This approach was demonstrated recently when Frontier and Spencer[11] found that exposure of vinyl alkoxyallenes to dimethyldioxirane (DMDO) initiated a Nazarov cyclization that produced cyclopentenones with adjacent stereocenters at C4 and C5 in high diastereoselectivity (Scheme 6).[12] A mechanistic rationale based on the results of several informative experiments was proposed which elucidates the observed diastereochemical outcomes of the reaction: when a vinylalkoxyallene substituted with a large group (RL) and a small group (RS) at the allene terminus reacts with DMDO, epoxidation occurs away from RL. When RL>>RS, the diastereoselectivity of this epoxidation is complete, resulting in allene oxide 29. When RL>RS, epoxidation is less diastereoselective, resulting in a mixture of allene oxide diastereomers (29'). Epoxide ring opening of 29 results in pentadienyl cation 30/31, which after electrocyclization yields a single diastereomer of the product cyclopentenone (32). The analogous process which occurs on diastereomeric allene oxide mixture 29' results in cyclopentenone 32' as a mixture of diastereomers. This transformation was used in a total synthesis of (±)-Rocaglamide.[13]
Scheme 6.
The Oxidation-Initiated Nazarov Cyclization.
Activation using transition metal catalysts
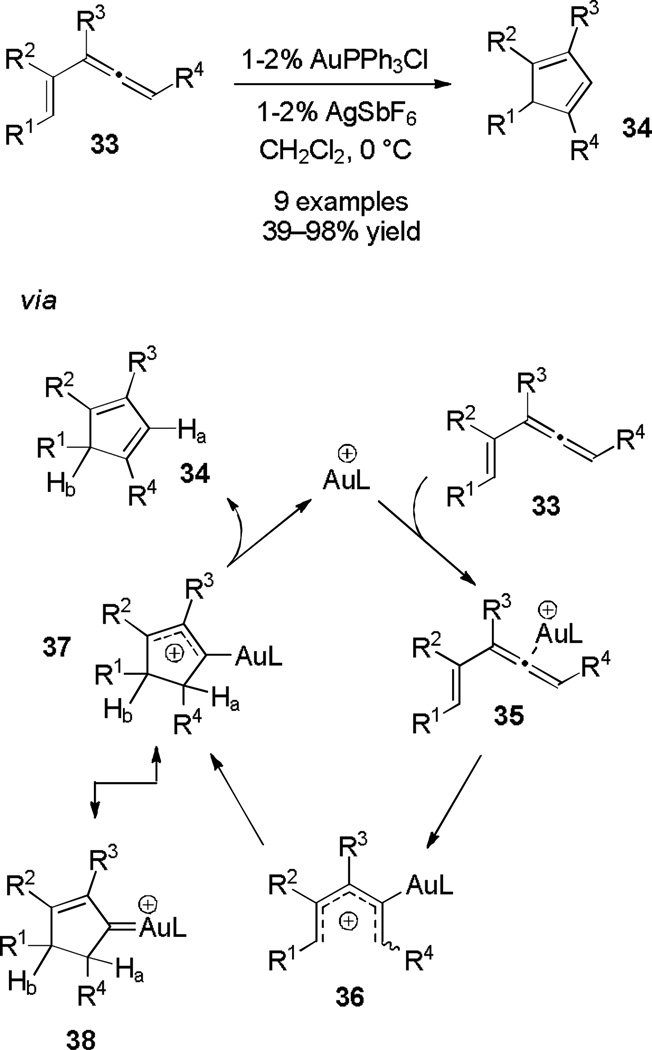
Toste and coworkers activated vinylallenes (33) with cationic Au(I), resulting in cyclopentadienes 34 (Scheme 7).[14] The group proposes the following catalytic cycle: activation of the allene produces pentadienyl cation 36, which undergoes electrocyclization to give allylic cation 37/38. A hydride shift then occurs (Ha shifts to the Au ipso position), followed by elimination to regenerate the active catalyst and yield a molecule of cyclopentadiene product 34. A similar variant initiated by platinum activation was published back-to-back with this work,[15] and an additional gold-catalyzed variant which generates a cyclopentadiene with different double bond regioselectivity was recently disclosed.[16]
Scheme 7.
Gold-catalyzed conversion of Vinylallenes to Cyclopentadienes.
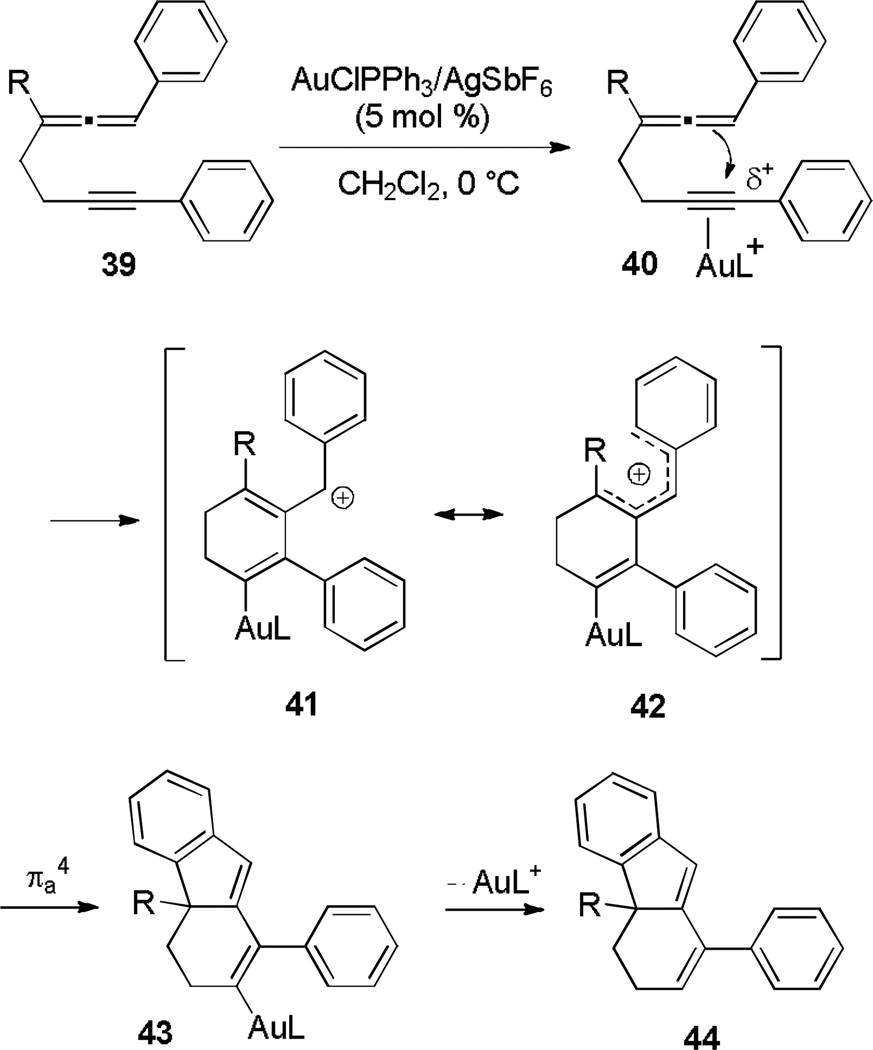
Cationic gold(I) activation of a phenylallene bonded with a homopropargylic moiety (39, Scheme 8) resulted in carbocyclic [6,5,6] systems (44).[17] Deuterium-labeling experiments supported an initial coordination of [AuPPh3]+ to the alkyne, followed by 6-endo-dig cyclization to form pentadienyl cation 41/42. Subsequent electrocyclization forms 43, which undergoes protonolysis of the C-Au bond to furnish polycycle 44.
Scheme 8.
6-endo-dig–Nazarov Cyclization to Form Tricycles.
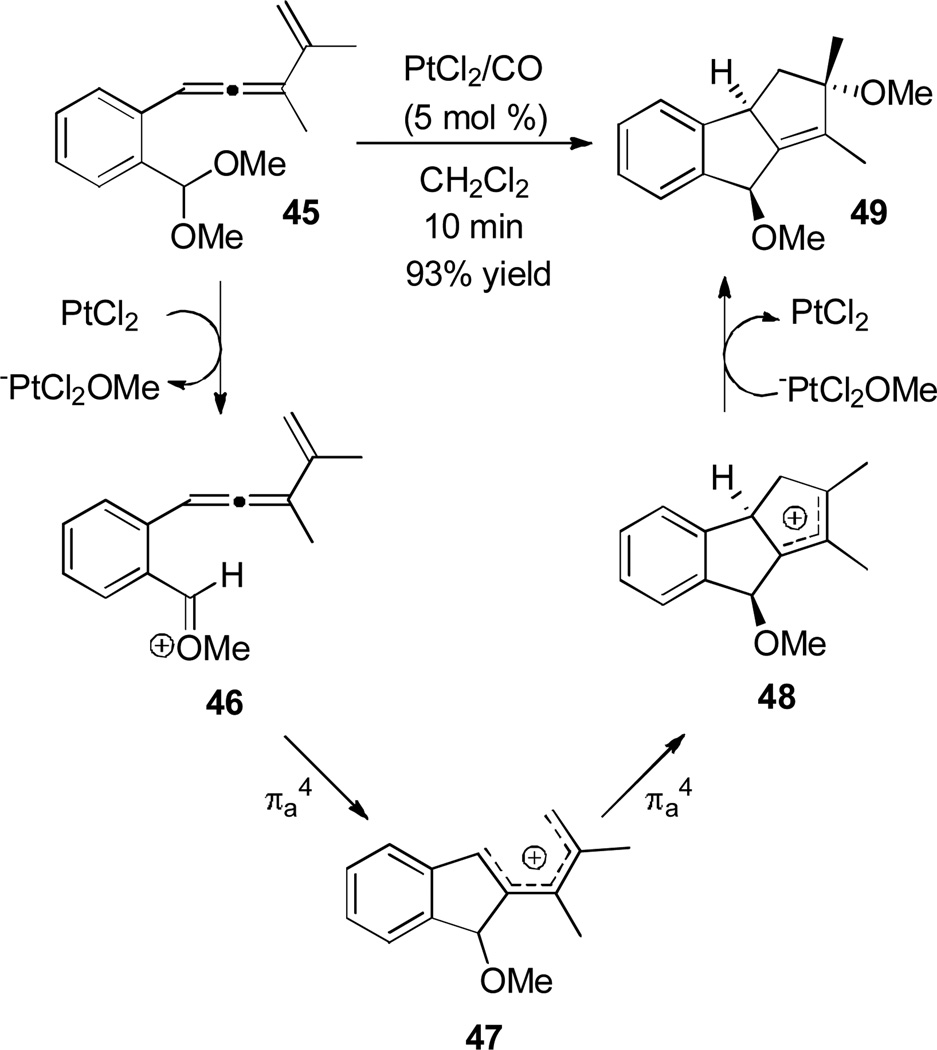
When allenes of type 45 were treated with PtCl2 under an atmosphere of CO,[18] an unexpected double Nazarov cyclization occurred (Scheme 9).[19] The proposed mechanism starts with the Pt(II)-catalyzed ionization of methoxide to give pentadienyl cation 46, which after a first Nazarov cyclization generates pentadienyl cation 47. A second Nazarov cyclization followed by methoxide trapping yields the product carbocycle 49.
Scheme 9.
Tandem Double Nazarov Cyclization to Form Tricycles.
4. Formation of Pentadienyl Cations Through 1,6-Conjugate Addition
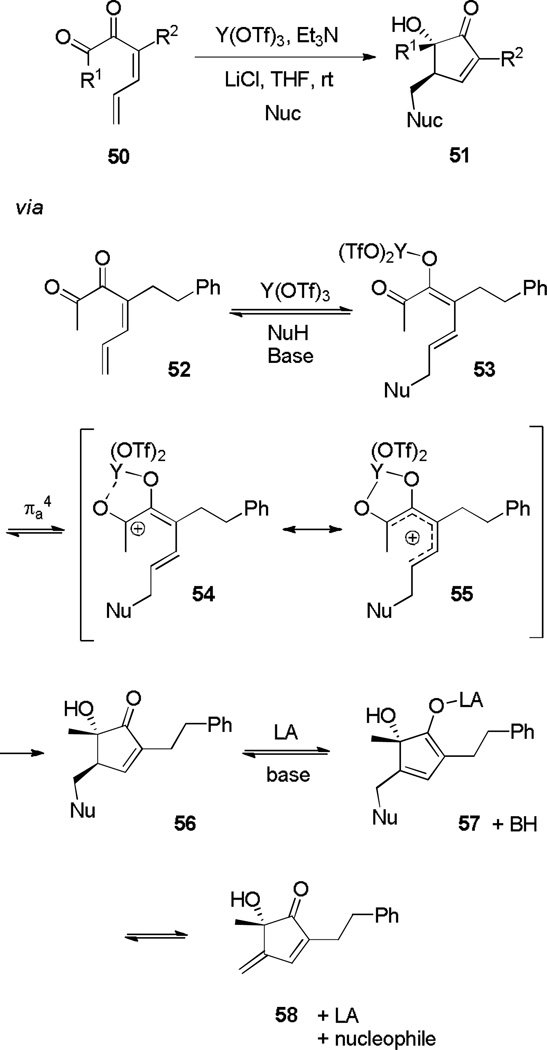
Frontier, Brooks, and Caruana[20] recently found that various carbon and nitrogen nucleophiles undergo a 1,6-conjugate addition on α-diketone substrates (50, Scheme 10). A subsequent Nazarov cyclization occurs to provide α-hydroxycyclopentenones (51),[21] which in the presence of Y(OTf)3 are isolated as a single diastereomer. If a catalytic amount of nucleophile is employed, exo-cyclopentadienones 58 are isolated instead of cyclopentenones 56. It is proposed that under the basic reaction conditions, γ-deprotonation of cyclopentenone 56 produces 57, which expels the nucleophile to form diene 58. This hypothesis was supported by the observation that when stoichiometric nucleophile was employed, extended reaction times led to epimerization of 56, presumably via either deprotonation/reprotonation (56/57) or retro-1,6-addition/ 1,6- addition (57/58).
Scheme 10.
Conjugate-Addition Initiated Nazarov Cyclization.
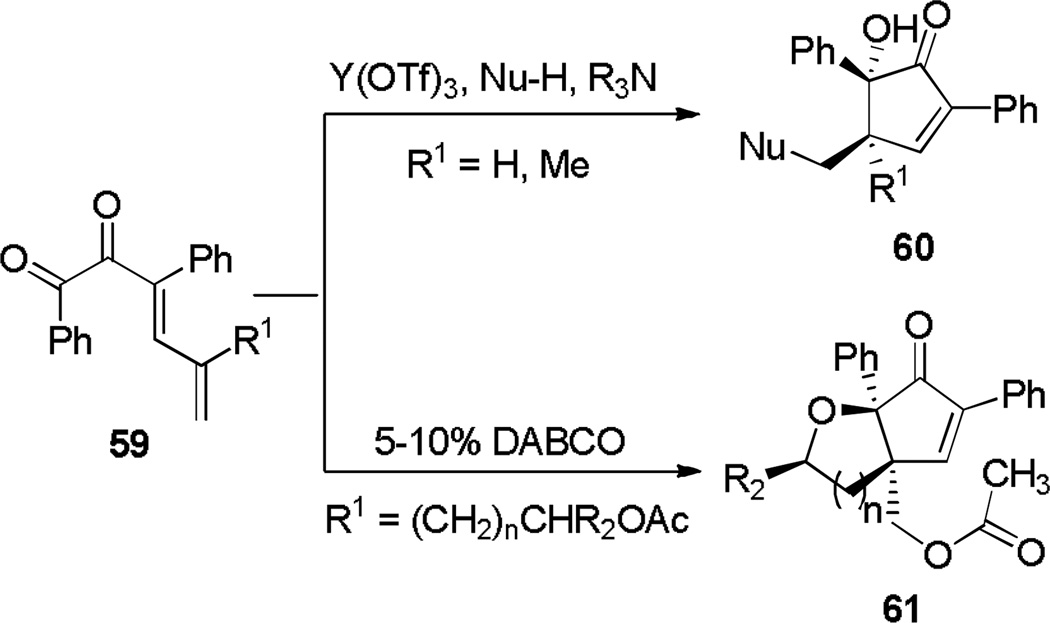
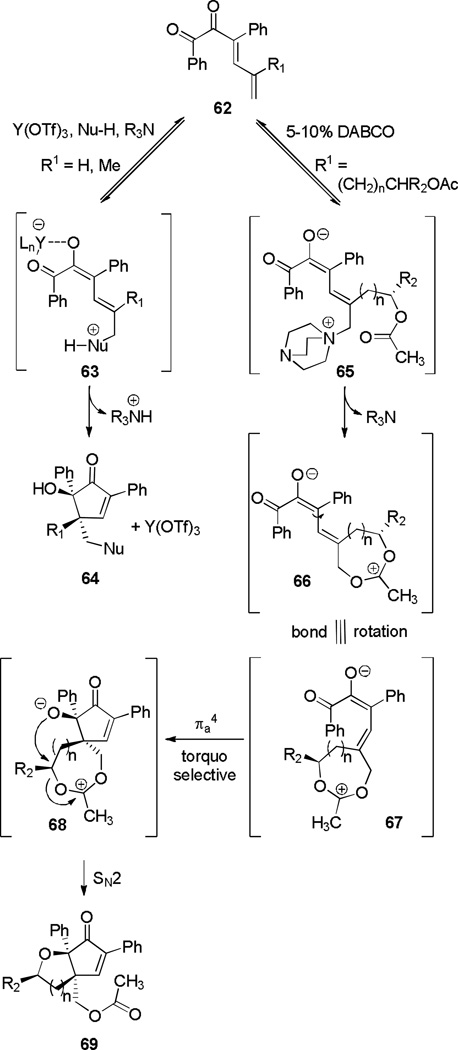
Extension to substrates containing a substituent R1 (59, Scheme 11) enabled the synthesis of products containing adjacent quaternary centers (60).[22] When R1 was a pendant acetate group (R1 = (CH2)nCHR2OAc) and exposed to conditions incorporating 1,4-diazabicyclo[2.2.2]octane (DABCO), diastereopure bicycle 61 was unexpectedly obtained. This result was rationalized by the conjugate addition of DABCO to dienyl ketones of type 62 to form zwitterion 65, which then undergoes an intramolecular SN2 reaction to form 7-membered intermediate 66 (Scheme 12). A Nazarov cyclization, torquoselective through the steric influence of the R2-substituted stereocenter, results in 68. A final SN2 displacement restores charge neutrality by forming bicycle 69.
Scheme 11.
Divergent Cyclization Pathways of Dienyl α-Diketones.
Scheme 12.
Mechanistic Proposal for Bicycle Formation.
5. Pentadienyl Cations from Transition Metal Catalyzed Rearrangements of Propargyl Acetates
The ability of propargylic alkanoates to undergo rearrangement chemistry with transition metals has been capitalized upon to generate pentadienyl cations when this moiety is appropriately substituted with a vinyl group.
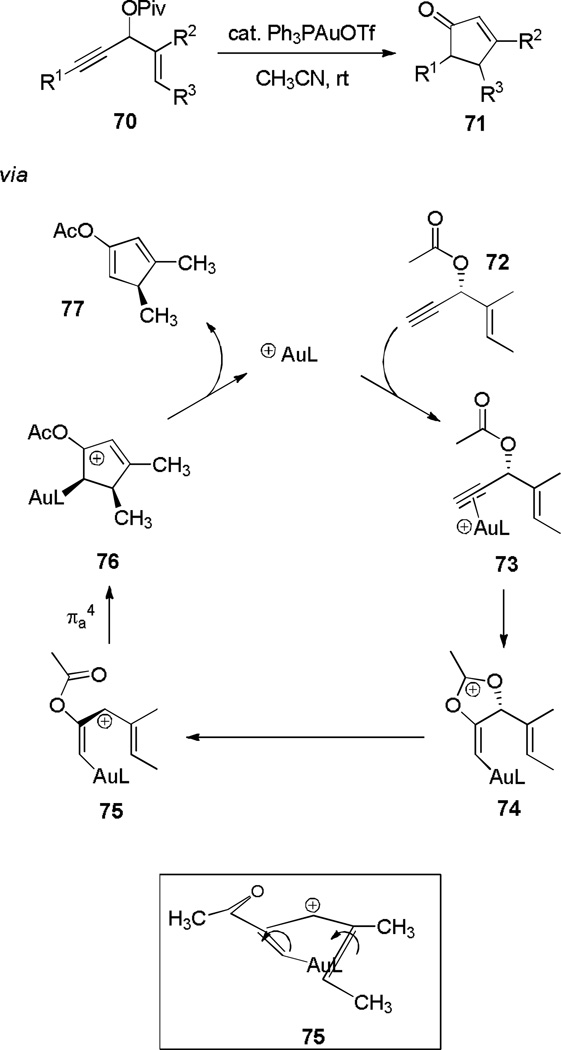
Toste and coworkers found that when propargylic pivalates (70) are substituted with a vinyl group at the propargylic position, they undergo a rearrangement under gold(I) catalysis to produce cyclopentenones 71,[23] in what is effectively a gold-catalyzed Rautenstrauch cyclization (Scheme 13).[24]
Scheme 13.
Gold-Catalyzed Rautenstrauch Cyclization.
The utility of the cyclization was further augmented when a series of chiral, enantiopure substrates yielded the corresponding cyclopentenone products enantioselectively. According to DFT calculations,[25] initial catalyst coordination to the triple bond of propargylic acetate 72 occurs, followed by intramolecular 1,2-addition of the ester onto the alkyne to form 74. Fragmentation then leads to a helically chiral pentadienyl cation 75, which undergoes electrocyclization faster than racemization to form 76. Elimination of 76 affords cyclopentenone surrogate 77 and regenerates the active catalyst. There is only one additional example[26] to our knowledge of memory of chirality[27] in the electrocyclization of a pentadienyl cation. Closely related versions of this cyclization using ruthenium[28] and palladium[29] catalysis have also been explored recently.
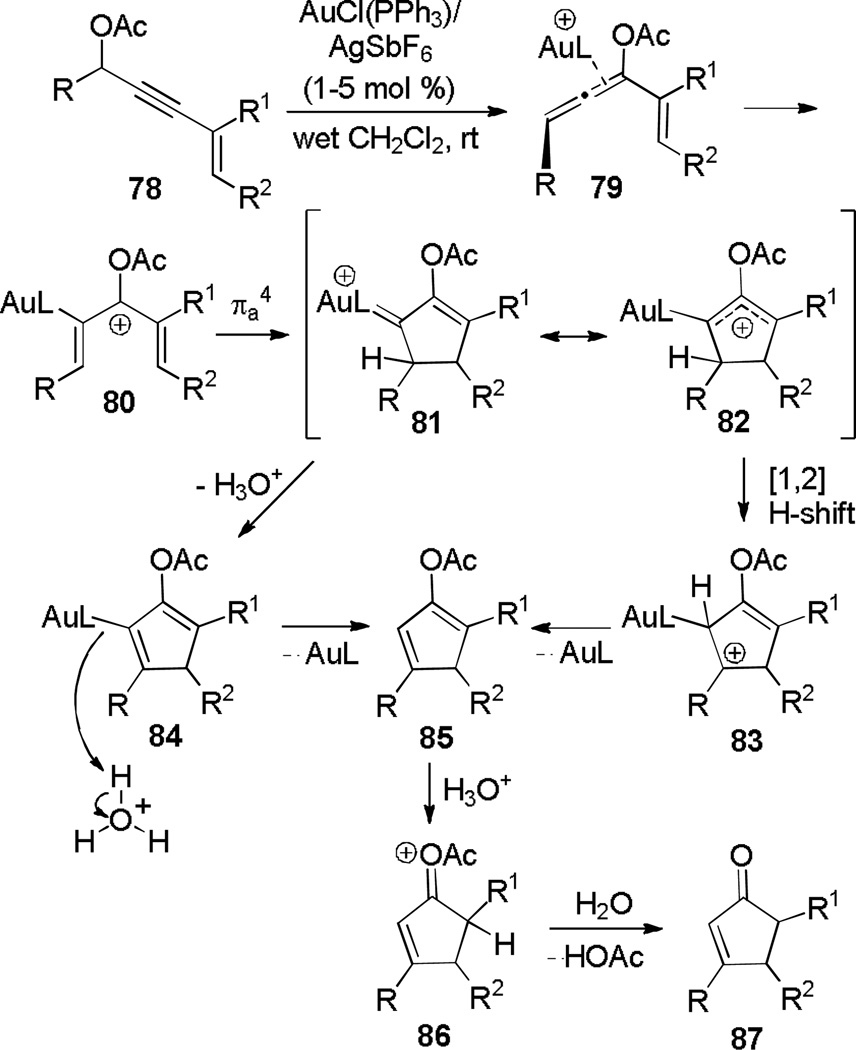
Remarkably, placement of the vinyl group at the alkyne terminus results in an entirely different mode of reactivity (Scheme 14).[30] Exposure of vinyl propargylic acetate of type 78 to catalytic AuPPh3Cl/AgSbF6 results in conversion to cyclopentenones of type 87. DFT calculations[31] suggest a formal 3,3-rearrangement of 78 to form allene 79, which is activated by the gold complex to form pentadienyl cation 80. Electrocyclization forms an allylic cation (81/82) which is converted to cyclopentadiene 85 in two ways. In dry CH2Cl2, the primary pathway is a 1,2-hydride shift to form 83, which eliminates to give 85. In wet CH2Cl2 however, 85 is formed through a two-step water-catalyzed deprotonation-protonation process in which water aggregates, stabilized by the acetoxy carbonyl, serve to shuttle protons. This water-catalyzed hydrogen transfer mechanism is lower in energy than the 1,2-hydride shift, supporting experimental observations demonstrating that the reaction is faster in wet CH2Cl2. A similar rearrangement was studied by Malacria and coworkers, involving a cyclopropanation of the intermediate gold alkylidene species with a pendant olefin.[32]
Scheme 14.
Tandem Gold(I)-Catalyzed 3,3-Rearrangement Followed by a Nazarov Cyclization.
6. Tautomerization / Nazarov Cyclization Sequences
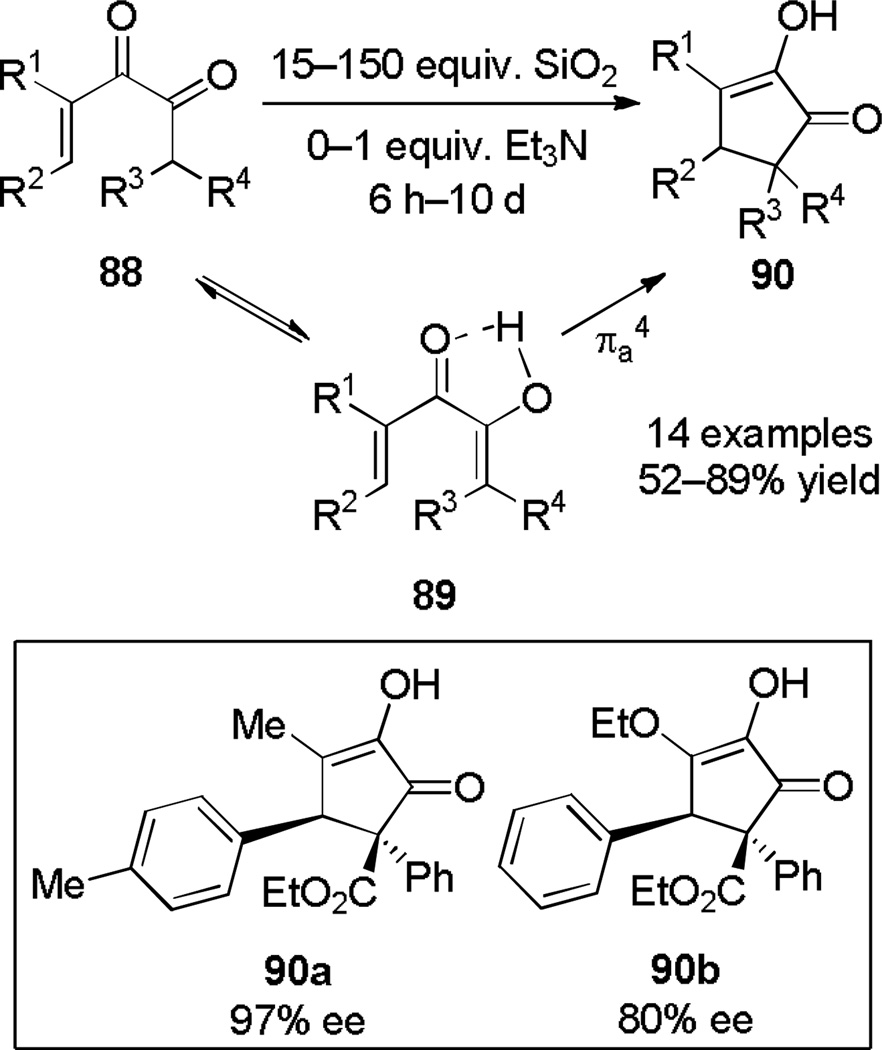
The Nazarov cyclization of α-diketones was first noted in 1965 by Muxfeldt[33] and then used by Weinreb in 1975 to synthesize Cephalotaxine.[34] Later, in an extension of his earlier studies on the palladium(II)-catalyzed cyclizations of α-alkoxydienes, Tius and coworkers explored the scope of the cyclization under both basic conditions using LiTMP and Lewis acidic conditions using Yb(OTf)3.[35] Shortly afterwards, they made the striking discovery that the reaction could simply be performed on activated silica in the absence of any other reagents or solvents (Scheme 15),[36] although triethylamine increased the reaction rate. The Tius group later developed asymmetric versions using a diamine promoter[37] and a bifunctional organocatalyst, allowing access to highly functionalized chiral cyclopentenones such as 90a and 90b.[38] Lewis-acid catalyzed variants have been used by Williams in the synthesis of Fusicoauritone[39] and by Harmata for preparation of an advanced intermediate in a synthetic strategy targeting Hamigeran B.[40]
Scheme 15.
Nazarov Cyclization of α-Ketoenones.
7. Electrocyclic Ring Opening / Nazarov Cyclization Sequences
An elegant and novel means to generate Nazarov substrates in situ is through a 4π or 6π electrocyclic ring opening. Subsequent Nazarov cyclization occurs under the same conditions to form the cyclopentenone product.
Hetero-enyne metathesis
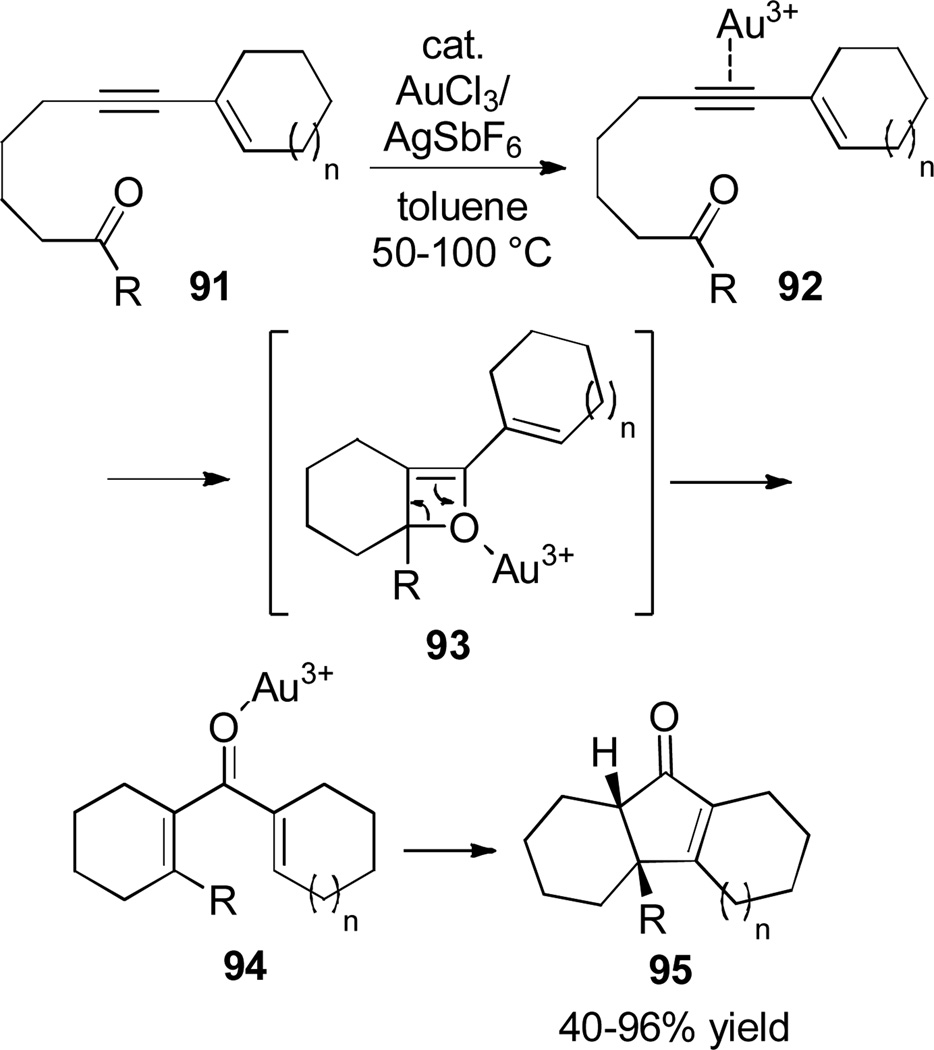
The use of a catalyst which plays dual roles in activating alkyne π bonds and carbonyl lone pairs has allowed the development of a tandem alkyne-aldehyde metathesis–Nazarov cyclization (Scheme 16).[41] Using a substrate containing both functional groups bridged by an appropriately sized tether, an initial metathesis enables formation of divinyl ketone substrate 94, which under the same catalytic conditions undergoes a Nazarov cyclization to regioselectively produce cyclopentenones of type 95, containing two new rings. An intermolecular version of the reaction in which the alkyne and aldehyde are in separate reactants has also been developed.[42]
Scheme 16.
Tandem Alkyne-Aldehyde Metathesis–Nazarov Cyclization.
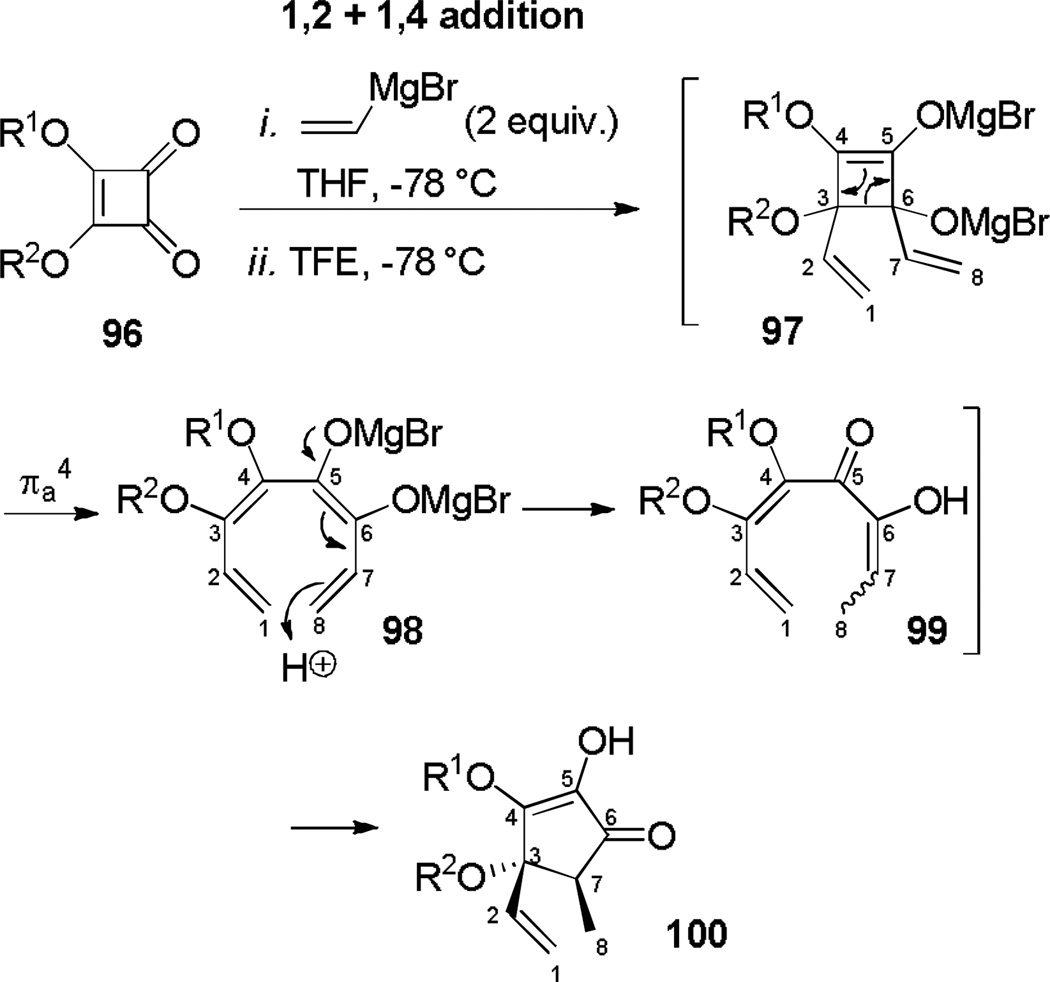
Treatment of squarate esters of type 96 at −78°C with two equivalents of vinylmagnesium bromide followed by quenching with a proton source at the same temperature allows regioselective formation of cyclopentenones 100 (Scheme 17).[43] The mechanism was proposed to initiate with a regioselective double 1,2–1,4 addition of vinylmagnesium bromide to form cyclobutadiene 97, which undergoes an electrocyclic ring opening to give tetraene 98. Regioselective protonation at the methylene terminus with the highest coefficient and electronic density occurs to yield divinyl ketone 99, which undergoes a 4π electrocyclization to furnish cyclopentenone 100 as a single diastereomer. DFT calculations show a transition state geometry and orbital topology compatible with a 4π conrotatory electrocyclization. Related cyclizations have been reported but whether or not these cyclopentannelations occur by a conrotatory electrocyclization is unclear.[44]
Scheme 17.
Formation of Cyclopententones Through Regioselective 1,2 ,4 Addition of Vinylmagnesium Bromide to Squarate Esters
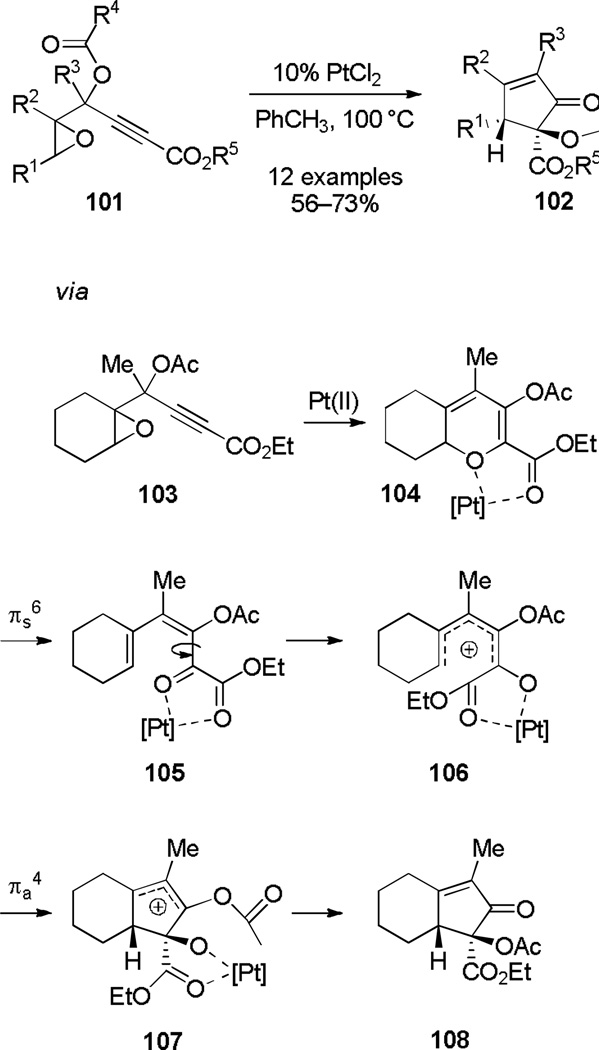
Sarpong and coworkers[45] have shown that propargylic acetates of type 101 undergo a PtCl2-catalyzed rearrangement to form cyclopentenones of type 102. DFT calculations[46] indicate the mechanism initiates with Pt-catalyzed formation of oxacycle 104. Electrocyclic ring opening of 104 leads to formation of pentadienyl cation 106, which undergoes a Pt-catalyzed Nazarov and acyl shift to form cyclopentenone 108 diastereospecifically in moderate-to-good yields (Scheme 18).
Scheme 18.
Oxa-6π Electrocyclic Ring Opening–Nazarov Cyclization.
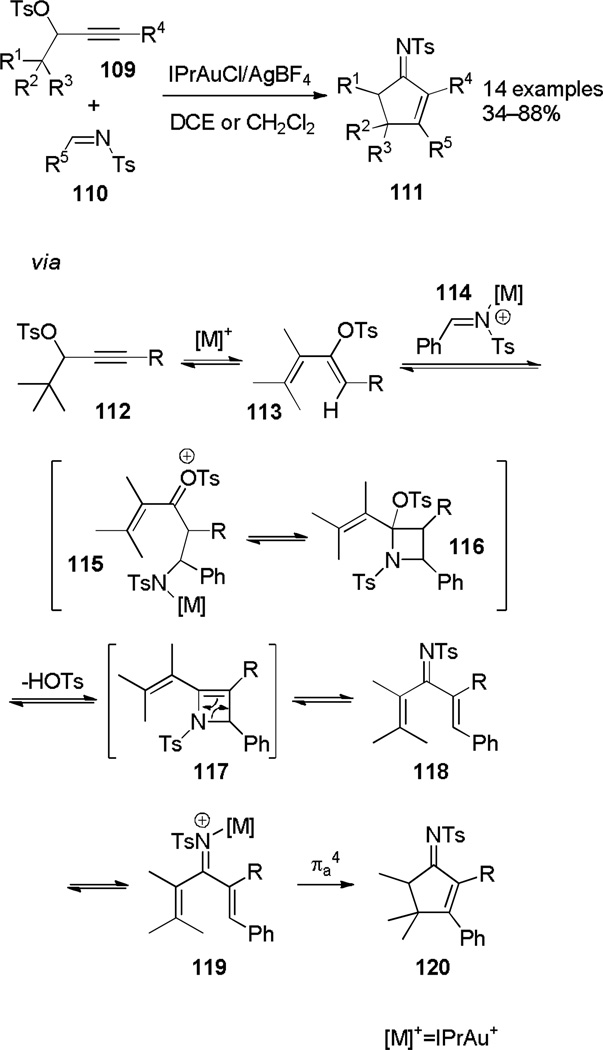
While screening propargylic derivatives for their reactivity toward N-tosylimines in the presence of gold catalysts, a IPrAuCl/AgBF4 catalyzed rearrangement using propargylic tosylates was found to completely reorganize both reactants into product carbocycle 111 (Scheme 19).[47] The reaction has a wide scope for variable propargylic tosylate and imine substitution. The mechanistic proposal involves an initial rearrangement of propargylic tosylate 112 to diene 113, which serves as a nucleophile for addition to activated imine 114. The resulting adduct (115) collapses to azetidine 116, which eliminates to form azete 117. Electrocyclic ring opening forms imino-Nazarov intermediate 118, which undergoes Nazarov cyclization to form cyclopentenone imine 120. Subjection of diene 113 to the reaction conditions resulted in 120, supporting this mechanistic hypothesis.
Scheme 19.
Rearrangement of Propargylic Tosylates and Imines to Form Cyclopentenone Imines.
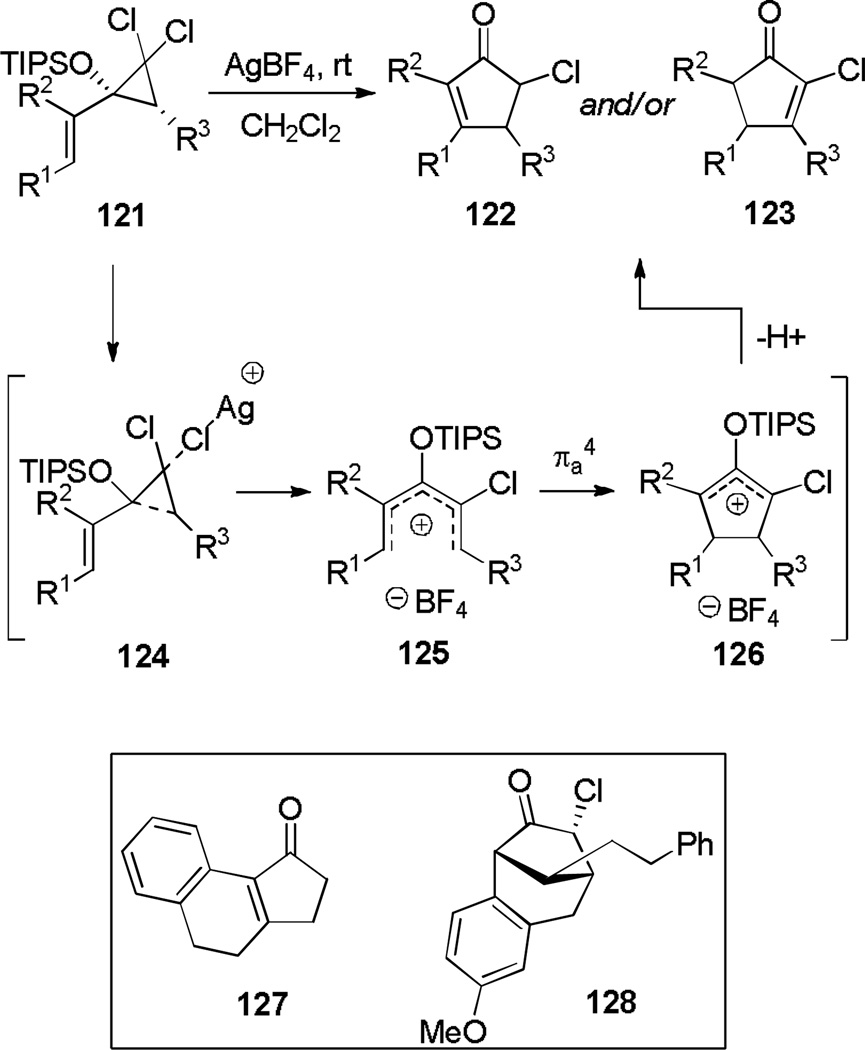
West and co-workers found that pentadienyl cation generation is possible through electrocyclic ring opening of a dichlorocyclopropane substituted with a vinyl group (121, Scheme 20).[48] An oxygen-containing substituent at the same position not only enables the formation of cyclopentenones after pentadienyl cation electrocyclization,[49] but according to computations enhances the rate of cyclization. The reaction is initiated by Ag(I), which ionizes a chloride to trigger electrocyclic ring opening. Substitution at R1 or R3 with a pendant aryl group allowed trapping of the oxyallyl cation to form fused or bridged tricyclic compounds such as 127 and 128.
Scheme 20.
Dichloropropane Ring Opening Nazarov Cyclization.
8. Formation of 1-Oxo-Pentadienyl Cations (Iso-Nazarov Cyclization)
4π electrocyclizations involving 1-oxo-pentadienyl cation intermediates, rather than the more typical 3-oxo-pentadienyl cation species, are sometimes termed “iso-Nazarov cyclizations.” Recently, some interesting strategies for generating and cyclizing 1-oxo-pentadienyl cation intermediates have been developed. Selected examples are described below.
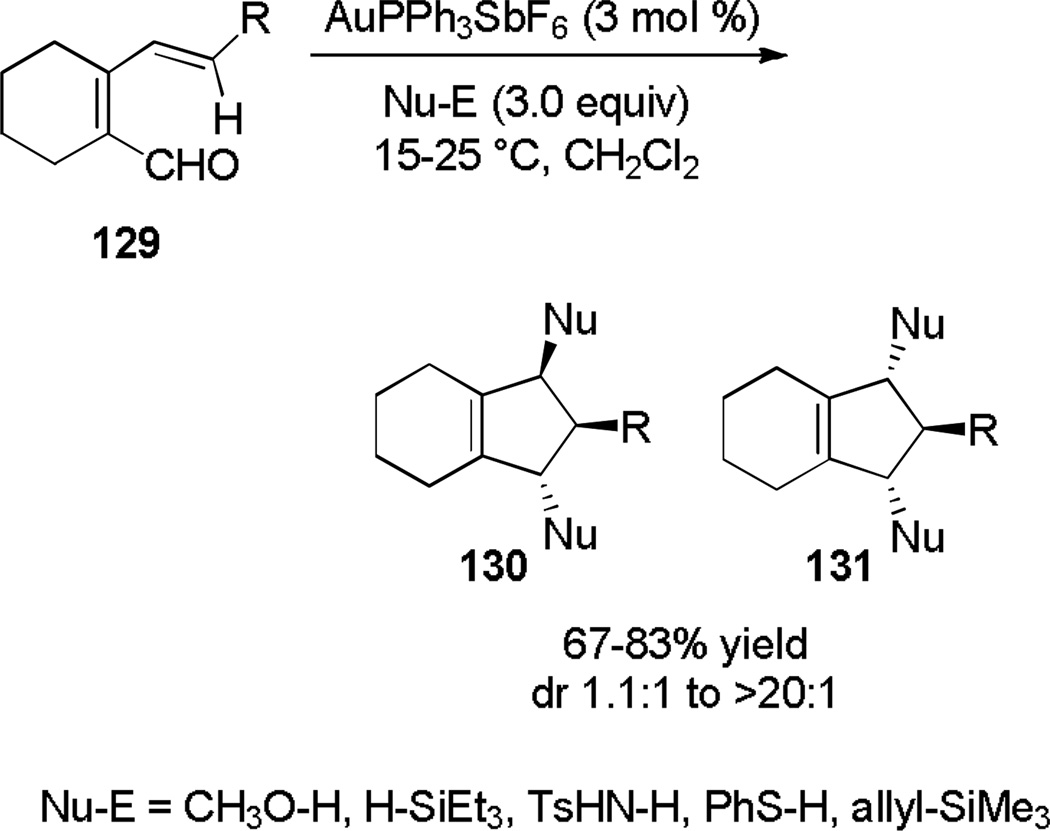
In the presence of an external nucleophile, AuPPh3SbF6 catalyzes a deoxygenative iso-Nazarov cyclization of dienals of type 129 with concomitant nucleophile trapping (Scheme 21).[50]
Scheme 21.
Deoxygenative Iso-Nazarov Cyclization.
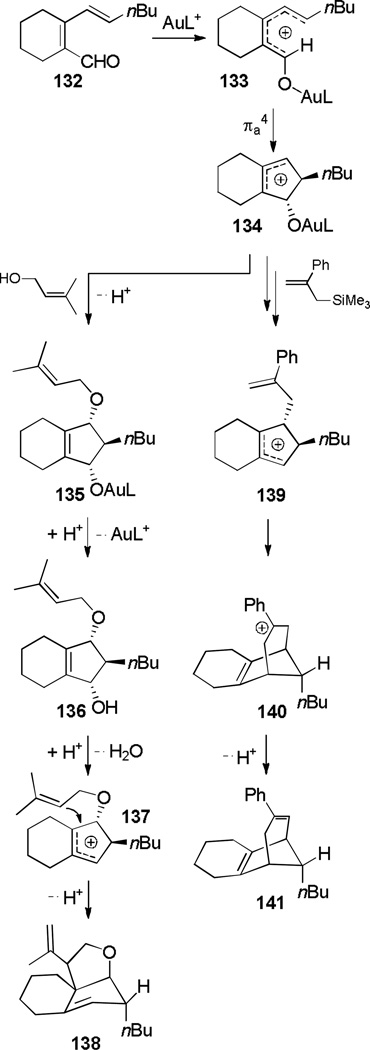
Extension to vinyl and aryl nucleophiles allowed the construction of polycyclic frameworks, some of which are shown in Scheme 22. The mechanism is proposed to occur by an initial trapping of allylic cation 134 formed from the Nazarov cyclization by an allylic alcohol or allylic silane, followed by ionization of the oxygen to form a second allylic cation (137 and 139). A subsequent intramolecular trapping by the double bond of each intermediate occurs, leading to fused ring system 138 or bridged ring system 141.
Scheme 22.
Assembly of Polycyclic Frameworks.
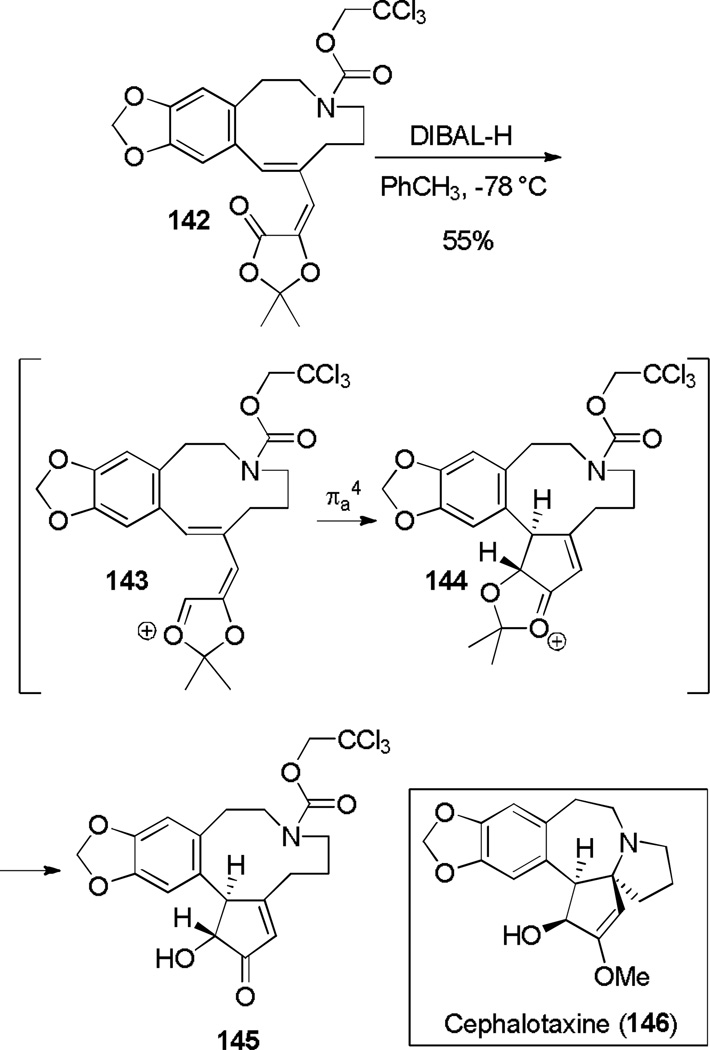
An iso-Nazarov-type cyclization was used in an expedient formal synthesis of the potent antileukemic natural product (±)-Cephalotaxine (Scheme 23).[51] Reduction of the ester carbonyl of dioxolanone 142 formed putative oxonium intermediate 143, which underwent facile electrocyclization to yield 145 as a single diastereomer. The cyclization was found to work on a number of additional substrates, including a torquoselective example.
Scheme 23.
Reductive Oxy-Nazarov Cyclization in the Formal Synthesis of (±)-Cephalotaxine
9. Formation of 1-Amino-Pentadienyl Cations (Imino-Nazarov Cyclization)
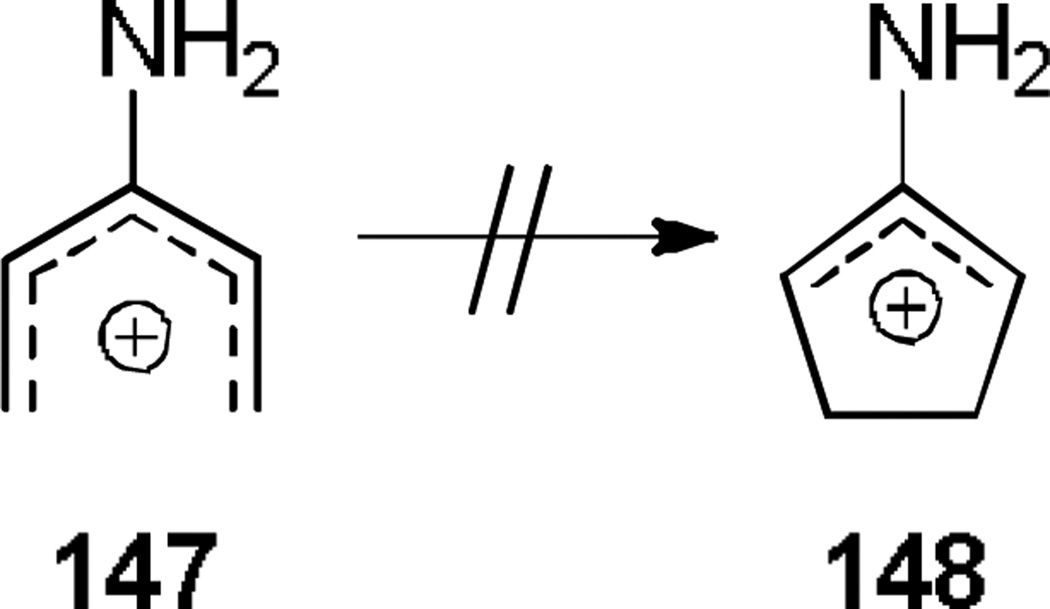
The canonical form of the imino-Nazarov cyclization has been determined by ab initio molecular orbital studies to be energetically disfavored due to stabilization of the pentadienyl cation (147) over the oxyallyl cation (148, Scheme 24).[52] However, 1-amino-pentadienyl cations have been shown to cyclize given a sufficient driving force.
Scheme 24.
Unfavorable Electrocyclization of 3-Amino Pentadienyl Cations.
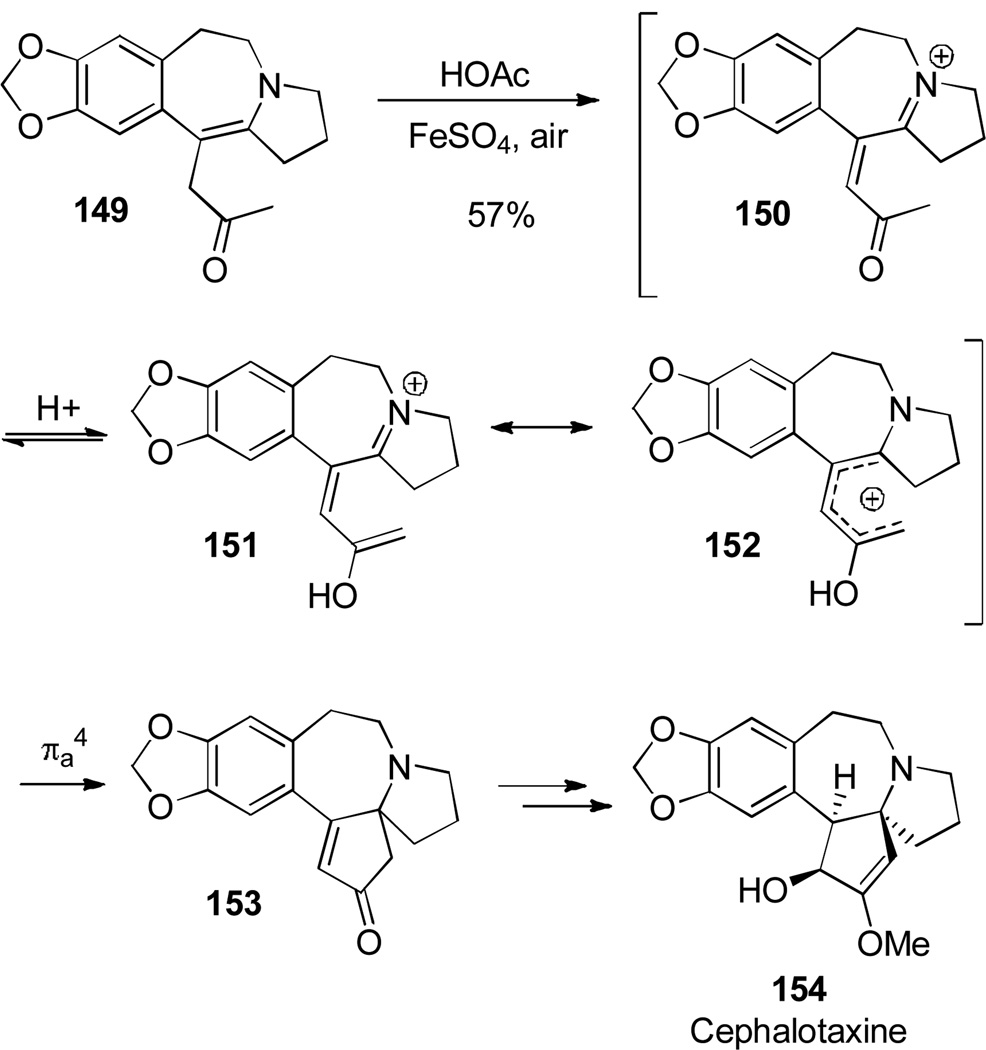
Li employed a novel 1-imino-type Nazarov cyclization to construct the E ring of (±)-Cephalotaxine (Scheme 25).[53] Exposure of enamine 149 to acetic acid and FeSO4 in air ensues an acid-catalyzed autoxidation process forming conjugated system 150. Acid-catalyzed tautomerization allows the formation of 151, in resonance with pentadienyl cation 152. Nazarov cyclization yields key intermediate 153.
Scheme 25.
Synthesis of the E ring of (±)-Cephalotaxine.
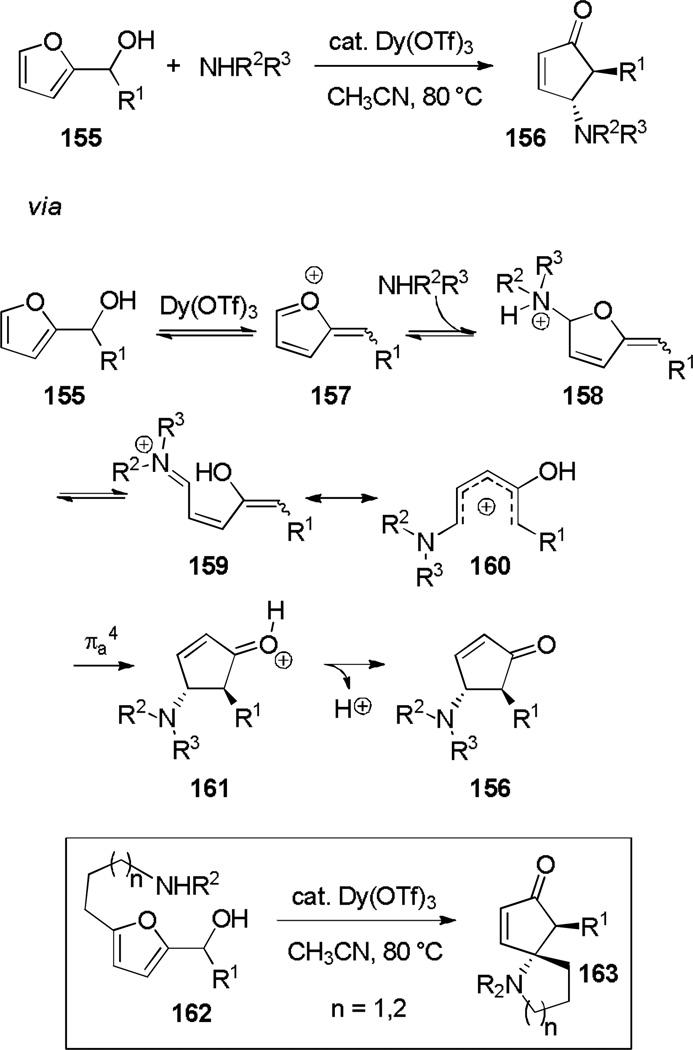
Over 36 years ago, Piancatelli noted that in the presence of water and an acid catalyst, 2-hydroxymethyl furans rearrange to 4-hydroxycyclopentenones.[54] Recently, Read de Alaniz and co-workers augmented the power of this cyclization by performing it in the presence of catalytic Dy(OTf)3 and an amine instead of water to produce the corresponding 4-amino-5-aryl/alkyl cyclopentenones (156) in excellent diastereoselectivity (Scheme 26).[55] The mechanism is thought to occur by the Dy(OTf)3-catalyzed ionization of hydroxide from 155, followed by trapping at the 5-position of resulting furyl cation 157 with an amine. Proton transfer and ring opening produce iminium ion 159, which is a resonance form of pentadienyl cation 160. Electrocyclization occurs to form 161, which yields cyclopentenone 156 upon elimination. The methodology was quite general for multiple variations of R1 and a variety of mono- and di-substituted aryl and alkylamines. It was later extended to the synthesis of spirocycles of type 163 by simple addition of a pendant amine to the furan α-position.[56] Batey and coworkers have studied a closely related cyclization that produces diastereopure 4,5-diamino cyclopentenones.[57]
Scheme 26.
Aza-Piancatelli Electrocyclization.
10. Conclusions
We hope this microreview on some of the recent unconventional extensions of the Nazarov cyclization offers perspective on the wealth of methods available for the generation of well-behaved pentadienyl cations. In particular, these advances demonstrate that if a pentadienyl cation can be generated, in most cases it will undergo efficient electrocyclization. Furthermore, pentadienyl cations with many different substitution patterns can be generated and cyclized, using the novel methods described in this review. for this reason, the cyclization has become appealing to chemists interested in many different chemical questions, ranging from catalysis to cationic reactivity to natural product synthesis. We expect that as the field continues to evolve, the versatility, generality and applicability that the Nazarov cyclization will achieve will further increase its synthetic utility.
Acknowledgments
We thank the NSF (CHE-0847851) and the NIGMS (R01 GM079364) for support of our programs.
Biographies

William T. Spencer III was born in Poughkeepsie, NY (United States) in 1979. He graduated with a Bachelor’s degree in chemistry from Rensselaer Polytechnic Institute in 2001, after which he held a position as a Research Scientist at AMRI, Inc. synthesizing small molecule analogs for biological testing until 2006. He then returned to academia to obtain his Master’s in chemistry from Rochester Institute of Technology under the direction of Professor Chrisitna G. Collison. After graduating in 2008, he enrolled in the Ph.D. program at the University of Rochester, where he earned his degree in 2012 under the supervision of Professor Alison J. Frontier performing methodology studies and natural product synthesis. He is currently a postdoctoral fellow at the same university in the laboratory of Professor Robert K. Boeckman synthesizing enzyme inhibitors for antifungal SAR.

Tulaza Vaidya is a native of Kathmandu, Nepal. She graduated with a Bachelor’s degree in Chemistry in 2007 from Lake Forest College, Lake Forest, IL, where she pursued research in palladium-catalyzed cross-coupling reactions under the direction of Professor William B. Martin. She joined the University of Rochester, Rochester, NY in 2007 and worked on electrophilicly-driven annulations of polarized Nazarov precursors using iridium and gold catalysts underthe joint supervision of Professor Alison J. Frontier and Professor Richard Eisenberg. After earning a Ph.D. degree in 2012, she moved to Cornell University, Ithaca, NY to explore polymer chemistry. She is currently a postdoctoral fellow working on olefin polymerization under the supervision of Professor Geoffrey W. Coates.

Alison J. Frontier grew up in Farmington, Michigan, a suburb of Detroit. She received her AB from Harvard in 1992, and then took a two-year position as an Associate Chemist at the Merck Research Laboratories in Rahway, NJ. She earned her PhD in 1999 from Columbia University, where she studied with Samuel Danishefsky, and then she did postdoctoral work with Barry Trost at Stanford University. She has been on the faculty at the University of Rochester since 2002. Her research interests focus on target molecule synthesis and reaction development using novel catalytic methods. Students in her research group are pursuing novel strategies for the synthesis of bioactive, structurally interesting natural products, as well as the development of cationic and neutral pericyclic reactions and multistep cyclization cascades.
References
- 1.Nazarov IN, Zaretskaya II. Izv. Akad. Nauk. SSSR, Ser. Khim. 1941:211–224. [Google Scholar]
- 2.Habermas KL, Denmark SE, Jones TK. Org. React. 1994;45:1–158. and references therein. [Google Scholar]
- 3.Reviews: Santellirouvier C, Santelli M. Synthesis. 2011:429–442. Pellissier H. Tetrahedron. 2011;61:6479–6517. Frontier AJ, Collison C. Tetrahedron. 2011;61:7577–7606. Tius MA. Eur. J. Org. Chem. 2005:2193–2206. Grant TN, Rieder CJ, West FG. Chem. Commun. 2011:5676–5688. doi: 10.1039/b908515g. Nakanishi WW, West FG. Curr. Opin. Drug Discovery Dev. 2011;12:732–751. Vaidya T, Eisenberg R, Frontier AJ. ChemCatChem. 2011;3:1531–1548.
- 4.(a) Halterman RL, Tretyakov A. Tetrahedron. 2011;51:4371–4382. [Google Scholar]; (b) Ohwada T, Suzuki T, Shudo K. J. Am. Chem. Soc. 120:4629–4637. [Google Scholar]; (c) Tius MA, Hu HP, Kawakami JK, Busch-Petersen J. J. Org. Chem. 2011;63:5971–5976. doi: 10.1021/jo980621z. [DOI] [PubMed] [Google Scholar]; (d) Langer P, Döring M, Seyferth D, Görls H. Chem. – Eur. J. 2011;7:573–584. doi: 10.1002/1521-3765(20010202)7:3<573::aid-chem573>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]; (e) Rupert KC, Liu CC, Nguyen TT, Whitener MA, Sowa JR. Organometallics. 2011;21:144–149. [Google Scholar]; (f) Tang JM, Bhunia S, Sohel SMA, Lin MY, Liao HY, Datta S, Das A, Liu RS. J. Am. Chem. Soc. 2011;129:15677–15683. doi: 10.1021/ja076175r. [DOI] [PubMed] [Google Scholar]; (g) Wan L, Tius MA. Org. Lett. 2011;9:647–650. doi: 10.1021/ol062919e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Cordier P, Aubert C, Malacria M, Lacote E, Gandon V. Angew. Chem. Int. Ed. 48:8757–8760. doi: 10.1002/anie.200903675. [DOI] [PubMed] [Google Scholar]; (i) Hastings CJ, Pluth MD, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2011;132:6938–6940. doi: 10.1021/ja102633e. [DOI] [PubMed] [Google Scholar]; (j) Rieder CJ, Winberg KJ, West FG. J. Org. Chem. 2011;76:50–56. doi: 10.1021/jo101497f. [DOI] [PubMed] [Google Scholar]
- 5.Singh R, Panda G. Org. Biomol. Chem. 2011;9:4782–4790. doi: 10.1039/c0ob00892c. [DOI] [PubMed] [Google Scholar]
- 6.Nair V, Bindu S, Sreekumar V, Chiaroni A. Org. Lett. 2002;4:2821–2823. doi: 10.1021/ol026010h. [DOI] [PubMed] [Google Scholar]
- 7.(a) de Lera AR, Rey JG, Hrovat D, Iglesias B, Lopez S. Tetrahedron Lett. 1997;38:7425–7428. [Google Scholar]; (b) Iglesias B, de Lera AR, Rodriguez-Otero J, Lopez S. Chem.—Eur. J. 2000;6:4021–4033. doi: 10.1002/1521-3765(20001103)6:21<4021::aid-chem4021>3.3.co;2-e. [DOI] [PubMed] [Google Scholar]
- 8.Reyes JCP, Romo D. Angew. Chem. Int. Ed. 2012;51:6870–6873. doi: 10.1002/anie.201200959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.(a) Occhiato EG, Prandi C, Ferrali A, Guarna A, Deagostino A, Venturello P. J. Org. Chem. 2002;67:7144–7146. doi: 10.1021/jo025930a. [DOI] [PubMed] [Google Scholar]; (b) Occhiato EG, Prandi C, Ferrali A, Guarna A, Venturello P. J. Org. Chem. 2003;68:9728–9741. doi: 10.1021/jo034939p. [DOI] [PubMed] [Google Scholar]; (c) Prandi C, Ferrali A, Guarna A, Venturello P, Occhiato EG. J. Org. Chem. 2004;69:7705–7709. doi: 10.1021/jo0489263. [DOI] [PubMed] [Google Scholar]; (d) Barluenga J, Barrio P, Vicente R, López LA, Tomás M. J. Organomet. Chem. 2004;689:3793–3799. [Google Scholar]; (e) Prandi C, Deagostino A, Venturello P, Occhiato EG. Org. Lett. 2005;7:4345–4348. doi: 10.1021/ol051464a. [DOI] [PubMed] [Google Scholar]; (f) Cavalli A, Masetti M, Recanatini M, Prandi C, Guarna A, Occhiato EG. Chem. – Eur. J. 2006;12:2836–2845. doi: 10.1002/chem.200501391. [DOI] [PubMed] [Google Scholar]; (g) Cavalli A, Pacetti A, Recanatini, Prandi C, Scarpi D, Occhiato EG. Chem. – Eur. J. 2008;14:9292–9304. doi: 10.1002/chem.200801030. [DOI] [PubMed] [Google Scholar]; (h) Barbero M, Cadamuro S, Deagostino A, Dughera S, Larini P, Occhiato EG, Prandi C, Tabasso S, Vulcano R, Venturello P. Synthesis. 2009:2260–2266. [Google Scholar]; (i) Bhattacharya C, Bonfante P, Deagostino A, Kapulnik Y, Larini P, Occhiato EG, Prandi C, Venturello P. Org. Biomol. Chem. 2009;7:3413–3420. doi: 10.1039/b907026e. [DOI] [PubMed] [Google Scholar]; (j) Eom D, Park S, Park Y, Ryu T, Lee PH. Org. Lett. 2012;14:5392–5395. doi: 10.1021/ol302271w. [DOI] [PubMed] [Google Scholar]
- 10.Related work on Nazarov cyclization of vinyl allenyl ketones:Stephen A, Hashmi K, Bats JW, Choi J-H, Schwarz L. Tetrahedron Lett. 1998;39:7491–7494. Tius MA. Acc. Chem. Res. 2003;36:284–290. doi: 10.1021/ar0200394. Bee C, Tius MA. Org. Lett. 2003;5:1681–1684. doi: 10.1021/ol034309+. Forest J, Bee C, Cordaro F, Tius MA. Org. Lett. 2003;5:4069–4072. doi: 10.1021/ol035498z. Banaag AR, Berger GO, Dhoro F, delos Santos DB, Dixon DD, Mitchell JP, Tokeshi BK, Tius MA. Tetrahedron. 2005;61:3419–3428. Berger GO, Tius MA. Org. Lett. 2005;7:5011–5013. doi: 10.1021/ol052015d. Dhoro F, Tius MA. J. Am. Chem. Soc. 2005;127:12472–12473. doi: 10.1021/ja053393g. delos Santos DB, Banaag AR, Tius MA. Org. Lett. 2006;8:2579–2582. doi: 10.1021/ol060816q. Banaag AR, Tius MA. J. Am. Chem. Soc. 2007;129:5328–5329. doi: 10.1021/ja069342g. Berger GO, Tius MA. The J. Org. Chem. 2007;72:6473–6480. doi: 10.1021/jo070923d. Dhoro F, Kristensen TE, Stockmann V, Yap GPA, Tius MA. J. Am. Chem. Soc. 2007;129:7256–7257. doi: 10.1021/ja0718873. Banaag AR, Tius MA. J. Org. Chem. 2008;73:8133–8141. doi: 10.1021/jo801503c. Basak AK, Tius MA. Org. Lett. 2008;10:4073–4076. doi: 10.1021/ol801587u.
- 11.Spencer WT, Levin MD, Frontier AJ. Org. Lett. 2011;13:414–417. doi: 10.1021/ol1027255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinylallene activation methods which produce similar cyclopentenone products: Delbecq F, Gore J. Tetrahedron Lett. 1976:3459–3460. Baudouy R, Delbecq F, Gore J. Tetrahedron. 1980;36:189–195. Wu Y-K, West FG. J. Org. Chem. 2010;75:5410–5413. doi: 10.1021/jo101112t.
- 13.(a) Malona JA, Cariou K, Frontier AJ. J. Am. Chem. Soc. 2009;131:7560–7561. doi: 10.1021/ja9029736. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Malona JA, Cariou K, Spencer WT, Frontier AJ. J. Org. Chem. 2012;77:1891–1908. doi: 10.1021/jo202366c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JH, Toste FD. Angew. Chem. Int. Ed. 2007;46:912–914. doi: 10.1002/anie.200604006. [DOI] [PubMed] [Google Scholar]
- 15.Funami H, Kusama H, Iwasawa N. Angew. Chem. Int. Ed. 2007;46:909–911. doi: 10.1002/anie.200603986. [DOI] [PubMed] [Google Scholar]
- 16.Chen B, Fan W, Chai G, Ma S. Org. Lett. 2012;14:3616–3619. doi: 10.1021/ol301408g. [DOI] [PubMed] [Google Scholar]
- 17.Lin GY, Yang CY, Liu RS. J. Org. Chem. 2007;72:6753–6757. doi: 10.1021/jo0707939. [DOI] [PubMed] [Google Scholar]
- 18.Carbon monoxide has been demonstrated to accelerate the rate of certain PtCl2-catalyzed skeletal rearrangements: Fürstner A, Davies PW, Gress T. J. Am. Chem. Soc. 2005;127:8244–8245. doi: 10.1021/ja050845g.
- 19.Bhunia S, Liu RS. J. Am. Chem. Soc. 2008;130:16488–16489. doi: 10.1021/ja807384a. [DOI] [PubMed] [Google Scholar]
- 20.Brooks JL, Caruana PA, Frontier AJ. J. Am. Chem. Soc. 2011;133:12454–12457. doi: 10.1021/ja205440x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.A cyclopentanellation which produces similar products: Zou W, Wang Z, Lacroix E, Wu S-H, Jennings HJ. Carbohydr. Res. 2001;334:223–231. doi: 10.1016/s0008-6215(01)00187-2. Zou W, Shao H, Wu S-H. Carbohydr. Res. 2004;339:2475–2485. doi: 10.1016/j.carres.2004.08.008.
- 22.Brooks JL, Frontier AJ. J. Am. Chem. Soc. 2012;134:16551–16553. doi: 10.1021/ja308451y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi X, Gorin DJ, Toste FD. J. Am. Chem. Soc. 2005;127:5802–5803. doi: 10.1021/ja051689g. [DOI] [PubMed] [Google Scholar]
- 24.Rautenstrauch V. J. Org. Chem. 1984;49:950–952. [Google Scholar]
- 25.Nieto Faza O, Lopez CS, Alvarez R, de Lera AR. J. Am. Chem. Soc. 2006;128:2434–2437. doi: 10.1021/ja057127e. [DOI] [PubMed] [Google Scholar]
- 26.Nieto Faza O, Lopez CS, de Lera AR. J. Org. Chem. 2011;76:3791–3796. doi: 10.1021/jo200090j. [DOI] [PubMed] [Google Scholar]
- 27.Reviews on memory of chirality: Kawabata T, Yahiro K, Fuji K. J. Am. Chem. Soc. 1991;113:9694–9696. Fuji K, Kawabata T. Chem. – Eur. J. 1998;4:373–376. Zhao HW, Hsu DC, Carlier PR. Synthesis. 2005;1–16
- 28.Nakanishi Y, Miki K, Ohe K. Tetrahedron. 2007;63:12138–12148. [Google Scholar]
- 29.Nakagawa D, Miyashita M, Tanino K. Tetrahedron Lett. 2010;51:2771–2773. [Google Scholar]
- 30.Zhang L, Wang S. J. Am. Chem. Soc. 2006;128:1442–1443. doi: 10.1021/ja057327q. [DOI] [PubMed] [Google Scholar]
- 31.Shi FQ, Li X, Xia Y, Zhang L, Yu ZX. J. Am. Chem. Soc. 2007;129:15503–15512. doi: 10.1021/ja071070+. [DOI] [PubMed] [Google Scholar]
- 32.(a) Lemiere G, Gandon V, Cariou K, Fukuyama T, Dhimane A-L, Fensterbank L, Malacria M. Org. Lett. 2007;9:2207–2209. doi: 10.1021/ol070788r. [DOI] [PubMed] [Google Scholar]; (b) Lemiere G, Gandon V, Cariou K, Hours A, Fukuyama T, Dhimane AL, Fensterbank L, Malacria M. J. Am. Chem. Soc. 2009;131:2993–3006. doi: 10.1021/ja808872u. [DOI] [PubMed] [Google Scholar]
- 33.Muxfeldt H, Weigele M, Van RV. J. Org. Chem. 1965;30:3573–3574. [Google Scholar]
- 34.Weinreb SM, Auerbach J. J. Am. Chem. Soc. 1975;97:2503–2506. [PubMed] [Google Scholar]
- 35.Yb(OTf)3 and LiTMP initiated. Batson WA, Sethumadhavan D, Tius MA. Org. Lett. 2005;7:2771–2774. doi: 10.1021/ol050970x. [DOI] [PubMed] [Google Scholar]
- 36.Uhrich EA, Batson WA, Tius MA. Synthesis. 2006:2139–2142. [Google Scholar]
- 37.Bow WF, Basak AK, Jolit A, Vicic DA, Tius MA. Org. Lett. 2010;12:440–443. doi: 10.1021/ol9025765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basak AK, Shimada N, Bow WF, Vicic DA, Tius MA. J. Am. Chem. Soc. 2010;132:8266–8267. doi: 10.1021/ja103028r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams DR, Robinson LA, Nevill CR, Reddy JP. Angew. Chem. Int. Ed. 2007;46:915–918. doi: 10.1002/anie.200603853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai Z-X, Harmata M. Org. Lett. 2010;12:5668–5670. doi: 10.1021/ol102478h. [DOI] [PubMed] [Google Scholar]
- 41.Jin T, Yamamoto Y. Org. Lett. 2008;10:3137–3139. doi: 10.1021/ol801265s. [DOI] [PubMed] [Google Scholar]
- 42.Saito A, Umakoshi M, Yagyu N, Hanzawa Y. Org. Lett. 2008;10:1783–1785. doi: 10.1021/ol800539a. [DOI] [PubMed] [Google Scholar]
- 43.Varea T, Alcalde A, Lopez dDC, Ramirez dAC, Cossio FP, Asensio G. J. Org. Chem. 2012;77:6327–6331. doi: 10.1021/jo300806y. [DOI] [PubMed] [Google Scholar]
- 44.(a) Paquette LA. Eur. J. Org. Chem. 1998:1709–1728. [Google Scholar]; (b) Varea T, Alcalde A, Grancha A, Lloret J, Asensio G, Lledos A. J. Org. Chem. 2008;73:6521–6533. doi: 10.1021/jo800617y. [DOI] [PubMed] [Google Scholar]
- 45.Pujanauski BG, Bhanu Prasad BA, Sarpong R. J. Am. Chem. Soc. 2006;128:6786–6787. doi: 10.1021/ja061549m. [DOI] [PubMed] [Google Scholar]
- 46.González-Pérez AB, Vaz B, Nieto Faza O, de Lera AR. J. Org. Chem. 1997;77:8733–8743. doi: 10.1021/jo301651r. [DOI] [PubMed] [Google Scholar]
- 47.Suárez-Pantiga S, Rubio E, Alvarez-Rúa C, Gonzaález JM. Org. Lett. 2009;11:13–16. doi: 10.1021/ol8025523. [DOI] [PubMed] [Google Scholar]
- 48.(a) Grant TN, West FG. J. Am. Chem. Soc. 2006;128:9348–9349. doi: 10.1021/ja063421a. [DOI] [PubMed] [Google Scholar]; (b) Grant TN, West FG. Org. Lett. 2007;9:3789–3792. doi: 10.1021/ol7015669. [DOI] [PubMed] [Google Scholar]
- 49.Other Nazarov cyclizations of chlorine-substituted pentadienyl cations: Hiyama T, Tsukanaka M, Nozaki H. J. Am. Chem. Soc. 1974;96:3713–3714. Hiyaina T, Shinoda M, Nozaki H. Tetrahedron Lett. 1978;19:771–774. Hiyama T, Shinoda M, Tsukanaka M, Nozaki H. Bull. Chem. Soc. Jpn. 1980;53:1010–1014.Cheletropic extrusion to generate divinyl gem-dichloride intermediate: Gaoni Y. Tetrahedron Lett. 1978;19:3277–3278.
- 50.(a) Lin CC, Teng TM, Odedra A, Liu RS. J. Am. Chem. Soc. 2007;129:3798–3798. doi: 10.1021/ja069171f. [DOI] [PubMed] [Google Scholar]; (b) Lin CC, Teng TM, Tsai CC, Liao HY, Liu RS. J. Am. Chem. Soc. 2008;130:16417–16423. doi: 10.1021/ja806415t. [DOI] [PubMed] [Google Scholar]
- 51.Li W-DZ, Duo W-G, Zhuang C-H. Org. Lett. 2011;13:3538–3541. doi: 10.1021/ol201390r. [DOI] [PubMed] [Google Scholar]
- 52.Smith DA, Ulmer CW., II J. Org. Chem. 1997;62:5110–5115. [Google Scholar]
- 53.Li W-DZ, Wang Y-Q. Org. Lett. 2003;5:2931–2934. doi: 10.1021/ol035098b. [DOI] [PubMed] [Google Scholar]
- 54.Seminal work: Piancatelli G, Scettri A, Barbadoro S. Tetrahedron Lett. 1976;17:3555–3558. Piancatelli G. Heterocycles. 1982;19:1735–1744. Piancatelli G, D'Auria M, D'Onofrio F. Synthesis. 1994:867–889. More recent studies: Rodríguez A, Nomen M, Spur BW, Godfroid J-J. Eur. J. Org. Chem. 1999;1999:2655–2662. D'Auria M. Heterocycles. 2000;52:185–194. Csaky AG, Contreras C, Mba M, Plumet J. Synlett. 2002:1451–1454. Csáky AG, Mba M, Plumet J. Synlett. 2003:2092–2094. Csákÿ AG, Mba M, Plumet J. Tetrahedron: Asymmetry. 2004;15:647–652. Computational study: Nieto Faza O, Lopez CS, Alvarez R, de Lera LAR. Chem. – Eur. J. 2004;10:4324–4333. doi: 10.1002/chem.200400037.
- 55.Veits GK, Wenz DR, Read de Alaniz dAJ. Angew. Chem. Int. Ed. 2010;49:9484–9487. doi: 10.1002/anie.201005131. [DOI] [PubMed] [Google Scholar]
- 56.Palmer LI, Read de Alaniz dAJ. Angew. Chem. Int. Ed. 2011;50:7167–7170. doi: 10.1002/anie.201102102. [DOI] [PubMed] [Google Scholar]
- 57.Li S-W, Batey RA. Chem. Commun. 2007:3759–3761. doi: 10.1039/b709337n. [DOI] [PubMed] [Google Scholar]