Abstract
HLA/peptide tetramers are frequently used for ex vivo monitoring of disease- or vaccine-induced T cell immune responses and for T cell epitope identification. However, when low-levels HLA/peptide tetramer-positive T cell populations are encountered, it is difficult to ascertain whether this represents a true T cell receptor (TCR)-mediated interaction or background signal. To address this issue, we have developed a method for both HLA class I and class II tetramer assays to confirm tetramer-binding to the TCR/CD3 complex. Preincubation of T cells with anti-CD3 mAb SPV-T3b and subsequent crosslinking interferes with the binding of HLA/peptide tetramers to the TCR/CD3 complex and thereby indicates to what extent HLA/peptide tetramer binds through interaction with TCR/CD3 complex. SPV-T3b pretreatment results in a 2- to 10-fold decrease in tetramer-binding intensity to antigen-specific CD8+ or CD4+ T cells, whereas background reactivity of HLA/peptide tetramers containing HIV-derived peptide in HIV-negative donors remained unchanged. SPV-T3b pretreatment forms a valuable tool to verify tetramer-based detection of antigen-specific T cells during the monitoring of immune responses in clinical studies.
Abbreviations: GAM-Ig, goat anti-mouse immunoglobulin; APC, allophycocyanin; PE, phycoerythrin; mAb, monoclonal antibody; MFI, mean fluorescence intensity
Keywords: Antigen-specificity, T lymphocytes, HLA/peptide tetramer, Immune response, Patients
1. Introduction
HLA/peptide tetramers have become widely applied tools to detect antigen-specific human T cells. These tetramers detect antigen-specific T cells by binding to specific T cell receptors (TCR) expressed by the T cells and reagents can be generated to detect either antigen-specific CD4 or antigen-specific CD8 T cells. In immunomonitoring studies of patients with cancer or viral infections, HLA/peptide tetramers have been proven valuable to monitor the presence of specific immune responses against tumor antigens or pathogens [8,12,17,19,23,24,28,31]. Furthermore, high-throughout screening of cytotoxic T cell epitopes in pathogen genomes or other disease-associated genes by HLA/peptide tetramer staining has become a realistic option [2,29]. However, HLA/peptide tetramer analyses consistently detect higher numbers of antigen-specific T cells than other methods of detection, such as ELISPOT, cytokine flow cytometry and limiting dilution analysis, which are based on the activation and/or proliferation of T cells in response to stimulation with antigen [28,40]. Discrepancies in the levels of antigen-specific T cells measured by HLA/peptide tetramers, as compared to the other methods might indicate that HLA/peptide tetramer analyses overestimate the size of the antigen-specific T cell population due to nonspecific reactivity of the HLA/peptide tetramer. On the other hand, the discrepancies may reveal an underestimation of the number of antigen-specific T cells detected by the other three methods due to unresponsiveness of T cells to stimulation, despite the expression of the specific TCR [16]. Therefore, validation of HLA/peptide tetramer analyses for reliable detection of antigen-specific T cells is essential for the applicability of HLA/peptide tetramer analyses in clinical studies [41,20].
We here describe a method to verify whether the detection of antigen-specific T cells by both HLA class I and class II tetramers, involves binding to the TCR/CD3 complex. We have investigated the ability of anti-CD3 and anti-TCR antibodies to interfere with the specific binding of HLA/peptide tetramers to T cells, and found that preincubation of T cells with monoclonal antibody (mAb) SPV-T3b and crosslinking with goat anti-mouse immunoglobulin (GAM-Ig) specifically decreases the specific binding of HLA/peptide tetramers. This treatment enables to distinguish binding of HLA/peptide tetramers to T cells by interaction with the TCR/CD3 complex from TCR-unrelated nonspecific binding of HLA/peptide tetramers. This method can be used to improve the accuracy of HLA/peptide tetramer-reactivity directly ex vivo, without in vitro expansion or activation.
2. Materials and methods
2.1. PBMC and HLA typing
Peripheral blood mononuclear cells (PBMC) were isolated from buffycoats from healthy donors of the Dutch Blood bank (Sanquin, Amsterdam, The Netherlands) by standard Ficoll density centrifugation. HLA-class I typing was performed by incubating PBMC with biotin-labeled antibodies (IgM) against HLA-A1, -A2 or -A3 (One Lambda, Canoga Park, CA) or control biotin-labeled IgM (BD Pharmingen, San Jose, CA) for 15 minutes on ice. Bound antibody was detected by incubation with Streptavidin-FITC (Immunotech, Beckman Coulter, Fullerton, CA) for 15 minutes, and flow cytometry analysis using a four-color FACS Calibur (Becton Dickinson, Pont de Claix, France). Dead cells were excluded by propidium iodide (PI) staining. HLA class II typing was performed by sequence based typing of HLA-DRA and HLA-DRB alleles (Sanquin, Amsterdam, The Netherlands).
2.2. Construction of HLA class I and class II/peptide tetramers
Synthetic peptides were produced at the Netherlands Cancer Institute by standard fluorenylmethoxycarbonyl chemistry and were >90% pure by analytical HPLC. Soluble allophycocyanin (APC)- or phycoerythrin (PE)-labeled HLA/peptide tetramers were generated as described [1]. HLA-A2 tetramers were produced containing the following HLA-A2-binding peptides: influenza A virus peptide (58–66) GILGFVFTL, modified MART-1 peptide (26–35, 27 A>L) ELAGIGILTV, gp100 peptide (280–288) YELPGPVTA, tyrosinase peptide (369–377) YMDGTMSQV, cytomegalovirus (CMV) peptide (495–503) NLVPMVATV. PE-labeled HLA-A2/HIV tetramer containing the p17 Gag derived SLYNTVATL peptide of the human immunodeficiency virus (HIV), kindly provided by dr. D. van Baarle (University Medical Center Utrecht, The Netherlands), was generated as described previously [34]. HLA-A1 tetramers were generated with the influenza A virus peptide (44–52) CTELKLSDY or tyrosinase peptide (243–251) KCDICTDEY. HLA-A3 tetramers were generated with influenza A virus NP peptide (265–273) ILRGSVAHK. HLA/peptide tetramers were tested for specific TCR binding using antigen-specific T cell clones and control T cell clones, as described [17]. HLA class II tetramers composed of HLA-DRA1⁎0101/DRB1⁎0401 molecules and influenza A virus hemagglutinin peptide (HA307-319, HLA-DR4/flu tetramer) were generated by the method described for murine MHC class II tetramers [25].
2.3. T cell culture
CD8+ cytotoxic T cell (CTL) clone INFA24 recognized the influenza A virus peptide (58–66) GILGFVFTL in HLA-A2 and was derived from the PBMC of an HLA-A2+ healthy donor by single cell sorting of T cells reactive with HLA-A2/flu tetramers, as described [36,42]. The CD8+ CTL clone AKR4D8 recognizes MART-1 (26–35) peptide EAAGIGILTV in HLA-A2 and was derived from the PBMC of an HLA-A2+ melanoma patient by in vitro stimulation with the autologous melanoma cell line, as described previously [11,36]. The CD8+ CTL line ZWI29 recognizes the gp100 (280–288) peptide YLEPGPVTA in HLA-A2 and was isolated from the PBMC of an HLA-A2+ melanoma patient by sorting of polyclonal T cells that bound HLA-A2/gp100 tetramer by MoFlo High speed sorter (Cytomation, Fort Collins, CO). After expansion of this population, a second round of sorting of HLA-A2/gp100 tetramer-positive cells was performed at a density of 10 cells/well into a 96 well plate by MoFlo High speed sorter. Both CTL clones and CTL line ZWI29 were cultured by weekly stimulation with a feeder cell mixture consisting of 106/ml irradiated (80 Gy) allogeneic PBMC and 105/ml irradiated JY cells, supplemented with 100 ng/ml PHA (HA16, Buroughs Wellcome, Bechenham, UK) or antigenic peptide and 20 IU/ml recombinant human IL-2 (Chiron, Amsterdam, The Netherlands) in Yssels medium as described [36,42]. CTL clone AKR4D8 and CTL line ZWI29 were immortalized by transduction with a retrovirus encoding the human telomerase reverse transcriptase (hTERT) gene, as described [11,18,36].
CD4+ T cells recognizing the influenza A virus hemagglutinin peptide (HA307-319) in HLA-DRA1⁎0101/DRB1⁎0401 molecules were cultured from the PBMC of an HLA-DRB1⁎0401+ healthy donor who had been immunized against influenza virus. PBMC were labeled with carboxy-fluorescein diacetate succinimidyl ester (CFSE) and cultured with the hemagglutinin peptide (HA307-319). CD4+ T cells recognizing the influenza A virus hemagglutinin peptide (HA307-319) in HLA-DRA1⁎0101/DRB1⁎0401 molecules were detected by binding the HLA-DR4/flu tetramer and decreased levels of CFSE labeling, as described [22]. Cloning of the tetramer-reactive CD4+ T cells was performed by single cell sorting of tetramer-reactive T cells and subsequent culture, as described for CD8+ T cell clones [36,42].
2.4. Antibodies and flow cytometry
Fluorochrome-labeled monoclonal antibodies (mAb) anti-CD3-FITC (Leu4, BD Pharmingen), anti-CD8α–FITC (Leu2a, BD Pharmingen), anti-CD8β–PE (Immunotech, Beckman Colter) and anti-CD4-APC (DAKO, Glostrup, Denmark) were used in flow cytometric analyses. Antibody incubations were performed PBS, 1% BSA, 0.05% sodium azide at 4 °C in 96 well round-bottom plates and cells were acquired in a four-color FACS Calibur. Viable lymphocytes were gated by forward and side scatter profile. Dead cells were excluded by propidium iodide (PI) staining. Data were analyzed with Cell Quest software (Immunocytometry systems, Becton Dickinson).
2.5. HLA/peptide tetramer-binding inhibition
HLA/peptide tetramer-binding inhibition assays were performed using purified anti-CD3 mAbs SPV-T3b [27] and OKT-3 (ATCC, Rockville, MD [21]) or anti-TCR mAbs WT31 [26] and T10B9 [37], or isotype control IgG (all obtained from BD Pharmingen). T cells or PBMC were preincubated with unlabeled SPV-T3b, OKT-3, WT31, T10B9 antibodies or with control IgG at concentrations ranging from 0.07–50 μg/ml for 15 minutes at 4 °C in 96 well round-bottom plate in PBS, 1% BSA, 0.05% Sodium Azide (PBS/BSA) supplemented with 1% normal mouse serum (NMS). Cells were washed and incubated with Goat anti-mouse immunoglobulins IgA, IgG, IgM (GAM-Ig, dilution 1:60, ICN Biomedicals, Zoetermeer, The Netherlands) for 15 minutes 4 °C. After washing, the cells were incubated with 500 ng HLA/peptide tetramer per 106 cells in PBS/BSA at 37 °C for 15 minutes. HLA/peptide tetramer-binding intensity was analyzed by flow cytometry. The levels of binding inhibition of tetramers by SPV-T3b pretreatment was analyzed by the decrease in mean fluorescence intensity (MFI) of the tetramer-reactive T cells. In analyses of PBMC, blocking of tetramer binding by SPV-T3b pretreatment resulted in a (partial) decrease in the percentage of tetramer-reactive cells in the tetramer-positive quadrant. To estimate the MFI of the total tetramer-positive population after SPV-T3b pretreatment (MFIc), the MFI value of the tetramer-reactive T cells was corrected for the fluorescence intensity of the cells, in which tetramer binding was fully blocked (FIneg). This MFIc was calculated by the formula: MFIc=(Tm1×MFI1+(Tm0-Tm1)×FIneg)/Tm0, in which Tm1 and MFI1 are the percentage of tetramer-positive cells and the MFI value after SPV-T3b pretreatment, respectively, Tm0 the percentage of tetramer-positive cells without SPV-T3b pretreatment or after control mIgG pretreatment and FIneg the fluorescence intensity (FI) of the quadrant setting discriminating tetramer-positive and negative cells. FIneg is taken as MFI of the tetramer-reactive T cells, in which tetramer-binding was fully blocked by SPV-T3b pretreatment.
3. Results
3.1. Identification of anti-CD3 or anti-TCR mAbs that compete with HLA/peptide tetramer-binding to human T cells
Bonafide identification of antigen-specific T cells using HLA/peptide tetramers requires the distinction between HLA/peptide tetramer binding to the TCR/CD3 complex and TCR-unrelated binding. To this end, we analyzed whether anti-TCR mAbs WT31 and T10B9, and anti-CD3 mAbs SPV-T3b and OKT3, which bind to the TCR/CD3 complex, interfered with HLA/peptide tetramer binding to antigen-specific T cells. All four antibodies bound to the HLA-A2/influenza-specific T cell clone INFA24 in a dose dependent fashion (Fig. 1A, left panel), which saturated at a concentration of 16 μg/ml, as measured by incubation of T cells with unlabeled antibody followed by goat anti-mouse (GAM) Ig-FITC antibody. When WT31, SPV-T3b or OKT3 mAbs were prebound to INFA24 T cells and cross-linked, subsequent HLA-A2/flu tetramer staining resulted in a decreased binding of HLA/peptide tetramer as compared to T cells without mAb preincubation (Fig. 1A, right panel). The extent of the tetramer-binding inhibition to the T-cells was dependent on the concentration of the TCR/CD3-reactive mAb during the preincubation. Although anti-CD3 mAb preincubation might induce T cell activation leading to activation-induced cell death, antibody preincubations did not affect the viability of the T cells, as measured by propidium iodide staining. mAb SPV-T3b was most effective in decreasing tetramer-binding to T cell clone INFA24, resulting in up to a four-fold decrease in mean fluorescence intensity (MFI). Preincubation with mAb T10B9 did not result in a decrease in tetramer binding, despite binding of this mAb to the T cells (Fig. 1A). These results show that binding of anti-CD3 mAbs SPV-T3b and OKT3 interfered more with HLA/peptide tetramer binding than anti-TCR mAb WT31, while anti-TCR mAb T10B9 did not affect HLA/peptide tetramer binding at all. Based on the superior level of HLA/peptide tetramer-binding inhibition by mAb SPV-T3b preincubation, further analyses were performed with anti-CD3 mAb SPV-T3b.
Fig. 1.
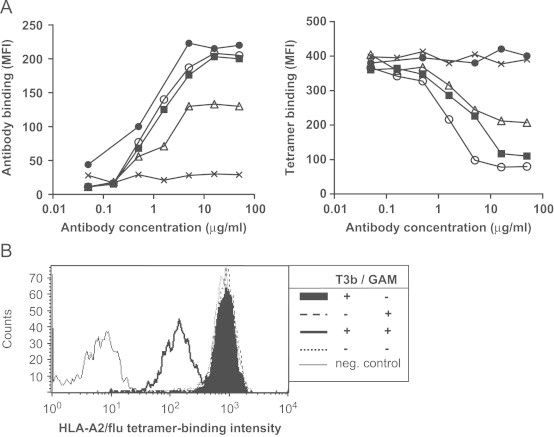
Inhibition of tetramer-binding to T cells by preincubation with anti-CD3 and anti-TCR antibodies. A, CTL INFA24 was preincubated with 3-fold increasing concentrations ranging from 0.07–50 μg/ml of anti-CD3 antibodies, SPV-T3b (○), OKT3 (■), and anti-TCR antibodies WT31 (▵) and T10B9 (●) or control IgG (X), followed by incubations with GAM-Ig and HLA-A2/flu tetramer. Left panel: binding intensity (MFI) of anti-CD3 or anti-TCR antibodies or control IgG to CTLINFA24 T cells. Right panel: Binding intensity of HLA-A2/flu tetramer to CTLINFA24 following preincubation with anti-CD3 or anti-TCR antibodies or control IgG and GAM-Ig. Data are representative of two independent experiments. B, Crosslinking of bound mAb is required for inhibition of tetramer-binding. Graph show the binding intensity of HLA-A2/flu tetramer to CTLINFA24 after preincubation with 5 μg/ml SPV-T3b mAb with or without crosslinking by GAM-Ig, preincubation with GAM-Ig alone, or without antibody preincubation. Graph shows representative results from three experiments.
Binding of HLA-A2/flu tetramers to the INFA24 T cells was not inhibited by preincubation with SPV-T3b alone but required subsequent cross-linking (Fig. 1B). Furthermore, HLA/peptide tetramer incubation prior to mAb incubation plus cross-linking did not significantly reduce tetramer binding intensity, which may be due to tetramer-induced internalization of the TCR/CD3 complex. SPV-T3b mAb pretreatment was most effective when SPV-T3b, GAM-Ig and HLA/peptide tetramer incubations were performed as three separate consecutive incubations. Since the incubations with anti-CD3 mAb and GAM-Ig were performed at 4°C and in the presence of sodium azide, anti-CD3 mAb-induced internalization of the TCR/CD3 complex is unlikely to occur. The reducing effect of SPV-T3b mAb pretreatment on HLA/peptide tetramer-binding may therefore result from sterical hindrance or a conformational change in the CD3 complex by the immune complexes of anti-CD3 mAb and GAM-Ig antibodies that inhibit tetramer-binding to the TCR/CD3 complex.
Next, we analyzed the effect of SPV-T3b mAb pretreatment on the binding of specific and control tetramers using CTL clone INFA24, CTL clone AKR4D8 and CTL line ZWI29, specific for influenza peptide, MART-1 peptide (27–35) or gp100 peptide (280–288) in HLA-A2, respectively. SPV-T3b mAb pretreatment resulted in decreased binding of specific tetramers, measured as a ten-fold decrease in mean fluorescence intensity (MFI) by all three clones, while the background reactivity of control tetramers remained unchanged (Fig. 2). Pretreatment with anti-TCR mAb T10B9 and GAM-Ig did not decrease specific tetramer-binding, indicating that for all three TCR specificities tested mAb SPV-T3b, but not T10B9, specifically interfered with the tetramer-binding capacity of the TCR/CD3 complex.
Fig. 2.
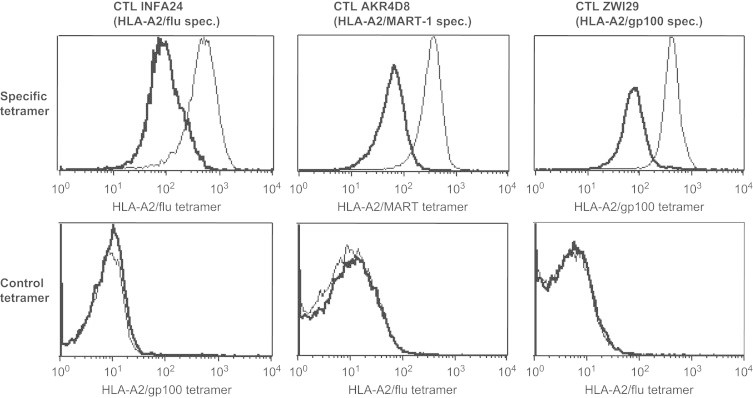
SPV-T3b pretreatment exclusively decreases the binding of tetramers to antigen-specific T cells. CTLINFA24, CTLAKR4D8 and CTLZWI29 were incubated with the specific tetramers, HLA-A2/flu, HLA-A2/MART-1 and HLA-A2/gp100(280) respectively (upper panel, thin lines), and the effect of preincubation with 5 μg/ml SPV-T3b mAb and GAM-Ig was measured on the tetramer-binding intensity (upper panel, bold lines). Lower panel: SPV-T3b and GAM-Ig preincubation (bold lines) did not affect the reactivity of the T cells with control non-specific tetramers (thin lines). Data are representative of three independent experiments. Preincubation with the anti-TCR mAb T10B9 and GAM-Ig gave similar levels of tetramer-binding as the tetramer-binding without antibody preincubation.
3.2. SPV-T3b mAb pretreatment enables distinction between HLA/peptide tetramer-binding to T cells involving the TCR/CD3 complex and TCR-unrelated binding
We investigated the ability of SPV-T3b mAb pretreatment to discriminate among PBMC the antigen-specific T cells that bind HLA/peptide tetramer through the TCR/CD3 binding from those T cells that bind the HLA/peptide tetramer nonspecifically. PBMC of an HLA-A2+ donor (donor A) containing a clearly detectable influenza virus-reactive T cell population (0.64% of CD8+ T cells) was mixed with PBMC of an HLA-A2+ donor without influenza-reactive T cells (donor B) at decreasing concentrations and the level of influenza-reactive T cells was tested by HLA-A2/flu tetramer analyses with SPV-T3b pretreatment or control IgG pretreatment (Table 1). As expected, the percentage of influenza-reactive T cells in donor A decreased with increasing dilution of the PBMC with donor B PBMC. SPV-T3b pretreatment of the samples prior to tetramer staining resulted in both a decrease in MFI and in the percentage of tetramer-positive cells in the upper right quadrant. The decrease in the percentage of tetramer-positive cells occurs when tetramer-binding was fully blocked and the MFI decreased to the level of tetramer-negative cells. To estimate the MFI of the total tetramer-positive population after SPV-T3b pretreatment (MFIc), the MFI value of the tetramer-reactive T cells was corrected for the fluorescence intensity of the cells, in which tetramer binding was fully blocked (FIneg), as described in the Methods section. As shown in Table 1, at the dilution of 12% donor A cells in the mixture, the tetramer staining indicated a level of 0.21%, which was blocked 3-fold by SPV-T3b pretreatment, whereas the background reactivity in 100% donor B (0.13%), in which no specific T cells are present, was not affected by SPV-T3b pretreatment (1.1-fold). Table 1 shows that SPV-T3b pretreatment can be used to detect small populations of antigen-specific T cells in PBMC samples. SPV-T3b pretreatment decreases the MFI about 2-fold in samples with a known tetramer-reactive population, which justifies a 2-fold decrease in MFI as a threshold in checking the TCR/CD3-mediated tetramer-binding by SPV-T3b pretreatment.
Table 1.
Sensitivity of detection of TCR/CD3-mediated tetramer binding in PBMC by SPV-T3b pretreatment.
| Tetramer-reactivity in the CD8+ T cell population | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No blocking |
SPV-T3b pretreatment |
||||||||
| % PBMC donor A | % PBMC donor B | Tetrameer | Tm0a | MFI0b | Tm1 | MFI1 | FIq | MFInegc | Fold decrease |
| 100 | 0 | A2/flu | 0.64 | 172 | 0.35 | 132 | 31 | 86 | 2.0 |
| 50 | 50 | A2/flu | 0.44 | 142 | 0.17 | 69 | 31 | 46 | 3.1 |
| 25 | 75 | A2/flu | 0.26 | 167 | 0.11 | 51 | 31 | 40 | 4.2 |
| 12 | 88 | A2/flu | 0.21 | 131 | 0.13 | 47 | 31 | 41 | 3.2 |
| 6 | 94 | A2/flu | 0.12 | 115 | 0.11 | 42 | 31 | 41 | 2.8 |
| 3 | 97 | A2/flu | 0.11 | 144 | 0.11 | 77 | 31 | 75 | 1.9 |
| 0 | 100 | A2/flu | 0.13 | 152 | 0.18 | 108 | 31 | 138 | 1.1 |
Tm0 and Tm1 indicate the percentages of tetramer-positive T cells of the CD8+ population without (Tm0) or with SPV-T3b pretreatment (Tm1), respectively.
MFI0 and MFI1 indicate the mean fluorescence intensity of the tetramer-positive T cells without (MFI0) or with SPV-T3b pretreatment (MFI1), respectively.
MFIc indicates the mean fluorescence intensity of the tetramer-reactive T cell population after SPV-T3b pretreatment corrected for T cells with fully blocked tetramer-reactivity (FIneg).
We extended our SPV-T3b pretreatment analyses to check the detection of antigen-specific T cells in PBMC of 5 donors, using HLA-A1, HLA-A2 or HLA-A3 tetramers containing peptides derived from influenza A virus (flu), cytomegalovirus (CMV), MART-1, tyrosinase (Fig. 3 and Table 2). Fig. 3 shows a decrease in MFI of the tetramer-reactive cells in donor 1 for HLA-A2/CMV-, HLA-A2/MART-1- or HLA-A2/tyrosinase-reactive CD8+ T cells, whereas the reactivity with the HLA-A2/flu tetramer was not changed by the SPV-T3b pretreatment. These results are consistent with the antigen-specific proliferation upon stimulation with CMV-, MART-1 or tyrosinase peptide, but not influenza peptide, seen in this donor. This antigen-specific proliferation was apparent from an increase in tetramer-reactive T cells in the culture over time, whereas tetramer-reactivity remained unchanged in control PHA-stimulated cultures. In the panel of donors containing antigen-specific T cells, as verified by in vitro peptide stimulation, SPV-T3b pretreatment blocked tetramer-reactivities of the CD8+ T cell population. SPV-T3b pretreatment did not affect the background tetramer-reactivity of the CD8 negative population (Table 2). This background reactivity is unlikely to be mediated by the TCR/CD3 complex, as the CD8-negative population mostly comprised CD4+ T cells that do not recognize peptides presented in HLA-class I.
Fig. 3.
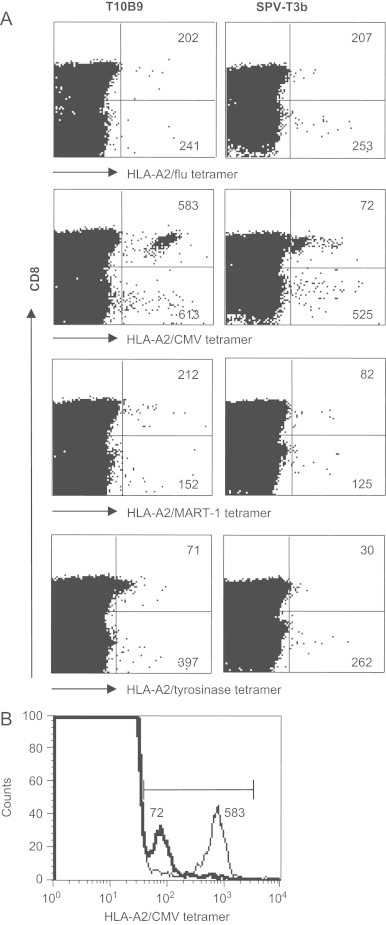
SPV-T3b pretreatment enables to distinguish tetramer-binding to the TCR/CD3 complex from TCR unrelated binding among PBMC. A., PBMC from healthy HLA-A2+ donors were incubated with HLA-A2/flu, HLA-A2/CMV, HLA-A2/MART-1 or HLA-A2/tyrosinase tetramers (horizontal axis), after preincubation with anti-TCR mAb T10B9 (left panel) or anti-CD3 mAb SPV-T3b (right panel), followed by GAM-Ig. After tetramer incubation the cells were incubated with FITC-labeled anti-CD8 mAb, which is depicted on the vertical axis. Numbers in the quadrants indicate the MFI of tetramer-binding CD8+ T cells (upper right quadrants), or the MFI of tetramer-binding CD8- T cells (lower right quadrants), respectively. Graphs show that blocking of HLA-A2/CMV, MART-1 and tyrosinase tetramer-reactivity is exclusively found in the CD8+ population, but not in the CD8- population, indicating that the latter does not involve the TCR/CD3 complex. HLA-A2/flu tetramer reactivity was not blocked in the depicted donor. Data are representative of three experiments performed on PBMC of two different HLA-A2+ donors, and of one HLA-A2 negative donor. B., Graph shows an example of the eight-fold decrease in MFI of the HLA-A2/CMV tetramer following SPV-T3b pretreatment.
Table 2.
Identification of HLA/peptide tetramer-binding T cells among donor PBMC by SPV-T3b pretreatment.
| Tetramer-reactivity in the CD8+ T cell population |
Tetramer-reactivity in the CD8-negative T cell population |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No blocking |
SPV-T3b pretreatment |
No blocking |
SPV-T3b pretreatment |
||||||||||||||
| Donor | HLA type | Tetramer | Tm0a | MFI0b | Tm1 | MFI1 | MFIneg | MFIcc | Fold decrease | Tm0 | MFI0 | Tm1 | MFI1 | MFIneg | MFIc | Fold decrease | |
| 1 | A2+ | A2/CMV | 1.425 | 583 | 1.500 | 72 | 43 | 72 | 8.1 | 0.147 | 613 | 0.129 | 591 | 43 | 525 | 1.2 | |
| 1 | A2+ | A2/MART-1 | 0.077 | 212 | 0.020 | 189 | 43 | 82 | 2.6 | 0.019 | 152 | 0.009 | 214 | 43 | 125 | 1.2 | |
| 1 | A2+ | A2/tyr | 0.553 | 71 | 0.022 | 66 | 29 | 30 | 2.4 | 0.024 | 397 | 0.023 | 262 | 29 | 262 | 1.5 | |
| 2 | A1+ A2+ | A1/flu | 0.743 | 539 | 0.130 | 286 | 132 | 159 | 3.4 | 0.008 | 350 | 0.003 | 316 | 132 | 207 | 1.7 | |
| 2 | A1+ A2+ | A2/flu | 0.340 | 400 | 0.151 | 83 | 40 | 59 | 6.8 | 0.175 | 113 | 0.292 | 199 | 40 | 199 | 0.6 | |
| 3 | A1+ | A1/flu | 0.606 | 319 | 0.189 | 359 | 38 | 138 | 2.3 | 0.015 | 674 | 0.013 | 837 | 38 | 742 | 0.9 | |
| 3 | A1+ | A1/tyr243 | 0.925 | 242 | 0.237 | 105 | 38 | 55 | 4.4 | 0.189 | 119 | 0.119 | 315 | 38 | 212 | 0.6 | |
| 4 | A3+ | A3/flu | 0.122 | 143 | 0.020 | 130 | 66 | 70 | 2.0 | 0.013 | 206 | 0.008 | 174 | 66 | 129 | 1.6 | |
| 5 | A1+ | A1/flu | 0.158 | 151 | 0.070 | 90 | 59 | 73 | 2.1 | 0.072 | 138 | 0.044 | 100 | 59 | 84 | 1.6 | |
| 5 | A1+ | A1/tyr243 | 0.118 | 93 | 0.000 | n.e. | 45 | 45 | 2.1 | 0.077 | 113 | 0.058 | 124 | 45 | 104 | 1.1 | |
| Average decrease: | 3.6 | Average decrease: | 1.2 | ||||||||||||||
Tm0 and Tm1 indicate the percentages of tetramer-positive T cells of the CD8+ population without (Tm0) or with SPV-T3b pretreatment (Tm1), respectively.
MFI0 and MFI1 indicate the mean fluorescence intensity of the tetramer-positive T cells without (MFI0) or with SPV-T3b pretreatment (MFI1), respectively.
MFIc indicates the mean fluorescence intensity of the tetramer-reactive T cell population after SPV-T3b pretreatment corrected for T cells with fully blocked tetramer-reactivity (FIneg).
To further verify whether SPV-T3b pretreatment discriminates between TCR-mediated binding and unrelated binding in the CD8+ T cell population, we tested the reactivity of HLA-A2 tetramers containing the HIV-derived p17 Gag (SLYNTVATL) peptide on PBMC of three HLA-A2+ HIV-negative healthy donors, in whom no T cell reactivity to HIV antigens is expected to be present. Upon acquisition of large cell samples comprising more than 1×106 PBMC, a small but detectable level of HLA/peptide tetramer-reactivity was found in these donors. Importantly, this reactivity remained unaffected by SPV-T3b pretreatment (Fig. 4). In parallel analyses, the MFI of the population of HLA-A2/flu tetramer-reactive T cells in these donors decreased by SPV-T3b pretreatment, indicating that SPV-T3b pretreatment efficiently discriminates true TCR/CD3-mediated binding from background HLA/peptide tetramer-reactivity. Furthermore, these data show that the low-level reactivity of HLA/peptide tetramers with CD8+ T cell populations that lack the relevant antigen-specificity is not mediated through binding to the TCR/CD3 complex.
Fig. 4.
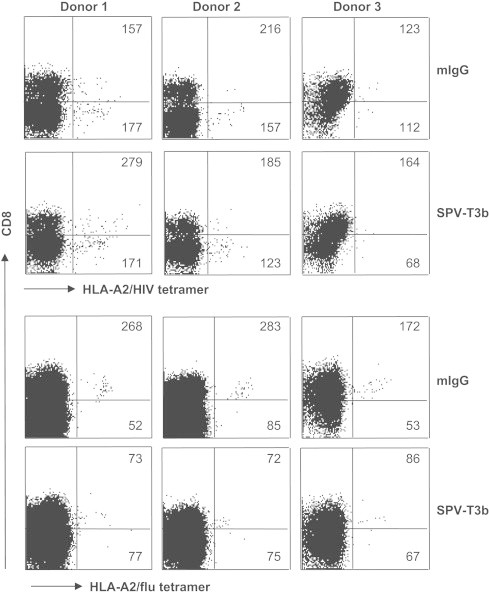
Validation of SPV-T3b pretreatment on HIV tetramer-reactivity in HIV-negative donors. One million PBMC from three healthy HIV-negative HLA-A2+ donors were incubated with HLA-A2/HIV or HLA-A2/flu tetramers (horizontal axis), after preincubation with control murine IgG (upper panels of each tetramer analysis) or anti-CD3 mAb SPV-T3b (lower panels), followed by GAM-Ig. After tetramer incubation the cells were incubated with FITC-labeled anti-CD8 mAb, which is depicted on the vertical axis. Numbers in the quadrants indicate the MFI of tetramer-binding CD8+ T cells (upper right quadrants), or the MFI of tetramer-binding CD8- T cells (lower right quadrants), respectively. Graphs show that the observed HLA-A2/HIV tetramer-reactivity is not affected by SPV-T3b pretreatment, whereas HLA-A2/flu reactivity is decreased. Data are representative of three independent experiments.
3.3. Testing of binding of HLA class II tetramers to the TCR/CD3 complex on T cells
Analyses of the immune response to vaccination are mostly performed based on the reactivity of the CD8+ T cells using HLA class I tetramers. However, successful activation of an effector CD8+ T cell response frequently involves the activation of CD4+ T cells. An increasing number of epitopes recognized by CD4+ T cells has been identified over the last years, and for which class II tetramers may be generated [3,5,14,30,43,44]. As the background reactivity of HLA-class II tetramer may vary [13], extra testing for TCR/CD3-mediated binding is useful to improve reliable detection of antigen-specific CD4+ T cells.
We have tested our SPV-T3b pretreatment method on HLA class II tetramers, composed of the influenza virus hemagglutinin peptide (HA307-319) bound to HLA-DRA1⁎0101/DRB1⁎0401 molecules (HLA-DR4/flu tetramers). As a source of PBMC containing antigen-specific T cells, we used PBMC of a DRA1⁎0101/DRB1⁎0401-positive donor stimulated with the influenza virus hemagglutinin peptide (HA307-319), which resulted in an increase in HLA-DR4/flu tetramer-reactive T cells [22]. When the PBMC were labeled with CFSE prior to peptide stimulation, the decreased CFSE intensity of the HLA-DR4/flu tetramer-positive CD4+ T cells indicated that these cells had proliferated following peptide stimulation (not shown). SPV-T3b pretreatment decreased the MFI of HLA-DR4/flu tetramer-reactive cells, indicating that mAb SPV-T3b also interfered with binding of HLA class II tetramers (Fig. 5A). As was observed for the HLA class I tetramers, pretreatment with mAb T10B9 did not inhibit HLA-class II tetramer-binding. To study the inhibition of tetramer-binding at the level of a single TCR specificity, we generated T cell clones from the HLA-DR4/flu tetramer-reactive T cell population. As shown in Fig. 5B, binding intensity of HLA-DR4/flu tetramers to T cell clones is almost ten-fold decreased by SPV-T3b pretreatment, indicating that HLA-DR4/flu tetramer bound to the T cells by interaction with the TCR/CD3 complex.
Fig. 5.
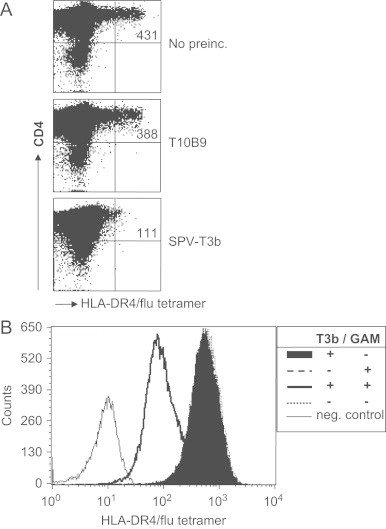
SPV-T3b pretreatment decreases the binding intensity of HLA class II tetramers. (A) PBMC of an HLA-DRB1*0401+ healthy donor, which had been cultured with the Influenza virus hemagglutinin peptide (HA307-319), were incubated with the HLA-DR4/flu tetramer-reactive T cells (upper panel), or after preincubation with anti-TCR mAb T10B9 (middle panel), or anti-CD3 mAb SPV-T3b (lower panel) and GAM-Ig. After tetramer incubation the cells were incubated with APC-labeled anti-CD4 mAb, which is depicted on the vertical axis. Numbers in the upper right quadrants indicate the MFI of tetramer-binding CD4+ T cells. (B) Crosslinking of bound anti-CD3 mAb is required for inhibition of class II tetramer-binding. HLA-DR4/flu tetramer-reactive T cells from the PBMC in A. were cloned by single cell sorting and tested for specific HLA-DR4/flu tetramer-binding after preincubation with 5 μg/ml SPV-T3b mAb with or without crosslinking by GAM-Ig, preincubation with GAM-Ig alone, or without antibody preincubation. Graph shows typical results of two experiments of a representative CD4+ T cell clone.
Taken together, the method of SPV-T3b mAb pretreatment described here allows one to determine whether HLA-class I and class II tetramer-binding to T cells are TCR-mediated, and our method is therefore a valuable tool for screening of polyclonal T cell populations in patient-derived samples.
4. Discussion
Monitoring of T cell responses in cancer patients following vaccination with tumor antigens frequently involves the analysis of small populations of tetramer-reactive T cells. In case of low frequency HLA/peptide tetramer-reactive T cells, the validity of the observed HLA/peptide tetramer staining is difficult to prove without in vitro stimulation and/or additional in vitro expansion [19]. We describe here a new method, SPV-T3b pretreatment, to test for binding of HLA class I/ or class II/peptide tetramers to the TCR/CD3 complex on T lymphocytes. This method enables to discriminate TCR/CD3-related binding of HLA/peptide tetramers from TCR-unrelated tetramer-binding to T cells, and thereby enhances reliable detection of antigen-specific T cells by HLA/peptide tetramer analyses. SPV-T3b pretreatment consists of preincubation of T cells with anti-CD3 mAb SPV-T3b followed by incubation with GAM-Ig prior to HLA/peptide tetramer incubations. Blocking of tetramer-binding by SPV-T3b pretreatment indicates the involvement of the TCR/CD3 complex in tetramer-binding.
HLA/peptide tetramers have mostly been validated by their specific binding to antigen-specific T cell clones or lines, and the absence of binding to HLA-matched control T cell clones [23,24,28]. However, analyses of polyclonal T cells, including PBMC, showed that HLA/peptide tetramer reactivity may also occur in a non-antigen specific fashion [15]. The antigen-specificity of HLA/peptide tetramer-binding T cells can be confirmed by in vitro peptide stimulation of the T cells, inducing proliferation of tetramer-reactive T cells, or by flow cytometric sorting of tetramer-binding T cells [16,33,32,12]. These validation methods depend on the in vitro growth capacity of the HLA/peptide tetramer-positive T cells, which may vary among antigen-specific T cells and introduce a bias in the T cell population analyzed. Alternatively, control samples of HLA mismatched donors have been used to estimate the nonspecific reactivity of HLA/peptide tetramers [23,28,35]. However, allospecificity of T cells in HLA-mismatched control samples may result in HLA/peptide tetramer-binding. Results of HLA mismatched control samples may therefore overestimate background levels of tetramer reactivity that is not fully relevant for analyses in HLA-matched patients, which do not have such alloreactivity. These observations underline the need for additional control measurements in HLA/peptide tetramer analyses to verify the antigen-specific binding of HLA/peptide tetramers. The new method of SPV-T3b pretreatment we describe here discriminates binding of HLA/peptide tetramers to the TCR/CD3 complex from TCR-unrelated binding, and thereby represents an internal control for HLA/peptide tetramer analyses.
The mechanism by which SPV-T3b pretreatment interferes with the binding of tetramers to T cells may include sterical hindrance of tetramers for binding the TCR/CD3 complex by bound anti-CD3 antibody immune complexes or the induction of a conformational change in the CD3 complex that reduces tetramer-binding. The observations that SPV-T3b pretreatment is effective at 4°C and in the presence of metabolic inhibitor sodium azide, and is not enhanced at 37°C indicate that blocking of internalization of TCR/CD3 complexes is unlikely to be responsible for the inhibitory effect of SPV-T3b pretreatment. Moreover, sterical hindrance or stabilization of tetramer-binding by bound antibodies has been shown for antibodies against the coreceptor CD8, which can either decrease or enhance CD8-dependent binding of certain MHC class I tetramers to human or murine T cells [4,6,7,10]. Surprisingly, antibodies T10B9 and WT31, which have been described to bind the TCRαβ heterodimer and not to the CD3 complex [37,38], did not interfere with tetramer-binding upon crosslinking. For the anti-CD3-specific antibodies that were analyzed, interference with tetramer-binding depends on the mAb that is used. Unfortunately, as it is presently unknown which subunit of the CD3 complex forms the ligand of these anti-CD3 mAbs, it remains presently unknown which CD3 chain is the most effective target for tetramer-binding inhibition. Binding intensities of FITC-labeled anti-human CD3 mAbs HIT3a and UCHT1 were shown to be approximately 2-fold decreased when HLA/peptide tetramer were prebound to human antigen-specific T cells in one study [9]. However, only minor inhibition of HLA/peptide tetramer binding was observed after preincubation with the anti-CD3 mAbs, and the competing effect of mAb UCHT1 was not confirmed by others [7]. We have found that crosslinking of the anti-CD3 mAb is required for its effect on specific tetramer-binding performed at 37 °C, which was also reported to enhance the specificity of tetramer-binding, as compared to 4 °C [39].
In conclusion, our method of SPV-T3b pretreatment can contribute valuable data to studies of immunomonitoring of vaccinated patients, in which reactivity of T cells with HLA/peptide tetramers that can be blocked by SPV-T3b pretreatment represents the total antigen-specific T cell population, including unresponsive T cells.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We thank A. Pfauth, G. Sotthewes, M. Toebes, R. Gomez and E. Taanman-Kueter for excellent technical support and dr. D. van Baarle for kindly providing the HLA-A2/HIV tetramers. R.M. Luiten was supported by the Dutch Cancer Society (grants NKI99-2048 and UVA2006-3606) and The Netherlands Organization for Scientific Research (NWO-Vidi grant 917.56.337).
References
- 1.Altman J.D., Moss P.A., Goulder P.J., Barouch D.H., McHeyzer-Williams M.G., Bell J.I. Phenotypic analysis of antigen-specific T lymphocytes. Science (New York, NY) 1996;274:94. doi: 10.1126/science.274.5284.94. [DOI] [PubMed] [Google Scholar]
- 2.Bakker A.H., Hoppes R., Linnemann C., Toebes M., Rodenko B., Berkers C.R. Conditional MHC class I ligands and peptide exchange technology for the human MHC gene products HLA-A1, -A3, -A11 and -B7. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3825. doi: 10.1073/pnas.0709717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron T.O., Cochran J.R., Yassine-Diab B., Sekaly R.P., Stern L.J. Cutting edge: detection of antigen-specific CD4+ T cells by HLA-DR1 oligomers is dependent on the T cell activation state. Journal of Immunology (Baltimore, MD: 1950) 2001;166:741. doi: 10.4049/jimmunol.166.2.741. [DOI] [PubMed] [Google Scholar]
- 4.Campanelli R., Palermo B., Garbelli S., Mantovani S., Lucchi P., Necker A. Human CD8 co-receptor is strictly involved in MHC-peptide tetramer-TCR binding and T cell activation. International Immunology. 2002;14:39. doi: 10.1093/intimm/14.1.39. [DOI] [PubMed] [Google Scholar]
- 5.Chaux P., Vantomme V., Stroobant V., Thielemans K., Corthals J., Luiten R.M. Identification of MAGE-3 epitopes presented by HLA-DR molecules to CD4(+) T lymphocytes. The Journal of Experimental Medicine. 1999;189:767. doi: 10.1084/jem.189.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clement M., Ladell K., Ekeruche-Makinde J., Miles J.J., Edwards E.S., Dolton G. Anti-CD8 antibodies can trigger CD8+ T cell effector function in the absence of TCR engagement and improve peptide-MHCI tetramer staining. Journal of Immunology (Baltimore, MD: 1950) 2011;187:654. doi: 10.4049/jimmunol.1003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denkberg G., Cohen C.J., Reiter Y. Critical role for CD8 in binding of MHC tetramers to TCR: CD8 antibodies block specific binding of human tumor-specific MHC-peptide tetramers to TCR. Journal of Immunology (Baltimore, MD: 1950) 2001;167:270. doi: 10.4049/jimmunol.167.1.270. [DOI] [PubMed] [Google Scholar]
- 8.Dunbar P.R., Smith C.L., Chao D., Salio M., Shepherd D., Mirza F. A shift in the phenotype of melan-A-specific CTL identifies melanoma patients with an active tumor-specific immune response. Journal of Immunology (Baltimore, MD: 1950) 2000;165:6644. doi: 10.4049/jimmunol.165.11.6644. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann T.K., Donnenberg V.S., Friebe-Hoffmann U., Meyer E.M., Rinaldo C.R., DeLeo A.B. Competition of peptide-MHC class I tetrameric complexes with anti-CD3 provides evidence for specificity of peptide binding to the TCR complex. Cytometry. 2000;41:321. [PubMed] [Google Scholar]
- 10.Holman P.O., Walsh E.R., Jameson S.C. Characterizing the impact of CD8 antibodies on class I MHC multimer binding. Journal of Immunology (Baltimore, MD: 1950) 2005;174:3986. doi: 10.4049/jimmunol.174.7.3986. [DOI] [PubMed] [Google Scholar]
- 11.Hooijberg E., Ruizendaal J.J., Snijders P.J., Kueter E.W., Walboomers J.M., Spits H. Immortalization of human CD8+ T cell clones by ectopic expression of telomerase reverse transcriptase. Journal of Immunology (Baltimore, MD: 1950) 2000;165:4239. doi: 10.4049/jimmunol.165.8.4239. [DOI] [PubMed] [Google Scholar]
- 12.Jager E., Nagata Y., Gnjatic S., Wada H., Stockert E., Karbach J. Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:4760. doi: 10.1073/pnas.97.9.4760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James E.A., LaFond R., Durinovic-Bello I., Kwok W. Visualizing antigen specific CD4+ T cells using MHC class II tetramers. Journal of Visualized Experiments. 2009 doi: 10.3791/1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwok W.W., Liu A.W., Novak E.J., Gebe J.A., Ettinger R.A., Nepom G.T. HLA-DQ tetramers identify epitope-specific T cells in peripheral blood of herpes simplex virus type 2-infected individuals: direct detection of immunodominant antigen-responsive cells. Journal of Immunology (Baltimore, MD: 1950) 2000;164:4244. doi: 10.4049/jimmunol.164.8.4244. [DOI] [PubMed] [Google Scholar]
- 15.Lee K.H., Wang E., Nielsen M.B., Wunderlich J., Migueles S., Connors M. Increased vaccine-specific T cell frequency after peptide-based vaccination correlates with increased susceptibility to in vitro stimulation but does not lead to tumor regression. Journal of Immunology (Baltimore, MD: 1950) 1999;163:6292. [PubMed] [Google Scholar]
- 16.Lee P.P., Yee C., Savage P.A., Fong L., Brockstedt D., Weber J.S. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nature Medicine. 1999;5:677. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 17.Luiten R.M., Kueter E.W., Mooi W., Gallee M.P., Rankin E.M., Gerritsen W.R. Immunogenicity, including vitiligo, and feasibility of vaccination with autologous GM-CSF-transduced tumor cells in metastatic melanoma patients. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology. 2005;23:8978. doi: 10.1200/JCO.2005.01.6816. [DOI] [PubMed] [Google Scholar]
- 18.Luiten R.M., Pene J., Yssel H., Spits H. Ectopic hTERT expression extends the life span of human CD4+ helper and regulatory T cell clones and confers resistance to oxidative stress-induced apoptosis. Blood. 2003;101:4512. doi: 10.1182/blood-2002-07-2018. [DOI] [PubMed] [Google Scholar]
- 19.Lyerly H.K. Quantitating cellular immune responses to cancer vaccines. Seminars In Oncology. 2003;30:9. doi: 10.1016/s0093-7754(03)00230-6. [DOI] [PubMed] [Google Scholar]
- 20.Maecker H.T., Hassler J., Payne J.K., Summers A., Comatas K., Ghanayem M. Precision and linearity targets for validation of an IFNgamma ELISPOT, cytokine flow cytometry, and tetramer assay using CMV peptides. BMC Immunology. 2008;9:9. doi: 10.1186/1471-2172-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marano N., Holowka D., Baird B. Bivalent binding of an anti-CD3 antibody to Jurkat cells induces association of the T cell receptor complex with the cytoskeleton. Journal of Immunology (Baltimore, MD: 1950) 1989;143:931. [PubMed] [Google Scholar]
- 22.Novak E.J., Liu A.W., Nepom G.T., Kwok W.W. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. The Journal of Clinical Investigation. 1999;104:R63–R67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero P., Dunbar P.R., Valmori D., Pittet M., Ogg G.S., Rimoldi D. Ex vivo staining of metastatic lymph nodes by class I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T lymphocytes. The Journal of Experimental Medicine. 1998;188:1641. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roy M.J., Wu M.S., Barr L.J., Fuller J.T., Tussey L.G., Speller S. Induction of antigen-specific CD8+ T cells, T helper cells, and protective levels of antibody in humans by particle-mediated administration of a hepatitis B virus DNA vaccine. Vaccine. 2000;19:764. doi: 10.1016/s0264-410x(00)00302-9. [DOI] [PubMed] [Google Scholar]
- 25.Schepers K., Toebes M., Sotthewes G., Vyth-Dreese F.A., Dellemijn T.A., Melief C.J. Differential kinetics of antigen-specific CD4+ and CD8+ T cell responses in the regression of retrovirus-induced sarcomas. Journal of Immunology (Baltimore, MD: 1950) 2002;169:3191. doi: 10.4049/jimmunol.169.6.3191. [DOI] [PubMed] [Google Scholar]
- 26.Spits H., Borst J., Tax W., Capel P.J., Terhorst C., De Vries J.E. Characteristics of a monoclonal antibody (WT-31) that recognizes a common epitope on the human T cell receptor for antigen. Journal of Immunology (Baltimore, MD: 1950) 1985;135:1922. [PubMed] [Google Scholar]
- 27.Spits H., Keizer G., Borst J., Terhorst C., Hekman A., De Vries J.E. Characterization of monoclonal antibodies against cell surface molecules associated with cytotoxic activity of natural and activated killer cells and cloned CTL lines. Hybridoma. 1983;2:423. doi: 10.1089/hyb.1983.2.423. [DOI] [PubMed] [Google Scholar]
- 28.Tan L.C., Gudgeon N., Annels N.E., Hansasuta P., O'Callaghan C.A., Rowland-Jones S. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. Journal of Immunology (Baltimore, MD: 1950) 1999;162:1827. [PubMed] [Google Scholar]
- 29.Toebes M., Coccoris M., Bins A., Rodenko B., Gomez R., Nieuwkoop N.J. Design and use of conditional MHC class I ligands. Nature Medicine. 2006;12:246. doi: 10.1038/nm1360. [DOI] [PubMed] [Google Scholar]
- 30.Touloukian C.E., Leitner W.W., Topalian S.L., Li Y.F., Robbins P.F., Rosenberg S.A. Identification of a MHC class II-restricted human gp100 epitope using DR4-IE transgenic mice. Journal of Immunology (Baltimore, MD: 1950) 2000;164:3535. doi: 10.4049/jimmunol.164.7.3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valmori D., Dutoit V., Lienard D., Lejeune F., Speiser D., Rimoldi D. Tetramer-guided analysis of TCR beta-chain usage reveals a large repertoire of melan-A-specific CD8+ T cells in melanoma patients. Journal of Immunology (Baltimore, MD: 1950) 2000;165:533. doi: 10.4049/jimmunol.165.1.533. [DOI] [PubMed] [Google Scholar]
- 32.Valmori D., Dutoit V., Lienard D., Rimoldi D., Pittet M.J., Champagne P. Naturally occurring human lymphocyte antigen-A2 restricted CD8+ T-cell response to the cancer testis antigen NY-ESO-1 in melanoma patients. Cancer Research. 2000;60:4499. [PubMed] [Google Scholar]
- 33.Valmori D., Pittet M.J., Vonarbourg C., Rimoldi D., Lienard D., Speiser D. Analysis of the cytolytic T lymphocyte response of melanoma patients to the naturally HLA-A⁎0201-associated tyrosinase peptide 368–376. Cancer Research. 1999;59:4050. [PubMed] [Google Scholar]
- 34.van Baarle D., Kostense S., Hovenkamp E., Ogg G., Nanlohy N., Callan M.F. Lack of Epstein-Barr virus- and HIV-specific CD27- CD8+ T cells is associated with progression to viral disease in HIV-infection. AIDS (London, England) 2002;16:2001. doi: 10.1097/00002030-200210180-00004. [DOI] [PubMed] [Google Scholar]
- 35.van Oijen M., Bins A., Elias S., Sein J., Weder P., de Gast G. On the role of melanoma-specific CD8+ T-cell immunity in disease progression of advanced-stage melanoma patients. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2004;10:4754. doi: 10.1158/1078-0432.CCR-04-0260. [DOI] [PubMed] [Google Scholar]
- 36.Verra N.C., Jorritsma A., Weijer K., Ruizendaal J.J., Voordouw A., Weder P. Human telomerase reverse transcriptase-transduced human cytotoxic T cells suppress the growth of human melanoma in immunodeficient mice. Cancer Research. 2004;64:2153. doi: 10.1158/0008-5472.can-03-1339. [DOI] [PubMed] [Google Scholar]
- 37.Waid T.H., Lucas B.A., Amlot P., Janossy G., Yacoub M., Cammisuli S. T10B9.1A-31 anti-T-cell monoclonal antibody: preclinical studies and clinical treatment of solid organ allograft rejection. American Journal of Kidney Diseases : The Official Journal of the National Kidney Foundation. 1989;14:61. [PubMed] [Google Scholar]
- 38.Weiss A, Newton M, Crommie D. Expression of T3 in association with a molecule distinct from the T-cell antigen receptor heterodimer. Proceedings of the National Academy of Sciences United States of America. 1986;83:6998. doi: 10.1073/pnas.83.18.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whelan J.A., Dunbar P.R., Price D.A., Purbhoo M.A., Lechner F., Ogg G.S. Specificity of CTL interactions with peptide-MHC class I tetrameric complexes is temperature dependent. Journal of Immunology (Baltimore, MD: 1950) 1999;163:4342. [PubMed] [Google Scholar]
- 40.Whiteside T.L., Zhao Y., Tsukishiro T., Elder E.M., Gooding W., Baar J. Enzyme-linked immunospot, cytokine flow cytometry, and tetramers in the detection of T-cell responses to a dendritic cell-based multipeptide vaccine in patients with melanoma. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2003;9:641. [PubMed] [Google Scholar]
- 41.Xu Y., Theobald V., Sung C., DePalma K., Atwater L., Seiger K. Validation of a HLA-A2 tetramer flow cytometric method, IFNgamma real time RT-PCR, and IFNgamma ELISPOT for detection of immunologic response to gp100 and MelanA/MART-1 in melanoma patients. Journal of Translational Medicine. 2008;6:61. doi: 10.1186/1479-5876-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yssel H., De Vries J.E., Koken M., Van Blitterswijk W., Spits H. Serum-free medium for generation and propagation of functional human cytotoxic and helper T cell clones. Journal of Immunological Methods. 1984;72:219. doi: 10.1016/0022-1759(84)90450-2. [DOI] [PubMed] [Google Scholar]
- 43.Zarour H.M., Kirkwood J.M., Kierstead L.S., Herr W., Brusic V., Slingluff C.L., Jr. Melan-A/MART-1(51-73) represents an immunogenic HLA-DR4-restricted epitope recognized by melanoma-reactive CD4(+) T cells. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:400. doi: 10.1073/pnas.97.1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng G., Wang X., Robbins P.F., Rosenberg S.A., Wang R.F. CD4(+) T cell recognition of MHC class II-restricted epitopes from NY-ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO-1 antibody production. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:3964. doi: 10.1073/pnas.061507398. [DOI] [PMC free article] [PubMed] [Google Scholar]


