Keywords: Plasmodium falciparum, dhfr/dhps, Pyrimethamine, Resistance
Highlights
-
•
Prevalence of dhfr/dhps mutations increased significantly between 2003 and 2011.
-
•
Triple mutant dhfr 51I/59R/108N increased, from 40% in 2003 to 93% in 2011.
-
•
Quadruple mutant dhfr and dhps 437G increased, from 20% to 66% then down.
-
•
A strong correlation between ex vivo response to pyrimethamine and dhfr genotype.
Abstract
Resistance to sulfadoxine–pyrimethamine (SP) in Plasmodium falciparum malaria parasites is associated with mutations in the dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) genes, and these mutations have spread resistance worldwide. SP, used for several years in Senegal, has been recommended for intermittent preventive treatment for malaria in pregnancy (IPTp) and has been widely implemented since 2003 in this country. There is currently limited data on SP resistance from molecular marker genotyping, and no data on pyrimethamine ex vivo sensitivity in Senegal. Molecular markers of SP resistance and pyrimethamine ex vivo sensitivity were investigated in 416 parasite samples collected from the general population, from the Thies region between 2003 and 2011. The prevalence of the N51I/C59R/S108N triple mutation in dhfr increased from 40% in 2003 to 93% in 2011. Furthermore, the prevalence of the dhfr N51I/C59R/S108N and dhps A437G quadruple mutation increased, from 20% to 66% over the same time frame, then down to 44% by 2011. There was a significant increase in the prevalence of the dhfr triple mutation, as well as an association between dhfr genotypes and pyrimethamine response. Conversely, dhps mutations in codons 436 and 437 did not show consistent variation between 2003 and 2011. These findings suggest that regular screening for molecular markers of antifolate resistance and ex vivo drug response monitoring should be incorporated with ongoing in vivo efficacy monitoring in areas where IPTp-SP is implemented and where pyrimethamine and sulfa drugs are still widely administered in the general population.
1. Introduction
Plasmodium falciparum malaria continues to be a major global cause of mortality and morbidity. Malaria treatment and control has been complicated by the emergence of resistance to widespread antimalarial drug use. The most common method for measuring antimalarial resistance is estimating the in vivo efficacy of the antimalarial, such as sulfadoxine–pyrimethamine (SP). Since 2003, SP has been used in the intermittent preventive treatment for pregnant women (IPTp-SP) in many Sub-Saharan African countries, including in Senegal since 2003 (WHO, 2004). sulfadoxine–pyrimethamine in combination with amodiaquine was also recently recommended by the WHO for seasonal malaria chemoprevention (SMC) in some malaria-endemic countries (WHO Global Malaria Programme, 2012). Due to the recent recommendation to use artemisinin combination therapies (ACTs) for the treatment of uncomplicated malaria (WHO, 2010), it is no longer acceptable to carry out in vivo efficacy studies of SP used alone for the treatment of uncomplicated malaria. Nonetheless, it is critical to assess parasite SP resistance in order to monitor the efficacy of SP use in IPTp and SMC.
Antimalarial drug sensitivity testing provides information on the frequency of resistant phenotypes among the populations of parasites being transmitted, as well as the possible cross-resistance patterns of antimalarial drugs. Isolates are defined as resistant to pyrimethamine when the 50-percent inhibitory concentration (IC50) is greater than 2000 nM (Aubouy et al., 2003). In vitro methods to measure parasite resistance to individual components is a useful adjunct to in vivo studies (Desjardins et al., 1979; Smilkstein et al., 2004; Baniecki et al., 2007; Laufer et al., 2007; Kurth et al., 2009; Ndiaye et al., 2010). In vivo and in vitro drug sensitivity tests present numerous technical and cost limitations, and these limitations have led to a search for genetic markers of resistance.
As in vivo drug efficacy cannot be routinely monitored in IPTp-SP, an alternative method to track SP resistance is to study the frequency of molecular markers that are associated with SP resistance in the parasite population. The mechanism of action of SP is well documented: point mutations at codons 50, 51, 59, 108, and 164 in the dhfr gene (Bzik et al., 1987; Cowman et al., 1988; Peterson et al., 1988, 1990; Foote et al., 1990; Basco et al., 1995; Reeder et al., 1996) are found to confer resistance to pyrimethamine, while mutations at codons 437, 540, 581, and 613 of the dhps gene confer resistance to sulfadoxine (Brooks et al., 1994; Triglia and Cowman, 1994; Bickii et al., 1998; Warhurst, 2001; Warsame et al., 2001). The single dhfr 108 mutation can increase in vitro resistance to pyrimethamine by 100-fold relative to wild-type (Reeder et al., 1996; Sirawaraporn et al., 1997), and the progressive addition of mutations altering Cys50 to Arg (C50R), Asn51 to Ile (N51I), Cys59 to Arg (C59R), and Ile164 to Leu (I164L) in the gene can yield higher levels of SP resistance both in vitro and in vivo (Reeder et al., 1996; Sirawaraporn et al., 1997). The triple dhfr mutant genotype consisting of N51I, C59R, and S108N shows in vitro resistance to pyrimethamine that is 225 times higher than a wild-type lab strain (Basco et al., 1995; Nzila-Mounda et al., 1998), and has a strong association with in vivo SP treatment failure (Basco et al., 1998; Kublin et al., 2002; Happi et al., 2005). Sulfadoxine is the most common of the sulfones and sulfonamide class of drugs used in prophylaxis and/or treatment for human malaria caused by P. falciparum. A change at codon A437G in dhps is the first step in resistance to sulfa drugs, followed by sequential mutations at K540E, A581G, and A613S/T, which cause a further increase in drug resistance (Triglia et al., 1997). The quintuple mutant genotype consisting of the double dhps mutant genotype (A437G, K540E) in combination with the dhfr triple mutant genotype (S108N, N51I, C59R) also predicts clinical failure (Omar et al., 2001; Kublin et al., 2002; Mugittu et al., 2004; Staedke et al., 2004; Alker et al., 2008).
In Senegal, malaria remains a formidable public health issue, causing significant morbidity and mortality in infants and pregnant women (WHO Malaria Report, 2012). In the absence of an effective vaccine, the National Malaria Control Program has followed WHO recommendations for IPTp-SP since 2003. The rapid spread of SP-resistant parasites highlights the need for regular monitoring of ex vivo parasite sensitivity to pyrimethamine and dhfr/dhps mutations in countries like Senegal, where SP has been widely used for several years.
2. Materials and methods
2.1. Study population
This study was conducted from 2003 to 2011 at the Service de Lutte Anti-Parasitaire (SLAP) clinic, in the Thies region of Senegal. Thies is an urban area, 70 km from the capital city of Dakar, where malaria is hypoendemic (1 < EIR < 5) (Trape et al., 1992; Faye et al., 1995; Thomas et al., 2002). Individuals seeking treatment for uncomplicated P. falciparum malaria at the SLAP clinic in Thies were tested for malaria infection by microscopy and rapid diagnostic test (RDT). Patients that presented with symptoms consistent with mild malaria, including fever and a positive blood slide with only P. falciparum, were offered enrollment into the study. Exclusion criteria included severe disease and/or history of recent treatment with anti-malarial drugs. The Human Subjects Committee of Harvard School of Public Health in Boston, (protocol #P10256-127) and the Ethics Committee of the Senegal Ministry of Health in Dakar (Protocol #16330) both approved the protocols used in these studies.
2.2. Blood sample collection
For screening, thin and thick blood films were performed for parasite detection and identification of malaria parasite species and parasite counts by light microscopy using Giemsa stain (10% dilution). Blood samples were collected either on Whatman FTA filter papers (Whatman catalog #WB120205) or via venous blood draw from consenting patients, who were then treated with the first line treatment regimen according to the guidelines of the Senegalese Ministry of Health. Filter papers alone were collected in 2003 for genotyping, while both filter papers for genotyping and venous blood for the ex vivo drug assay were collected from 2008–2011. Blood samples were collected after written informed consent was obtained from each patient or their parent/guardian.
2.3. DNA extraction and genotyping
DNA extraction was performed from filter paper blood spots using a QIAamp DNA Minikit (Qiagen #51183) following manufacturer’s instructions (Thomas et al., 2002). In 2003, dhfr and dhps mutations were analyzed using the PCR-RFLP protocol (Ndiaye et al., 2005), with primers used to amplify the region that includes codons 50, 51, 59, 108, and 164 in dhfr and codons 436, 437, 540, 581, and 613 in dhps. In 2008, 2009, 2010, and 2011, haplotypes were analyzed using High Resolution Melting genotyping (Daniels et al., 2012) (Table 1).
Table 1.
Primer and probe sequences used for PCR-RFLP and high resolution melting assay.
| Technique | Gene/SNP ID | Forward primer 5’ > 3’ | Reverse primer 5’ > 3’ | Probe 5’ > 3’ |
|---|---|---|---|---|
| PCR–RFLP | dhfr.1 | ATG GAA CAA GTC TGC GAC GTT TTC | ATG ACA TGT ATC TTT GTC ATC ATT | |
| dhfr.2 | ATG GAA CAA GTC TGC GAC GTT TTC | ATT GTT ACT AGT ATA TAC ATC GCT | ||
| dhps.1 | CCATTCCTCATGTGTATA CAACAC | CATCTG AAACATCCAATTGTGT GA | ||
| dhps.2 | TATGATTCTTTT TCAGAT GGAGGT | CATCTGAAACATCCAATTGTG TGA | ||
| HRM | N51/C59 | ACATTTAGAGGTCTAGGAAATAAAGGAGT | ATATTTACATCTCTTATATTTCAATTTTTC ATATTT TGATTCATTCAC | AAATGTAATTCCCTAGATATGAAATATTTTTGTG CAG-block |
| I164 | ACAAAGTTGAAGATCTAATAGTTTTACTTGGG | CTGGAAAAAATACATCACATTCATATGTACTATTTATTCTA | AATGTTTTATTATAGGAGGTTCCG-block | |
| S108 | CTGTGGATAATGTAAATGATATGCCTAATTCTA | GACAATATAACATTTATCCTATTGCTTAAAGGT | GGAAGAACAAGCTGGGAAAGCAT-block | |
| S436/A437 | GAATGTTTGAAATGATAAATGAAGGTGCTA | CAGGAAACAGCTATGACGAAATAATTGTAATACAGG TACTACTAAATCTCT | ATCCTCTGGTCCTTTTGTTATACC-block | |
| K540 | GTGTTGATAATGATTTAGTTGATATATTAAATGATATTAGTGC | GTTTATCCATTGTATGTGGATTTCCTCTT | TAATCCAGAAATTATAAAATTATTAAAAAAAAA AAAC-block | |
| A581 | CTTGTATTAAATGGAATACCTCGTTATAGGA | AGTGGATACTCATCATATACATGTATATTTTGTAAG | TTGGATTAGGATTTGCGAAGAAACATGAT CA-block | |
| A613 | CTCTTACAAAATATACATGTATATGATGAGTATCCACTT | CATGTAATTTTTGTTGTGTATTTATTACAACATTTTGA | AAGATTTATTGCCCATTGCATGA-block | |
2.4. Ex vivo drug assay
Parasites were drug tested using the ex vivo DAPI assay (Ndiaye et al., 2010). Briefly, 180 μL of media plus parasitized erythrocytes at 2% hematocrit and parasitemia between 0.4% and 1% were distributed into 96-well plates preloaded with 20 μL of serially diluted pyrimethamine, prepared in duplicate wells. Pyrimethamine was obtained from Sigma (catalog #P7771) and stock solutions were prepared in DMSO. Drug plates were prepared in a single batch and frozen prior to use, and lab strain controls (3D7 and Dd2) were used to validate each plate batch. Two sets of serial dilutions were prepared in unsupplemented RPMI and distributed in duplicate into 96 well black plates: a series with high pyrimethamine concentrations from 295 to 2.7 μM, followed by a series of low pyrimethamine concentrations from 1366.6 to 3.3 nM. Samples that had parasitemia greater than 1% were diluted with leukocyte-free O+ donor red blood cells resulting in a final parasitemia of 0.4–1%. Parasites were cultured for 48–72 h at 37° Celsius in standard gas conditions (1% O2, 5% CO2, and 94% N2) before addition of 4′,6-diamidino-2-phenylindole (DAPI) solution, as previously described (Ndiaye et al., 2010). After culture, drug plates were read using a fluorescent plate reader. The 50% inhibitory concentration (IC50) was calculated using GraphPad Prism v5.0, estimated by non-linear regression analysis of log10-transformed dose-response curves.
2.5. Statistical analysis
Two-tailed Fisher’s exact test was used to determine whether mutant allele frequencies increased by year (2003 versus 2011). Mann–Whitney U test was used to determine whether median IC50 values differed for parasites with wild-type and mutant alleles. GraphPad Prism was used to analyze IC50s for pyrimethamine. For all statistical tests, alpha = 0.05.
3. Results
3.1. Patient ages and parasite densities
We monitored 416 Senegalese patients from 2003–2011 with ages ranging from 2 to 65 years. Patient parasitemia increased between 2003 and 2008 (p < 0.002) (Table 2).
Table 2.
Ages and parasitemias of patients included in this study from 2003 to 2011.
| 2003 | 2008 | 2009 | 2010 | 2011 | |
|---|---|---|---|---|---|
| Number (n) | 15 | 93 | 84 | 94 | 130 |
| Median age (years) | 17.7 (7–54) | 23 (2–55) | 23.5 (4–61) | 17 (3–65) | 16.5 (3–59) |
| Median parasitemia (lowest–highest) asexual parasite/μL | 12,216 (1019–110,000) | 18,000 (4500–135,000) | 22,500 (2250–351,000) | 23,400 (450–585,000) | 22,500 (3150–315,000) |
3.2. Prevalence of dhfr and dhps point mutations
A total of 416 P. falciparum samples collected between 2003 and 2011 were successfully genotyped for the following mutations: dhfr C50R, N51I, C59R, S108N, and I164L, and dhps S436A, A437G, K540E, A581G, and A613S/T. We did not detect the following mutations: dhfr C50R and I164L, and dhps K540E, A581G, and A613S/T. Fig. 1 shows that the prevalence of mutations in dhfr in 2003 was between 40% (N51I and C59R) and 67% (S108N), and rose to 93% or greater in 2011, resulting in a significant increase (Fischer’s exact, p = 0.0002) from 2003 to 2011. Dhps mutations individually fluctuated (no significant change) between 2003 and 2011. The dhps mutations at codons 436 and 437 did not show significant variation between 2003 and 2011 (p = 0.08), but rather fluctuated between 2% and 23% (S436A) and between 20% and 67% (A437G). Among all isolates, no more than 6 isolates had mixed alleles at any given dhfr or dhps locus.
Fig. 1.
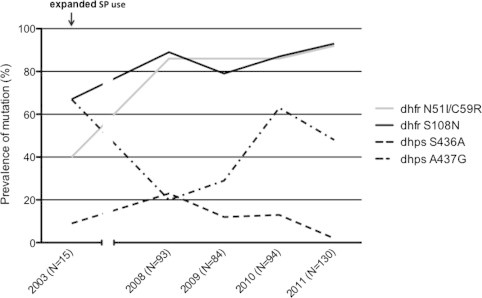
Evolution of dhfr N51I/C59R/S108N and dhps mutation prevalence after expanded SP use in Thies, Senegal. The prevalence of the dhfr mutant alleles for both 51I/59R and 108N increased significantly between 2003 and 2011 (Fischer’s exact, p = 0.0002). Dhps mutation individually fluctuated (no significant change) between 2003 and 2011 (Ndiaye et al., 2005), (Daniels et al., 2012).
We observed that the prevalence of the dhfr 51I/59R/108N triple mutant genotype increased significantly from 40% in 2003 to 93% in 2011 (Fisher’s exact, p = 0.0002); and the prevalence of the dhfr 51I/59R/108N and dhps 437G quadruple mutant genotype also increased from 20% to 44% over the same time period (Fig. 2). We did not observed the appearance of the dhfr 51I/59R/108N and dhps 437G/540E quintuple mutant genotype. The quadruple mutant genotype increased between 2003 and 2008, and decreased between 2008 and 2011.
Fig. 2.
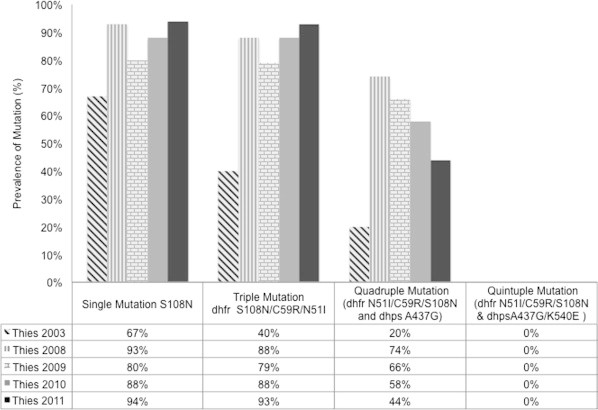
Haplotype frequencies and number of mutations in dhfr codons 51, 59, 108 and dhps 436 and 437, present in P. falciparum isolates from Thies between 2003 and 2011. Haplotype frequencies were determined by HRM (in 2003) or PCR-RFLP (in 2008–2011). Significant increases were detected using Fisher’s exact test to detect differences between 2003 and 2011. The prevalence of the dhfr 51I/59R/108N triple mutant genotype increased from 40% in 2003 to 93% in 2011 (Fischer’s exact, p = 0.0002); and the prevalence of the dhfr 51I/59R/108N and dhps 437G quadruple mutant genotype increased from 20% to 44% over the same time period. 2003 data was previously reported in Ndiaye et al. (2005).
3.3. Ex vivo susceptibility of Senegalese P. falciparum isolates to pyrimethamine
A DAPI-based ex vivo assay was used to test pyrimethamine sensitivity in 66 parasite isolates from 2011 (Ndiaye et al., 2010). 3D7 and Dd2 parasites were used as control strains; their IC50s were 47.2 and 49,464 nM, respectively. Out of the 66 isolates, 56 (84.8%) were found to be resistant to pyrimethamine with IC50s greater than 2000 nM. The median pyrimethamine IC50 was 25,125 nM with a minimum of 2.4 nM and a maximum of 11,107 nM (Table 3). The median IC50 among sensitive isolates and resistant isolates were 247.8 (2.4–1503) nM, and 30,705 (2259–201,046) nM, respectively (Table 4).
Table 3.
Pyrimethamine IC50 values and percentage of resistant parasites tested in 2011.
| Drug tested | IC50 median (nM) (CI 95%) | Range |
Resistant isolates (%) (n/N) | |
|---|---|---|---|---|
| Min | Max | |||
| Pyrimethamine (n = 66) | 11,107 (20,104–27,046) | 2.4 | 201,046 | 84.8% (56/66) |
Table 4.
Ex vivo pyrimethamine IC50 values for resistant and sensitive isolates.
| Isolates tested | Pyrimethamine sensitive isolates (n = 10) | Pyrimethamine resistant isolates (n = 56) | (Mann–Whitney U test) p-value |
|---|---|---|---|
| Field isolates (n = 66) median (lowest–highest) IC50 (nM) | 15.5 (2.4–1503) | 15,725 (2259–201,046) | 0.0001 |
| 3D7 | 47.2 | – | |
| Dd2 | – | 49,464 |
3.4. Correlation between dhfr polymorphisms and pyrimethamine ex vivo susceptibility
The correlation between the dhfr mutation and resistance to pyrimethamine measured ex vivo was verified by our study. We found significant increases in the geometric means of the IC50 values for ex vivo pyrimethamine susceptibility among parasites bearing single mutations within dhfr (Fig. 3, Mann–Whitney U test, p = 0.0001). In the pyrimethamine resistant isolates, the mean IC50 for parasites with the mutant 108N allele (N = 58, mean IC50 = 31,181 nM, CI95% 30,002–32,606) was 324 times higher than the mean IC50 for parasites with the wild-type S108 allele (N = 9, mean IC50 = 96 nM, CI95% 94.7–97). The mean IC50 for parasites with the mutant 51I allele (N = 56, mean IC50 = 31,540 nM, CI95% 30,160–32,100) was 240 times higher than the mean IC50 for parasites with the wild-type N51 allele (N = 6, mean IC50 = 131 nM, CI95% 130.7–133.6). Likewise, the mean IC50 for parasites with the mutant 59R allele (N = 57, mean IC50 = 31,021 nM, CI95% 30,259–32,119) was 5640 times higher than the mean IC50 for parasites with the wild-type C59 allele (N = 2, mean IC50 = 5.5 nM, CI95% 5.2–5.8) (Fig. 3a). We observed a mean IC50 1000 times higher between wild type and triple mutant dhfr 51I/59R/108N parasites, (Mann–Whitney U test, p = 0.0002), as well as between wild type and dhfr 51I/59R/108N and dhps 437G quadruple mutant parasites (Mann–Whitney U test, p = 0.0002) (Fig. 3b). Four of the 10 isolates that were sensitive to pyrimethamine also had the mutation at dhfr 108, while all six parasites with the dhfr S108 wild-type allele were sensitive to pyrimethamine. The six isolates with the wild-type allele at dhfr C51 were also sensitive to pyrimethamine, and the same observation was made for isolates with the wild-type dhfr C59 allele. This illustrates that all parasites with the wild-type alleles at dhfr 51, 59 and 108 were sensitive to pyrimethamine, and the correlation between dhfr mutation and phenotypes was statistically significant (Fisher exact, p = 0.00001).
Fig. 3.
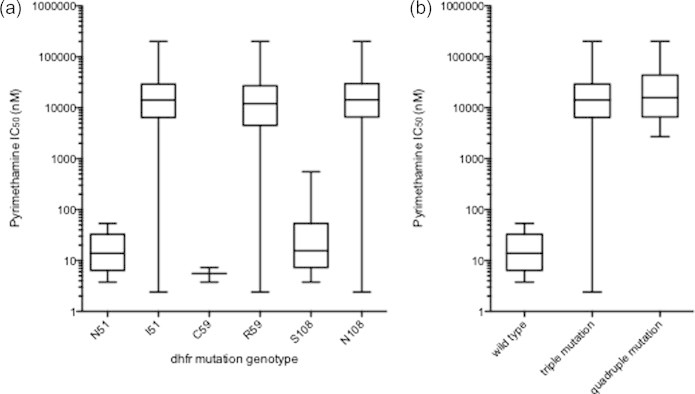
IC50 (nM) comparisons between mutant and wild type alleles at codons 51, 59, and 108 in dhfr and comparison between parasites with wild type versus dhfr N51I/C59R/S108N triple mutation and dhfr N51I/C59R/S108N and dhps A437G quadruple mutation. (a) We found significant increases in the geometric mean IC50 values for ex vivo pyrimethamine susceptibility between mutant and wild type alleles (Mann–Whitney U test, p = 0.0001). Pyrimethamine IC50s were measured ex vivo in 2011 using the DAPI drug assay. (b) IC50s were different between wild type and dhfr N51I/C59R/S108N triple mutation parasites, as well as between wild type and dhfr N51I/C59R/S108N and dhps A437G quadruple mutation parasites (Mann–Whitney U test, p = 0.0002 for both comparisons).
4. Discussion
In 2003, Senegal adopted intermittent preventive treatment for pregnant women (IPTp) using sulfadoxine–pyrimethamine (SP). At the same time, between 2003 and 2004, Senegal switched to sulfadoxine–pyrimethamine with amodiaquine as the first-line therapy for uncomplicated malaria in response to increasing chloroquine resistance (WHO Roll Back Malaria Focus on Senegal, 2010). In 2005, Senegal adopted artemisinin combination therapies (ACTs) as first line treatment for uncomplicated malaria. The results reported here were obtained from samples collected from the general population in an urban site with expanded SP use, and this is one of the few reports that includes both dhfr/dhps polymorphisms and ex vivo drug phenotype data. Previous studies carried out in Senegal and other West African countries have focused on rural sites and studied P. falciparum polymorphisms without assessment of corresponding ex vivo phenotypes.
Our results show an increase in the prevalence of parasites bearing individual dhfr mutations at codons 51, 59, and 108 in an interval of eight years. Furthermore, the number of parasites with all three dhfr mutations increased from 40% in 2003 (Ndiaye et al., 2005), to 93% in 2011. Emergence of the dhfr 51I/59R/108N triple mutant has been observed in countries using sulfadoxine–pyrimethamine alone or in combination, as first line treatment for uncomplicated malaria as reported in Africa and elsewhere (Bwijo et al., 2003; Griffin et al., 2010; Malisa et al., 2010; Raman et al., 2010; Yusuf et al., 2010; Zakeri et al., 2010; Mula et al., 2011; Mombo-Ngoma et al., 2011; Naidoo and Ropper, 2011). A similar increase in the dhfr N51I/C59R/S108N triple mutation has been observed after IPT in children in southern Senegal (Faye et al., 2011), as well as in rural regions in Mali (Dicko et al., 2010) and southern Mozambique (Enosse et al., 2008), with dhfr mutations being an important predictive risk factor of in vivo resistance (Boumbou-Moukoko et al., 2009; Picot et al., 2009). Dhps mutations individually fluctuated (no significant change) between 2003 and 2011 in this study, but when considered in combination with dhfr mutations, the number of parasites with an additional mutation at dhps 437 (dhfr N51I/C59R/S108N and dhps A437G quadruple mutation) increased from 2003 to 2008 and then steadily decreased until 2011. Interestingly, we found that mutations at dhps codons 436 and 437 were not always inherited together, despite residing very close to each other on the chromosome (Bwijo et al., 2003; Bouyou-Akotet et al., 2010).
The quintuple mutant dhfr 51I/59R/108N and dhps 437G/ 540E has not been previously observed in Senegal (Ndiaye et al., 2005, 2006; Henry et al., 2006; Faye et al., 2011), or in Mali (Dicko et al., 2010). The dhfr I164L mutation was also not found in this study. The combination of dhfr C59R and dhps K540E mutations, which predict clinical failure of sulfadoxine–pyrimethamine (Basco et al., 2000; Kublin et al., 2002; Talisuna et al., 2004; McCollum et al., 2012), were also not found in our study.
We found a correlation between the dhfr S108N single mutation and pyrimethamine resistance, and a correlation between the dhfr N51I/C59R/S108N triple mutation, as well as the dhfr N51I/C59R/S108N and dhps A437G quadruple mutation, and pyrimethamine resistance. Overall, we found a significant difference in the geometric mean IC50 values for pyrimethamine (p = 0.0009) between parasites possessing wild-type and resistant alleles in dhfr, as has been reported by others (Andriantsoanirina et al., 2011). We confirmed the existence of an association between the dhfr genotype and chemosensitivity to pyrimethamine in P. falciparum isolates from Thies, as the increase in the number of mutations was associated with an increase in ex vivo resistance to pyrimethamine, similar to what has been observed in Gabon (Aubouy et al., 2003), Central African Republic (Menard et al., 2006), and Cote D’Ivoire (Djaman et al., 2007). However, some parasites harbored the N51I, C59R, and S108N mutations in dhfr but were still susceptible to pyrimethamine as reported in isolates from Brazil (Petersen et al., 1991), and Gabon (Aubouy et al., 2003) for the dhfr S108N mutation and Papua New Guinea (Reeder et al., 1996) for dhfr S108N and dhfr C59R. Further sequencing of these parasites for possible compensatory mutations may explain this finding. The ex vivo assay data does not permit strong conclusions because we obtained ex vivo pyrimethamine data from only 1 year; however, the high rates of pyrimethamine ex vivo resistance in this study are correlated with high rates of the dhfr N51I/C59R/S108N triple mutation.
The use of SP in IPTp may not be the only driver of parasite polymorphisms in this population, because Senegal has used sulfadoxine and/or pyrimethamine in the national antimalarial treatment plan for many years, and furthermore these drugs are still being used in antibacterial combination therapy. Nonetheless, this type of general population survey could form part of the monitoring system for IPTp as an alert strategy plan, because the genetic and phenotypic diversity among parasites infecting the general population in very low transmission areas like Thies (EIR < 5), likely reflect the parasites circulating among pregnant women.
Our study is not without limitations. The small number of patients recruited in 2003 was due to logistical constraints, which were addressed in the following years and allowed for deeper sampling in 2008–2011. The intervening years were also spent developing the DAPI ex vivo drug assay (Ndiaye et al., 2010) and High Resolution Melting genotyping (Daniels et al., 2012). The latter technology is a reliable, adaptable, and accessible tool that provides comparable results to PCR-RFLP. Future studies will strengthen the present data set and will provide valuable information for the Senegalese National Malaria Control Program.
In conclusion, our results show an increasing prevalence of dhfr N51I/C59R/S108N triple and dhfr N51I/C59R/S108N and dhps A437G quadruple mutations between 2003 and 2011 in Thies, Senegal. This study suggests that intermittent drug pressure with SP is selecting parasites with mutant alleles. The use of SP is not only implemented in IPTp, but also recently for seasonal malaria chemoprevention in children, thus surveillance of molecular markers of drug resistance and ex vivo drug sensitivity assays should be an integral part of planned malaria control programs, so that resistance dynamics can be assessed and the most effective treatment can be selected or modified.
Acknowledgements
The work was supported by a grant (D43 TW001503) to D.F.W. from the National Institutes of Health. The authors are very grateful to Dan Milner, Ambroise Ahouidi, Younouss Diedhiou, Lamine Ndiaye, Amadou Mactar Mbaye, Moussa Dieng Sarr, Ngayo Sy, SLAP patients, and the SLAP health staff.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Alker A.P., Kazadi W.M., Kutelemeni A.K., Bloland P.B., Tshefu A.K., Meshnick S.R. Dhfr and dhps genotype and sulfadoxine–pyrimethamine treatment failure in children with falciparum malaria in the Democratic Republic of Congo. Trop. Med. Int. Health. 2008;13:1384–1391. doi: 10.1111/j.1365-3156.2008.02150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriantsoanirina V., Durand R., Pradines B., Baret E., Bouchier C., Ratsimbasoa A., Menard D. In vitro susceptibility to pyrimethamine of dhfr I164L single mutant Plasmodium falciparum. Malar. J. 2011;10:283. doi: 10.1186/1475-2875-10-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubouy A., Jafari S., Huart V., Migot-Nabias F., Mayombo J., Durand R., Bakary M., Le Bras J., Deloron P. DHFR and DHPS genotypes of Plasmodium falciparum isolates from Gabon correlate with in vitro activity of pyrimethamine and cycloguanil, but not with sulfadoxine–pyrimethamine treatment efficacy. J. Antimicrob. Chemother. 2003;53:43–49. doi: 10.1093/jac/dkg294. [DOI] [PubMed] [Google Scholar]
- Baniecki M.L., Wirth D.F., Clardy J. High-throughput Plasmodium falciparum growth assay for malaria drug discovery. Antimicrob. Agents Chemother. 2007;51:716–723. doi: 10.1128/AAC.01144-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basco L.K., De Pecoulas P.E., Wilson C.M., Le Bras J., Mazabraud A. Point mutations in the dihydrofolate reductase thymidylate synthase gene and pyrimethamine and cycloguanil resistance in Plasmodium falciparum. Mol. Biochem. Parasitol. 1995;69:135–138. doi: 10.1016/0166-6851(94)00207-4. [DOI] [PubMed] [Google Scholar]
- Basco L.K., Tahar R., Ringwald P. Molecular basis of in vivo resistance to sulfadoxine–pyrimethamine in African adult patients infected with Plasmodium falciparum malaria parasites. Antimicrob. Agents Chemother. 1998;42:1811–1814. doi: 10.1128/aac.42.7.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basco L.K., Tahar R., Keundjian A., Ringwald P. Sequence variations in the genes encoding dihydropteroate synthase and dihydrofolate reductase and clinical response to sulfadoxine- pyrimethamine in patients with acute uncomplicated falciparum malaria. J. Infect. Dis. 2000;182:624–628. doi: 10.1086/315731. [DOI] [PubMed] [Google Scholar]
- Bickii J., Basco L.K., Ringwald P. Assessment of three in vitro tests and an in vivo test for chloroquine resistance in Plasmodium falciparum clinical isolates. J. Clin. Microbiol. 1998;36:243–247. doi: 10.1128/jcm.36.1.243-247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumbou-Moukoko E.C., Bogreau H., Briolant S., Pradines B., Rogier C. Molecular markers of Plasmodium falciparum drug resistance. Med. Trop. 2009;69:606–612. [PubMed] [Google Scholar]
- Bouyou-Akotet M.K., Mawili-Mboumbat D.P., Tchantchou T., Kombila M. High prevalence of sulfadoxine/pyrimethamine-resistant alleles of Plasmodium falciparum isolates in pregnant women at the time of introduction of intermittent preventive treatment with sulfadoxine/pyrimethamine in Gabon. J. Antimicrob. Chemother. 2010;65:438–441. doi: 10.1093/jac/dkp467. [DOI] [PubMed] [Google Scholar]
- Brooks D.R., Wang P., Read M., Watkins W.M., Sims P.F. Sequence variation of the hydroxymethyldihydropterin pyrophosphokinase: dihydropteroate synthase gene in lines of the human malaria parasite, Plasmodium falciparum, with differing resistance to sulfadoxine. Eur. J. Biochem. 1994;224:397–405. doi: 10.1111/j.1432-1033.1994.00397.x. [DOI] [PubMed] [Google Scholar]
- Bwijo B., Kaneko A., Takechi A., Zungu I.L., Moriyama Y. High prevalence of quintuple mutant dhps/dhfr genes in Plasmodium falciparum infections seven years after introduction of sulfadoxine and pyrimethamine as first first line treatment in Malawi. Acta Trop. 2003;85:363–373. doi: 10.1016/s0001-706x(02)00264-4. [DOI] [PubMed] [Google Scholar]
- Bzik D.J., Li W.B., Hon T., Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase–thymidylate synthase gene. Proc. Natl. Acad. Sci. USA. 1987;84:8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman A.F., Morry M.J., Biggs B.A., Cross G.A., Foote S.J. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase–thymidylate synthase gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA. 1988;85:9109–9113. doi: 10.1073/pnas.85.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels R., Ndiaye D., Wall M., McKinney J., Sene P.D., Sabeti P.C., Volkman S., Mboup S., Wirth D.F. Rapid, field-deployable method for genotyping and discovery of single-nucleotide polymorphisms associated with drug resistance in Plasmodium falciparum. Antimicrob. Agents Chemother. 2012;56(6):2976–2986. doi: 10.1128/AAC.05737-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins R.E., Canfield C.J., Haynes J.D., Chulay J.D. Quantitative assessment of antimalarial activity in vitro by a semi automated microdilution technique. Antimicrob. Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djaman J.A., Mazabraud A., Basco L. sulfadoxine–pyrimethamine susceptibilities and analysis of the dihydrofolate reductase and dihydropteroate synthase of Plasmodium falciparum isolates from Cote d’Ivoire. Ann. Trop. Med. Parasitol. 2007;101(2):103–112. doi: 10.1179/136485907X154584. [DOI] [PubMed] [Google Scholar]
- Dicko A., Sagara I., Djimbde A.A., Toure S.O., Traore M., Dama S., Diallo A.I., Barry A., Dicko M., Coulibaly O.M., Rogier C., De Sousa A., Doumbo O.K. Molecular markers of resistance to sulfadoxine-pyriemethamine one year after implementiion of intermittent preventive treatment of malaria in infants in Mali. Malar. J. 2010;10(9):9. doi: 10.1186/1475-2875-9-9. http://dx.doi.org/10.1186/1475-2875-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faye B., Ndiaye M., Ndiaye J.L., Annie A., Tine R.C., Lo A.C., Ndiaye M., Sow D., De Sousa A., Gaye O. Prevalence of molecular markers of Plasmodium falciparum resistance to sulfadoxine-pyrimethamine during the intermittent preventive treatment in infants coupled with the expanded program immunization in Senegal. Parasitol. Res. 2011;109:133–138. doi: 10.1007/s00436-010-2236-9. [DOI] [PubMed] [Google Scholar]
- Faye O., Gaye O., Fontenille D. . Comparison of the transmission of malaria in two epidemiological patterns in Senegal: the Sahel border and the Sudan-type savanna. Dakar Med. 1995;40:1–7. [PubMed] [Google Scholar]
- Foote S.J., Galatis D., Cowman A.F. Amino acids in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum involved in cycloguanil resistance differs from those involved in pyrimethamine resistance. Proc. Natl. Acad. Sci. USA. 1990;87:3014–3017. doi: 10.1073/pnas.87.8.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J.T., Cairns M., Ghani A.C., Roper C., Schellenberg D., Carneiro I., Newman R.D. Protective efficacy of Intermittent Preventive Treatment of Malaria in infants (IPTi) using sulfadoxine-pyrimethamine and parasite resistance. Plos One. 2010;5(9):e12618. doi: 10.1371/journal.pone.0012618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happi C.T., Gbotosho G.O., Folarin O.A., Akinboye D.O., Yusuf B.O., Ebong O.O., Sowunmi A., Kyle D.E., Milhous W., Wirth D.F., Oduola A.M. Polymorphisms in Plasmodium falciparum dhfr and dhps genes and age related in vivo sulfadoxine–pyrimethamine resistance in malaria-infected patients from Nigeria. Acta Trop. 2005;95:183–193. doi: 10.1016/j.actatropica.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Henry M., Diallo I., Bordes J., Ka S., Pradines B., Diatta B., Mbaye P.S., Sane M., Thiam M., Gueye P.M., Wade B., Touze J.E., Debonne J.M., Rogier C., Fusai T. Urban malaria in Dakar, Senegal. Am. J. Trop. Med. Hyg. 2006;75(1):146–151. [PubMed] [Google Scholar]
- Kublin J.G., Dzinjalamala F.K., Kamwendo D.D., Malkin E.M., Cortese J.F., Martino L.M., Mukadam R.A., Rogerson S.J., Lescano A.G., Molyneux M.E., Winstanley P.A., Chimpeni P., Taylor T.E., Plowe C.V. Molecular markers for failure of sulfadoxine–pyrimethamine and chlorproguanil-dapsone treatment of Plasmodium falciparum malaria. J. Infect. Dis. 2002;185:380–388. doi: 10.1086/338566. [DOI] [PubMed] [Google Scholar]
- Kurth F., Pongratz P., Belard S., Mordmuller B., Kremsner P.G., Ramharter M. In vitro activity of pyronaridine against Plasmodium falciparum and comparative evaluation of anti-malarial drug susceptibility assays. Malar. J. 2009;23(8):79. doi: 10.1186/1475-2875-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer M.K., Djimbde A.A., Plowe C.C. Monitoring and dettering drug resistance malaria in the area of combination therapy. Am. J. Trop. Med. Hyg. 2007;77(Suppl. 6):160–169. [PubMed] [Google Scholar]
- Malisa A.L., Pearce R.J., Abdulla S., Mshinda H., Kachur P.S., Bloland P., Roper C. Drug coverage in treatment of malaria and the consequence for resistance evolution-evidence from the use of sulfadoxine/pyrimethamine. Malar. J. 2010;9:190. doi: 10.1186/1475-2875-9-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollum A.M., Schneider K.A., Griffing S.M., Zhou Z. Differences in selective pressure on dhps and dhfr drug resistant mutations in western Kenya. Malar. J. 2012;11:77. doi: 10.1186/1475-2875-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard D., Yapou F., Manirakiza A., Djalle D., Matsika-Claquin M.D., Talarmin A. Polymorphism in pfcrt, pfmdr1, dhfr genes and in vitro responses to antimalarials in Plasmodium falciparum isolates from Bangui, Central African Republic. Am. J. Trop. Med. Hyg. 2006;75(3):381–387. [PubMed] [Google Scholar]
- Mombo-Ngoma G., Oyakhirome S. High prevalence of dhfr triple mutatnt and correlation with high rates of sulfadoxine–pyrimethamine treatment failures in vivo in Gabonese children. Malar. J. 2011;10:123. doi: 10.1186/1475-2875-10-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugittu K., Ndejembi M., Malisa A., Lemnge M., Premji Z., Mwita A., Nkya W., Kataraihya J., Abdulla S., Beck H.P., Mshinda H. Therapeutic efficacy of sulfadoxine–pyrimethamine and prevalence of resistance markers in Tanzania prior to revision of malaria treatment policy: Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase mutations in monitoring in vivo resistance. Am. J. Trop. Med. Hyg. 2004;71:696–702. [PubMed] [Google Scholar]
- Mula P., Fernandez-Martinez A., De Lucio A., Ramos J.M., Reyes F., Gonzalez V., Benito A., Berzosa P. Detection of high levels of mutations involved in anti-malarial drug resistance in Plasmodium falciparum and Plasmodium vivax at rural hospital in southern Ethiopia. Malar. J. 2011;10:214. doi: 10.1186/1475-2875-10-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidoo I., Ropper C. Drug resistance maps to guide intermittent preventive treatment of malaria in African infants. Parasitology. 2011;138(12):1469–1479. doi: 10.1017/S0031182011000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiaye D., Daily J.P., Sarr O., Ndir O., Gaye O., Mboup S., Wirth D.F. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase genes in Senegal. Trop. Med. Int. Health. 2005;10(11):1176–1179. doi: 10.1111/j.1365-3156.2005.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiaye D., Daily J.P., Sarr O., Ndir O., Gaye O., Mboup S., Roper C., Wirth D.F. Defining the origin of Plasmodium falciparum resistant dhfr isolates in Senegal. Acta Trop. 2006;99(1):106–111. doi: 10.1016/j.actatropica.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndiaye D., Patel V. A non-radioactive DAPI-based high-throughput in vitro assay to assess Plasmodium falciparum responsiveness to antimalarials increased sensitivity of P. falciparum to chloroquine in Senegal. Am. J. Trop. Med. Hyg. 2010;82(2):228–230. doi: 10.4269/ajtmh.2010.09-0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nzila-Mounda A., Mberu E.K., Sibley C.H., Plowe C.V., Winstanley P.A., Watkins W.M. Kenyan Plasmodium falciparum field isolates: correlation between pyrimethamine and chlorcycloguanil activity in vitro and point mutations in the dihydrofolate reductase domain. Antimicrob. Agents Chemother. 1998;42:164–169. doi: 10.1128/aac.42.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar S.A., Adagu I.S., Warhurst D.C. Can pretreatment screening for dhps and dhfr point mutations in Plasmodium falciparum infections be used to predict sulfadoxine–pyrimethamine treatment failure? Trans. R. Soc. Trop. Med. Hyg. 2001;95:315–319. doi: 10.1016/s0035-9203(01)90250-0. [DOI] [PubMed] [Google Scholar]
- Peterson D.S., Walliker D., Wellems T.E. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc. Natl. Acad. Sci. USA. 1988;85:9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D.S., Milhous W.K., Wellems T.E. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc. Natl. Acad. Sci. USA. 1990;87:3018–3022. doi: 10.1073/pnas.87.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen D.S., Di Santi S.M., Povoa M., Calvosa V.S., Do Rosario V.E., Wellems T.E. Prevalence of the dihydrofolate reductase Asn-108 mutation as the basis for pyrimethamine-resistant falciparum malaria in the Brazilian Amazon. Am. J. Trop. Med. Hyg. 1991;45(4):492–497. doi: 10.4269/ajtmh.1991.45.492. [DOI] [PubMed] [Google Scholar]
- Picot S., Olliaro P., De Monbrison F., Bienvenu A.L., Price R.N., Ringwald P. A systematic review and meta-analysis of evidence for correlation between molecular markers of parasite resistance and treatment outcome in falciparum malaria. Malar. J. 2009;8:89. doi: 10.1186/1475-2875-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman J., Little F., Roper C., Kleinschmidt I., Cassam Y., Maharaj R., Barnes K.I. Five years of large –scale dhfr and dhps mutation surveillance following the phased implementation of artesunate plus sulfadoxine-pyrimethamine in Maputo province, southern Mozambique. Am. J. Trop. Med. Hyg. 2010;82(5):788–794. doi: 10.4269/ajtmh.2010.09-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeder J.C., Rieckmann K.H., Genton B., Lorry K., Wines B., Cowman A.F. Point mutations in the dihydrofolate reductase and dihydropteroate synthetase genes and in vitro susceptibility to pyrimethamine and cycloguanil of Plasmodium falciparum isolates from Papua New Guinea. Am. J. Trop. Med. Hyg. 1996;55:209–213. doi: 10.4269/ajtmh.1996.55.209. [DOI] [PubMed] [Google Scholar]
- Sirawaraporn W., Sathitkul T., Sirawaraporn R., Yuthavong Y., Santi D.V. Antifolate-resistant mutants of Plasmodium falciparum dihydrofolate reductase. Proc. Natl. Acad. Sci. USA. 1997;94:1124–1129. doi: 10.1073/pnas.94.4.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilkstein M., Sriwilaijaroen N., Kelly J.X., Wilairat P., Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004;48:1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staedke S.G., Sendagire H., Lamola S., Kamya M.R., Dorsey G. Relationship between age, molecular markers, and response to sulphadoxine–pyrimethamine treatment in Kampala, Uganda. Trop. Med. Int. Health. 2004;9:624–629. doi: 10.1111/j.1365-3156.2004.01239.x. [DOI] [PubMed] [Google Scholar]
- Talisuna A.O., Nalunkuma-Kazibwe A., Langi P., Mutabingwa T.K., Watkins W.W., Marck E.V., Egwang T.G., D’Alessandro U. Two mutations in dihydrofolate reductase combined with one in the dihydropteroate synthase gene predict sulphadoxine-pyrimethamine parasitological failure in Uganda children with uncomplicated falciparum malaria. Infect. Genet. Evol. 2004;4:321–327. doi: 10.1016/j.meegid.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Thomas S.M., Ndir O., Dieng T. In vitro chloroquine susceptibility and PCR analysis of pfcrt and pfmdr1 poly-morphisms in Plasmodium falciparum isolates from Senegal. Am. J. Trop. Med. Hyg. 2002;66:474–480. doi: 10.4269/ajtmh.2002.66.474. [DOI] [PubMed] [Google Scholar]
- Trape J.F., Lefebvre-Zante E., Legros F. Vector density gradients and the epidemiology of urban malaria in Dakar, Sengal. Am. J. Trop. Med. Hyg. 1992;147:181–189. doi: 10.4269/ajtmh.1992.47.181. [DOI] [PubMed] [Google Scholar]
- Triglia T., Cowman A.F. Primary structure and expression of the dihydropteroate synthetase gene of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA. 1994;91:7149–7153. doi: 10.1073/pnas.91.15.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triglia T., Menting J.G., Wilson C., Cowman A.F. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA. 1997;94:13944–13949. doi: 10.1073/pnas.94.25.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warhurst D.C. A molecular marker for chloroquine resistant falciparum malaria. N. Engl. J. Med. 2001;344:299–302. doi: 10.1056/NEJM200101253440411. [DOI] [PubMed] [Google Scholar]
- Warsame M., Wernsdorfer W.H., Payne D., Bjorkman A. Susceptibility of Plasmodium falciparum in vitro to chloroquine, mefloquine, quinine and sulfadoxine/pyrimethamine in Somalia: relationships between the responses to the different drugs. Trans. R. Soc. Trop. Med. Hyg. 2001;85:565–569. doi: 10.1016/0035-9203(91)90343-w. [DOI] [PubMed] [Google Scholar]
- WHO, 2004. A strategic framework for Malaria Prevention and Control during Pregnancy in the African Region. Brazzaville, World Health Organization Regional Office for Africa, AFR/MAL/04/01.
- WHO, 2010. Guidelines for the treatment of malaria. 1. Malaria – Drug Therapy. 2. Malaria – Diagnosis. 3. Antimalarials – Administration and Dosage. 4. Drug Therapy, Combination. 5. Guidelines, second ed. World Health Organization.
- WHO, 2012. Malaria report. Global Malaria Programme World Health Organization 20, Avenue Appia CH-1211, Geneva 27. Available from: <www.who.int/malaria>, e-mail: infogmp@who.int.
- WHO Global Malaria Programme, 2012. WHO Policy Recommendation: Seasonal Malaria Chemoprevention (SMC) for Plasmodium falciparum Malaria Control in Highly Seasonal Transmission Areas of the Sahel Sub-region in Africa.
- WHO, 2010. Focus on Senegal. Roll Back Malaria Progress and Impact Series, vol. 4. Geneva, pp. 1–56.
- Yusuf R.U., Omar S.A., Ngure R.M. The effect of point mutations in dihydrofolate reductase genes and the multidrug resistance gene 1–86 on treatment of falciparum malaria in Sudan. J. Infect. Dev. Ctries. 2010;4(2):61–69. doi: 10.3855/jidc.630. [DOI] [PubMed] [Google Scholar]
- Zakeri S., Farahani M.S., Afsharpad M., Salehi M., Raeisi A., Djadid N.D. High Prevalence of the 437G mutation associated with sulfadoxine resistance among Plasmodium falciparum clinical isolates from Iran, three years after the introduction of sulfadoxine–pyrimethamine. Int. J. Infect. Dis. 2010;14S:e123–e128. doi: 10.1016/j.ijid.2009.11.035. [DOI] [PubMed] [Google Scholar]


