Graphical abstract
Highlights
► We compared effects of abamectin on motility and feeding of adult Haemonchus contortus worms in vitro. ► Subtle changes in movement occur at lower concentrations than inhibition of feeding. ► Body musculature therefore appears to be a more sensitive target for this ML drug. ► Greater sensitivity of our motility measurements may explain the difference between this and previous studies on relative ML effects.
Keywords: Abamectin, Feeding, Motility, Haemonchus contortus
Abstract
Macrocyclic lactone (ML) drugs inhibit pharyngeal pumping, motility and egg laying in parasitic nematodes. Previous work has indicated that in vitro effects on worm feeding occurred at lower ivermectin concentrations than effects on worm motility, suggesting that the pharynx musculature was a more important target site for the ML drugs than somatic musculature. We have reassessed this issue of relative sensitivity by examining the response of drug-susceptible and -resistant adult Haemonchus contortus worms to abamectin in vitro using both feeding and motility assays. The motility assay involved observation of changes in the form and degree of movement of individual worms in response to the drug. A comparison of the data from the two assays indicated that worm motility was affected at drug concentrations below those required to inhibit feeding. Analysis of the motility data using different levels of sensitivity (varying in the degree to which they accounted for subtle vs. more profound changes in worm motility) provided an explanation as to why earlier reports had observed feeding to be the more sensitive target. Motility IC50 values shifted from being less than feeding IC50s to being greater than the feeding IC50s as the motility assay analysis method became less sensitive. The present study indicates that when sensitive worm motility assessment methods are utilised, worm motility is affected at lower abamectin concentrations than worm feeding, and hence highlights somatic musculature as a more important target site for this ML drug, and most likely for ML drugs in general.
1. Introduction
Macrocyclic lactones (MLs) are broad spectrum anthelmintics used to control nematode parasites of animals and humans. They increase the permeability of muscle cell membranes to chloride ions by opening glutamate-gated chloride channels, resulting in inhibition of pharyngeal pumping, motility and egg laying (Martin, 1997; Köhler, 2001). However, the distribution of ML target sites within the worm body is unclear. There has been a deal of debate over the years as to the relative importance of inhibition of pharyngeal pumping or motility in the anthelmintic activity of the drugs. Geary et al. (1993) showed that feeding by Haemonchus contortus adults in vitro was reduced at concentrations much lower than those required to inhibit motility, as measured using a motility metre. The greater sensitivity of pharyngeal pumping to the effects of the drug suggested that this was the more important site of action of the drug. Similarly, Richards et al. (1995) reported that the EC50s for effects of ivermectin on ingestion by Ancylostoma ceylanicum and Necator americanus adult worms in vitro were approximately one order of magnitude less than for motility (as measured by classing worms as either active or inactive) within each species. These observations on pharyngeal sensitivity to MLs were subsequently supported by studies which located ivermectin-sensitive glutamate gated chloride ion channels in the pharynx musculature of various nematode species (Martin, 1996; Laughton et al., 1997), as well as direct measurements of inhibition of pharyngeal pumping activity (Brownlee et al., 1997; Sheriff et al., 2002).
This difference in feeding and motility sensitivity allows ivermectin to be used as a chemical ligature. Worms remain alive and show significant levels of movement in the presence of ivermectin at concentrations that completely inhibited pharyngeal pumping. A number of studies with H. contortus have used this ligature approach to study cuticular uptake of various materials (Sims et al., 1996; Kotze and McClure, 2001; Colgrave et al., 2010).
The suggestion that the pharynx was the most important target site for MLs was, however, questioned by observations on worm expulsion kinetics by Gill and Lacey (1998). They noted that H. contortus and Trichostrongylus colubriformis worms were expelled from sheep between 8 and 10 h post treatment with ivermectin. This rapid expulsion suggested that paralysis of the somatic musculature was the most important factor leading to expulsion, as effects of worm feeding would be expected to take much longer to deplete energy reserves sufficiently to cause expulsion. Indeed, Sheriff et al. (2005) found that adult H. contortus worms were feeding at normal levels within sheep during the period 4–5 h after the host animal was treated with ivermectin, that is, just 3 h before expulsion was expected to occur. This further questioned the role of feeding inhibition in the in vivo action of MLs.
In comparing adult worm in vitro motility data (Geary et al., 1993) with the in vivo effects of MLs on adult worms within host animals (Gill and Lacey, 1998), it was apparent that a relationship between observed reduction in degree of movement in vitro and the ability of a worm to maintain its position within the host intestinal tract had not been established. That is, it was unclear what would be the effect on the ability of a worm to maintain its position in the gut after exposure to a drug concentration that had been shown to cause a certain percentage reduction in motility in vitro as measured by a motility metre, as used by Geary et al. (1993). Hence, the ability of in vitro motility assays to reflect the consequence of drug exposure in vivo was unclear. This raised a question as to the sensitivity of in vitro motility measurements, and whether assumptions of relative in vivo effects based on relative sensitivities in in vitro motility and feeding assays were valid. While complete, or almost complete, paralysis was easily observable in vitro, and would be expected to be associated with worm expulsion in vivo, more subtle effects on worm motility which may have significant consequences in vivo may not be detectable by standard in vitro motility assays.
The present study therefore aimed to observe the effects of the ML abamectin on movement of individual worms in vitro by careful observation of subtle changes in both the degree of movement and its distribution along the body of the worm in response to the drug. Such observations were then compared to the effect of the drug on worm feeding levels. In order to compare our motility assessment method to the outputs of previous motility studies, we examined the effects of changing the sensitivity of the motility assay data analysis on motility/feeding comparisons.
2. Materials and methods
2.1. Recovery of adult H. contortus worms
The use of sheep to provide adult worms for in vitro assays was approved by the DEEDI animal ethics committee, Queensland Government (approval number SA 2010/07/321). Sheep were infected with 5,000 infective stage larvae of either the drug-susceptible Kirby isolate of H. contortus (Albers and Burgess, 1988) or the multi-drug-resistant Wallangra 2003 isolate (Love et al., 2003). This isolate has been selected further using a full dose of moxidectin over at least five generations since it was originally isolated from the field. The sheep were euthanized 5–7 weeks after infection and the adult worms were gently scraped from the lining of the abomasum into 0.1% (w/v) agar in PBS, containing 20 U/ml penicillin sodium, 20 μg/ml streptomycin sulphate, and 0.1% (w/v) glucose, on a warming tray set at 42 °C. The worms were picked individually into another dish, and then into a further dish in order to separate them from any digesta material. They were finally transferred to an RPMI-based medium (modified from Kotze and McClure, 2001) and held in this solution for at least 2 h at 37 °C. The medium consisted of: RPMI-1640 (with l-glutamine) 10.4 g/L, 10 mM HEPES, 10 mM NaHCO3, 0.8% (w/v) glucose, 200 U/ml penicillin sodium, 200 μg/ml streptomycin sulphate, 2.5 μg/ml amphotericin B, 8 μg/ml tylosin, at pH 7.0.
Worms were then placed into a dish containing fresh medium on a warming tray. Individual worms moving in a smooth sinusoidal fashion were transferred to 6-well plates containing 2.88 ml of culture medium per well (=the medium described above plus 20% (v/v) foetal bovine serum, Gibco). The numbers of worms added to each well was either 5, 10 or 15, depending on the numbers available on each experimental day. The wells then received either 6 μl DMSO (no-drug control wells) or 6 μl of various serially-diluted abamectin solutions in DMSO (final DMSO concentration 0.2%). The final abamectin assay concentrations consisted of 8-fold dilutions over the range: 0.0055–11,500 nM. Plates were placed into a modular incubator (volume 10 L), and a gas mixture (5% O2, 20% CO2, in nitrogen) was passed through for 3 min. The incubators were sealed and placed at 37 °C for 24 h. In order to quantify worm feeding levels over the 24–48 h incubation interval, the assay plates were removed from the incubator at the 24 h time point, and a feeding substrate was added to each well. A solution of fluorescein isothiocyanate (FITC)-dextran (average molecular weight 40,000 Da) (Sigma) was prepared at 25 mg/ml in culture medium, and 120 ul was added to each assay well, except for a small number of fluorescence-control (no dextran) wells. The plates were re-gassed and returned to the incubator for a further 24 h.
A number of separate experiments were performed as follows: the effects of abamectin on feeding levels were assessed in four or three separate experiments for the Kirby and Wallangra isolates, respectively; effects of abamectin on motility were assessed in two of these experiments for both the Kirby and Wallangra isolates. That is, two separate motility experiments were performed with fresh batches of worms for both the Kirby and Wallangra isolates. Each experiment consisted of 2–3 assay wells at each drug concentration, alongside 4–6 control wells for feeding, or 6–12 control wells for motility.
2.2. Worm motility assay
After a total incubation period of 48 h, worms were picked from each assay well and placed into a dish held on the warming tray containing medium without serum, and viewed under a 12 cm diameter illuminated magnifying lens. Any clumped worms were gently teased apart using two sharpened probes. The operator then scored the motility of each individual worm using the system described in Table 1. Firstly, the worms moving freely in a sinusoidal fashion, and rising up from the bottom of the dish (score 4) were counted and removed to a rinsing dish containing medium without serum. The remaining worms were moved into the four quadrants of the dish corresponding to those moving with scores of 3, 2, 1 or 0 as described in Table 1. Those showing significant movement at a score 3 level were transferred to the rinse dish, allowing further clear visualisation of remaining less motile worms. These worms were then observed for a further 30–40 s, and their placement into the 2, 1, and 0 score categories was confirmed. Finally, all the worms were picked into the rinse dish. The worms were then rinsed in two more dishes of medium, and finally in PBS, before being transferred to a 2 ml screw-top plastic tube containing approximately 0.3 g of 1 mm diameter zirconia beads (BioSpec Products) to be further processed for feeding level measurements (described below). All worms used in this study were scored for motility by the same operator. This operator scored the assays in a blinded fashion, with assay wells labelled only with sequential numbers, not treatment descriptions.
Table 1.
Motility scoring system.
| Motility score | Description |
|---|---|
| 4 | Rapid sinusoidal movement in whole body or in >90% of body |
| Worm able to rise up from the base of the dish | |
| 3 | Significant movement: |
| movement spread over >75% of the body; worm on base of dish | |
| no tight coiling (tight coil ⩽ 2 mm diameter) | |
| 2 | Limited movement: |
| <75% of body moving; | |
| movement limited to one or both ends of body, central area of body immotile; | |
| or, coiled: | |
| tight coils observed, regardless of overall degree of body movement; | |
| constant coiling or transient formation of at least one tight coil during observation period | |
| 1 | Minimal movement: |
| minor movement at either end of body only, | |
| <10% of body moving (most commonly seen as minimal movement at anterior end only) | |
| 0 | No movement |
2.3. Worm feeding levels
Feeding levels in worms during the 24–48 h interval were quantified by measuring the amount of FITC-dextran taken up by the worms during this time period of the assay. The processing of worms for feeding measurements was undertaken after they had been scored for motility. Borate buffer (0.5 ml of 10 mM Na2B4O7·10H2O, pH 9.0) was added to each screw top vial, and the worms were homogenised by shaking the vials in a FastPep-24 sample preparation system (MP Biomedicals) for 40 s at full speed. The vials were then spun at 10,000g for 10 min, and 0.3 ml of supernatant was transferred to the wells of a 96-well black-sided fluorescence microplate (Greiner Bio-One). Fluorescence was measured at excitation and emission wavelengths of 485 and 535 nm, respectively, using a Tecan Spectrafluor Plus.
2.4. Data analysis
The number of worms at each motility score in drug-treated or control wells was expressed as a percentage of the total number of worms in the well. These % values were then averaged across the two replicate experiments (separately for each isolate). The mean worm %s at each motility score where then compared in control and drug-treated assays using two way ANOVA (GraphPad Prism 5, P = 0.05), with motility score and abamectin concentration as factors, in order to determine if abamectin exposure altered the % of worms within each motility score compared to controls.
In order to define motility dose responses, and hence be able to compare feeding and motility dose responses, we expressed the number of worms showing certain degrees of movement (motility scores) in drug-treated assays as a % of the number of worms showing the same degree of movement in control assays. In aiming not only to compare feeding and motility responses, but also to examine the relationship between the sensitivity of our motility assay and the motility/feeding comparisons, we examined the motility scores in three ways:
-
•
Most sensitive: only the number of score 4 worms in each assay well was considered in comparing drug-treated assays to controls. In this way we examined the ability of abamectin to reduce the motility of worms from a level 4 score to any of the lower scores compared to control assays. This represented abamectin concentrations having effects ranging from extreme inhibition of motility (an increase in the frequency of scores 2, 1, 0 in drug treated assays compared to controls), as well as mild inhibition of motility (any transition of worms from score 4 to 3 in response to drug treatment).
-
•
Intermediate sensitivity: the numbers of worms showing scores 4 and 3 were combined, and then compared between control and drug-treated wells. Hence, this analysis examined just the ability of abamectin to reduce worms to a level of limited, minimal or zero movement (scores 2, 1, 0) compared to the numbers of worms showing score 4 and 3 levels of motility in drug-treated and control assays, and did not detect the scores 4 to 3 transition.
-
•
Least sensitive: worm numbers for scores 4, 3 and 2 in each assay well were combined, and then compared in control and drug-treated wells. Hence, this analysis measured the ability of abamectin to reduce worms to a level of minimal movement (score 1) or no movement (0) compared to the numbers of worms showing score 4, 3 and 2 levels of motility in drug-treated and control assays. This type of assessment did not take into account the subtle changes in movement which would have otherwise been highlighted by considering scores 4 and 3 separately.
For quantification of feeding levels, the fluorescence present in drug-treated worms at each drug concentration (after correction for auto-fluorescence in no-dextran controls) was expressed as a % of the fluorescence measured in control worms (also after correction using no-dextran controls). Feeding and motility dose response data were analysed using non-linear regression to generate IC50 values with 95% confidence intervals (CIs) (GraphPad Prism 5). Differences between IC50 values were evaluated using non-overlap of 95% CIs as the criterion.
3. Results
Dose responses describing the effects of abamectin on worm feeding levels are shown in Fig. 1. There was a clear separation between the drug-susceptible Kirby and -resistant Wallangra isolates, with a resistance ratio at the IC50 of 559-fold (Table 2).
Fig. 1.
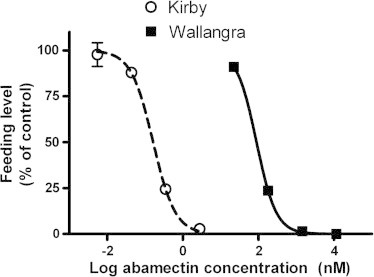
Effects of abamectin on uptake of FITC-dextran by Kirby and Wallangra adult H. contortus in vitro. Each data point represents mean ± SE, n = 4 or 3 separate experiments for Kirby and Wallangra, respectively, each with 2–3 assay wells at each drug concentration, and 4–6 control (no drug) wells. Where SEs are not visible, they are smaller than the data point symbols.
Table 2.
Dose response data describing the effects of abamectin on feeding and motility (analysed using three different levels of sensitivity) of Kirby and Wallangra adult H. contortus in vitro.
| Assay/assessment method | Kirby |
Wallangra |
RRa | ||
|---|---|---|---|---|---|
| IC50 nM | 95% CI | IC50 nM | 95% CI | ||
| Feeding | 0.16 | 0.13–0.21 | 89.4 | 81.9–97.6 | 559 |
| Motility/most sensitive | 0.015 | 0.0088–0.0257 | 19.9 | ndb | 1,327 |
| Motility/intermediate sensitivity | 0.010 | 0.0047–0.0222 | 28.5 | 9.3–86.8 | 2,850 |
| Motility/least sensitive | 0.40 | 0.10–1.58 | >11,500 | ndc | >29,000 |
The distribution patterns for motility scores in control assays for the Kirby and Wallangra isolates are shown in Fig. 2. Both isolates showed the presence of approximately equal numbers of worms with motility scores of 4, 3, 2 or 0. There were lower numbers of worms with scores of 1. Within each isolate, there were no significant differences between numbers of worms showing each motility score (P > 0.05).
Fig. 2.
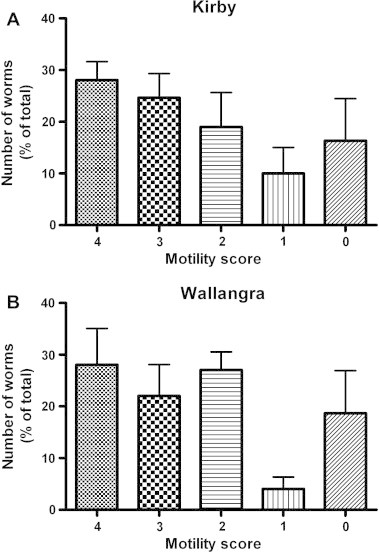
Frequency distributions of motility scores for Kirby and Wallangra adult H. contortus in control (no drug) assays. Each column represents mean ± SE, n = 2 separate experiments for each isolate, each with 6–12 replicate control wells (parts A and D) or 2–3 replicate drug-treated wells (parts B, C, E and F).
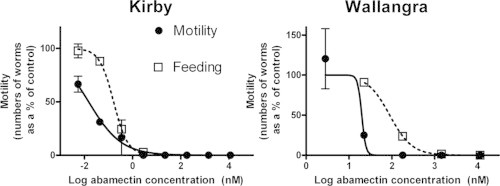
Fig. 3 shows the effect of increasing concentrations of abamectin on the numbers of worms showing each motility score, compared to the numbers of worms in control wells showing an equivalent motility score. The effect of increasing drug concentration is indicated by moving from left (control) to right (increasing drug) within each motility score. The abamectin concentrations shown here were chosen after consideration of the feeding data from Fig. 1. The three abamectin concentrations examined in Fig. 3 for each isolate represented: firstly, a level at which feeding was not impacted in Kirby (0.0055 nM, lowest point in Fig. 1), followed by the concentration giving an approximately 10% inhibition of feeding (IC10) (0.044 nM, from Fig. 1), followed by the approximate feeding IC75 concentration (0.35 nM, from Fig. 1). The relationship was similar for Wallangra, although at higher abamectin concentrations: firstly, a level below the approximate IC10 (2.9 nM, 8-fold lower than the lowest concentration tested in Fig. 1), the approximate IC10 point (22 nM, from Fig. 1), and the approximate IC75 point (180 nM). Hence, the concentrations examined in Fig. 3 represented a range from no effect on feeding, up to a feeding inhibition of approximately 75%. As the abamectin concentration increased, the numbers of Kirby worms showing motility 4 and 3 scores decreased, alongside an increase in score 1 worms. The mean % of total worms showing a motility score of four decreased 7-fold (24.5% down to 3.5%) relative to controls as the abamectin concentration reached the feeding IC75, while the % of worms showing a motility score of three decreased from 27% to zero. Similarly, the numbers of score 4 Wallangra worms decreased, while score 2 worms increased, as the abamectin concentration increased. The highest abamectin concentration resulted in a complete absence of Wallangra worms showing score 4 motility compared to a 30% presence in control wells. These trends were in many cases not statistically significant, most likely associated with the large standard errors, particularly at the lower motility scores, and an n value of only two (duplicate experiments for each isolate). Despite this, a shift towards lower motility scores as abamectin concentration increased was apparent. Thus, Fig. 3 indicates that abamectin concentrations that inhibited feeding by up to 75% had marked inhibitory effects on the degree and form of movement shown by the worms in both isolates.
Fig. 3.
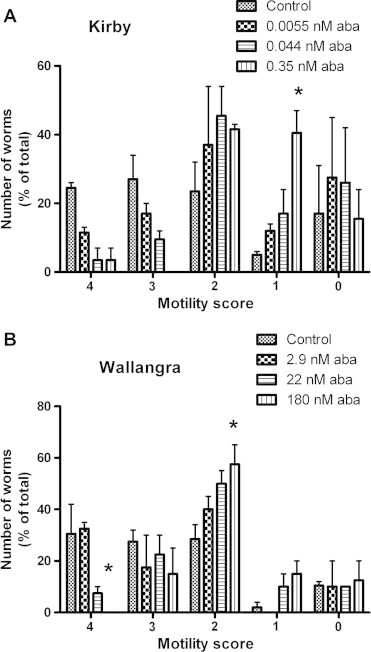
Effects of abamectin on motility of Kirby and Wallangra adult H. contortus in vitro. Each column represents mean ± SE, n = 2 separate experiments for each isolate, each with 6–12 replicate control wells, and 2–3 replicate drug-treated wells. Within an isolate, and within a motility score, columns labelled with ∗ are significantly different from the corresponding control assay (P < 0.05).
In order to examine the relationship between the sensitivity of our motility assay scoring system and the feeding assay, we examined the motility scores by pooling data from the different scoring levels (representing different assay sensitivity levels). Fig. 4 shows motility dose responses derived from analysis of the motility data using the different levels of sensitivity. The feeding assay data from Fig. 1 is also shown here for comparison. Table 2 shows the IC50 (and 95% CIs) values for the motility data at each of the three sensitivity levels. For both Kirby and Wallangra, the dose response for the most sensitive and intermediate sensitivity motility analyses lay to the left of the feeding response (Fig. 4, parts A, B, D and E). As the motility scoring system became less sensitive (through consideration of only more pronounced effects on motility) the motility dose responses shifted to the right of the feeding responses for both isolates (parts C and F). This was particularly marked for Wallangra which showed very little change in motility over a wide range of abamectin concentrations using the least sensitive motility assessment method (part F). Motility IC50 values derived from the intermediate and most sensitive motility analysis methods were 16- and 11-fold lower than the feeding IC50 for the Kirby isolate (Table 2). These IC50 values were significantly less than the feeding IC50 for the Kirby isolate (as judged by non-overlap of 95% CIs). The equivalent comparisons for Wallangra showed 3- and 4.5-fold lower motility values compared to feeding, however these values were not significantly different between the two assay types (CIs could not be calculated for the most sensitive motility assessment). Both assays produced high resistance ratios in comparing Kirby and Wallangra responses, with the ratios determined by each of the motility scoring systems being greater than that generated from the feeding assay.
Fig. 4.
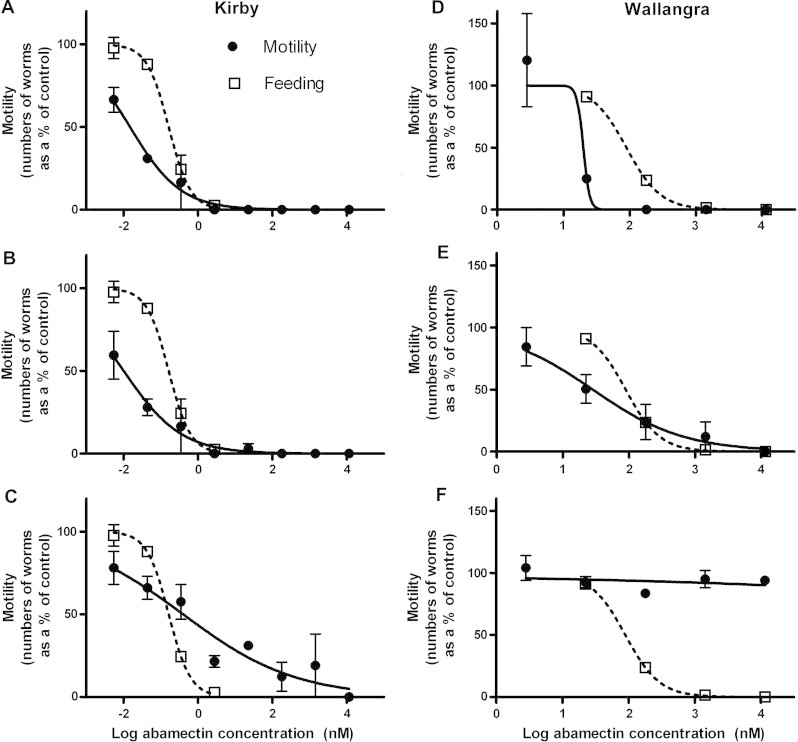
Effects of abamectin on motility of Kirby and Wallangra adult H. contortus in vitro, alongside feeding assay dose response data (from Fig. 1). Worm motility data was analysed at different levels of sensitivity (see text for details): most sensitive (A, D), intermediate sensitivity (B, E), or least sensitive (C, F). Each data point represents mean ± SE, n = 2 separate experiments for each isolate, each with 6–12 replicate control wells, and 2–3 replicate drug-treated wells. Where SEs are not visible, they are smaller than the data point symbols.
4. Discussion
This study indicates that previous suggestions of greater sensitivity of worm feeding to the effects of MLs in vitro compared to motility may be incorrect. Firstly, we have shown that worm motility is reduced by abamectin concentrations below those required to inhibit feeding. Secondly, by examining the effect of imposing different degrees of sensitivity on the analysis of motility assay data we have demonstrated why previous studies comparing relative sensitivities of feeding and motility may have found the former to be affected at lower drug concentrations than the latter. This may be explained by a lack of sensitivity to detect subtle effects of drug on worm motility using techniques such as motility metres (Geary et al., 1993) or observation of general levels of movement by groups of worms confined in bioassay vials (O’Grady and Kotze, 2004), compared to our observations on the form and degree of movement in individual worms. The habit of adult H. contortus to clump in vitro may partly explain the differences in sensitivity between our assessment methods and the use of a motility metre or visual observation of groups of worms in vials. The total amount of movement detectable in worms showing any signs of clumping may be less than if the worms are teased apart and hence are completely free to move (as in the present study). Geary et al. (1993) noted that paralysis by ivermectin was restricted to the mid-body region, while the head and tail showed apparently normal motility. This is also reflected in our scoring system from Table 1. Similarly, clumped worms tend to thrash the ends of their body around while the central part remains less motile as part of the clump. Hence, if the worms are clumped to some degree, the effects of MLs on the mid body region will be masked, alongside continued uninhibited movement of the body head and tail regions. Thus, movement in control vials showing some clumping would not be as distinguishable as it should be from ML-treated worms. Movement in both cases would be restricted in the mid body region, by clumping in the former, and drug action alongside clumping in the latter.
It is clear that while motility is affected at drug concentrations lower than those required to inhibit feeding, worms are able to survive and continue to show some movement at drug concentrations that cause a complete cessation of feeding during the 24–48 h time period used in the present study (from Fig. 4, using the least sensitive motility analysis method). Thus the use of MLs as in vitro chemical ligatures, as first suggested by Geary et al. (1993), remains unchallenged by the present study as long as some subtle reductions in motility, particularly in the mid-body region, are understood to be a consequence of the ligation.
We used abamectin in this study, in contrast to most studies on the action of ML drugs against parasitic nematodes in vitro which have used ivermectin, including Geary et al. (1993). Hence our study specifically indicates the relative sensitivity of feeding and body musculature to inhibition by abamectin alone. While avermectins as well as milbemycins are thought to act on the same target, namely, glutamate gated chlorine ion channels (Martin, 1996, 1997), there are some differences in the relative sensitivities of worms to the different analogues, suggesting that different receptor subsets may interact with the different analogues. Gill et al. (1995) found that abamectin was more active (slightly lower IC50 values) than ivermectin in in vitro larval development assays with drug-susceptible isolates of H. contortus. Resistance patterns to the two drugs can also vary; for example, Gill et al. (1991) showed that resistance ratios (at the IC50) towards abamectin in in vitro larval motility assays with different isolates of H. contortus were in some cases considerably higher than shown towards ivermectin. Abamectin is generally more effective than ivermectin against ML-resistant worms in vivo at the equivalent dose rate (0.2 mg/kg) (for example, Love et al., 2003, H. contortus on Farm N; Little et al., 2010, Teladorsagia sp. in Trial 9). Hence, while the present study specifically demonstrates the relative effects of abamectin on feeding and motility in adult H. contortus, and therefore most likely represents a phenomenon generally applicable to ML drugs given their common mode of action, this remains to be tested experimentally using a variety of ML analogues as it remains possible that the observed relative feeding/motility effects may vary between drugs due to interactions with different receptor subsets.
Very high resistance ratios were obtained for the Wallangra isolate compared to Kirby in both the feeding and motility assays. The relationships between these in vitro resistance levels and in vivo changes in drug efficacy are unclear, however, the study by Ranjan et al. (2002) provides a means to compare the Wallangra/Kirby in vitro and in vivo resistance levels to some extent. This report described data from in vivo dose titrations with ivermectin and moxidectin against a susceptible isolate of H. contortus as well as two isolates which had been pressured separately with one of the drugs over 22 generations. At the end of this process the ivermectin-selected resistant isolate showed some resistance to ivermectin (efficacy 71%) although no resistance to moxidectin (100% efficacy at a half dose). The in vivo ivermectin IC50 was increased 61-fold in the ivermectin-selected isolate compared to the parent susceptible isolate. That is, a reduced ivermectin efficacy of 71%, alongside complete susceptibility to moxidectin, was associated with a 61-fold shift in in vivo IC50. At the time of its isolation from the field, the Wallangra isolate was not affected by a full dose of ivermectin (0% efficacy), while abamectin efficacy was just 19%, and moxidectin efficacies of 67% and 84% were reported for two trials (Love et al., 2003). Since that time, the isolate has been selected at least five times using a full dose of moxidectin. It is now largely unaffected by moxidectin treatment as judged through its ability to establish infections at equivalent levels to the susceptible Kirby isolate in sheep of similar age and weight despite receiving a full dose of moxidectin 14 days after the worm dose (Kotze unpublished, animal-house egg count data). Hence, the Wallangra isolate used in the present study shows much higher levels of resistance to MLs than the ivermectin-pressured isolate of Ranjan et al. (2002) (zero vs. 71% ivermectin efficacy, very low vs. 100% moxidectin efficacy). Therefore it may not be unrealistic to consider that the 61-fold shift in in vivo IC50 described by Ranjan et al. (2002) could be extended by another order of magnitude for the highly resistant Wallangra isolate. This would bring it to a level approximating the in vitro resistance ratios from the present study (559 for feeding, and 1,327–2,850 for motility). On the other hand, the >29,000 resistance ratio for the least sensitive motility assay assessment method in the present can be discounted from this in vitro/in vivo comparison as it most likely represents a level of assay sensitivity removed from that which is relevant to worm expulsion in vivo. The unrealistic nature of this resistance ratio further suggests that the more subtle motility scoring methods applied in the present study represent a more accurate appraisal of motility effects that may be significant in vivo.
An important point in regard to the high resistance ratios shown in Table 2 is that very high resistance ratios do not represent fold increases over the standard full dose of the drug that would be required to impact on the worm population in vivo. Ranjan et al. (2002) showed that the full dose of ivermectin (0.2 mg/kg) was 111-fold higher than the IC50 for their drug susceptible isolate, indicating that significant shifts in in vivo IC50 can occur before there is any impact on the ability of the standard drug dose to remove the infection
The in vitro IC50 values shown in Table 2 can be compared to some extent with the expected concentrations of abamectin in sheep following in vivo drug treatment. Cerkvenik-Flajs et al. (2007) reported a maximal abamectin plasma concentration (Cmax) of 35 nM at a tmax of 1.7 days in ewes following subcutaneous administration. This plasma concentration is at least 88-fold higher than the in vitro feeding and motility IC50 values (from Table 2) for the Kirby isolate which would be expected to be removed from sheep by drug treatment in vivo. On the other hand, the maximum plasma concentration is comparable to, or less than, the in vitro feeding and motility IC50 values for the Wallangra isolate (not including the least sensitive motility assessment method), as may be expected given the inability of a standard dose of the drug to remove a worm population from sheep (abamectin efficacy 19%, Love et al., 2003).
While we analysed the motility data in the present study using different levels of sensitivity, and found this to profoundly affect the assay output, we did not examine changes in the sensitivity of our feeding assay through, for example, using feeding substrates differing significantly in molecular size. However, it is likely that there is little difference in feeding assay sensitivity using different feeding substrates as long as the dimensions of the feed material do not become prohibitive for uptake through the worm’s mouth and pharynx. The relative feeding/motility relationship described for ivermectin by Geary et al. (1993) was apparent using Escherichia coli, blue dextran and inulin as feeding substrates alongside the single motility assessment method (motility metre). This represented feeding substrate molecular radii ranging from uM (E. coli) to nm (inulin). The dimensions of the FITC-dextran used for the present study (radius 4.5 nm) lies at the lower end of this range.
Two observations on the assays used in this study are worthy of noting. Firstly, the motility assay described here should be viewed as being useful only for the study of subtle drug effects rather than having any general application in drug studies. It is far too laborious and time-consuming to have any application in general drug discovery studies. It represents a step away from recent efforts to develop high throughput drug screening methods for drug discovery (for example, Buckingham and Sattelle, 2009; Smout et al., 2010). However, in challenging the previous motility/feeding balance of ML action, the present study has illustrated the difference in drug action assay outputs between an observation-based assay and a higher throughput motility metre-based assay. Secondly, the feeding assay described here should be viewed as being of general use for examining worm ‘well being’ in response to any drug, as distinct from its use here as a direct measure of ML action in inhibiting glutamate-gated chloride ion channels in the pharynx musculature. A significant difference between the assays is illustrated by the much larger SEs present on the motility data compared to the feeding assay data (comparison of Figs. 1 and 4). The subjective and time consuming nature of the motility assay contrasts clearly with the ability of the feeding assay to readily quantify anthelmintic effects.
It remains unclear whether the subtle effects on worm motility that the present study indicates will occur at relatively low ML drug concentrations are sufficient to cause worm expulsion in vivo, or whether higher drug concentrations causing feeding inhibition and more severe motility defects are required. However, it may be expected that inability to move in a normal sinusoidal fashion, and even mild paralysis in the body mid-section, as seen in the score 4 to 3, and 3 to 2 transitions in the present study, could affect the ability of a worm to maintain its position in the host gut. Despite this uncertainty as to the extent of paralysis (body or pharyngeal musculature) required for worm expulsion, the present study suggest that motility will most likely be affected before feeding as ML drug concentrations increase in the worm’s environment following treatment of host animals.
Acknowledgements
Andrew Kelly is thanked for assistance with all animal husbandry practices. Funding for this study was provided by Pfizer Animal Health.
References
- Albers G.A., Burgess S.K. Serial passage of Haemonchus contortus in resistant and susceptible sheep. Vet. Parasitol. 1988;28:303–306. doi: 10.1016/0304-4017(88)90077-5. [DOI] [PubMed] [Google Scholar]
- Brownlee D.J., Holden-Dye L., Walker R.J. Actions of the anthelmintic ivermectin on the pharyngeal muscle of the parasitic nematode, Ascaris suum. Parasitology. 1997;115:553–561. doi: 10.1017/s0031182097001601. [DOI] [PubMed] [Google Scholar]
- Buckingham S.D., Sattelle D.B. Fast, automated measurement of nematode swimming (thrashing) without morphometry. BMC Neurosci. 2009;10:84. doi: 10.1186/1471-2202-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerkvenik-Flajs V., Grabnar I., Kozuh Erzen N., Marc I., Antonić J., Vergles-Rataj A., Kuzner J., Pogacnik M. Kinetics of abamectin disposition in blood plasma and milk of lactating dairy sheep and suckling lambs. J. Agric. Food Chem. 2007;55:9733–9738. doi: 10.1021/jf071941z. [DOI] [PubMed] [Google Scholar]
- Colgrave M.L., Huang Y.H., Craik D.J., Kotze A.C. Cyclotide interactions with the nematode external surface. Antimicrob. Agents Chemother. 2010;54:2160–2166. doi: 10.1128/AAC.01306-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary T.G., Sims S.M., Thomas E.M., Vanover L., Davis J.P., Winterrowd C.A., Klein R.D., Ho N.F., Thompson D.P. Haemonchus contortus: ivermectin-induced paralysis of the pharynx. Exp. Parasitol. 1993;77:88–96. doi: 10.1006/expr.1993.1064. [DOI] [PubMed] [Google Scholar]
- Gill J.H., Redwin J.M., van Wyk J.A., Lacey E. Detection of resistance to ivermectin in Haemonchus contortus. Int. J. Parasitol. 1991;21:771–776. doi: 10.1016/0020-7519(91)90144-v. [DOI] [PubMed] [Google Scholar]
- Gill J.H., Redwin J.M., van Wyk J.A., Lacey E. Avermectin inhibition of larval development in Haemonchus contortus – effects of ivermectin resistance. Int. J. Parasitol. 1995;25:463–470. doi: 10.1016/0020-7519(94)00087-5. [DOI] [PubMed] [Google Scholar]
- Gill J.H., Lacey E. Avermectin/milbemycin resistance in trichostrongyloid nematodes. Int. J. Parasitol. 1998;28:863–877. doi: 10.1016/s0020-7519(98)00068-x. [DOI] [PubMed] [Google Scholar]
- Köhler P. The biochemical basis of anthelmintic action and resistance. Int. J. Parasitol. 2001;31:336–345. doi: 10.1016/s0020-7519(01)00131-x. [DOI] [PubMed] [Google Scholar]
- Kotze A.C., McClure S.J. Haemonchus contortus utilises catalase in defence against exogenous hydrogen peroxide in vitro. Int. J. Parasitol. 2001;31:1563–1571. doi: 10.1016/s0020-7519(01)00303-4. [DOI] [PubMed] [Google Scholar]
- Laughton D.L., Lunt G.G., Wolstenholme A.J. Reporter gene constructs suggest that the Caenorhabditis elegans avermectin receptor beta-subunit is expressed solely in the pharynx. J. Exp. Biol. 1997;200:1509–1514. doi: 10.1242/jeb.200.10.1509. [DOI] [PubMed] [Google Scholar]
- Little P.R., Hodges A., Watson T.G., Seed J.A., Maeder S.J. Field efficacy and safety of an oral formulation of the novel combination anthelmintic, derquantel–abamectin, in sheep in New Zealand. NZ Vet. J. 2010;58:121–129. doi: 10.1080/00480169.2010.67513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S.C.J., Neilson F.J.A., Biddle A.J., McKinnon R. Moxidectin -resistant Haemonchus contortus in sheep in northern New South Wales. Aust. Vet. J. 2003;81:359–360. doi: 10.1111/j.1751-0813.2003.tb11514.x. [DOI] [PubMed] [Google Scholar]
- Martin R.J. An electrophysiological preparation of Ascaris suum pharyngeal muscle reveals a glutamate-gated chloride channel sensitive to the avermectin analogue, milbemycin D. Parasitology. 1996;112:247–252. doi: 10.1017/s0031182000084833. [DOI] [PubMed] [Google Scholar]
- Martin R.J. Modes of action of anthelmintic drugs. Vet. J. 1997;154:11–34. doi: 10.1016/s1090-0233(05)80005-x. [DOI] [PubMed] [Google Scholar]
- O’Grady J., Kotze A.C. Haemonchus contortus: in vitro drug screening assays with the adult life stage. Exp. Parasitol. 2004;106:164–172. doi: 10.1016/j.exppara.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Ranjan S., Wang G.T., Hirschlein C., Simkins K.L. Selection for resistance to macrocyclic lactones by Haemonchus contortus in sheep. Vet. Parasitol. 2002;103:109–117. doi: 10.1016/s0304-4017(01)00551-9. [DOI] [PubMed] [Google Scholar]
- Richards J.C., Behnke J.M., Duce I.R. In vitro studies on the relative sensitivity to ivermectin of Necator americanus and Ancylostoma ceylanicum. Int. J. Parasitol. 1995;25:1185–1191. doi: 10.1016/0020-7519(95)00036-2. [DOI] [PubMed] [Google Scholar]
- Sheriff J.C., Kotze A.C., Sangster N.C., Martin R.J. Effects of macrocyclic lactone anthelmintics on feeding and pharyngeal pumping in Trichostrongylus colubriformis in vitro. Parasitology. 2002;125:477–484. doi: 10.1017/s0031182002002251. [DOI] [PubMed] [Google Scholar]
- Sheriff J.C., Kotze A.C., Sangster N.C., Hennessy D.R. Effect of ivermectin on feeding by Haemonchus contortus in vivo. Vet. Parasitol. 2005;128:341–346. doi: 10.1016/j.vetpar.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Sims S.M., Ho N.F., Geary T.G., Thomas E.M., Day J.S., Barsuhn C.L., Thompson D.P. Influence of organic acid excretion on cuticle pH and drug absorption by Haemonchus contortus. Int. J. Parasitol. 1996;26:25–35. doi: 10.1016/0020-7519(95)00113-1. [DOI] [PubMed] [Google Scholar]
- Smout M.J., Kotze A.C., McCarthy J.S., Loukas A. A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PLoS Negl. Trop. Dis. 2010;4:e885. doi: 10.1371/journal.pntd.0000885. [DOI] [PMC free article] [PubMed] [Google Scholar]


