Abstract
The aim of this study is to investigate the long-term immunogenicity of inactivated split-virion 2009 pandemic influenza A H1N1 vaccine after a single immunization. We recruited 480 adults, aged 18–60 years, for a placebo-controlled, observer-masked, single-center clinical study. We randomly assigned subjects into four groups: 15 μg, 30 μg and 45 μg of hemagglutinin (HA) dosage groups, and a placebo control group. Finally, 259 subjects completed the entire study. The rates of seroconversion and seroprotection and the geometric mean increase (GMI) fulfilled the criteria of the European Medicines Agency (EMEA) for influenza vaccine for 180 days after vaccination in all three dosage groups. However, the seroprotection rates of all dosage groups were below 70% at day 360 post vaccination, while the seroconversion rates and the GMI continued to meet the licensure criteria at this time point. In conclusion, a single dose of 15 μg HA vaccine could induce a protective immune response persisting for at least six months in adults. This study could be beneficial for the future development of influenza vaccines conferring long-term immunity.
Keywords: Hemagglutinin (HA), H1N1, Influenza, Long-term, Immunogenicity
Highlights
▸ We recruited 480 adults (aged 18–60 years) administered with different doses of influenza A H1N1 vaccine without adjuvant. ▸ We collected and analyzed the serum samples on day 28, 90, 180 and 360 post vaccination. ▸ Although a single dose of vaccine could induce long-term immunity persisting for at least six months protection waned significantly one year after vaccine suggesting the need for a booster vaccine.
1. Introduction
Influenza is a serious public health problem, causing severe illness and death in humans. In April 2009, a new swine-origin influenza A (H1N1) virus emerged in Mexico and the United States, and then spread rapidly to more than 200 countries and regions, causing human infections and tens of thousands of deaths throughout the world [1,2]. This novel H1N1 virus is responsible for the first influenza pandemic of the 21st century [3].
Vaccines are considered to be one of the most effective tools, not only to prevent the spread of influenza virus but also to mitigate the severity of illness and the impact of the disease [4]. The risk presented by the pandemic influenza A (H1N1) virus prompted a new monovalent vaccine to be actively developed and clinically assessed by several vaccine manufacturers throughout the world, and mass immunization programs have been implemented by many countries. A variety of vaccines are being thoroughly evaluated for their safety and immunogenicity in humans, including inactivated whole virus vaccines, split vaccines with or without adjuvant and live attenuated vaccines [5,6]. The results of clinical trials showed that these vaccines had good levels of safety and that single-dose vaccination could induce strong immune responses in healthy people [7–11]. Furthermore, the indicators all met the EU criteria for assessing seasonal influenza vaccines [12]. It has now been reported by many studies that 2009 pandemic influenza A H1N1 vaccine can provide effective protection in humans [7–11]. Clinical trials were completed in China in August 2009. In these clinical trials, 15 μg of hemagglutinin antigen as a two-dose regimen was administered to vaccine subjects of different age groups and the results showed that the vaccine was safe and effective [13]. Despite the fact that the current influenza epidemic has reached a peak in many areas and that the incidence rate is now declining, the influenza A H1N1 (2009) virus continues to cause a threat and remains the predominant cause of seasonal influenza virus infection [14]. The WHO has added the 2009 pandemic influenza A H1N1 virus to the recommended composition of influenza virus vaccines for use as a seasonal influenza vaccine candidate [15]. Although the WHO has announced the end of the pandemic of influenza A H1N1 (2009) virus, we cannot rule out the possibility of local epidemics of this virus. The WHO has also advised the continued administration of the influenza A H1N1 vaccine. It is important to study the long-term immunogenicity of the 2009 pandemic influenza A H1N1 vaccine and to determine the potential need for re-vaccination during extended epidemics.
The primary aim of this study was to investigate the immune responses and the persistence of immunogenicity induced by a single dose of the 2009 pandemic influenza A H1N1 monovalent split-virion vaccine among adults aged 18–60 years. We also compared the effects of dosage on the long-term immunogenicity and efficacy of the split-virion H1N1 vaccine, with the aim of determining the optimum dosage and regimen for the vaccine for long-term immunization.
2. Methods
2.1. Study design
From July 2009 to July 2010, we carried out randomized, double-blind, single-center clinical trial in Hengdong County of Hunan Province (China) on 480 subjects. The Center for Disease Control and Prevention (CDC) in Hunan Province was responsible for the clinical trial and the CDC in Hengdong County participated in the clinical trial. The study was sponsored by the Shanghai Institute of Biological Products (China). The CDC in Hunan Province and Hengdong County were mainly responsible for data collection during the clinical trial. The Central South University (Changsha, Hunan, China) was responsible for data analysis and statistical processing. All of the pilot programs, clinical manuals and other materials used in this study were consistent with the Declaration of Helsinki and the quality control requirements for clinical trials, and were approved by the Ethics Committee of Hunan Province.
All 480 participants received a single dose injection of the vaccine or a placebo. The immunologic end points were determined by detecting the hemagglutination-inhibition (HI) antibody positive rates on day 28, day 90, day 180 and day 360. After serum samples were collected on day 180, the subjects in control group were received a supplementary injection of vaccine and the serum samples were not collected on day 360. The subjects were monitored and their systemic and local reactions were recorded after vaccination.
2.2. Vaccines
The inactivated split-virion vaccine against the H1N1 (2009) virus was developed by the Shanghai Institute of Biological Products, and the seed virus was prepared from the reassortant vaccine virus A/California/7/2009 NYMC X-179A, as recommended by the WHO [16,17]. The vaccine was prepared in embryonated chicken eggs according to the standard techniques used in the production of seasonal influenza vaccine. In brief, the virus was amplified in chicken embryos, then harvested and inactivated with formaldehyde. The viral cultures were then concentrated and purified for use as the final vaccine. The vaccine was approved for clinical use by the Chinese National Institute for the Control of Pharmaceutical and Biological Products (China).
The experimental vaccines were split-virus products containing 15 μg, 30 μg or 45 μg of hemagglutinin antigen per dose (0.5 ml). The placebo consisted of phosphate-buffered saline (PBS).
2.3. Participants
All subjects participated voluntarily in the clinical trials and their written informed consents were obtained once they fully understood the study procedures. All subjects were 18–60 years old. The main exclusion criteria included: a history of infection with the 2009 pandemic influenza A H1N1 virus; receipt of any influenza vaccine within six months; inoculated with any other prevention products in the last week; a history of allergy or contraindications of vaccination.
Injections were given intramuscularly in the deltoid muscle. After an on-site safety observation within 30 min of the injection, subjects were asked to record data on systemic and local adverse reactions at 6, 24, 48 and 72 h and on day 7, day 14 and day 21. Serum samples were collected on day 28, day 90, day 180 and day 360 after vaccination.
2.4. Randomization and masking
We recruited 480 subjects, aged from 18 to 60. Subjects were randomly divided into four treatment groups in a 1:1:1:1 ratio which were administered 15 μg, 30 μg or 45 μg of hemagglutinin or placebo, respectively. Each treatment group comprised 120 subjects with a male to female ratio of 1:1. The blind testing was designed by a third party at Central South University, who was not involved in other elements of the clinical trials.
2.5. Assays
The antibody titers against the vaccine strain were determined by HI assays of the anti-homologous strain of X-179A, performed in accordance with established measures using turkey erythrocytes [18,19]. In brief, sera were firstly treated with receptor destroy enzyme at 36°C for 16 h. The titers of HI antibody that were below the detection limit (i.e., <1:10) were recorded as a value of 1:5, and titers above 1:10,240 were recorded as a value of 1:10,240. The seroconversion rate represented a post-vaccination titer ≥1:40 in subjects with a pre-vaccination titer of <1:10 or a ≥4-fold titer increase in subjects with a pre-vaccination titer of ≥1:10. All serum samples were assayed in a blinded manner, in duplicate, and were checked in parallel by the Chinese National Institute for the Control of Pharmaceutical and Biological Products.
2.6. Statistical analysis
For safety assessments, the frequency, severity, mean time of appearance and duration of all the local and systemic adverse events were calculated in all groups in accordance with the requirements for influenza vaccines published by the Division of Microbiology and Infectious Diseases of the US National Institutes of Health [20,21].
For immunogenicity assessments, the seroconversion rate represented either a post-vaccination titer ≥1:40 (in accordance with the requirements for seasonal influenza vaccines by the European Committee for Proprietary Medicinal Products) in subjects with a pre-vaccination titer of <1:10 or a ≥4-fold titer increase in subjects with a pre-vaccination titer of ≥1:10. The seroprotection rate represented the proportion of subjects with a post-vaccination titer ≥1:40. A seroprotection rate >70% was considered to provide protection. In addition, the geometric mean increase (GMI) was the ratio of the titer after vaccination to the titer before vaccination. All the serum data analyzed in this research were from the subjects who received five times blood collections [22].
Hypothesis testing was conducted using two-sided tests, with an alpha value of 0.05 considered to indicate statistical significance. All statistical analyses were performed using the SPSS software package (version 11.5).
3. Results
3.1. Study participants
Recruitment visits were attended by 493 participants (Fig. 1). A total of 480 subjects between 18 and 60 years of age participated in the clinical trial and 480 serum samples were collected initially. Some subjects were gradually withdrawn from the clinical trial, so only 454 serum samples were collected on day 28, 416 serum samples were collected on day 90, 377 serum samples were collected on day 180. In addition, we only collected 259 serum samples in vaccine groups on day 360, because the subjects in control group were received a supplementary injection of vaccine on day 180 and the serum samples were not collected on day 360. In all vaccine groups, 259 subjects completed the entire study and provided five serum samples.
Fig. 1.
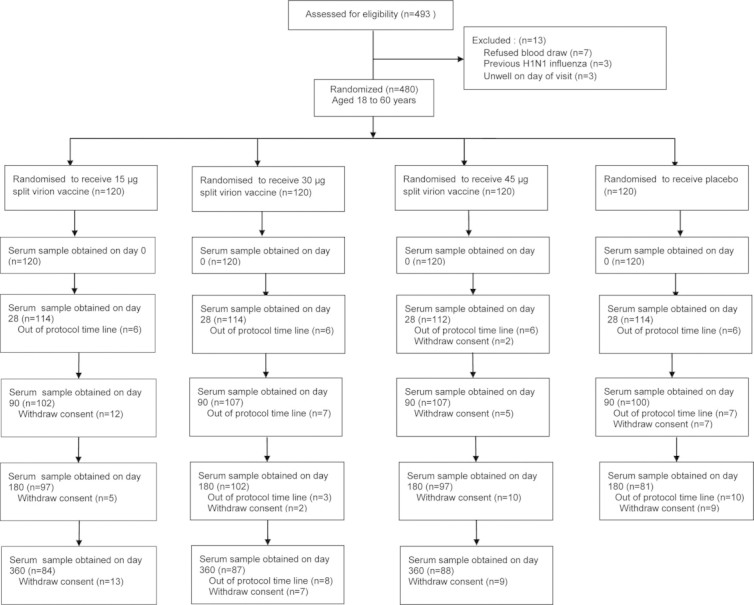
Enrollment and follow-up of study participants.
3.2. Safety of the vaccine
The safety and side effects of the vaccine have been reported previously [13]. Briefly, adverse reactions were only mild or moderate, and no serious adverse reaction was detected. In addition, pain was the most frequently reported adverse effect in the local response and fever was the most frequently reported adverse effect in the systemic response. No serious adverse event was reported during the entire study period [13].
3.3. Immune response
Before vaccination, the proportion of subjects showing HI ≥1:40 in all of the dosage groups was 2.27–4.94%. Immune responses were induced in all subjects after vaccination. On day 28 after vaccination, the rates of seroconversion and seroprotection in the 15 μg group were 96.43% and 95.24%, respectively, the rates in the 30 μg group were 98.85% and 97.70%, respectively, and the rates in the 45 μg group were 100.00% and 97.73%, respectively. On day 90 after vaccination, the rates of seroconversion and seroprotection declined in all of the vaccine groups. The rates in the 15 μg group were 89.29% and 85.71%, respectively, the rates in the 30 μg group were 91.95% and 90.80%, respectively, and the rates in the 45 μg group were 94.32% and 93.18%, respectively. On day 180 after vaccination, the rates of seroconversion and seroprotection were lower than the rates on day 28, but were similar to the rates on day 90. The seroconversion and seroprotection rates in the 15 μg group were 91.67% and 89.29%, respectively, the rates in the 30 μg group were 95.40% and 87.36%, respectively, and the rates in the 45 μg group were 95.40% and 90.91%, respectively. On day 360 after vaccination, the rates of seroconversion and seroprotection in all groups were significantly lower than the rates on day 180 ( P<0.01).The seroconversion and seroprotection rates in the 15 μg group were 70.24% and 46.43%, respectively, the rates in the 30 μg group were 74.71% and 49.43%, respectively, and the rates in the 45 μg group were 81.82% and 55.68%, respectively (Table 1).
Table 1.
Proportion of participants with seroprotection and seroconversion in the various groups.
| Dose | Baseline |
28 days after injection |
90 days after injection |
180 days after injection |
360 days after injection |
||||
|---|---|---|---|---|---|---|---|---|---|
| SP rate |
SC rate |
SP rate |
SC rate |
SP rate |
SC rate |
SP rate |
SC rate |
SP rate |
|
| Number of participants (Percentage, 95% CI) | |||||||||
| Placebo (n=81) | 4 (4.9, 1.4–12.2) | 4 (4.9, 1.4–12.2) | 6 (7.4, 2.8–15.4) | 2 (2.5, 0.3–8.6) | 4 (4.9, 1.4–12.2) | 17 (21.0, 12.7–31.5) | 19 (23.5, 14.8–34.2) | – | – |
| 15 μg (n=84) | 4 (4.8, 1.3–11.8) | 81 (96.4, 89.9–99.3)a | 80 (95.2, 88.3–98.7)a | 75 (89.3, 80.6–95.0)a | 72 (85.7, 76.4–92.4)a | 77 (91.7, 83.6–96.6)a | 75 (89.3, 80.6–95.0)a | 59 (70.2, 59.3–79.7) | 39 (46.4, 35.5–57.7) |
| 30 μg (n=87) | 3 (3.4, 0.7–9.8) | 86 (98.9, 93.8–100.0)a | 85 (97.7, 92.0–99.7)a | 80 (92.0, 84.1–96.7)a | 79 (90.8, 82.7–96.0)a | 83 (95.4, 88.6–98.7)a | 76 (87.4, 78.5–93.5)a | 65 (74.7, 64.3–83.4) | 43 (49.4, 38.5–60.4) |
| 45 μg (n=88) | 2 (2.3, 0.3–8.0) | 88 (100.0, 95.9–100.0)a | 86 (97.7, 92.0–99.7)a | 83 (94.3, 87.2–98.1)a | 82 (93.2, 85.8–97.5)a | 84 (95.5, 88.8–98.8)a | 80 (90.9, 82.9–96.0)a | 72 (95.5, 88.8–98.8) | 49 (55.7, 44.7–66.3) |
Seroprotection (SP) was defined as an HI titer of no less than 1:40; seroconversion (SC) was defined as an increase in the HI titer by a factor of four or more.
P<0.05 compared with the placebo.
On day 28, the geometric mean titer (GMT) of the HI antibody titers in the three vaccine groups had increased significantly compared with the GMT pre-vaccination ( P<0.01) and the GMT in the placebo group ( P<0.01). Moreover, the GMT in the 45 μg group showed a significant difference with that in the 15 μg group and the 30 μg group, respectively ( P<0.01), but there was no significant difference between the 15 μg group and the 30 μg group. On day 90, the GMT of the HI antibody titers in the three vaccine groups had declined significantly compared with that on day 28 ( P<0.01). Moreover, the GMT in the 45 μg group showed a significant difference with that of the 15 μg group ( P<0.01). On day 180, the GMT of the HI antibody titers was lower than that on day 90 in the three vaccine groups, but showed no significant differences. However, the GMT was significantly lower than that on day 28 ( P<0.01). On day 360, the GMT was significantly lower than before in all groups. The differences between groups on day 90, day 180 and day 360 were similar, with only the GMT between the 15 μg group and the 45 μg group showing a significant difference ( P<0.01) (Table 2; Figs. 2 and 3).
Table 2.
Geometric mean titer (GMT) and the geometric mean increase (GMI) in the various groups.
| Dose | Baseline |
28 days after injection |
90 days after injection |
180 days after injection |
360 days after injection |
||||
|---|---|---|---|---|---|---|---|---|---|
| GMT (95% CI) | GMT (95% CI) | GMI (95% CI) | GMT (95% CI) | GMI (95% CI) | GMT(95% CI) | GMI (95% CI) | GMT (95% CI) | GMI (95% CI) | |
| Placebo (n=81) | 6.1 (5.3–7.0) | 7.4 (6.3–8.7) | 1.2 (1.1–1.4) | 6.3 (5.5–7.2) | 1.4 (1.0–1.1) | 12.6 (9.6–16.5) | 2.0 (1.6–2.7) | – | – |
| 15 μg (n=84) | 6.6 (5.8–7.2) | 249.8 (189.6–329.2)a | 38.1 (28.6–50.6)a | 100.6 (75.1–134.8)a | 15.4 (11.5–20.6)a | 105.9 (81.7–137.4)a | 16.1 (12.4–21.0)a | 28.3 (23.9–33.5) | 4.3 (3.6–5.1) |
| 30 μg (n=87) | 6.0 (5.4–6.7) | 343.8 (259.5–455.5)a | 57.2 (43.5–75.4)a | 123.7 (93.3–164.1)a | 20.6 (15.6–27.3)a | 119.2 (90.3–157.3)a | 19.8 (15.3–25.8)a | 28.0 (23.3–33.6) | 4.7 (3.8–5.7) |
| 45 μg (n=88) | 6.2 (5.6–6.9) | 555.4 (427.5–721.6)abc | 89.1 (68.1–116.5)abc | 188.8 (143.9–247.9)abc | 30.3 (23.0–39.8)abc | 147.9 (114.6–190.9)a | 23.7 (18.1–31.1)a | 36.7 (30.7–43.8)bc | 5.9 (4.8–7.2)bc |
P<0.05 compared with the placebo.
P<0.05 compared with the 15 μg dose group.
P<0.05 compared with the 30 μg dose group.
Fig. 2.
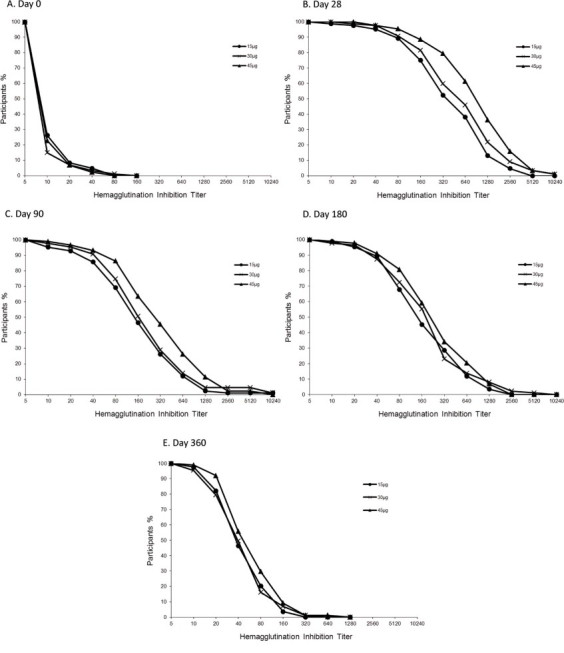
Reverse cumulative distribution curves of the antibody titers against H1N1 virus on 0, 28, 90,180 and 360 days after injection. The limit of detection was a titer of 1:5. Titers are expressed as the reciprocal of the dilution.
Fig. 3.
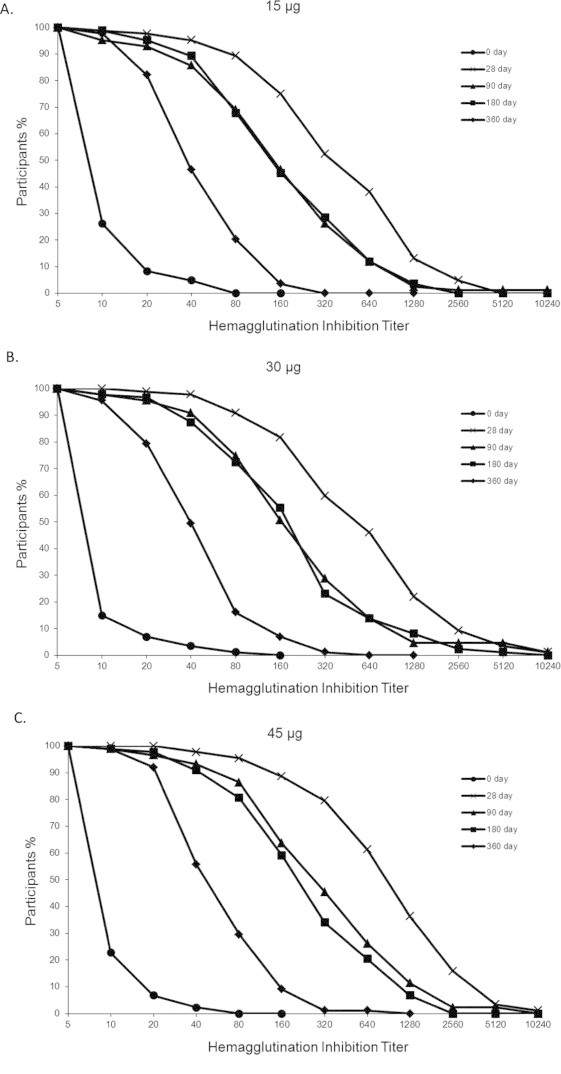
Reverse cumulative distribution curves of the antibody titers against H1N1 virus in the different dosage groups. The limit of detection was a titer of 1:5. Titers are expressed as the reciprocal of the dilution
In the placebo group, the GMT of the HI antibody titers on day 28 and day 90 showed no significant difference with the GMT pre-vaccination. However, the proportion of HI ≥1:40 (23.46%, 95% CI 14.75–34.18) and the GMT on day 180 had increased significantly compared with the values pre-vaccination and on day 28 and day 90 ( P<0.01). Meanwhile, the seroprotection rate was 4.94% on day 0; the rates of seroconversion and seroprotection were 4.94% and 7.41%, respectively, on day 28; the rates were 2.47% and 4.94%, respectively, on day 90 and the rates were 20.98% and 23.46%, respectively, on day 180. All of the above data suggested that about 20% of the subjects may have been infected the 2009 pandemic influenza A H1N1 virus during the entire clinical trial. In February 2010, the 2009 pandemic influenza A H1N1 virus was still a threat in China. Taking into account the safety of subjects, after the serum samples were collected on day 180, the subjects in control group were received a supplementary injection of vaccine (15 μg) and on day 360 after immunization, we did not collect the serum samples in control group.
In summary, in the subjects administered a single dose of vaccine, regardless of the dosage (15 μg, 30 μg or 45 μg), could all induce long-term immune response and six months after immunization, the rates of seroconversion and seroprotection and the GMI met the requirements specified by the European Medicines Agency (EMEA). The results showed that the vaccine could provide six months at least long-term protection, and provide only partial protection after twelve months.
4. Discussion
In this study, we investigated the long-term immunogenicity of inactivated split-virion 2009 pandemic influenza A H1N1 vaccine in adults aged 18–60 years, 360 days post a single immunization. We recruited 480 adults for a placebo-controlled, observer-masked, single-center clinical study. Finally, 259 subjects completed the entire study. This study demonstrated that a single dose of vaccine could induce long-term immunity persisting for at least six months, protection waned significantly 1 year after vaccination suggesting the need for a booster vaccine.
The influenza epidemic may continue for several years, it is important to explore the persistence of the antibody response induced by a single dose of the 2009 pandemic influenza A H1N1 vaccine in long term. Martin et al. [23] reported the generation of long-term immunity at the mucous membranes in a mouse model 120 days after immunization with the inactivated influenza vaccine containing adjuvant, and protection against challenge with a lethal dose of the virus. Ferguson et al. [24] demonstrated that a single dose of the 3.75 μg HA AS03A-adjuvanted H1N1 2009 influenza vaccine could induce long-term persistence of the immune response until at least six months after one dose in subjects aged 18–60 and >60 years. In addition, Nassim et al. [25] demonstrated that a single dose of 3.75 μg of MF59-adjuvanted influenza vaccine in adults can provide effective protection for upto ten months. In view of the influenza vaccines without adjuvant were used worldwide, so we carried out the clinical trial with different doses of influenza A H1N1 vaccine without adjuvant in the adult population for investigation on the long-term immune response.
In this study, the GMT of the HI antibody 90 days after immunization had decreased significantly in all of the vaccine groups compared with 28 days after immunization. Six months after immunization, although the GMT decreased significantly, the rates of seroconversion and seroprotection remained high, which were above the standards required by the EMEA (the percentage of subjects achieving seroconversion for HI antibody should be ≥40%; GMI should be >2.5; and the percentage of subjects achieving seroprotection for HI antibody should be >70%). The results in our study are consistent with the studies of Lai et al. [26] and Ferguson et al. [24]. In a randomized clinical trial by Lai et al. [26] 218 participants aged 18–60 years were recruited and were vaccinated with split-virion 2009 pandemic influenza vaccine without adjuvant. The results showed that the rate of seroprotection remained 76.8% of the participants who received a single dose of 15 μg hemagglutinin antigen six months post vaccine. Similar results of immune responses were also observed by Ferguson et al. [24].
However, the seroprotection rates of all dosage groups were below 70% on day 360 post vaccination, while the seroconversion rates and the GMI continued to meet the licensure criteria at this time point. In addition, the GMT of HI antibodies produced in the various groups increased in a dose-dependent manner, with the highest dose of 45 μg group induced the strongest HI antibody response during these twelve months, which demonstrated that a higher dose of vaccine can induce stronger antibody responses in humans. However, twelve months post-vaccination, the higher dose groups (30 μg and 45 μg) did not display much improvement in their seroprotection rates which did not fulfill the criteria of EMEA, indicating that vaccination once with a high dose could not improve the seroprotection rate effectively for long term. Therefore, healthy adults vaccinated with a 15 μg single dose of the 2009 pandemic influenza A H1N1 vaccine have the potential to resist virus infection after six months without booster immunizations.
With respect to the safety of the vaccine, the Chinese CDC have summarized the clinical adverse-reaction results of the H1N1 influenza virus split vaccine developed by the 10 domestic Chinese influenza vaccine manufacturers and reported them in a paper by Liang et al. [13] which includes the clinical adverse-reaction results of our vaccine. The results showed that, after vaccination, in all groups, adverse reactions were only mild or moderate, without serious adverse reactions, which proved our vaccine was safe. Since the purpose of this study is to investigate the long-term immunogenicity of the H1N1 influenza virus split vaccine, we did not describe the clinical adverse-reaction results of our vaccine in detail here.
Our investigations on the long-term protection induced by the 2009 pandemic influenza A H1N1 vaccine showed that large-scale vaccination with a 15 μg single dose of the split vaccine could provide protection in the human population during the epidemic period. The vaccine could induce sufficient protective immunity last for six months. However, one year after immunization, the three dosage groups (15 μg, 30 μg and 45 μg) all provided only partial protection. Since the 45 μg and 30 μg doses of the split vaccine also could not meet the requirement of EMEA, the guideline of a 15 μg dose of the split vaccine is correct and one year later, revaccination may be needed. The results of this study are therefore promising for the future development and production of influenza vaccines.
Funding
This work was supported by the following research funds: National High Technology Research and Development Program of China (863 Program 2010AA022905, 2010AA022908 and 2010AA022910); Program of Shanghai Subject Chief Scientist (type B, 10XD1422200); Shanghai Talent Development Foundation (2009030); Programs of Science and Technology Commission Foundation of Shanghai (
09DZ1908601).
Acknowledgments
We thank China State Food and Drug Administration for their critical suggestions on this study. We thank Chinese Center for Disease Control and Prevention and Hengdong County Center for Disease Control and Prevention for their help on this study. We also thank the volunteers for their participation in this study.
Footnotes
Clinical trials registration: NCT01336166.
References
- 1.Smith G.J., Vijaykrishna D., Bahl J., Lycett S.J., Worobey M., Pybus O.G. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 2.Pandemic (H1N1) 2009-update 112. WHO, <http://www.who.int/csr/don/2010_08_06/en/index.html>; 2009 [accessed 06.08.10].
- 3.Peiris J.S., Tu W.W., Yen H.L. A novel H1N1 virus causes the first pandemic of the 21st century. European Journal ofImmunology. 2009;39:2946–2954. doi: 10.1002/eji.200939911. [DOI] [PubMed] [Google Scholar]
- 4.Ferguson N.M., Cummings D.A., Fraser C., Cajka J.C., Cooley P.C., Burke D.S. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Girard M.P., Tam J.S., Assossou O.M., Kieny M.P. The 2009 A (H1N1) influenza virus pandemic:a review. Vaccine. 2010;28:4895–4902. doi: 10.1016/j.vaccine.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 6.Wacheck V., Egorov A., Groiss F., Pfeiffer A., Fuereder T., Hoeflmayer D. A novel type of influenza vaccine: safety and immunogenicity of replication-deficient influenza virus created by deletion of the interferon antagonist NS1. Journal of Infectious Diseases. 2010;201:354–362. doi: 10.1086/649428. [DOI] [PubMed] [Google Scholar]
- 7.Greenberg M.E., Lai M.H., Hartel G.F., Wichems C.H., Gittleson C., Bennet J. Response to a monovalent 2009 influenza A (H1N1) vaccine. New England Journal of Medicine. 2009;361:2405–2413. doi: 10.1056/NEJMoa0907413. [DOI] [PubMed] [Google Scholar]
- 8.Clark T.W., Pareek M., Hoschler K., Dillon H., Nicholson K.G., Groth N. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. New England Journal of Medicine. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 9.Zhu F.C., Wang H., Fang H.H., Yang J.G., Lin X.J., Liang X.F. A novel influenza A (H1N1) vaccine in various age groups. New England Journal of Medicine. 2009;361:2413–2423. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 10.Plennevaux E., Sheldon E., Blatter M., Reeves-Hoché M.K., Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 2010;375:41–48. doi: 10.1016/S0140-6736(09)62026-2. [DOI] [PubMed] [Google Scholar]
- 11.Vajo Z., Tamas F., Sinka L., Jankovics I. Safety and immunogenicity of a 2009 pandemic influenza A H1N1 vaccine when administered alone or simultaneously with the seasonal influenza vaccine for the 2009–10 influenza season: a multicentre, randomised controlled trial. Lancet. 2010;375:49–55. doi: 10.1016/S0140-6736(09)62039-0. [DOI] [PubMed] [Google Scholar]
- 12.Guideline on dossier structure and content for pandemic influenza vaccine marketing authorisation application. EMEA/ CPMP/ VEG/ 4717/ 2003- Rev 1. European Agency for the Evaluation of Medicinal Products (EMEA). Committee for Medicinal Products for Human Use (CHMP), <http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003869.pdf>; 2008 [accessed 18.12.08].
- 13.Liang X.F., Wang H.Q., Wang J.Z., Fang H.H., Wu J., Zhu F.C. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2010;375:56–66. doi: 10.1016/S0140-6736(09)62003-1. [DOI] [PubMed] [Google Scholar]
- 14.Butler D. Portrait of a year-old pandemic. Nature. 2010;464:1112–1113. doi: 10.1038/4641112a. [DOI] [PubMed] [Google Scholar]
- 15.Recommended viruses for influenza vaccines for use in the 2010–2011 northern hemisphere influenza season. WHO, <http://www.who.int/influenza/vaccines/virus/recommendations/recommendations2010_11north/en/>; 2010 [accessed 18.02.10]. [PubMed]
- 16.Availability of a candidate reassortant vaccine virus for the novel influenza A (H1N1) vaccine development X-179A. WHO, <http://www.who.int/csr/resources/publications/swineflu/candidates_X-179a/en/index.html>; 2009[accessed 27.05.09].
- 17.Summary of status of development and availability of A/California/7/2009 (H1N1)-like candidate vaccine viruses and potency testing reagents. WHO, <http://www.who.int/csr/resources/publications/swineflu/summary_candidate_vaccine.pdf>; 2010 [accessed 19.04.11].
- 18.Lin J., Zhang J., Dong X., Fang H., Chen J., Su N. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A(H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006;368:991–997. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 19.Wu J., Fang H.H., Chen J.T., Zhou J.C., Feng Z.J., Li C.G. Immunogenicity, safety, and cross-reactivity of an inactivated, adjuvanted, prototype pandemic influenza (H5N1) vaccine: a phase II, double-blind, randomized trial. Clinical Infectious Diseases. 2009;48:1087–1095. doi: 10.1086/597401. [DOI] [PubMed] [Google Scholar]
- 20.Division of Microbiology and Infectious Diseases. Adult toxicity table. National Institute of Allergy and Infectious Diseases (NIAID), <http://www.niaid.nih.gov/labsandresources/resources/dmidclinrsrch/pages/toxtables.aspx>; 2007 [accessed 30.11.07].
- 21.Division of Microbiology and Infectious Diseases. Pediatric toxicity table. National Institute of Allergy and Infectious Diseases (NIAID), <http://www.niaid.nih.gov/labsandresources/resources/dmidclinrsrch/pages/toxtables.aspx>; 2007 [accessed 30.11.07].
- 22.Note for guidance on harmonisation of requirements for influenza vaccines (CPMP/ BWP/ 214/ 96). European Agency for the Evaluation of Medicinal Products (EMEA). Committee for Proprietary Medicinal Products (CPMP), <http://www.ema.europa.eu/pdfs/human/bwp/021496en.pdf>; 1997 [accessed 28.01.10].
- 23.Martin Mdel P., Seth S., Koutsonanos D.G., Jacob J., Compans R.W., Skountzou I. Adjuvanted influenza vaccine administered intradermally elicits robust long-term immune responses that confer protection from lethal challenge. PloS One. 2010;5:e10897. doi: 10.1371/journal.pone.0010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson M., Risi G., Davis M., Sheldon E., Baron M., Li P. Safety and long-term humoral immune response in adults after vaccination with an H1N1 2009 pandemic influenza vaccine with or without AS03 adjuvant. Journal of Infectious Diseases. 2012;205:733–744. doi: 10.1093/infdis/jir641. [DOI] [PubMed] [Google Scholar]
- 25.Nassim C., Christensen S., Henry D., Holmes S., Hohenboken M., Kanesa-Thasan N. Identification of antigen and adjuvant doses resulting in optimal immunization with a pandemic A/H1N1 influenza vaccine in children 3 to < 9 years of age. Pediatric Infectious Disease Journal. 2012;31:e59–65. doi: 10.1097/INF.0b013e31824b9545. [DOI] [PubMed] [Google Scholar]
- 26.Lai Y.C., Yang K.C., Hsieh S.M., Yao C.A., Lee L.T., Huang K.C. Persistence of immunogenicity of a monovalent influenza virus A/H1N1 2009 vaccine in healthy volunteers. Clinical and Vaccine Immunology. 2012;3:429–435. doi: 10.1128/CVI.05528-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


