Graphical abstract
Highlights
► All treatments used for Dientamoeba fragilis infections are discussed. ► Most of current treatment data is based only on small sized case reports. ► Absence of large scale treatment studies highlight the need for further study. ► Recent studies have indicated the newer treatment options to be highly effective.
Keywords: Dientamoeba fragilis, Therapy, Drug Clinical efficacy
Abstract
Dientamoeba fragilis belongs to the trichomonad group of protozoan parasites and it has been implicated as a cause of gastrointestinal disease with world-wide prevalences ranging from 0.5% to 16%. The majority of patients with dientamoebiasis present with gastrointestinal complaints. Chronic symptoms are common with up to a third of patients exhibiting persistent diarrhoea. Numerous studies have successfully demonstrated parasite clearance, coupled with complete resolution of clinical symptoms following treatment with various antiparasitic compounds. Treatments reported to be successful for dientamoebiasis include carbarsone, diphetarsone, tetracyclines, paromomycin, erythromycin, hydroxyquinolines and the 5-nitroimidazoles, including metronidazole, secnidazole, tinidazole and ornidazole. It is of note that most current treatment data is based only on small number of case reports. No large scale double blind randomised placebo controlled trials testing the efficacy of antimicrobial agents against D. fragilis has been undertaken highlighting the need for further study. In addition there is very little in vitro susceptibility data available for the organism making some current treatment options questionable. The aim of this review is to critically discuss all treatment options currently available for dientamoebiasis.
1. Introduction
Dientamoeba fragilis is a trichomonad parasite which has been implicated as a cause of gastrointestinal disease. Despite the frequency of this organism being encountered it continues to be neglected as a significant pathogen with many laboratories not routinely performing adequate laboratory diagnostic testing for the parasite (Windsor and Johnson, 1999; Johnson et al., 2004; Stark et al., 2006, 2010; Barratt et al., 2011b). The prevalence of D. fragilis varies widely with recent studies finding D. fragilis to be the most common pathogenic protozoan found in stool when appropriate diagnostic methods are utilised (Crotti and D’Annibale, 2007; Rayan et al., 2007).
The clinical presentation of dientamoebiasis varies from asymptomatic carriage to symptoms ranging from altered bowel motions, abdominal discomfort, nausea and diarrhea with associated eosinophilia reported in up to 50% of paediatric and 10% of adult patients (Preiss et al., 1991; Cuffari et al., 1998; Stark et al., 2010). Studies have shown that dientamoebiasis may cause irritable bowel syndrome (IBS) – like symptoms (Stark et al., 2007b), and chronic symptoms ranging from weeks to months have been reported for general populations in the scientific literature.
The life cycle and mode of transmission of D. fragilis are poorly defined. Some researchers have suggested the mode of transmission occurs via a helminth vector, while others suggest direct transmission from infected patients the most likely route of transmission (Ockert and Schmidt, 1976; Stark et al., 2005, 2006; Girginkardesler et al., 2008). Currently transmission of Dientamoeba remains a mystery and further study is required to describe its complete life cycle (Barratt et al., 2011a).
Despite the abundance of reports in the scientific literature regarding infections with this parasite and the fact that it was discovered nearly 100 years ago, very little research has been conducted on the use of suitable antimicrobial compounds. The balance of scientific evidence currently supports the pathogenic potential of D. fragilis and various antimicrobial compounds have been shown to be effective for treating dientamoebiasis with both clearance of parasite and resolution of symptoms achievable. Compounds reported to be effective in treating dientamoebiasis include clioquinol (Bosman et al., 2004), doxycycline (Preiss et al., 1991), iodoquinol (Spencer et al., 1979b; Preiss et al., 1991; Cuffari et al., 1998; Stark et al., 2010), metronidazole (Preiss et al., 1991; Cuffari et al., 1998; Vandenberg et al., 2006; Kurt et al., 2008; Stark et al., 2010), ornidazole (Kurt et al., 2008), oxytetracycline (Preiss et al., 1991), paromomycin (Simon et al., 1967; Vandenberg et al., 2006, 2007; Stark et al., 2010) and secnidazole (Girginkardesler et al., 2003). However, it must be noted that most of these reports are based upon case studies and no large-scale randomised double blinded control trials have been undertaken on D. fragilis treatment regimens to date (Stark et al., 2010).
It is of note that no comprehensive review on the treatment options for D. fragilis infection has been published to date, although progress has been made in defining the clinical disease (Stark et al., 2010; Barratt et al., 2011b). The aim of this review article is to provide an overview of all antimicrobial compounds described in the scientific literature for the treatment of dientamoebiasis in order to aid healthcare professionals with the selection of current treatment options available.
2. Clinical aspect
Not long after D. fragilis was described as a non-pathogenic amoeba in 1918, researchers began to question the assumptions made by Jepps and Dobell regarding the pathogenic nature of the organism. A study in the Philippines less than a year later in 1919 found three cases of D. fragilis in 100 symptomatic children (Haughwout and Horrilleno, 1920). The following year Jepps described ten cases of D. fragilis from 971 symptomatic soldiers at a war hospital (Dobell, 1940). These reports led to an increased interest in the parasite and five years later D. fragilis was reported and implicated as a potential pathogen throughout the world (Taliaferro and Becker, 1924).
Wenrich et al. (1936) reported an incidence of 4.3% of D. fragilis from 1060 university students in the USA. They found that there was a higher rate of gastrointestinal symptoms in the students infected with D. fragilis than those infected with Entamoeba histolytica, with diarrhoea and abdominal pain present in the majority of cases. However, it was not known at this time that E. histolytica consisted of two species, E. histolytica and Entamoeba dispar, the latter of which is considered to be non-pathogenic and much more common than the former.
The same year Hakansson (1936) described a case of D. fragilis infection in a 48-year-old physician (himself) who complained of gastrointestinal symptoms including pain in the upper abdomen, mucoid stool, loss of appetite and irritation of the rectum. After 2 weeks of recurrent symptoms he was treated with carbarsone, which led to complete resolution of symptoms and negative post-therapy stool samples. A year following these findings, Hakansson (1937) undertook a follow-up study where 12 patients with D. fragilis infections were treated with carbarsone, which resulted in complete resolution of symptoms with clearance of parasites.
In support of early findings, numerous studies over the following 75 years have subsequently shown the pathogenic potential of D. fragilis and demonstrated that it is a commonly encountered enteropathogen associated with signs of clinical disease such as diarrhea and other gastrointestinal complaints (Windsor and Macfarlane, 2005; Vandenberg et al., 2006; Kurt et al., 2008; Stark et al., 2010). Crotti and D’Annibale (2007) analysed stool specimens from 1989 subjects and while Giardia intestinalis was present in 1.8% of subjects, D. fragilis was detected in 4.1%. It was also demonstrated that D. fragilis was more commonly associated with clinical symptoms than G. intestinalis. More recently, Stark et al. (2010) examined 750 symptomatic and asymptomatic patients, detecting D. fragilis at a prevalence of 5.2%, more common than G. intestinalis. Similarly, most of the infected patients exhibited clinical symptoms largely consisting of diarrhea (30/36); loose stools (26/36); and abdominal pain/discomfort (28/36). Shedding of the parasite was found to be highly variable. Complete resolution of symptoms was observed in the majority of patients following treatments including iodoquinol, paromomycin, metronidazole or combination therapy (Stark et al., 2010). Chronic infections are reported, with one study indicating 32% of patients infected with D. fragilis present with symptoms greater than 2 weeks in duration (Stark et al., 2005). A recent study has also shown high rates of D. fragilis infection amongst close household contacts of patients with dientamoebiasis. A total of 30% of close human contacts tested for D. fragilis harbored the parasite, and the majority of these contacts (n = 80%) were symptomatic (Stark et al., 2012).
Unfortunately, study into the pathological manifestations of D. fragilis infections is hampered by the lack of a suitable animal model, despite previous attempts using macaques, cats, chickens and rats, none of which have been reproducible (Dobell, 1940; Knoll and Howell, 1946; Barratt et al., 2011a). However no recent studies in the last 75 years have attempted to establish animal models for the further study of D. fragilis. In addition, while a large proportion of infected individuals present with gastrointestinal illnesses, clinical presentations of D. fragilis frequently show variability and asymptomatic carriage can occur. Intermittent shedding of the organism is common among patients therefore care must be taken and correct diagnostic procedures used for definitive diagnosis. Numerous studies have also reported that treatments which eliminate the organism lead to clinical improvement (cited by Windsor and Johnson, 1999; cited by Johnson et al., 2004; Stark et al., 2010) and as such, D. fragilis needs to be included as a part of routine laboratory diagnostics for the differential detection of enteric protozoa.
3. Treatment options
3.1. Historic treatment regimes
A number of early case reports demonstrated that the anti-amoebic compounds, including emetine-bismuth-iodide and the arsenic compound carbarsone to be effective for the treatment D. fragilis infections with clinical improvements in the majority of treated cases (Gittings and Waltz, 1927; Hakansson, 1936, 1937; Knoll and Howell, 1946). One of the earliest studies undertaken in the late 1920s (Wenyon, 1926a,b) reported the elimination of D. fragilis following administration of emetine, resulting in the resolution of clinical symptoms and this was supported by subsequent studies of the administration of emetine or carbarsone (Gittings and Waltz, 1927; Hakansson, 1936, 1937; Mollari and Anzulovic, 1938).
First isolated by Pelletier and Magendie in 1822, emetine is an oral agent and is an alkaloid originally derived from ipecac (dried rhizome and roots of ipecacuanha plant); it inhibits protein synthesis by restricting movement of ribosomes along mRNA, however it has significant toxicity with a number of side effects, including cardiac arrhythmia, gastrointestinal toxicity and neutromuscular reactions (Khaw and Panosian, 1995).
Carbarsone oxide (p-carbamidophenyl arsenous oxide) is an arsenic-based antiprotozoal compound, particularly known for its use as an anti-amoebic treatment (Epstein, 1936). As with other arsenic compounds however, accumulation can lead to arsenic poisoning which ultimately leads to a variety of adverse health effects and in extreme circumstances, death (Rahman et al., 2009).
Keystone et al. (1983) reported on one of the earliest toxicological study of the arsenical compound, diphetarsone. Diphetarsone (1,2,di-(4arsonophenylamino ethane decahvdrate)) is a polar pentavalent arsenical compound. The first report of efficacy was against cysts of E. histolytica (Schneider and Dupoux, 1953). The exact mechanism of action for this drug is unknown; however it is thought that it acts directly by conversion to an active arsenoxide, leading to inhibition of sulphydryl enzymes (Schneider, 1957). A total of nine patients with known D. fragilis infections were treated with 500 mg of diphetarsone, thrice daily for 10 days and parasite clearance was demonstrated in all patients (Keystone et al., 1983). It was widely used as a first-line treatment for intestinal amoebiasis in France for over 25 years; however its use was reviewed due to the concerns over encephalopathy, polyneuritis, visual disturbances and severe dermatitis, all of which have been associated with the use of arsenicals (Keystone et al., 1983). Due to the side effects associated with these early treatments and the discovery of newer, less toxic alternative compounds these antimicrobials are no longer routinely used in clinical practise.
Erythromycin is a macrolide antibiotic which prevents protein biosynthesis by binding to the 50S ribosomal subunit and thus interferes with the elongation process of polypeptide chains (Weisblum, 1995). There has only been one study to date which investigated the use of erythromycin for treatment of dientamoebiasis. A total of six paediatric patients were treated with 50 mg/kg/day of erythromycin for 10 days; 50% of the patients reported resolution of clinical symptoms and parasite clearance (Preiss et al., 1991).
The use of erythromycin is associated with a number of side effects including abdominal pain, diarrhoea, nausea, vomiting, dizziness, stomach irritation and skin rash. In addition, jaundice, heart arrhythmias, Stevens-Johnson syndrome and tinnitus have been reported in rare cases as severe side effects. The use of erythromycin is contraindicated in pregnant women.
As the report by Preiss et al. (1991) was based only on single case report, erythromycin cannot be recommended as a first-line treatment. Further study is necessary to determine the efficacy in treating D. fragilis infections.
4. Current treatment regimes
4.1. Tetracyclines
Tetracyclines are a group of antimicrobial compounds that are active protein synthesis inhibitors (Agwuh and MacGowan, 2006). This is achieved by preventing the attachment of aminoacyl-tRNA binding to the ribosomal acceptor (A) site (Chopra and Roberts, 2001).
The first report of tetracycline in treating D. fragilis infections was by Spencer et al. (1979b). Despite the deleterious effects in children, one paediatric patient with gastrointestinal complaints was given a course of tetracycline (250 mg twice a day (bid) for 5 days); however its outcome is unclear and authors do not state whether tetracycline was an effective treatment (Spencer et al., 1979b).
Following these findings, Dardick (1983) treated a 35 years old male suffering from watery stools for several months with a course of tetracycline (500 mg PO four times a day (qid) for 10 days) after two courses of metronidazole (no dosage given, for 10 days) had failed to clear D. fragilis infection. Parasite clearance and clinical improvements were observed immediately upon initiating tetracycline treatment and as such it was concluded that tetracycline was a safer alternative than using metronidazole, iodoquinol or cabarsone with which there are a number of known associated side effects.
Additionally, a recent case study consisting of three symptomatic adults suffering from Dientamoeba-associated diarrhoea lasting between 5 days to over 1 month were treated with a course of tetracycline (no dosage given) with complete elimination of D. fragilis and clinical improvements observed in all patients (Stark et al., 2007a).
Tetracycline is an agent that is currently recommended as a treatment option by the centres for Disease Control (CDC; see Table 1). Such recommendations however, are based only on three case reports each comprising of small patient populations therefore one must question the scientific validity of the therapeutic efficacy of these agents.
Table 1.
List of treatment options for dientamoebiasis recommended by the centre for Disease Control as of 2012.a
| Drug of choice | Alternative drugs |
|---|---|
| Iodoquinol | Paromomycin |
| Adults: 650 mg PO tid × 20 days | Adults: 25–35 mg/kg/day PO in 3 doses × 7 days |
| Paediatric: 30–40 mg/kg/day (max. 2 g) PO in 3 doses × 20 days | Paediatric: 25–35 mg/kg/day PO in 3 doses × 7 days |
| Tetracycline | |
| Adults: 500 mg PO qid × 10 days | |
| Paediatric: 40 mg/kg/day (max. 2 g) PO in 4 doses × 10 days | |
| Metronidazole | |
| Adults: 500–750 mg PO tid × 10 days | |
| Paediatric: 35–50 mg/kg/day PO in 3 doses × 10 days |
The information provided by CDC Health Information for International Travel 2012: The Yellow Book.
Additionally, two compounds closely related to tetracycline, oxytetracycline which has a better absorption profile, and doxycycline with a longer elimination half-life of 16 h (Agwuh and MacGowan, 2006), have been reported to be effective for dientamoebiasis. Butler, 1996 treated a single patient with 100 mg of doxycycline twice daily for 10 days. Within 36 h nausea and diarrhoea resolved. Notably, Preiss et al. (1990) demonstrated the use of oxytetracycline (n = 8) and doxycycline (n = 4) in paediatric patients with known D. fragilis infections. Patients were given either drug at different dosages for 10 days (see Table 2). The patients treated with oxytetracycline had clinical improvement and clearance of the parasite in 90% (8/9) of patients while 75% (3/4) of patients treated with doxycline reported clinical improvement and clearance of the parasite (Preiss et al., 1990). Once again the sample size is small and it is difficult to interpret the clinical efficacy.
Table 2.
Previous studies using recommended treatments to date for D. fragilis infections, study size, reported treatment efficacy and dosage used are summarised.
| Recommended treatment | Study size (n) | Treatment efficacy | Dosage used | References |
|---|---|---|---|---|
| Diphetarsone | 9 | 100% | 500 mg tida × 10 days | (Keystone et al., 1983) |
| Clioquinol | 27 | 81.5% | 40 mg/kg/day × 10–21 days | (Bosman et al., 2004) |
| 12 | 83% | 250 mg tid × 7 days | (van Hellemond et al., 2012) | |
| Iodoquinol | 3 | 100% | 650 mg POb daily × 7–10 days | (Stark et al., 2010) |
| 12 | 83.3% | 650 mg PO tid × 20 days | (Millet et al., 1983a,b) | |
| 5 | 80% | 40 mg/kg/day × 20 days | (Cuffari et al., 1998) | |
| 5 | 20% | 20 mg/kg/day × 10 days | (Preiss et al., 1991) | |
| 12 | N/A | 30–40 mg/kg/day × 21 days | (Spencer et al., 1979a,b) | |
| Paromomycin | 5 | 100% | 8–12 mg/kg PO daily × 7–10 days | (Stark et al., 2010) |
| 15 | 80% parasite clearance/87% clinical improvement | 25–35 mg/kg daily × 7 days | (Vandenberg et al., 2007) | |
| 4 | 100% | 25–35 mg/kg/day PO tid × 7 days | (Vandenberg et al., 2006) | |
| 21 | 100% | 25–35 mg/kg, daily × 4–5 days | (Simon et al., 1967) | |
| 61 | 98% | 500 mg tid × 7 days | (van Hellemond et al., 2012) | |
| Tetracycline | 1 | 100% | 500 mg qidc × 10 days | (Dardick, 1983) |
| 1 | N/A | 250 mg PO bidd × 5 days | (Spencer et al., 1979a,b) | |
| Oxytetracycline | 9 | 90% | 30–40 mg/kg/day PO qid × 7 to 30 days | (Preiss et al., 1991) |
| Doxycycline | 4 | 75% | 2 mg/kg/day PO × 10 days | (Preiss et al., 1991) |
| 1 | 100% | 100 mg PO bid × 10 days | (Butler, 1996) | |
| Erythromycin | 6 | 50% | 50 mg/kg/day PO × 10 days | (Preiss et al., 1991) |
| Metronidazole | 35 | 80% | 400–750 mg PO every 8 h or daily × 3–10 days | (Stark et al., 2010) |
| 56 | 69.6% parasite eradication/76.8% clinical improvement | 20 mg/kg for children; 1.5 g for adults, daily | (Kurt et al., 2008) | |
| 6 | 83.3% | N/A | (Cuffari et al., 1998) | |
| 15 | 66.7% | 500–750 mg PO tid × 10 days | (Vandenberg et al., 2006) | |
| 91 | 70% | 30 mg/kg/day PO × 10 days | (Preiss et al., 1991) | |
| 5 | N/A | 250 mg PO tid × 7 days | (Spencer et al., 1979a,b) | |
| 32 | 12.5% parasite clearance/37.5% reduced or recurring symptoms | N/A | (Norberg et al., 2003) | |
| 3 | 66.7% | 500–750 mg PO tid × 10 days | (Oxner et al., 1987) | |
| Metronidazole/Tinidazole | 16 | 68.8% | N/A | (Bosman et al., 2004) |
| Secnidazole | 35 | 97% parasite eradication/100% clinical improvement (27-disappeared; 8-decreased) | 30 mg/kg for children; 2 g for adults, single dose (second treatment required in one case) | (Girginkardesler et al., 2003) |
| Ornidazole | 56 | 92.9% parasite eradication/96.4% clinical improvement | 30 mg/kg for children; 2 g for adults, single dose | (Kurt et al., 2008) |
Bosman et al. (2004), Butler (1996), Cuffari et al. (1998), Dardick (1983), Girginkardesler et al. (2003), Keystone et al. (1983), Kurt et al. (2008), Millet et al. (1983a,b), Norberg et al. (2003), Oxner et al. (1987), Preiss et al. (1991), Simon et al. (1967), Spencer et al. (1979a, b), Stark et al. (2010), van Hellemond et al. (2012), Vandenberg et al. (2006, 2007).
“tid” = “ter in die”; “three times a day”.
PO = Perorally.
“qid” = “quater in die”; “four times a day”.
“bid” = “bis in die”; “twice a day”.
The use of tetracyclines is however, associated with a number of potential side effects including photosensitivity, skin reactions, phototoxicity and gastrointestinal upsets. Deleterious effect on dental development has also been described and use of tetracycline is not recommended for children under the age of eight and for women during pregnancy (Dardick, 1983; Turner, 1985).
Based on the small number of case report studies it is not possible to recommend the use of tetracyclines for the treatment of D. fragilis. Tetracycline is still recommended by the CDC (see Table 3) but it needs to be reconsidered as first-line treatment option.
Table 3.
Chemical structures of treatment options for D. fragilis infections.
| Treatment options | Chemical formula | Molecular structure | Side effects/contraindications | Recommended dosagea |
|---|---|---|---|---|
| Carbarsone | C7H9AsN2O4 | Long-term exposure to As has been associated with bladder and kidney cancer; contraindicated for patients with severe hepatic disease | 75 mg/kg × 10 days | |
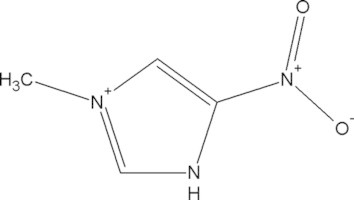
| Diphetarsone | C14H18As2N2O6 | 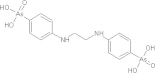 |
Transient hepatic abnormalities, contraindicated for patients with severe hepatic disease | 500 mg POb tidc × 10 days |
| Tetracycline | C22H24N2O8 | 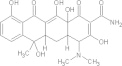 |
Detrimental effects on dental development/contraindicated for children under the age of 5 yrs old, renal impaired patients and during pregnancy | 500 mg PO qidd × 10 days |
| 40 mg/kg/day (max. 2 g) PO qid × 10 days | ||||
| Oxytetracycline | C22H24N2O9 | 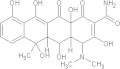 |
Discolouration of teeth; affects foetal skeletal development/contraindicated for children under 8 yrs old, renal impaired and during pregnancy | General: 250–500 mg PO qid × 10 days |
| For severe infections: 250–500 mg PO qid × 7–30 days | ||||
| Doxycycline | C22H24N2O8 | 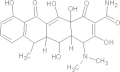 |
Gastrointestinal disturbances; esophageal ulceration; photosensitising agent/contraindicated for children under the age of 8 yrs old, renal impaired patients and during pregnancy | 100–200 mg PO qde × 7–14 days |
| 1–2 mg/kg/day PO tid | ||||
| Iodoquinol (hydroxyquinoline) | C5H5I2NO | 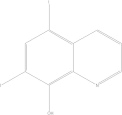 |
Nausea; vomiting; abdominal cramps; diarrhoea; skin irritation; fever; chills; headache; dizziness | 650 mg PO tid × 7–10 days |
| 30–40 mg/kg/day PO in three doses × 7–10 days | ||||
| Clioquinol | C9H5CIINO | 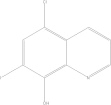 |
Subacute Myelo-Optico-Neuropathy (SMON) | 250 mg PO tid × 7 days |
| 40 mg/kg/day PO × 10 days | ||||
| Erythromycin | C37H67NO13 | 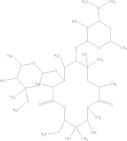 |
GI disturbances; arrhythmia; neurological reactions; contraindicated for women during pregnancy | 500 mg PO bidf × 10–14 days |
| 30–100 mg/kg/day × 10–14 days | ||||
| Paromomycin | C23H47N5O18S. H2SO4 | 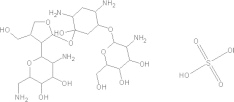 |
Diarrhoea; nausea; stomach cramps/seek medical attention for severe allergic reactions | 25–35 mg/kg/day PO tid × 7 daysg |
| Metronidazole | C6H9N3O3 | 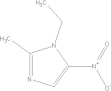 |
Loss of appetite; metallic taste; headache; insomnia; vertigo; anorexia; vomiting/seek medical attention if experiencing severe adverse effects | 500–750 mg PO tid × 10 days |
| 35–50 mg/kg/day PO tid × 10 days | ||||
| Secnidazole | C7H11N3O3 | 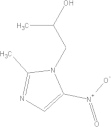 |
GI disturbances; nausea | 2 g qd PO |
| 30 mg/kg qd PO | ||||
| Ornidazole | C7H10CIN33 | 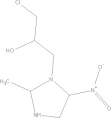 |
GI disturbances; nausea; headache; dizziness | 2 g qd PO |
| 30 mg/kg qd PO | ||||
| Tinidazole | C8H13N3O4S | 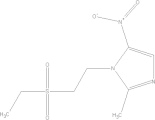 |
GI disturbances; bitter taste; diarrhea; itchiness | 2 gg qd PO × 3 days |
| 50 mg/kg qd × 3 days |
Adult and paediatric dosages shown where applicable, paediatric dosage is shown below adult dosage.
PO = Perorally.
“tid” = “ter in die”; “three times a day”.
“qid” = “quater in die”; “four times a day”.
“qd” = “quaque die”; “once a day”.
“bid” = “bis in die”; “twice a day”.
Universal dosage for adults and children.
4.2. Iodoquinol
Introduced in the early 1960s, iodoquinol, a poorly absorbed, halogenated hydroxyquinoline formerly known as diiodohydroxyquin is a chelating agent for ferrous ions that are essential for amoebic metabolism (Knight, 1980). It acts as a luminal amoebicide but the exact mechanism of action is not known.
One of the earliest studies which reported iodoquinol treatment of dientamoebiasis was of 32 paediatric patients (Spencer et al., 1979b), who presented with symptomatic D. fragilis infections in the absence of other gastrointestinal pathogens. Twelve were treated with iodoquinol (30 mg/kg/day) for 21 days (see Table 2) while the others were given metronidazole. Although it was concluded that treatment with either metronidazole or iodoquinol led to clinical improvement, respective therapeutic efficacy was not confirmed (Spencer et al., 1979b).
Millet et al. (1983a,b) reported on twelve symptomatic patients treated with 650 mg of iodoquinol thrice daily for 20 days; with parasite clearance observed in ten. In comparison to these findings, Preiss et al. (1991) used iodoquinol for five children with D. fragilis infection, but found it to be effective in only one patient. Such findings however, may be attributed to the lower dosage of 20 mg/kg/day of iodoquinol for 10 days, as compared with the dosage of 40 mg/kg/day for 20–21 days previously described by Spencer et al. (1979b).
Five patients were treated with 40 mg/kg/day for 20 days in another study by Cuffari and colleagues, with 4/5 patients (80%) exhibiting clinical improvement (Cuffari et al., 1998).
In another small case series, three symptomatic patients were treated with iodoquinol (650 mg perorally (PO), daily for 10–12 days) with clinical and parasitological cure in all patients (Stark et al., 2010). A recent case study reported clinical and parasitological cure in two paediatric patients treated with iodoquinol (no dosage given), after the initial treatment with metronidazole had failed (Banik et al., 2011).
For treating dientamoebiasis, iodoquinol is usually given orally at a dosage of 650 mg PO thrice daily for 20 days in adults and 40 mg/kg/day PO in three doses (max. 2 g) for 20 days in children. There have been a number of studies reporting the side effects associated with the use of hydroxyquinolines. The iodine component of iodoquinol in particular, is associated with toxicity and there have been cases of neuropathy and blindness following prolonged administration (Khaw and Panosian, 1995). Ingestion of a large amount of the drug over a short period of time or a long-term treatment can lead to toxic encephalopathy in the form of drowsiness, mental confusion, disorientation, hallucinations and headache with subsequent amnesia (Baumgartner et al., 1979).
Given the conflicting results described in the literature following iodoquinol treatment and the fact that once again the studies have been small case series with no control groups, caution must be used when using this agent for the treatment of Dientamoeba, despite it being a CDC recommended drug for treatment (see Table 1).
4.3. Clioquinol
Clioquinol (Iodochlorhydroxyquin), a structurally related compound of iodoquinol is a member of halogenated 8-hydroxyquinolines which has been shown to possess antiprotozoal activity (Mao and Schimmer, 2008). It was used in the 1950s to 1970s as an oral anti-parasitic treatment for intestinal amoebiasis; however it was withdrawn from the market due to the 10,000 estimated cases of neurotoxicity in Japan, a condition known as subacute myelo-optico neuropathy or SMON (Tsubaki et al., 1971). It has been indicated though, that the post-war diet in Japan may have lacked in vitamin B12 intake and may have been a significant contributing factor in the SMON incidence (Tabira, 2001).
It acts as a luminal amoebicide and is bacteriostatic however the exact mechanism of action is unknown. It has been demonstrated though, that clioquinol is a potent inhibitor of the proteasome (Daniel et al., 2005; Ding et al., 2005; Chen et al., 2007; Mao et al., 2009) and has the ability to act as a zinc and copper chelator (Cuajungco et al., 2000).
In addition to iodoquinol, clioquinol was reported to be parasitologically and clinically effective for D. fragilis infections in 27 out of 33 (n = 82%) paediatric patients, when used at a dosage of 40 mg/kg/day for 10–21 days (Bosman et al., 2004).
4.4. Paromomycin
Paromomycin is an aminoglycoside antibiotic with a broad-spectrum activity, first isolated from Streptomyces krestomuceticus in the 1950s. Currently it is recommended for use in amoebiasis as a luminal agent, giardiasis and for treating Cyrptosporidium and microsporidia (Gupta et al., 2004; Davidson et al., 2009).
The earliest study of paromomycin for dientamoebiasis was reported by Simon et al. (1967). All 21 cases of D. fragilis infection were cured by administering paromomycin at 25–35 mg/day for 4–5 days.
A recent study by Vandenberg et al. (2006) reported the treatment of four symptomatic paediatric patients with paromomycin (no dosage given) and it was shown to be parasitologically and clinically effective in all cases after a follow-up triple faeces test 1 month later. A year later, Vandenberg et al. (2007) evaluated the use of paromomycin in 15 paediatric patients (25–35 mg/kg/day for 7 days) with known D. fragilis infections. Parasitic elimination and clinical improvements were observed in all patients after 1 month follow-up and in addition, no major side-effects were reported. Such findings led authors to recommend paromomycin as a first-line treatment option for D. fragilis infections.
Another study by Stark et al. (2010) reported on treatment of five symptomatic patients with paromomycin (8–12 mg/kg PO, daily for 7–10 days). All patients cleared the infection, and reported clinical improvement with resolution of symptoms.
A larger cohort of 93 symptomatic adult patients were included in the retrospective study from the Netherlands (van Hellemond et al., 2012) where these patients were treated with paromomycin (n = 61; three daily doses of 500 mg for 7 days), along with other drugs including clioquinol (n = 12; three daily doses of 250 mg for 7 days), metronidazole (n = 7; three daily doses of 500 mg for 7–10 days) and doxycycline in combination with drugs mentioned (no dosage given; with paromomycin n = 27; clioquinol n = 2; metronidazole n = 1). Paromomycin was found to be the most effective treatment and higher eradication rates of 98% was reported, in comparison to 83% and 57% for clioquinol and metronidazole, respectively.
Paromomycin is administered orally at a dosage of 25–35 mg/kg/day, usually in three divided doses for a total of 5–10 days. Paromomycin is poorly absorbed so is unsuitable for use in systemic infections. Similar to other aminoglycosides, side effects associated with the use of paromomycin include vestibular, cochlear and renal toxicity. All of these are considered very rare due to the poor absorption of the drug (Davidson et al., 2009).
While the in vitro susceptibility testing for paromomycin has previously found it to be ineffective for D. fragilis with Minimal Lethal Concentration (MLC) of 500 μg/mL (Nagata et al., 2012), the majority of clinical data currently supports the fact that paromomycin may be an effective treatment option (Simon et al., 1967; Vandenberg et al., 2006, 2007; Stark et al., 2010; van Hellemond et al., 2012). Most case reports have demonstrated successful treatment of dientamoebiasis with paromomycin, coupled with low incidences of adverse events. Despite this, the number of studies using paromomycin for treatment of dientamoebiasis is surprisingly small and further studies are required. However it is still recommended as a treatment option by the CDC (see Table 1).
5. 5-Nitroimidazoles
5.1. Metronidazole
Developed in 1962, metronidazole is an oral synthetic antiprotozoal and antibacterial compound, originally indicated for management of trichomoniasis (Löfmark et al., 2010; Chaudhari and Singh, 2011). It is a prodrug, which forms active metabolites upon reduction by flavin enzymes within the cytoplasm of trophozoites.
Reduction of the parent compound leads to either a single electron transfer reduction product, a nitroimidazole nitroradical anion or further reduced reactive intermediates, i.e. nitrosoimidazole or hydroxyamineimidazole (Moreno and Docampo, 1985). Such reduction takes place in microaerophilic, anaerobic and even in aerobic conditions (Perez-Reyes et al., 1980; Viode et al., 1999). However the presence of oxygen in aerobic condition leads to reoxidation of reactive intermediates back into the parent compound, a redox cycling effect termed “futile cycle” (Lloyd and Pedersen, 1985). It is both a luminal and tissue amoebicide as it is active in both the intestinal lumen/wall, and at extra-intestinal sites following oral administration.
There have been mixed reports in the literature regarding the efficacy of metronidazole for the treatment of D. fragilis infection. While it has been shown to be effective in some studies, others report treatment failures and relapses.
In a retrospective study of 35 paediatric patients, D. fragilis was found to be the only parasite in the gastrointestinal tract in 32 individuals with clinical symptoms. Peripheral blood eosinophilia was present in half of children examined (Spencer et al., 1979b). Eighteen patients were treated with either iodoquinol (n = 12), tetracycline (n = 1) or metronidazole (n = 5). Twelve patients completed the course of therapy and returned for follow-up evaluation. Re-infection was reported in two cases (Spencer et al., 1979b). The authors conclude that all treatments led to symptomatic relief and parasitic clearance, however therapeutic efficacy for each drug was not provided and it is not known which drug was associated with failure to complete the treatment course.
Another study described treatment of three symptomatic patients with a course of metronidazole (dosage not given). One patient subsequently required a further course of combination treatment consisting of metronidazole and oxytetracycline for successful elimination of the organism (Oxner et al., 1987).
Preiss et al. (1991) was the first to examine metronidazole in a larger sample size. The authors used metronidazole, 30 mg/kg/day for 10 days in children and found it to be effective in 70% out of the 91 cases treated. As 30% of the cases required up to three follow-up treatments for complete resolution of parasites and symptoms, it was suggested by the authors that metronidazole should be given 10 days at the recommended dosage, followed by oxytetracycline, doxycycline or erythromycin.
Another study described the treatment of 32 D. fragilis infections with different doses and duration of therapy with metronidazole (Norberg et al., 2003). Clinical improvements were observed in 16 cases. However no specific data was given in regards to the dosages used and the duration of therapy.
Vandenberg et al. (2006) treated 15 patients with a ten day course of metronidazole. Of these, 12 patients returned for a follow-up triple faeces test a month after treatment and eight patients had parasitological and clinical cure. Once again no dosage information was given.
Successful treatment of four symptomatic adults with a course of metronidazole (no dosage given), combined with tetracycline in three patients was reported, with follow up stool samples negative in all cases (Stark et al., 2007a). Following this preliminary study 35 patients were treated with metronidazole treatment at different dosages and duration (Stark et al., 2010). While the treatment was found to be effective in 80% (n = 28) of cases, a high rate of treatment failures/relapses (6/28; 21.4%) were associated with the use of metronidazole. The majority of treatment failures were associated with a three day course of metronidazole and were less likely with a longer duration of therapy.
Recently 41 paediatric patients diagnosed with D. fragilis infection were treated with metronidazole (no dosage given). Complete resolution of symptoms and parasite clearance was observed in 85% (Banik et al., 2011). Treatment failures occurred in 15% (n = 6) who required an additional course of metronidazole (4/6) or iodoquinol (2/6). All became negative on follow-up stool samples (Banik et al., 2011).
Metronidazole is typically administered at 500–750 mg three times daily for 10 days for adults and 35–50 mg/kg/day three times a day for 10 days in paediatric patients. The safety profile of metronidazole is well known and the majority of side effects are considered to be mild to moderate in severity. A variety of adverse events however, have been described including loss of appetite, metallic taste, headache, insomnia, vertigo, anorexia and vomiting. Rarely convulsive seizures and peripheral neuropathy have been reported following prolonged treatments (Gupta et al., 2004; Löfmark et al., 2010). Notably, metronidazole inhibits the metabolism of alcohol in some patients, often leading to intolerance (Löfmark et al., 2010).
Despite the inconsistency in clinical efficacy of metronidazole with clearance of D. fragilis ranging from 66.75% to 100%, overall metronidazole has been shown to be reasonably effective in treating dientamoebiasis (Preiss et al., 1991; Kurt et al., 2008; Stark et al., 2010; Banik et al., 2011). However treatment failures or relapses may require prolonged therapy with the potential for significant side effects.
6. Possible novel treatment regimes
6.1. Secnidazole
Recently newer 5-nitroimidazole derivatives with a single oral dose schedule such as secnidazole have been used for the treatment of D. fragilis. It has a longer elimination half-life of approximately 17–29 h (compared with six to seven hours for metronidazole (Gupta et al., 2004)). Secnidazole has been used for the treatment of giardiasis, trichomoniasis and all symptomatic forms of amoebiasis with recorded parasitological cure rates of 80–100%, similar to the response rates achieved through multiple doses of metronidazole or tinidazole (Gillis and Wiseman, 1996).
Girginkardesler et al. (2003) screened 400 stool samples for the presence of pathogenic protozoans, with D. fragilis detected in 35. All patients were treated with a single oral dose of secnidazole and D. fragilis was eradicated in 34 patients. A second dose was required in one patient, who was given an identical dose and a follow-up evaluation showed parasitic clearance seven days after the second treatment. These findings, coupled with the mild nausea reported in two patients the only side effect led the authors to recommend secnidazole as an effective therapeutic option.
6.2. Ornidazole
Ornidazole is similar in efficacy to metronidazole; however it possesses a longer half-life and is given as a single oral dose. The side effect profile of the drug is more favourable than metronidazole, with lesser side effects including nausea and bitter taste in mouth (Gupta et al., 2004).
Kurt et al. (2008) undertook one of the few randomised and double-blinded studies, comparing the efficacy of metronidazole and ornidazole in 112 patients with D. fragilis infection, who were randomised into two treatment groups: group 1 (n = 56), who received metronidazole, 20 mg/kg/day for children; 1.5 g/day for adults for 5 days; and group 2 (n = 56), who received a single oral dose of ornidazole (30 mg/kg for children; 2 g for adults). Stool examinations were undertaken 7 and 14 days following treatment. Ornidazole resulted in clinical cure in 54 patients (96.4%) with parasite eradication in 52/56 (92.9%). In comparison the clinical cure rate for metronidazole was only 76.8% with parasite eradication in 69.6% (Kurt et al., 2008). Only minor side-effects were recorded for six patients in the ornidazole group. These consisted of nausea, headache and dizziness. In contrast 18 patients complained of numerous side-effects when treated with metronidazole, including nausea, metallic taste, vomiting, anorexia, dizziness, insomnia, vertigo and dry mouth (Kurt et al., 2008). These results led the authors to recommend ornidazole as a novel agent for the treatment of dientamoebiasis.
6.3. Tinidazole
A structural analogue of metronidazole, tinidazole has been used in Europe, Australia and in a number of developing countries for decades, and it was recently approved by the Food and Drug Administration for the treatment of trichomoniasis, giardiasis, amoebiasis and amoebic liver abscess (Fung and Doan, 2005). In addition to a longer half-life (12 h) it is reported to be better tolerated than metronidazole and the cure rates for protozoan infections are higher (Gupta et al., 2004).
A retrospective study of 23 paediatric patients with symptomatic D. fragilis infections, who were treated with metronidazole (50 mg/kg/day bid for 7 days) or tinidazole (single dose of 50 mg/kg, maximum of 2 g) was undertaken. Therapeutic efficacy were compared with those of a control group (n = 41), consisting of untreated patients (cited in Bosman et al., 2004). While clinical resolution or improvements were observed in 60.9% of treated group, individual treatment efficacy was not described. Therefore it is difficult to determine the impact of tinidazole in this study.
Recommended dosages of secnidazole and ornidazole are 2 g as a single dose for adults and 30 mg/kg a day for children, while tinidazole is given in dosages of 2 g a day for adults and 50 mg/kg/day (maximum of 2 g) for children, for a total of 3 days (Gupta et al., 2004). The pharmacokinetic profiles of these nitroimidazole derivatives are similar to those of metronidazole, but they have longer elimination half-lives and in most cases demonstrate clinical improvements with only one to three doses of the drug (Lau et al., 1992).
The newer 5-nitroimidazole derivatives have been shown to be more effective treatment options available for D. fragilis. Additionally, uses of such treatments are associated with far fewer side effects when compared with metronidazole and may be considered as drugs of choice for the treatment of D. fragilis. As such, they should be considered the first-line treatment option for cases of symptomatic infections if no other possible pathogens are present.
6.4. Nitazoxanide
Nitazoxanide (2-acetolyloxy-N-(5-nitro-2-thiazolyl benzamide) was first introduced in 1984 as a human cestocidal drug (Rossignol and Maisonneuve, 1984). It is the parent compound of a class of drugs collectively named thiazolides (Gilles and Hoffman, 2002; White, 2004; Fox and Saravolatz, 2005). In contrast to the nitroimidazoles, recent studies have indicated that nitazoxanide inhibits pyruvate:ferredoxin oxidoreductase (PFOR) directly and is not dependent on flavin metabolism (Gilles and Hoffman, 2002; Hemphill et al., 2006; Leitsch et al., 2010). In vitro studies have demonstrated nitazoxanide inhibits Trichomonas vaginalis (Adagu et al., 2002; Cedillo-Rivera et al., 2002) and it has been shown to be a noncompetitive inhibitor of the PFOR of T. vaginalis, E. histolytica and G. intestinalis (Hoffman et al., 2007).
Clinical trials have shown nitazoxanide to be effective in the treatment of diarrhoea caused by E. histolytica, G. intestinalis and Cryptosporidium parvum, in particular organisms displaying high levels of resistance to metronidazole (Abboud et al., 2001; Hemphill et al., 2006). Adverse effects associated with nitazoxanide have been investigated and are uncommon, with the incidence reported to be lower than metronidazole, albendazole or praziquantel (Gilles and Hoffman, 2002).
The number of reports of nitazoxanide as treatment for dientamoebiasis is limited. A single case report has shown nitazoxanide in combination with secnidazole and doxycycline to be effective for treatment (n = 2), with total parasitological clearance and complete resolution of symptoms (Stark et al., 2009). However both patients complained of side effects and no dosage data is available, thus it is difficult to determine whether side effects were due to nitazoxanide alone or other components of the combination therapy. It is surprising that no larger studies have been undertaken to determine the therapeutic potential of this compound given the low toxicity and potential clinical efficacy.
Nitazoxanide is administered as an oral suspension of 20 mg/mL or in tablet formulation at a dosage of 500 mg. The recommended dosage for adults this is 500 mg/day (Hemphill et al., 2006). Following oral administration, the drug is absorbed from the gastrointestinal tract and absorption is doubled when taken with food (Stockis et al., 2002). Side effects are generally mild and transient and may include abdominal pain, diarrhoea and nausea. More than 2000 patients have participated in a variety of clinical trials with less than 1% experiencing more severe symptoms, including anorexia, flatulence, increased appetite, enlarged salivary glands and dizziness (Hemphill et al., 2006).
Although nitazoxanide appears to be a viable candidate for future treatment options of D. fragilis infections there has only been one case study to date, thus it is impossible to determine the effectiveness of this agent. Further studies are required.
6.5. Furazolidone
Furazolidone (N-5-nitro-2-furfurylidene amino-2-oxazolidine) is a synthetic nitrofuran derivative used for the treatment of a broad range of bacterial and protozoal infections. In particular, furazolidone has activity against E. histolytica and Giardia and it is considered to be an alternative compound in the case of treatment failure of first line agents such as the 5-nitroimidazole compounds (Escobedo et al., 2009; Lalle, 2010). Like metronidazole, furazolidone is activated by the reduction in trophozoites; however it is likely to be mediated by NADH oxidase (Brown et al., 1996; Upcroft and Upcroft, 1998). It is also more efficient than nitazoxanide in the in vitro reduction of cyst production and possibly affects the mechanism of endocytosis of the Giardia cells (Hausen et al., 2006).
For treatment of giardiasis, furazolidone is administered at 100 mg for adults and 1.25–2 mg/kg for children, four times a day for 7–10 days. Although it is generally well-tolerated, a minority of patients have reported gastrointestinal symptoms including nausea, vomiting and abdominal pain. Brown discolouration of the urine and hemolysis can occur in G6PDH-deficient patients (Gardner and Hill, 2001).
As there have been no studies to date which tested on the efficacy of furazolidone for treatment of dientamoebiasis, further studies are required before its use in a clinical setting. However this agent may have a role in treating D. fragilis infection.
7. Antibiotic susceptibility testing
The first antimicrobial studies on D. fragilis were performed in the 1950s by Balamuth (1953). A mono-phasic medium containing egg yolk/liver infusion capable of supporting the growth of D. fragilis was developed and used to study the effects of six antimicrobial compounds: emetine-bismuth-iodide; vioform; carbarsone oxide; prodigiosin; aureomycin; and a dithio-derivative of carbarsone oxide, known as C.C. no. 914 (Balamuth, 1953). It was suggested by the author that the use of arsenical compounds or prodigiosin were the best options for treatment of D. fragilis infection. However none of these compounds are in use today.
In vitro antimicrobial susceptibility testing for current treatment options using the ATCC strain of D. fragilis (ATCC 30948), grown in a dixenic culture was undertaken by Chan et al. (1994). The minimal amoebicidal concentrations for iodoquinol, paromomycin, tetracycline and metronidazole were determined as 128, 16, 32 and 32 μg/mL, respectively. It may be difficult to relate these results to situations in vivo, as there have been no reports of clinical infections caused by ATCC strain of D. fragilis, known to be genotype 2, while nearly all clinical isolates of D. fragilis that have undergone genotyping are genotype 1, the predominant strain worldwide (Peek et al., 2004; Stark et al., 2005; Bart et al., 2008).
More recently, susceptibility testing of a number of potential therapeutic agents has been undertaken. Compounds tested include the newer 5-nitroimidazole derivatives and a number of previously untested compounds: diloxanide furoate; furazolidone; nitazoxanide and ronidazole using four clinical isolates of D. fragilis. The long acting 5-nitroimidazoles were found to be the most effective, with MLCs for ornidazole, tinidazole, ronidazole, metronidazole and secnidazole of 16, 16, 31, 31 and 63 μg/mL, respectively (Nagata et al., 2012). While these findings, particularly MLCs obtained for metronidazole were in agreement with the study by Chan et al. (1994), conflicting results were obtained for a number of agents tested. For example, Chan and colleagues reported that the minimal amoebicidal concentrations for iodoquinol, paromomycin and tetracycline were 128, 16 and 32 μg/mL, in comparison to the recent study with 500, 500 and 250 μg/mL, respectively which can be explained in part by methodology differences (Nagata et al., 2012). Additionally, previously untested compounds demonstrated minimal inhibition of D. fragilis with MLCs obtained for diloxanide furoate, furazolidone and nitazoxanide of >500, 250–500 and 63 μg/mL, respectively.
As such studies are undertaken in the presence of bacterial flora, the absence of axenic culture for D. fragilis makes interpretation of in vitro susceptibility testing difficult, especially for clinical isolates. Elimination of certain and/or the majority of the bacterial flora may indirectly result in detrimental effects to D. fragilis trophozoites, as they have been long known to utilise them as a food source (Nagata et al., 2012).
8. Conclusion
Given the number of reports linking gastrointestinal illnesses with D. fragilis, there is little doubt concerning the pathogenic potential of this parasite. Indeed, a number of studies have shown that D. fragilis is often more prevalent than G. intestinalis in cases of diarrhoeal disease (Crotti et al., 2005; Vandenberg et al., 2006; Crotti and D’Annibale, 2007; Stark et al., 2010). Moreover, treatment of patients harboring D. fragilis can eradicate the organisms and results in complete resolution of clinical symptoms. As such, D. fragilis should be included as part of a routine laboratory diagnostic investigation and symptomatic patients should be treated in the absence of other possible etiological agents.
Currently the use of iodoquinol, paromomycin, metronidazole, tetracycline or a combination therapy is given as recommended treatments (Stark et al., 2010). Recent case reports and non-randomised studies have indicated the newer 5-nitroimidazole derivatives, namely ornidazole and secnidazole to be effective options for treatment (Girginkardesler et al., 2003; Kurt et al., 2008). In addition in vitro susceptibility testing of clinical isolates has indicated a number of 5-nitroimidazole derivatives, including ornidazole, ronidazole and tinidazole, to be potentially effective therapy treatment, with the MLC for some isolates as low as 8 μg/mL (Nagata et al., 2012).
Despite the number of small studies and case series showing clinical improvement with treatment, there is little information available concerning the optimal therapeutic options for D. fragilis infections. Although some of these agents are now unavailable due to their toxicity and adverse effects, the treatments reported to be successful for dientamoebiasis to date include: carbarsone (Knoll and Howell, 1946; Kean and Malloch, 1966); diphetarsone (Desser and Yang, 1976; Keystone et al., 1983); tetracyclines (Kean and Malloch, 1966; Dardick, 1983; Preiss et al., 1990; Butler, 1996; Stark et al., 2007a); iodoquinol (Spencer et al., 1979a, 1982; Millet et al., 1983a,b; Shein and Gelb, 1983; Cuffari et al., 1998); paromomycin (Cuffari et al., 1998); erythromycin (Preiss et al., 1991); and metronidazole (Spencer et al., 1979a; Cuffari et al., 1998). All treatment options that have previously been administered are summarized (see Table 3).
It is notable that no randomised double-blind, placebo controlled trials have been undertaken for the evaluation of treatment of dientamoebiasis. Additionally, a number of studies have used relatively small sample sizes ranging from one or two patients to over 50 (median = 17), with no control groups. Given that the rate of spontaneous eradication of the parasite and the ‘self-limiting’ characteristics of D. fragilis infections is unknown further adds to the confusion when evaluating potential drugs for therapy of dientamoebiasis if an appropriate control group is not utilised. Notably, one study has reported spontaneous eradication in 41% of untreated cases of D. fragilis infections (van Hellemond et al., 2012). Many if not all studies on the efficacy of antimicrobial compounds against D. fragilis infections utilised microscopy for the screening of Dientamoeba which given the intermittent shedding of the parasite may lack sensitivity. The use of molecular techniques with higher specificity and sensitivity such as PCR could clarify therapeutic success (or failure) by demonstrating the presence or absence of the parasite.
There have been reported cases of treatment failure and relapse in the treatment of D. fragilis infection and the emergence of drug resistance may be a concern, especially for the compounds such as metronidazole or furazolidone. Such findings have been reported and resistance has been induced successfully for G. intestinalis and T. vaginalis in vitro (Cerkasovova et al., 1988; Townson et al., 1992, 1994; Kulda et al., 1993; Upcroft and Upcroft, 1993; Brown et al., 1999; Rasoloson et al., 2002). It should be noted that treatment failure may also be attributed to poor compliance due to side effects or inadequate drug dosage. Clinicians also need to exclude re-infection.
In summary while a number of drugs have been shown to be effective for treating D. fragilis infection and antimicrobial agents such as metronidazole, paromomycin, iodoquinol and tetracycline and are among those recommended by the CDC (see Table 1) such recommendations, are based only on small numbers of non-randomised studies. Until large scale treatment trials incorporating properly randomised control groups are conducted, physicians should carefully monitor the efficacy and toxicity of current therapeutic regimes.
References
- Abboud P., Lemee V., Gargala G., Brasseur P., Ballet J.J., Borsa-Lebas F., Caron F., Favennec L. Successful treatment of metronidazole- and albendazole-resistant giardiasis with nitazoxanide in a patient with acquired immunodeficiency syndrome. Clinical Infectious Diseases. 2001;32:1792–1794. doi: 10.1086/320751. [DOI] [PubMed] [Google Scholar]
- Adagu I.S., Nolder D., Warhurst D.C., Rossignol J.F. In vitro activity of nitazoxanide and related compounds against isolates of Giardia intestinalis, Entamoeba histolytica and Trichomonas vaginalis. Journal of Antimicrobial Chemotherapy. 2002;49:103–111. doi: 10.1093/jac/49.1.103. [DOI] [PubMed] [Google Scholar]
- Agwuh K.N., MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. Journal of Antimicrobial Chemotherapy. 2006;58:256. doi: 10.1093/jac/dkl224. [DOI] [PubMed] [Google Scholar]
- Balamuth W. Comparative action of selected amebicidal agents and antibiotics against several species of human intestinal amebae. The American Journal of Tropical Medicine and Hygiene. 1953;2:191. doi: 10.4269/ajtmh.1953.2.191. [DOI] [PubMed] [Google Scholar]
- Banik, G.R., Barratt, J.L., Marriott, D., Harkness, J., Ellis, J.T., Stark, D., 2011. A case-controlled study of Dientamoeba fragilis infections in children. Parasitology 138, 1–5. [DOI] [PubMed]
- Barratt J.L., Harkness J., Marriott D., Ellis J.T., Stark D. The ambiguous life of Dientamoeba fragilis: the need to investigate current hypotheses on transmission. Parasitology. 2011;138:557–572. doi: 10.1017/S0031182010001733. [DOI] [PubMed] [Google Scholar]
- Barratt J.L., Harkness J., Marriott D., Ellis J.T., Stark D. A review of Dientamoeba fragilis carriage in humans: several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes. 2011;2:3–12. doi: 10.4161/gmic.2.1.14755. [DOI] [PubMed] [Google Scholar]
- Bart A., van der Heijden H.M., Greve S., Speijer D., Landman W.J., van Gool T. Intragenomic variation in the internal transcribed spacer 1 region of Dientamoeba fragilis as a molecular epidemiological marker. Journal of Clinical Microbiology. 2008;46:3270–3275. doi: 10.1128/JCM.00680-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner G., Gawel M., Kaeser H., Pallis C., Rose F., Schaumburg H., Thomas P., Wadia N. Neurotoxicity of halogenated hydroxyquinolines: clinical analysis of cases reported outside Japan. Journal of Neurology, Neurosurgery & Psychiatry. 1979;42:1073. doi: 10.1136/jnnp.42.12.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosman D., Benninga M., van de Berg P., Kooijman G., van Gool T. Dientamoeba fragilis: possibly an important cause of persistent abdominal pain in children. Nederlands tijdschrift voor geneeskunde. 2004;148:575. [PubMed] [Google Scholar]
- Brown D.M., Upcroft J.A., Upcroft P. A H2O-producing NADH oxidase from the protozoan parasite Giardia duodenalis. European Journal of Biochemistry. 1996;241:155–161. doi: 10.1111/j.1432-1033.1996.0155t.x. [DOI] [PubMed] [Google Scholar]
- Brown D.M., Upcroft J.A., Dodd H.N., Chen N., Upcroft P. Alternative 2-keto acid oxidoreductase activities in Trichomonas vaginalis. Molecular and Biochemical Parasitology. 1999;98:203–214. doi: 10.1016/s0166-6851(98)00169-8. [DOI] [PubMed] [Google Scholar]
- Butler W.P. Dientamoeba fragilis. An unusual intestinal pathogen. Digestive Diseases and Sciences. 1996;41:1811–1813. doi: 10.1007/BF02088750. [DOI] [PubMed] [Google Scholar]
- Cedillo-Rivera R., Chavez B., Gonzalez-Robles A., Tapia A., Yepez-Mulia L. In vitro effect of nitazoxanide against Entamoeba histolytica, Giardia intestinalis and Trichomonas vaginalis trophozoites. Journal of Eukaryotic Microbiology. 2002;49:201–208. doi: 10.1111/j.1550-7408.2002.tb00523.x. [DOI] [PubMed] [Google Scholar]
- Cerkasovova A., Novák J., Cerkasov J., Kulda J., Tachezy J. Metabolic properties of Trichomonas vaginalis resistant to metronidazole under anaerobic conditions. Acta Universitatis Carolinae Biologica. 1988;30:505–512. [Google Scholar]
- Chan F.T., Guan M.X., Mackenzie A.M., Diaz-Mitoma F. Susceptibility testing of Dientamoeba fragilis ATCC 30948 with iodoquinol, paromomycin, tetracycline, and metronidazole. Antimicrobial Agents and Chemotherapy. 1994;38:1157–1160. doi: 10.1128/aac.38.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari H.S., Singh P.P. Comparative drug susceptibility study of five clonal strains of Trichomonas vaginalis in vitro. Asian Pacific Journal of Tropical Medicine. 2011;4:50–53. doi: 10.1016/S1995-7645(11)60031-X. [DOI] [PubMed] [Google Scholar]
- Chen D., Cui Q.C., Yang H., Barrea R.A., Sarkar F.H., Sheng S., Yan B., Reddy G.P., Dou Q.P. Clioquinol, a therapeutic agent for Alzheimer’s disease, has proteasome-inhibitory, androgen receptor-suppressing, apoptosis-inducing, and antitumor activities in human prostate cancer cells and xenografts. Cancer Research. 2007;67:1636–1644. doi: 10.1158/0008-5472.CAN-06-3546. [DOI] [PubMed] [Google Scholar]
- Chopra, I., Roberts, M., 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiology and Molecular Biology Reviews 65, 232–260 (second page, table of contents). [DOI] [PMC free article] [PubMed]
- Crotti D., D’annibale M., Fonzo G., Lalle M., Caccio S., Pozio E. Dientamoeba fragilis is more prevalent than Giardia duodenaus in children and adults attending a day care centre in central Italy. Parasite. 2005;12:165–170. doi: 10.1051/parasite/2005122165. [DOI] [PubMed] [Google Scholar]
- Crotti D., D’Annibale M. Intestinal infections caused by Dientamoeba fragilis and Giardia duodenalis in our experience. Recenti Progressi in Medicina. 2007;98:361. [PubMed] [Google Scholar]
- Cuajungco M.P., Faget K.Y., Huang X., Tanzi R.E., Bush A.I. Metal chelation as a potential therapy for Alzheimer’s disease. Annals of the New York Academy of Sciences. 2000;920:292–304. doi: 10.1111/j.1749-6632.2000.tb06938.x. [DOI] [PubMed] [Google Scholar]
- Cuffari C., Oligny L., Seidman E.G. Dientamoeba fragilis masquerading as allergic colitis. Journal of Pediatric Gastroenterology and Nutrition. 1998;26:16–20. doi: 10.1097/00005176-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Daniel K.G., Chen D., Orlu S., Cui Q.C., Miller F.R., Dou Q.P. Clioquinol and pyrrolidine dithiocarbamate complex with copper to form proteasome inhibitors and apoptosis inducers in human breast cancer cells. Breast Cancer Research: BCR. 2005;7:R897–R908. doi: 10.1186/bcr1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardick K.R. Tetracycline treatment of Dientamoeba fragilis. Connecticut Medicine. 1983;47:69–70. [PubMed] [Google Scholar]
- Davidson R.N., den Boer M., Ritmeijer K. Paromomycin. Transactions of the Royal Society of Tropical Medicine and Hygien. 2009;103:653–660. doi: 10.1016/j.trstmh.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Desser S., Yang Y. Letter: Dientamoeba fragilis in idiopathic gastrointestinal disorders. Canadian Medical Association Journal. 1976;114:290. [PMC free article] [PubMed] [Google Scholar]
- Ding W.Q., Liu B., Vaught J.L., Yamauchi H., Lind S.E. Anticancer activity of the antibiotic clioquinol. Cancer Research. 2005;65:3389–3395. doi: 10.1158/0008-5472.CAN-04-3577. [DOI] [PubMed] [Google Scholar]
- Dobell C. Researches on the intestinal protozoa of monkeys and man. Parasitology. 1940;32:417–459. doi: 10.1017/s0031182000084225. [DOI] [PubMed] [Google Scholar]
- Epstein E. Toxicity of carbarsone. Journal of the American Medical Association. 1936;106:769. [Google Scholar]
- Escobedo A.A., Almirall P., Alfonso M., Cimerman S., Rey S., Terry S.L. Treatment of intestinal protozoan infections in children. Archives of Disease in Childhood. 2009;94:478–482. doi: 10.1136/adc.2008.151852. [DOI] [PubMed] [Google Scholar]
- Fox L.M., Saravolatz L.D. Nitazoxanide: a new thiazolide antiparasitic agent. Clinical Infectious Diseases. 2005;40:1173–1180. doi: 10.1086/428839. [DOI] [PubMed] [Google Scholar]
- Fung H.B., Doan T.L. Tinidazole: a nitroimidazole antiprotozoal agent. Clinical Therapeutics. 2005;27:1859–1884. doi: 10.1016/j.clinthera.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Gardner T.B., Hill D.R. Treatment of giardiasis. Clinical Microbiology Reviews. 2001;14:114. doi: 10.1128/CMR.14.1.114-128.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles H.M., Hoffman P.S. Treatment of intestinal parasitic infections: a review of nitazoxanide. Trends in Parasitology. 2002;18:95–97. doi: 10.1016/s1471-4922(01)02205-x. [DOI] [PubMed] [Google Scholar]
- Gillis J., Wiseman L. Secnidazole: a review of its antimicrobial activity, pharmacokinetic properties and therapeutic use in the management of protozoal infections and bacterial vaginosis. Drugs. 1996;51:621–638. doi: 10.2165/00003495-199651040-00007. [DOI] [PubMed] [Google Scholar]
- Girginkardesler, N., Coşkun, S., Balcioğlu, C., Ertan, P., Ok, Ü., 2003. Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole. Clinical Microbiology and Infection 9, 110–113. [DOI] [PubMed]
- Girginkardesler N., Kurt O., Kilimcioglu A.A., Ok U.Z. Transmission of Dientamoeba fragilis: evaluation of the role of Enterobius vermicularis. Parasitology International. 2008;57:72–75. doi: 10.1016/j.parint.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Gittings J., Waltz A. Dientamoeba fragilis. Archives of Pediatrics and Adolescent Medicine. 1927;34:542. [Google Scholar]
- Gupta Y., Gupta M., Aneja S., Kohli K. Current drug therapy of protozoal diarrhoea. Indian Journal of Pediatrics. 2004;71:55–58. doi: 10.1007/BF02725657. [DOI] [PubMed] [Google Scholar]
- Hakansson E. Dientamoeba fragilis, a cause of illness: report of case. The American Journal of Tropical Medicine and Hygiene. 1936;1:175. [Google Scholar]
- Hakansson E. Dientamoeba fragilis: some further observations. The American Journal of Tropical Medicine and Hygiene. 1937;1:349. [Google Scholar]
- Haughwout F., Horrilleno F. The intestinal animal parasites found in one hundred sick Filipino children. Philippine Journal of Science. 1920;16:1–73. [Google Scholar]
- Hausen M.A., Freitas J.C., Jr., Monteiro-Leal L.H. The effects of metronidazole and furazolidone during Giardia differentiation into cysts. Experimental Parasitology. 2006;113:135–141. doi: 10.1016/j.exppara.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Hemphill A., Mueller J., Esposito M. Nitazoxanide, a broad-spectrum thiazolide anti-infective agent for the treatment of gastrointestinal infections. Expert Opinion on Pharmacotherapy. 2006;7:953–964. doi: 10.1517/14656566.7.7.953. [DOI] [PubMed] [Google Scholar]
- Hoffman P.S., Sisson G., Croxen M.A., Welch K., Harman W.D., Cremades N., Morash M.G. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrobial Agents and Chemotherapy. 2007;51:868–876. doi: 10.1128/AAC.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, E.H., Windsor, J.J., Clark, C.G., 2004. Emerging from obscurity: biological, clinical, and diagnostic aspects of Dientamoeba fragilis. Clinical Microbiology Reviews 17, 553–570 (table of contents). [DOI] [PMC free article] [PubMed]
- Kean B., Malloch C. The neglected ameba: dientamoeba fragilis. A report of 100 “pure” infections. The American Journal of Digestive Diseases. 1966;11:735. doi: 10.1007/BF02239427. [DOI] [PubMed] [Google Scholar]
- Keystone J., Proctor E., Glenn C., McIntyre L. Safety and efficacy of diphetarsone in the treatment of amoebiasis, non-pathogenic amoebiasis and trichuriasis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1983;77:84–86. doi: 10.1016/0035-9203(83)90022-6. [DOI] [PubMed] [Google Scholar]
- Khaw M., Panosian C.B. Human antiprotozoal therapy: past, present, and future. Clinical Microbiology Reviews. 1995;8:427–439. doi: 10.1128/cmr.8.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R. The chemotherapy of amoebiasis. Journal of Antimicrobial Chemotherapy. 1980;6:577. doi: 10.1093/jac/6.5.577. [DOI] [PubMed] [Google Scholar]
- Knoll E.W., Howell K.M. Studies on Dientamoeba fragilis; its incidence and possible pathogenicity. Medical Women’s Journal. 1946;53:40–46. [PubMed] [Google Scholar]
- Kulda J., Tachezy J., Cerkasovova A. In vitro induced anaerobic resistance to metronidazole in Trichomonas vaginalis. The Journal of Eukaryotic Microbiology. 1993;40:262–269. doi: 10.1111/j.1550-7408.1993.tb04915.x. [DOI] [PubMed] [Google Scholar]
- Kurt O., Girginkardesler N., Balcioglu I.C., Ozbilgin A., Ok U.Z. A comparison of metronidazole and single-dose ornidazole for the treatment of dientamoebiasis. Clinical Microbiology and Infection. 2008;14:601–604. doi: 10.1111/j.1469-0691.2008.02002.x. [DOI] [PubMed] [Google Scholar]
- Löfmark S., Edlund C., Nord C.E. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clinical Infectious Diseases. 2010;50:S16. doi: 10.1086/647939. [DOI] [PubMed] [Google Scholar]
- Lalle M. Giardiasis in the post genomic era: treatment, drug resistance and novel therapeutic perspectives. Infectious Disorders Drug Targets. 2010;10:283–294. doi: 10.2174/187152610791591610. [DOI] [PubMed] [Google Scholar]
- Lau A.H., Lam N.P., Piscitelli S.C., Wilkes L., Danziger L.H. Clinical pharmacokinetics of metronidazole and other nitroimidazole anti-infectives. Clinical Pharmacokinetics. 1992;23:328–364. doi: 10.2165/00003088-199223050-00002. [DOI] [PubMed] [Google Scholar]
- Leitsch D., Kolarich D., Duchêne M. The flavin inhibitor diphenyleneiodonium renders Trichomonas vaginalis resistant to metronidazole, inhibits thioredoxin reductase and flavin reductase, and shuts off hydrogenosomal enzymatic pathways. Molecular and Biochemical Parasitology. 2010;171:17–24. doi: 10.1016/j.molbiopara.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Lloyd, D., Pedersen, J., 1985. Metronidazole radical anion generation in vivo in Trichomonas vaginalis: oxygen quenching is enhanced in a drug-resistant strain. Microbiology (Reading, England) 131, 87. [DOI] [PubMed]
- Mao X., Schimmer A.D. The toxicology of clioquinol. Toxicology Letters. 2008;182:1–6. doi: 10.1016/j.toxlet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Mao, X., Li, X., Sprangers, R., Wang, X., Venugopal, A., Wood, T., Zhang, Y., Kuntz, D.A., Coe, E., Trudel, S., Rose, D., Batey, R.A., Kay, L.E., Schimmer, A.D., 2009. Clioquinol inhibits the proteasome and displays preclinical activity in leukemia and myeloma. Leukemia: official journal of the Leukemia Society of America, Leukemia Research Fund, UK 23, 585–590. [DOI] [PubMed]
- Millet V., Spencer M.J., Chapin M., Stewart M., Yatabe J.A., Brewer T., Garcia L.S. Dientamoeba fragilis, a protozoan parasite in adult members of a semicommunal group. Digestive Diseases and Sciences. 1983;28:335–339. doi: 10.1007/BF01324950. [DOI] [PubMed] [Google Scholar]
- Millet V., Spencer M. Intestinal protozoan infection in a semicommunal group. The American Journal of Tropical Medicine and Hygiene. 1983;32(1):54. doi: 10.4269/ajtmh.1983.32.54. [DOI] [PubMed] [Google Scholar]
- Mollari M., Anzulovic J. Cultivation and pathogencity of Dientamoeba fragilis, with a case report. Journal of Tropical Medicine and Hygiene. 1938;41:246–247. [Google Scholar]
- Moreno S., Docampo R. Mechanism of toxicity of nitro compounds used in the chemotherapy of trichomoniasis. Environmental Health Perspectives. 1985;64:199. doi: 10.1289/ehp.8564199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata N., Marriott D., Harkness J., Ellis J.T., Stark D. In vitro susceptibility testing of Dientamoeba fragilis. Antimicrobial Agents and Chemotherapy. 2012;56:487–494. doi: 10.1128/AAC.05125-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg A., Nord C., Evengård B. Dientamoeba fragilis—a protozoal infection which may cause severe bowel distress. Clinical Microbiology and Infection. 2003;9:65–68. doi: 10.1046/j.1469-0691.2003.00459.x. [DOI] [PubMed] [Google Scholar]
- Ockert G., Schmidt T. On the epidemiology of Dientamoeba fragilis Jepps and Dobell 1918. Fourth communication: evidence of Dientamoeba fragilis in enterobius eggs using isoelectric point determination. Journal of Hygiene, Epidemiology, Microbiology, and Immunology. 1976;20:76. [PubMed] [Google Scholar]
- Oxner R., Paltridge G., Chapman B., Cook H., Sheppard P. Dientamoeba fragilis: a bowel pathogen? The New Zealand Medical Journal. 1987;100:64. [PubMed] [Google Scholar]
- Peek R., Reedeker F.R., van Gool T. Direct amplification and genotyping of Dientamoeba fragilis from human stool specimens. Journal of Clinical Microbiology. 2004;42:631–635. doi: 10.1128/JCM.42.2.631-635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E., Kalyanaraman B., Mason R.P. The reductive metabolism of metronidazole and ronidazole by aerobic liver microsomes. Molecular Pharmacology. 1980;17:239–244. [PubMed] [Google Scholar]
- Preiss U., Ockert G., Brömme S., Otto A. Dientamoeba fragilis infection, a cause of gastrointestinal symptoms in childhood. Klinische Padiatrie. 1990;202:120–123. doi: 10.1055/s-2007-1025503. [DOI] [PubMed] [Google Scholar]
- Preiss U., Ockert G., Broemme S., Otto A. On the clinical importance of Dientamoeba fragilis infections in childhood. Journal of Hygiene, Epidemiology, Microbiology, and Immunology. 1991;35:27. [PubMed] [Google Scholar]
- Rahman M.M., Ng J.C., Naidu R. Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environmental Geochemistry and Health. 2009;31:189–200. doi: 10.1007/s10653-008-9235-0. [DOI] [PubMed] [Google Scholar]
- Rasoloson, D., Vanacova, S., Tomkova, E., Razga, J., Hrdy, I., Tachezy, J., Kulda, J., 2002. Mechanisms of in vitro development of resistance to metronidazole in Trichomonas vaginalis. Microbiology (Reading, England) 148, 2467–2477. [DOI] [PubMed]
- Rayan H., Ismail O., El Gayar E. Prevalence and clinical features of Dientamoeba fragilis infections in patients suspected to have intestinal parasitic infection. Journal of the Egyptian Society of Parasitology. 2007;37:599. [PubMed] [Google Scholar]
- Rossignol J.F., Maisonneuve H. Nitazoxanide in the treatment of Taenia saginata and Hymenolepis nana infections. American Journal of Tropical Medicine and Hygiene. 1984;33:511–512. doi: 10.4269/ajtmh.1984.33.511. [DOI] [PubMed] [Google Scholar]
- Schneider J., Dupoux R. Bis-(p-arsonophenylamino) 1–2-ethane therapy of intestinal amebiasis. Bulletin de la SociÊtÊ de pathologie exotique et de ses filiales. 1953;46:550. [PubMed] [Google Scholar]
- Schneider, J., 1957. Treatment of intestinal amebiasis & amebic colitis. Medicina Contemporânea (Lisbon, Portugal) 75, 479. [PubMed]
- Shein R., Gelb A. Colitis due to Dientamoeba fragilis. The American Journal of Gastroenterology. 1983;78:634–636. [PubMed] [Google Scholar]
- Simon M., Shookhoff H.B., Terner H., Weingarten B., Parker J.G. Paromomycin in the treatment of intestinal amebiasis; a short course of therapy. American Journal of Gastroenterology. 1967;48:504–511. [PubMed] [Google Scholar]
- Spencer M., Garcia L., Chapin M. Dientamoeba fragilis: an intestinal pathogen in children? Archives of Pediatrics and Adolescent Medicine. 1979;133:390. [PubMed] [Google Scholar]
- Spencer M.J., Garcia L.S., Chapin M.R. Dientamoeba fragilis. An intestinal pathogen in children? American Journal of Diseases of Children. 1979;133:390–393. [PubMed] [Google Scholar]
- Spencer M., Chapin M., Garcia L. Dientamoeba fragilis: a gastrointestinal protozoan infection in adults. The American Journal of Gastroenterology. 1982;77:565–569. [PubMed] [Google Scholar]
- Stark D., Beebe N., Marriott D., Ellis J., Harkness J. Prospective study of the prevalence, genotyping, and clinical relevance of Dientamoeba fragilis infections in an Australian population. Journal of Clinical Microbiology. 2005;43:2718–2723. doi: 10.1128/JCM.43.6.2718-2723.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark D.J., Beebe N., Marriott D., Ellis J.T., Harkness J. Dientamoebiasis: clinical importance and recent advances. Trends in Parasitology. 2006;22:92–96. doi: 10.1016/j.pt.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Stark D., Beebe N., Marriott D., Ellis J., Harkness J. Dientamoeba fragilis as a cause of travelers’ diarrhea: report of seven cases. Journal of Travel Medicine. 2007;14:72–73. doi: 10.1111/j.1708-8305.2006.00100.x. [DOI] [PubMed] [Google Scholar]
- Stark D., van Hal S., Marriott D., Ellis J., Harkness J. Irritable bowel syndrome: a review on the role of intestinal protozoa and the importance of their detection and diagnosis. International Journal for Parasitology. 2007;37:11–20. doi: 10.1016/j.ijpara.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Stark D., Barratt J., Ellis J., Harkness J., Marriott D. Repeated Dientamoeba fragilis infections: a case report of two families from Sydney, Australia. Infectious Disease Reports. 2009;1:e4. doi: 10.4081/idr.2009.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark D., Barratt J., Roberts T., Marriott D., Harkness J., Ellis J. A review of the clinical presentation of dientamoebiasis. American Journal of Tropical Medicine and Hygiene. 2010;82:614–619. doi: 10.4269/ajtmh.2010.09-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark D., Roberts T., Marriott D., Harkness J., Ellis J.T. Detection and transmission of Dientamoeba fragilis from environmental and household samples. The American Journal of Tropical Medicine and Hygiene. 2012;86:233–236. doi: 10.4269/ajtmh.2012.11-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockis A., Allemon A.M., De Bruyn S., Gengler C. Nitazoxanide pharmacokinetics and tolerability in man using single ascending oral doses. International Journal of Clinical Pharmacology and Therapeutics. 2002;40:213–220. doi: 10.5414/cpp40213. [DOI] [PubMed] [Google Scholar]
- Tabira T. Clioquinol’s return: cautions from Japan. Science. 2001;292:2251–2252. doi: 10.1126/science.292.5525.2251. [DOI] [PubMed] [Google Scholar]
- Taliaferro, W.H., Becker, E.R., 1924. A note on the human intestinal amoeba, Dientamoeba fragilis. Reprinted from: The American Journal of Hygiene, Vol. 4, no. 1.
- Townson S.M., Laqua H., Upcroft P., Boreham P.F., Upcroft J.A. Induction of metronidazole and furazolidone resistance in Giardia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1992;86:521–522. doi: 10.1016/0035-9203(92)90095-t. [DOI] [PubMed] [Google Scholar]
- Townson S.M., Boreham P.F., Upcroft P., Upcroft J.A. Resistance to the nitroheterocyclic drugs. Acta Tropica. 1994;56:173–194. doi: 10.1016/0001-706x(94)90062-0. [DOI] [PubMed] [Google Scholar]
- Tsubaki T., Honma Y., Hoshi M. Neurological syndrome associated with clioquinol. Lancet. 1971;1:696–697. doi: 10.1016/s0140-6736(71)92699-7. [DOI] [PubMed] [Google Scholar]
- Turner J.A. Giardiasis and infections with Dientamoeba fragilis. Pediatric Clinics of North America. 1985;32:865–880. doi: 10.1016/s0031-3955(16)34859-3. [DOI] [PubMed] [Google Scholar]
- Upcroft J.A., Upcroft P. Drug resistance and Giardia. Parasitology Today. 1993;9:187–190. doi: 10.1016/0169-4758(93)90144-5. [DOI] [PubMed] [Google Scholar]
- Upcroft J., Upcroft P. My favorite cell: Giardia. Bioessays. 1998;20:256–263. doi: 10.1002/(SICI)1521-1878(199803)20:3<256::AID-BIES9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- van Hellemond J.J., Molhoek N., Koelewijn R., Wismans P.J., van Genderen P.J.J. Is paromomycin the drug of choice for eradication of Dientamoeba fragilis in adults? International Journal for Parasitology: Drugs and Drug Resistance. 2012;2:162–165. doi: 10.1016/j.ijpddr.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg O., Peek R., Souayah H., Dediste A., Buset M., Scheen R., Retore P., Zissis G., van Gool T. Clinical and microbiological features of dientamoebiasis in patients suspected of suffering from a parasitic gastrointestinal illness: a comparison of Dientamoeba fragilis and Giardia lamblia infections. International Journal of Infectious Diseases. 2006;10:255–261. doi: 10.1016/j.ijid.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Vandenberg O., Souayah H., Mouchet F., Dediste A., van Gool T. Treatment of Dientamoeba fragilis infection with paromomycin. The Pediatric Infectious Disease Journal. 2007;26:88–90. doi: 10.1097/01.inf.0000247139.89191.91. [DOI] [PubMed] [Google Scholar]
- Viode C., Bettache N., Cenas N., Krauth-Siegel R.L., Chauviere G., Bakalara N., Perie J. Enzymatic reduction studies of nitroheterocycles. Biochemical Pharmacology. 1999;57:549–557. doi: 10.1016/s0006-2952(98)00324-4. [DOI] [PubMed] [Google Scholar]
- Weisblum B. Erythromycin resistance by ribosome modification. Antimicrobial Agents and Chemotherapy. 1995;39:577. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenrich D., Stabler R., Arnett J. Endamoeba histolytica and other intestinal protozoa in 1060 college freshmen. The American Journal of Tropical Medicine and Hygiene. 1936;1:331. [Google Scholar]
- Wenyon C. Wm. Wood & Co.; New York: 1926. Protozoology, vol. II. p. 1244. [Google Scholar]
- Wenyon C.M. William Wood; 1926. Protozoology. [Google Scholar]
- White C.A., Jr. Nitazoxanide: a new broad spectrum antiparasitic agent. Expert Review of Anti-Infective Therapy. 2004;2:43–49. doi: 10.1586/14787210.2.1.43. [DOI] [PubMed] [Google Scholar]
- Windsor J.J., Johnson E.H. Dientamoeba fragilis: the unflagellated human flagellate. British Journal of Biomedical Science. 1999;56:293–306. [PubMed] [Google Scholar]
- Windsor J., Macfarlane L. Irritable bowel syndrome: the need to exclude Dientamoeba fragilis. The American Journal of Tropical Medicine and Hygiene. 2005;72:501. [PubMed] [Google Scholar]


