Graphical abstract
Highlights
► EGCG inhibits TbACC activity in lysates. ► EGCG induces an increase in TbACC phosphorylation in lysates. ► EGCG inhibits growth of Trypanosoma brucei in culture with an EC50 of ∼30 μM. ► Intra-peritoneal administration of EGCG did not reduce virulence in mice.
Abbreviations: ACC, acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; BF, bloodstream form; DMSO, dimethyl sulfoxide; EGCG, (−)-epigallocatechin gallate; PF, procyclic form; RNAi, RNA interference; SA-HRP, streptavidin conjugated to horseradish peroxidase
Keywords: Trypanosoma brucei, Epigallocatechin gallate, Acetyl-CoA carboxylase, Phosphorylation
Abstract
The current pharmacopeia to treat the lethal human and animal diseases caused by the protozoan parasite Trypanosoma brucei remains limited. The parasite’s ability to undergo antigenic variation represents a considerable barrier to vaccine development, making the identification of new drug targets extremely important. Recent studies have demonstrated that fatty acid synthesis is important for growth and virulence of Trypanosoma brucei brucei, suggesting this pathway may have therapeutic potential. The first committed step of fatty acid synthesis is catalyzed by acetyl-CoA carboxylase (ACC), which is a known target of (−)-epigallocatechin-3-gallate (EGCG), an active polyphenol compound found in green tea. EGCG exerts its effects on ACC through activation of AMP-dependent protein kinase, which phosphorylates and inhibits ACC. We found that EGCG inhibited TbACC activity with an EC50 of 37 μM and 55 μM for bloodstream form and procyclic form lysates, respectively. Treatment with 100 μM EGCG induced a 4.7- and 1.7- fold increase in TbACC phosphorylation in bloodstream form and procyclic lysates. EGCG also inhibited the growth of bloodstream and procyclic parasites in culture, with a 48 h EC50 of 33 μM and 27 μM, respectively, which is greater than the EGCG plasma levels typically achievable in humans through oral dosing. Daily intraperitoneal administration of EGCG did not reduce the virulence of an acute mouse model of T. b. brucei infection. These data suggest a reduced potential for EGCG to treat T. brucei infections, but suggest that EGCG may prove to be useful as a tool to probe ACC regulation.
1. Introduction
The protozoan parasite Trypanosoma brucei (sub-species gambiense and rhodesiense) is the causative agent of African sleeping sickness, a fatal human disease that ranges across sub-Saharan Africa. In addition to causing substantial morbidity and mortality in humans, a third sub-species, Trypanosoma brucei brucei is responsible for causing nagana, a livestock disease that results in wasting and death. Nagana imposes a tremendous economic burden on the region, causing 4.5 billion dollars in economic losses each year (FAO, 2007). Vaccine development is confounded by the parasite’s ability to switch its surface coat through antigenic variation (Horn and McCulloch, 2010). Chemotherapeutics are relied upon to battle the disease, yet currently approved drugs cause undesirable side effects, are subject to failure, and can be too expensive for citizens of economically depressed regions (Castillo et al., 2010). To meet this urgent need, investigation of existing compounds are an important avenue to identify potential new drugs that are effective, safe, and economical.
Green tea is amongst the most widely consumed beverages worldwide and is often touted for its wealth of medicinal effects (Moon et al., 2007; Khan and Mukhtar, 2008; Thielecke and Boschmann, 2009; Ahmed, 2010). The best-studied active components of green tea are the catechins, of which (−)-epigallocatechin-3-gallate (EGCG) is one of the most abundant (Lin et al., 2003). In addition to numerous other pathways, EGCG has been demonstrated to inhibit fatty acid synthesis (Wang and Tian, 2001; Brusselmans et al., 2003) through its effect on the regulation of acetyl-CoA carboxylase (ACC) (Huang et al., 2009). ACC catalyzes the first committed step in fatty acid synthesis: the ATP-dependent carboxylation of acetyl-CoA, which provides the two-carbon donor, malonyl-CoA, for fatty acid synthesis (Tong and Harwood, 2006). ACC is negatively regulated by phosphorylation by AMP-activated protein kinase (AMPK), a key regulator of cellular energy metabolism (Barber et al., 2005; Brownsey et al., 2006). EGCG treatment activates AMPK, leading to increased phosphorylation of human ACC, which resulted in its inhibition (Moon et al., 2007; Huang et al., 2009).
In T. b. brucei, both the cytoplasmic and mitochondrial fatty acid synthesis pathways are important for growth in culture and virulence in mouse models (Lee et al., 2006, 2007; Stephens et al., 2007; Vigueira and Paul, 2011). We have recently demonstrated that RNA interference (RNAi)-mediated gene knock-down of ACC in T. b. brucei reduced fatty acid elongation activity in intact cells and reduced virulence in a mouse model of infection (Vigueira and Paul, 2011). In addition, treatment with thiolactomycin, an inhibitor of fatty acid synthetase, inhibited fatty acid synthesis activity and growth of T. b. brucei in culture (Morita et al., 2000). Taken together, these results suggest that fatty acid synthesis has the potential to be an effective drug target in T. brucei. Here, we examined the effect of EGCG on T. b. brucei and found that physiologically relevant levels of EGCG had no effect on growth in culture and did not reduce virulence in a mouse model of infection, while higher levels of EGCG decreased TbACC activity, likely through an increase in TbACC phosphorylation. These data suggest that although EGCG inhibits T. b. brucei ACC activity, EGCG is a questionable candidate for further development as a potential cure for T. brucei infection. However, EGCG may be a useful pharmacological tool to investigate the signaling pathways governing phospho-regulation of TbACC.
2. Material and methods
2.1. Materials, cell lines, and media
Most chemicals and reagents were purchased from Thermo Fisher Scientific and Sigma, including EGCG (E4143), which was prepared and frozen in single-use aliquots, either as a 100X stock in dimethyl sulfoxide (DMSO) or in sterile water for use in mice. EGCG stocks were freshly thawed just before use. Minimum essential medium and Iscove’s modified Dulbecco’s medium, was from Invitrogen. Serum Plus was from JRH Biosciences. [14C]NaHCO3 (14.9 mCi mmol−1) was from American Radiolabeled Chemicals. Wild-type (WT) T. b. brucei 427 bloodstream form (BF) and tsetse midgut procyclic form (PF) cell lines were a kind gift of Dr. Paul Englund (Johns Hopkins School of Medicine). BFs were grown at 37°C/5% CO2 in HMI-9 media containing 10% heat-inactivated fetal bovine serum (FBS) and 10% Serum Plus (Hirumi and Hirumi, 1989). PFs were grown at 28°C/5% CO2 in SDM-79 media containing 10% heat-inactivated FBS and 7.5 μg ml−1 hemin (Brun and Shonenberger, 1979). PF ACC-myc cells are tagged in one genomic locus with a c-terminal fusion to the c-myc epitope and have been described previously (Vigueira and Paul, 2011). BF ACC-myc cells were generated in this study with the same tagging construct used to make the PF ACC-myc cell line. ACC-myc cell lines are maintained in the appropriate growth media supplemented with 2.5 μg ml−1 phleomycin.
2.2. Effect of EGCG on T. b. brucei growth
WT BF T. b. brucei were diluted into fresh media containing 0.1–1 μM EGCG or 1% DMSO as the solvent control and the cell culture densities measured every 2 days for 6 days by flow cytometry (BD FACScan). Following each cell count, cultures were diluted to maintain logarithmic phase growth, and EGCG/DMSO was added to maintain experimental concentrations. Doubling times were calculated from the growth curves. To determine EC50 for growth, WT BF and PF T. brucei were diluted into fresh media containing 5–50 μM EGCG or 1% DMSO as solvent control and cell culture densities determined after 2 days.
2.3. ACC activity assays
Hypotonic lysates were desalted and TbACC activity was assayed essentially as described (Vigueira and Paul, 2011). Briefly, ACC activity was determined by measuring the incorporation of the [14C]CO2 from [14C]NaHCO3 into the acid resistant [14C]malonyl-CoA product, which is quantified by scintillation counting. To test the effect of EGCG on TbACC activity, 5-100 μM EGCG or 1% DMSO only (solvent control) was added to the lysates with or without 1X HALT phosphatase inhibitor cocktail (Thermo Scientific Pierce) just prior to assaying for TbACC activity.
2.4. ACC phosphorylation and blotting
Hypotonic lysates from BF and PF ACC-myc cells were prepared in the presence of 1X HALT phosphatase inhibitor cocktail. Lysates were supplemented with 5 mM ATP, divided, with one aliquot treated with 100 μM EGCG and the other with 1% DMSO as solvent control. Lysates were then incubated at 30 °C for 30 min. under constant mixing (Eppendorf Thermomixer, 500 rpm). ACC-myc was immunoprecipitated from the lysates using the ProFound c-myc IP/Co-IP kit (Thermo Scientific Pierce) according to the manufacturer’s instructions and eluted in 150 mM glycine at pH 2.2 followed by immediate neutralization with 9.5 M Tris–HCl, pH 9.0.
To detect phosphorylated ACC-myc, approximately equal quantities of immunoprecipitated ACC-myc (∼8.8 × 107 cell equivalents/lane for PF and ∼1.5 × 108 cell equivalents/lane for BF) were resolved by SDS–10% PAGE, followed by gel staining with Pro Diamond Q phosphoprotein gel stain (Invitrogen) according to the manufacturer’s instructions. Phospho-stained gels were imaged under UV using the Gel Doc XR Imaging System (BioRad). To detect total ACC, identically loaded non-stained gels were transferred to nitrocellulose and probed for ACC as described (Vigueira and Paul, 2011) with streptavidin conjugated to horseradish peroxidase (SA-HRP), which detects the ACC biotin prosthetic group. Densitometric quantitation was performed on gels and appropriately exposed blots (in the linear range of detection) using Image J software (NIH). Phosphorylated ACC-myc was normalized to the total amount of ACC in the ACC-myc immunoprecipitates and lysates.
2.5. Mouse infection
To test the ability of EGCG to clear a T. b. brucei infection in mice, we chose a daily dose of 4.13 mg/kg EGCG prepared in sterile H2O and administered daily by IP injection. This EGCG concentration had been previously determined not to cause mouse liver damage, though actual serum levels of EGCG were not determined (Güida et al., 2007). For the EGCG-treated group, 12 week old Swiss mice were pre-treated with EGCG for 2 days prior to infection and EGCG treatment was continued throughout the course of infection. Both the EGCG-treated (n = 5) and untreated controls (n = 5) were infected with 1 × 105 BF wild-type 427 parasites as previously described (Bacchi et al., 2009). Tail blood samples were examined by microscopy to confirm successful infection, and time to death (or humane end-point) was determined. All work with animals was carried out in compliance with a protocol approved by the Clemson University Institutional Animal Care and Use Committee.
3. Results and discussion
To investigate whether EGCG could be used to target ACC in T. b. brucei, we first tested EGCG for its ability to inhibit TbACC enzymatic activity in whole cell lysates from two different life cycle stages: the procyclic form (PF) found in the fly midgut, and the mammalian bloodstream form (BF). Because of the known mode of action of EGCG on mammalian ACC phosphorylation, we sought to preserve any potential changes in phosphorylation caused by EGCG by adding HALT, a broad-spectrum phosphatase inhibitor, to the cell lysate. In the presence of HALT, EGCG reduced ACC activity in both BF and PF lysates, with an EC50 of 29 ± 1.3 μM (n = 3) and 55 ± 2.4 μM (n = 3), respectively (Fig. 1A and B). HALT treatment alone had no effect on ACC activity (data not shown). However, in the absence of HALT, we observed no inhibition of TbACC activity in lysates by EGCG. Thus, EGCG treatment inhibited ACC activity only in the presence of phosphatase inhibitors. Although the effect of EGCG on ACC activity in lysates has not been previously measured, our observations are consistent with the reported mode of action of EGCG on ACC by increasing ACC phosphorylation (Collins et al., 2007; Hwang et al., 2007; Moon et al., 2007; Huang et al., 2009; Murase et al., 2009). That no effect was observed in the absence of HALT rules out a more general inhibitory “tannin” effect on the ACC enzyme (Wink, 2008). Thus, we propose that HALT, by inhibiting the endogenous phosphatases in the lysate, preserved an EGCG-driven increase in ACC phosphorylation, resulting in decreased TbACC activity.
Fig. 1.
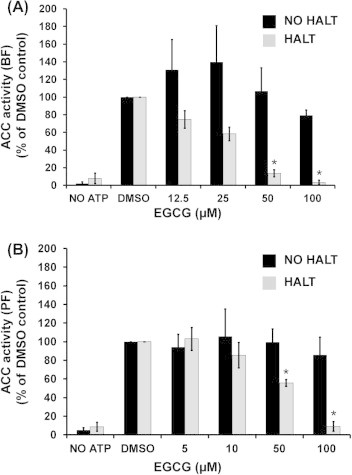
Inhibition of TbACC activity by EGCG. TbACC activity in lysates of (A) BF and (B) PF trypanosomes was measured in the presence of 5–100 μM EGCG in the absence (black bars) or presence (grey bars) of HALT phosphatase inhibitor cocktail. As a negative control, ATP was omitted from the reaction (No ATP). Values are expressed as a percentage of the DMSO solvent control. DMSO concentrations were maintained at 1% v/v for all conditions. The mean of 3 independent experiments is shown. Error bars indicate ±SEM. The * indicates P < 0.01 for the difference between DMSO control and EGCG-treated conditions (student’s t-test).
To more directly examine the effect of EGCG treatment on TbACC phosphorylation, we used BF and PF cell lines in which the C-terminus of one TbACC allele was fused to the c-myc epitope (TbACC-myc), which allows for expression of TbACC-myc at endogenous levels under the native promoter (Vigueira and Paul, 2011; and this study). BF and PF TbACC-myc lysates were treated with 100 μM EGCG or DMSO as solvent control in the presence of HALT. After EGCG treatment, TbACC-myc immunoprecipitates were resolved by SDS–PAGE and phosphorylated TbACC detected by in-gel phospho-staining with Pro Q Diamond stain (Fig. 2A and B, upper panels, respectively). As a loading control, identically-loaded gels were transferred to nitrocellulose and total ACC detected by blotting with streptavidin-HRP (Fig. 2A and B, lower panels). Densitometry of the phospho-stained gels revealed that EGCG treatment resulted in a statistically significant 4.7 ± 0.12-fold (P < 0.00001; n = 3) and 1.7 ± 0.20-fold (P < 0.05; n = 3) increase in phosphorylation of TbACC in BFs and PFs, respectively. This increase in phosphorylation is correlated with the observed inhibition of TbACC activity in BF and PF lysates (Fig. 1A and 1B). It is possible that ACC-myc is inefficiently or aberrantly phosphorylated compared to native ACC, which might mask the true response of ACC to EGCG treatment. However, the facts that immunoprecipitated ACC-myc was also highly active and ACC-myc exhibited the same subcellular fractionation as native ACC (Vigueira and Paul, 2011) argue against this idea. In addition, similar increases in ACC phosphorylation were observed in mammalian cells after treatment with similar concentrations of EGCG (50–400 μM) (Hwang et al., 2007; Moon et al., 2007; Huang et al., 2009; Murase et al., 2009). Taken together, the results shown in Figs. 1 and 2 indicate that ACC is phosphorylated in both BFs and PFs of T. brucei, and that EGCG treatment leads to increased TbACC phosphorylation and correspondingly decreased TbACC activity. It is unknown how EGCG treatment leads to phosphorylation and inactivation of TbACC. In mammalian cells, how EGCG activates AMPK is not well understood, but occurs via activation of one or more upstream kinases (e.g. liver kinase B1 (LKB1) and calmodulin-dependent protein kinase kinase (CaMKK)) in a process that may involve the production of reactive oxygen species (ROS) via oxidation of EGCG (Sang et al., 2005; Hou et al., 2005; Hwang et al., 2007; Collins et al., 2007; Moon et al., 2007; Murase et al., 2009). Future work will be needed to determine the identity of the upstream TbACC kinase(s) and the role of ROS in their signaling.
Fig. 2.
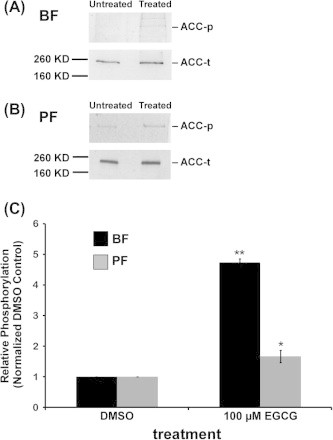
EGCG induces phosphorylation of TbACC. BF and PF ACC-myc lysates were treated with 100 μM EGCG or DMSO as solvent control in the presence of HALT phosphatase inhbitor cocktail. (A) BF and (B) PF TbACC-myc immunoprecipitates (∼5 × 108 cell equivalents/lane) were resolved by SDS–PAGE and stained with Pro Q Diamond phosphoprotein gel stain to detect phosphorylated ACC (ACC-p; upper panels). Identically loaded gels were blotted to nitrocellulose and probed for total ACC with SA-HRP (ACC-t; bottom panels). Representative gels and blots are shown. Unlabeled upper band in panel A is an unknown phosphorylated contaminant. (C) Densitometric quantitation of phosphorylated ACC from phospho-stained gels normalized to total ACC detected by SA-HRP blotting. The mean of 3 independent experiments is shown. Error bars indicate the ±SEM. The * indicates P < 0.05 and ** indicates P < 0.00001 for the difference between DMSO control and EGCG-treated conditions (student’s t-test).
We next examined the effect of EGCG on T. b. brucei growth in culture. Orally administered EGCG is rapidly cleared from the body (Ullmann et al., 2003; Chow et al., 2005). In humans, repeated daily oral administration of EGCG can achieve a maximum plasma concentration of ∼1 μM for 5–6 h (Ullmann et al., 2004). A 6 day treatment of BF cells at these physiological EGCG concentrations (0.1–1 μM) caused no change in growth or doubling time (Fig. 3A and B). Next, we tested higher EGCG concentrations (5–50 μM), and observed a statistically significant reduction in cell growth over 48 h (Fig. 3C and D), with an EC50 of 34 ± 2.4 μM and 25 ± 2.7 μM for BF and PF parasites, respectively (P < 0.01, n = 4). These values are consistent with the trypanocidal activity of EGCG (IC50 20.2 μM at 72 h) previously reported for T. b. rhodesiense (Tasdemir et al., 2006). Our previous data showed that TbACC is largely unnecessary in BF parasites cultured in vitro and is only required when PF parasites are cultured in low-lipid media (Vigueira and Paul, 2011). Thus, we contend that inhibition of TbACC by EGCG should have little consequence on T. b. brucei growth in culture. Consequently, we attribute the reduction in growth observed with EGCG concentrations >5 μM to other previously described target(s) of EGCG (Ahmed, 2010; Singh et al., 2011), rather than to its inhibition of TbACC.
Fig. 3.
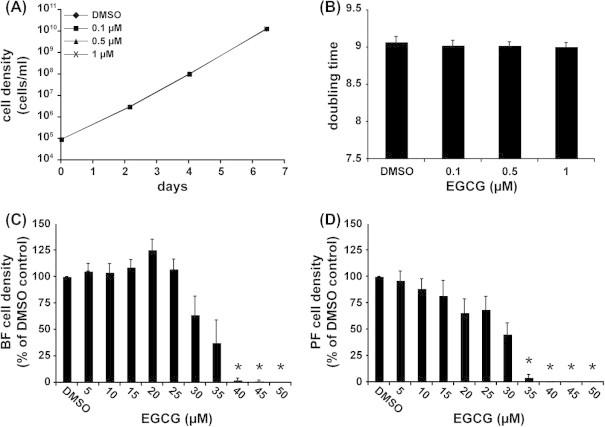
Effect of EGCG on in vitro growth of T. brucei. (A and B) WT BF cells were diluted back to 1 × 106 cells/ml into fresh media containing 0.1–1 μM EGCG or DMSO as the solvent control and culture cell densities were monitored for 6 days. (A) Representative growth curve showing cumulative culture densities. (B) Mean culture doubling times of 3 independent experiments. Error bars show ±SEM. (C) WT BF and (D) WT PF cells were back-diluted into fresh media containing 5–50 μM EGCG or DMSO control. Culture cell densities were determined after 48 h. Values are expressed as a percentage of DMSO control. The mean of 4 independent experiments is shown. Error bars show ±SEM. The * indicates P < 0.01 for the difference between DMSO and EGCG-treated conditions (student’s t-test).
Finally, we examined the effect of EGCG treatment on the course of T. b. brucei infection in mice. Although the EC50 values for EGCG for growth in culture are greater than the achievable physiological concentrations in humans, there were three reasons to justify testing EGCG in a mouse model of infection. First, T. b. brucei exhibited condition-specific essentiality in the case of both TbACC and enoyl-CoA reductase (EnCAR). PF ACC and EnCAR RNAi cell lines exhibited slowed growth only when exogenous lipids were limited, and a TbACC RNAi line had attenuated virulence in mice (Lee et al., 2006; Vigueira and Paul, 2011). Second, studies in rats showed that intravenous administration of EGCG resulted in greater plasma half-life and in higher maximal plasma concentrations than seen with the oral route of administration (Isbrucker et al., 2006; Misaka et al., 2012). Third, previous studies of EGCG in mice have yielded promising results for treatment of trypanosomiasis. T. cruzi mortality in mice and growth in culture was reduced with EGCG treatment (Paveto et al., 2004; Güida et al., 2007). In addition, inflammation caused by T. b. brucei infection was reduced and survivorship was extended with oral green tea supplementation (Karori et al., 2008). Taken together, these observations suggest that first, parenteral administration of EGCG might achieve therapeutic plasma concentration of EGCG, and second, the requirement for ACC and thus the parasite’s sensitivity to EGCG could be higher in vivo.
To test the ability of EGCG to clear a T. b. brucei infection in mice, we administered daily IP injections of 4.13 mg/kg EGCG in sterile H2O two days prior to infection and over the course of the trial. This EGCG concentration had been previously determined not to cause mouse liver damage, though the actual EGCG serum concentration was not determined (Güida et al., 2007). After 2 days of EGCG pre-treatment, Swiss mice were then infected with 1 × 105 BF wild-type 427 parasites as previously described (Bacchi et al., 2009), and time until death (or humane end-point) was determined. In this infection model, EGCG treatment had no effect on infection duration or mouse mortality. Mean time until death was 3.5 days for both treatment and control groups (data not shown). Thus, although EGCG has trypanocidal activity at supraphysiological concentrations, the compound did not attenuate virulence in our acute infection mouse model. EGCG concentrations may not have been sufficiently high in the mouse bloodstream to affect the parasite, however a much lower intraperitoneal dose (0.8 mg/kg/day) led to a significant decrease in parasitemia and increased survivorship of mice infected with T. cruzi (Güida et al., 2007). Alternatively, it is possible that the course of infection was too rapid to allow EGCG to exert its effects. If so, a chronic infection model might be better suited to examine the efficacy of EGCG as a possible anti-trypanosomiasis therapy.
In summary, we found that EGCG inhibits TbACC activity in lysates, and this inhibition was correlated with an increase in ACC phosphorylation. This suggests that EGCG may be a useful tool for studying the effects of phosphorylation on TbACC activity. We also demonstrated that EGCG kills both PF and BF parasites in culture. However, EGCG treatment did not affect T. b. brucei virulence in a mouse model of acute infection, which reduces its promise as a therapeutic candidate to treat T. brucei infection.
Acknowledgements
We thank Jim Morris for critical reading of the manuscript. We thank Marilyn Parsons, Bryan Jensen, and members of the Clemson Eukaryotic Pathogen Club for helpful discussions and comments. This work was supported by funding from the National Institutes of Health (R15AI081207) and Clemson University. The sponsors had no role in the conduct, analysis, or publication of this research. The authors declare no competing interests.
Contributor Information
Patrick A. Vigueira, Email: pvigueir@dom.wustl.edu.
Ben A. Martin, Email: bemartin@georgiahealth.edu.
Marianne M. Ligon, Email: mmligon@uga.edu.
Kimberly S. Paul, Email: kpaul@clemson.edu.
References
- Ahmed S. Green tea polyphenol epigallocatechin 3-gallate in arthritis: progress and promise. Arthritis Res. Ther. 2010;12:208. doi: 10.1186/ar2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacchi C.J., Barker R.H., Jr., Rodriguez A., Hirth B., Rattendi D., Yarlett N., Hendrick C.L., Sybertz E. Trypanocidal activity of 8-methyl-5′-{[(Z)-4-aminobut-2-enyl]-(methylamino)}adenosine (Genz-644131), an adenosylmethionine decarboxylase inhibitor. Antimicrob. Agents Chemother. 2009;53:3269–3272. doi: 10.1128/AAC.00076-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber M.C., Price N.T., Travers M.T. Structure and regulation of acetyl-CoA carboxylase genes of metazoa. Biochim. Biophys. Acta. 2005;1733:1–28. doi: 10.1016/j.bbalip.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Brownsey R.W., Boone A.N., Elliott J.E., Kulpa J.E., Lee W.M. Regulation of acetyl-CoA carboxylase. Biochem. Soc. Trans. 2006;34:223–227. doi: 10.1042/BST20060223. [DOI] [PubMed] [Google Scholar]
- Brun R., Shonenberger M. Cultivation and in vitro cloning of procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Acta Trop. 1979;36:289–292. [PubMed] [Google Scholar]
- Brusselmans K., De Schrijver E., Heyns W., Verhoeven G., Swinnen J.V. Epigallocatechin-3-gallate is a potent natural inhibitor of fatty acid synthase in intact cells and selectively induces apoptosis in prostate cancer cells. Int. J. Cancer. 2003;106:856–862. doi: 10.1002/ijc.11317. [DOI] [PubMed] [Google Scholar]
- Castillo E., Dea-Ayuela M.A., Bolas-Fernandez F., Rangel M., Gonzalez-Rosende M.E. The kinetoplastid chemotherapy revisited: current drugs, recent advances and future perspectives. Curr. Med. Chem. 2010;17:4027–4051. doi: 10.2174/092986710793205345. [DOI] [PubMed] [Google Scholar]
- Chow H.H., Hakim I.A., Vining D.R., Crowell J.A., Ranger-Moore J., Chew W.M., Celaya C.A., Rodney S.R., Hara Y., Alberts D.S. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clin. Cancer Res. 2005;11:4627–4633. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- Collins Q.F., Liu H.Y., Pi J., Liu Z., Quon M.J., Cao W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J. Biol. Chem. 2007;282:30143–30149. doi: 10.1074/jbc.M702390200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO, 2007. Ethiopian fly factory guns for “poverty insect”. In: FAO newsroom. Food and agricultural organization of the United Nations. <http://www.fao.org/newsroom/en/focus/2007/1000511/article_1000514en.html> (accessed 09.07.2012.).
- Güida M.C., Esteva M.I., Camino A., Flawia M.M., Torres H.N., Paveto C. Trypanosoma cruzi: in vitro and in vivo antiproliferative effects of epigallocatechin gallate (EGCg) Exp. Parasitol. 2007;117:188–194. doi: 10.1016/j.exppara.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Hirumi H., Hirumi K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989;75:985–989. [PubMed] [Google Scholar]
- Horn D., McCulloch R. Molecular mechanisms underlying the control of antigenic variation in African trypanosomes. Curr. Opin. Microbiol. 2010;13:700–705. doi: 10.1016/j.mib.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z., Sang S., You H., Lee M.-J., Hong J., Chin K.-V., Yang C.S. Mechanism of action of (−)-epigallocatechin-3-gallate: auto-oxidation–dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–8056. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- Huang C.H., Tsai S.J., Wang Y.J., Pan M.H., Kao J.Y., Way T.D. EGCG inhibits protein synthesis, lipogenesis, and cell cycle progression through activation of AMPK in p53 positive and negative human hepatoma cells. Mol. Nutr. Food Res. 2009;53:1156–1165. doi: 10.1002/mnfr.200800592. [DOI] [PubMed] [Google Scholar]
- Hwang J.-T., Ha J., Park I.-J., Lee S.-K., Baik H.W., Kim Y.M., Park O.J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007;247:115–121. doi: 10.1016/j.canlet.2006.03.030. [DOI] [PubMed] [Google Scholar]
- Isbrucker R.A., Bausch J., Edwards J.A., Wolz E. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 1: genotoxicity. Food Chem. Toxicol. 2006;44:626–635. doi: 10.1016/j.fct.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Karori S.M., Ngure R.M., Wachira F.N., Wanyoko J.K., Mwangi J.N. Different types of tea products attenuate inflammation induced in Trypanosoma brucei infected mice. Parasitol. Int. 2008;57:325–333. doi: 10.1016/j.parint.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Khan N., Mukhtar H. Multitargeted therapy of cancer by green tea polyphenols. Cancer Lett. 2008;269:269–280. doi: 10.1016/j.canlet.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.H., Stephens J.L., Paul K.S., Englund P.T. Fatty acid synthesis by elongases in trypanosomes. Cell. 2006;126:691–699. doi: 10.1016/j.cell.2006.06.045. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Stephens J.L., Englund P.T. A fatty-acid synthesis mechanism specialized for parasitism. Nat. Rev. Microbiol. 2007;5:287–297. doi: 10.1038/nrmicro1617. [DOI] [PubMed] [Google Scholar]
- Lin Y.-S., Tsai Y.-J., Tsay J.-S., Lin J.-K. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J. Agric. Food Chem. 2003;51:1864–1873. doi: 10.1021/jf021066b. [DOI] [PubMed] [Google Scholar]
- Misaka, S., Kawabe, K., Onoue, S., Werba, J.P., Giroli, M., Kimura, J., Watanabe, H., Yamada, S., 2012. Development of rapid and simultaneous quantitative method for green tea catechins on the bioanalytical study using UPLC/ESI-MS. Biomed. Chromatogr. http://dx.doi.org/10.1002/bmc.2740. [Epub ahead of print]. [DOI] [PubMed]
- Moon H.S., Lee H.G., Choi Y.J., Kim T.G., Cho C.S. Proposed mechanisms of (−)-epigallocatechin-3-gallate for anti-obesity. Chem. Biol. Interact. 2007;167:85–98. doi: 10.1016/j.cbi.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Morita Y.S., Paul K.S., Englund P.T. Specialized fatty acid synthesis in African trypanosomes: myristate for GPI anchors. Science. 2000;288:140–143. doi: 10.1126/science.288.5463.140. [DOI] [PubMed] [Google Scholar]
- Murase T., Misawa K., Haramizu S., Hase T. Catechin-induced activation of the LKB1/AMP-activated protein kinase pathway. Biochem. Pharmacol. 2009;78:78–84. doi: 10.1016/j.bcp.2009.03.021. [DOI] [PubMed] [Google Scholar]
- Paveto C., Guida M.C., Esteva M.I., Martino V., Coussio J., Flawia M.M., Torres H.N. Anti-Trypanosoma cruzi activity of green tea (Camellia sinensis) catechins. Antimicrob. Agents Chemother. 2004;48:69–74. doi: 10.1128/AAC.48.1.69-74.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang S., Lee M.-J., Hou Z., Ho C.-T., Yang C.S. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005;53:9478–9484. doi: 10.1021/jf0519055. [DOI] [PubMed] [Google Scholar]
- Singh B.N., Shankar S., Srivastava R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens J.L., Lee S.H., Paul K.S., Englund P.T. Mitochondrial fatty acid synthesis in Trypanosoma brucei. J. Biol. Chem. 2007;282:4427–4436. doi: 10.1074/jbc.M609037200. [DOI] [PubMed] [Google Scholar]
- Tasdemir D., Kaiser M., Brun R., Yardley V., Schmidt T.J., Tosun F., Ruedi P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: in vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006;50:1352–1364. doi: 10.1128/AAC.50.4.1352-1364.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielecke F., Boschmann M. The potential role of green tea catechins in the prevention of the metabolic syndrome – a review. Phytochemistry. 2009;70:11–24. doi: 10.1016/j.phytochem.2008.11.011. [DOI] [PubMed] [Google Scholar]
- Tong L., Harwood H.J., Jr. Acetyl-coenzyme A carboxylases: versatile targets for drug discovery. J. Cell. Biochem. 2006;99:1476–1488. doi: 10.1002/jcb.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann U., Haller J., Decourt J.P., Girault N., Girault J., Richard-Caudron A.S., Pineau B., Weber P. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J. Int. Med. Res. 2003;31:88–101. doi: 10.1177/147323000303100205. [DOI] [PubMed] [Google Scholar]
- Ullmann U., Haller J., Decourt J.D., Girault J., Spitzer V., Weber P. Plasma-kinetic characteristics of purified and isolated green tea catechin epigallocatechin gallate (EGCG) after 10 days repeated dosing in healthy volunteers. Int. J. Vitam. Nutr. Res. 2004;74:269–278. doi: 10.1024/0300-9831.74.4.269. [DOI] [PubMed] [Google Scholar]
- Vigueira P.A., Paul K.S. Requirement for acetyl-CoA carboxylase in Trypanosoma brucei is dependent upon the growth environment. Mol. Microbiol. 2011;80:117–132. doi: 10.1111/j.1365-2958.2011.07563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Tian W. Green tea epigallocatechin gallate: a natural inhibitor of fatty-acid synthase. Biochem. Biophys. Res. Commun. 2001;288:1200–1206. doi: 10.1006/bbrc.2001.5923. [DOI] [PubMed] [Google Scholar]
- Wink M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Curr. Drug Metab. 2008;9:996–1009. doi: 10.2174/138920008786927794. [DOI] [PubMed] [Google Scholar]


