Graphical abstract
Keywords: Dirofilaria immitis, In vitro, Macrocyclic lactone, Third-stage larva
Highlights
-
•
We optimized a larval migration inhibition assay for use with Dirofilaria immitis.
-
•
Consistent and reproducible dose–response data were generated.
-
•
High control migration rates (>90%) were observed.
-
•
The IC50 is the preferred parameter for this assay.
-
•
IC50 and IC95 values were better defined with eprinomectin than ivermectin.
Abstract
For more than 20 years, anthelmintics of the macrocyclic lactone (ML) drug class have been widely and effectively used as preventives against the canine heartworm, Dirofilaria immitis. However, in recent years an increased number of lack of efficacy (LOE) cases are being reported, in which dogs develop mature heartworm infections despite receiving monthly prophylactic doses of ML drugs. While this situation is raising concerns that heartworms may be developing resistance to MLs, compelling evidence for this is still lacking. Resolution of this dilemma requires validated biological or molecular diagnostic assays, but, unfortunately, no such tests currently exist. To address this need, we developed and optimized a larval migration inhibition assay (LMIA) for use with D. immitis third-stage larvae. The LMIA was used to measure the in vitro dose–response of two ML drugs (ivermectin and eprinomectin) on a known ML-susceptible laboratory strain of D. immitis. A nonlinear regression model was fit to the dose–response data, from which IC50 values were calculated; the mean IC50 and 95% confidence interval for IVM was 4.56 μM (1.26–16.4 μM), greater than that for EPR at 2.02 μM (1.68–2.42 μM), and this difference was significant (p = 0.0428). The R2 value for EPR assays (0.90) was also greater than that for IVM treatment (0.71). The consistency and reproducibility of the dose–response data obtained with this assay suggests that it may be a useful technique for investigating the relative susceptibilities to ML drugs in other D. immitis populations.
1. Introduction
The canine heartworm (Dirofilaria immitis) is an important filarial nematode parasite of dogs, cats, and other animals. The definitive host becomes infected through the bite of mosquitoes harboring infective third-stage larvae (L3), which penetrate the bite wound and invade the subcutaneous tissues. Over a period of approximately 2 months, the larval stages of D. immitis migrate through the subcutaneous tissues and musculature before penetrating into the vasculature. Once in the vasculature, immature adult worms migrate to the pulmonary arteries and the right side of the heart, where they mature to adults (Kotani and Powers, 1982). Tissue-migrating larval stages are not associated with any recognizable pathology; however, once in the vasculature, worms can cause significant damage and life-threatening disease (Bowman and Atkins, 2009).
Treatment of mature heartworm infection is costly and can cause serious adverse side effects, thus prevention by monthly administration of drugs that interrupt larval development is widely used (Bowman and Atkins, 2009). All compounds currently used for prevention of adult heartworm infection are members of the macrocyclic lactone (ML) drug class. In 1987, ivermectin became the first ML drug approved for use as a heartworm preventive (Campbell, 1989), and since then, products containing other ML drugs (milbemycin oxime, selamectin, and moxidectin) have been introduced. The US Food and Drug Administration (FDA) requires that all heartworm preventives achieve 100% efficacy when administered according to label directions (Hampshire, 2005). Therefore, when owner compliance with label directions is exact, no adult worms should develop in a dog administered one of these products. However, in recent years the FDA has received increasing reports of cases where mature worms develop in dogs despite evidence that monthly preventives were administered in a compliant fashion (Hampshire, 2005). These lack of efficacy (LOE) cases, are raising doubts that ML drugs achieve 100% protection (Geary et al., 2011). The two most obvious and common explanations for these LOE cases are (1) a lack of full compliance by the pet owners, which cannot be properly tested, or (2) that D. immitis has developed resistance to the ML drugs.
Resistance to the ML drugs is highly prevalent throughout the world in numerous species of gastrointestinal nematodes of livestock (Kaplan, 2004; Kaplan and Vidyashankar, 2012). Several in vitro bioassays have been developed and validated to detect ML resistance in many of these species (Taylor et al., 2002). One of these, the larval migration inhibition assay (LMIA) has already been successfully applied to several important species of nematode pathogens of sheep and cattle for diagnosis of ML resistance (Kotze et al., 2006; Demeler et al., 2010) and appears particularly amenable for use with D. immitis.
Evidence suggests that ML drugs exert their anthelmintic effects on nematodes by binding to glutamate-gated chloride channels (GluCls) and hyperpolarizing motor neurons, resulting in paralysis and death (Wolstenholme and Rogers, 2005). This paralytic action of the ML drugs is the rationale behind the development of the LMIA, which measures the ability of larvae to migrate across a fine mesh sieve following incubation with increasing concentrations of drug. Motility of the infective L3 of heartworms is critical for both the initial infection process, as well as for migration through the subcutaneous and muscular tissues of the canine host in order to gain access to the vasculature. Furthermore, the L3 is one of the targets (along with the fourth-stage larvae) of monthly preventive treatment with ML drugs. Since L3 can be produced in large numbers in properly equipped laboratories, this appears to be the ideal life cycle stage of D. immitis for use in an in vitro bioassay, and the LMIA appears to be the most suitable bioassay for quantifying the susceptibility of L3 to the paralytic effects of ML drugs.
In this study, we optimized the LMIA for use with D. immitis L3. We then measured the ML susceptibility of a laboratory heartworm strain in the LMIA using both ivermectin and eprinomectin. This generated reproducible dose–response data, to which we applied a variable-slope nonlinear regression model, enabling us to calculate IC50 values as a means of characterizing the in vitro susceptibility to these drugs. Validation of the LMIA for identifying reduced susceptibility in heartworm populations would provide an extremely valuable tool for monitoring the emergence and spread of anthelmintic resistance in D. immitis.
2. Materials and methods
2.1. Parasites
An ML-susceptible strain of D. immitis (2005 Missouri strain) maintained and passaged in beagle dogs at the University of Georgia (Athens, GA) was used in this study. This strain was originally collected in 2000 from a dog at a Missouri animal pound, and while this donor dog was not treated with any MLs thereafter, prior treatment records are not available. Third-stage larvae were obtained by feeding microfilaremic blood to Aedes aegypti mosquitoes (black-eyed Liverpool strain) using artificial blood feeders, as previously described (McCall, 1981). Fourteen days after feeding, L3 were obtained by gently crushing the infected mosquitoes, rinsing them onto a 32 μm mesh sieve set in either a Petri dish or a Baermann apparatus, and soaking them in warm Hanks’ balanced salt solution. The Brugia pahangi L3 (FR3 strain) used in some of the optimization experiments were obtained in the same manner, and are also maintained in beagle dogs at the University of Georgia.
2.2. Drug solutions
ML stock solutions (10 mM) were prepared by dissolving powdered ivermectin or eprinomectin (Sigma, St. Louis, MO) in DMSO (>99.5%; Sigma). Stock solutions were diluted with additional DMSO into 100× working solutions ranging from 0.0312 to 2.0 mM. These were then added to culture media at a rate of 1% (v/v) to yield the concentrations used in the LMIA (0.312–20 μM in 1% DMSO). For each drug, the maximum concentration tested was restricted by its solubility in 1% DMSO.
2.3. Larval migration assay optimization
2.3.1. Media
RPMI-1640 (Lonza BioWhittaker, Basel, Switzerland) media were prepared with 100 U/ml penicillin (Gibco-Invitrogen, Carlsbad, CA), 100 mg/ml streptomycin (Gibco-Invitrogen), 40 μg/ml gentamicin (Sigma). For use in the assays, ivermectin or eprinomectin in the concentrations detailed above were added to the RPMI, or only DMSO (1%) in the no-drug controls. NCTC/IMDM (NI) media were prepared from equal parts NCTC medium (Sigma) and IMDM (Sigma) with l-glutamine (200 mM; Sigma) added to a concentration of 2 mM and with 100 U/ml penicillin (Gibco-Invitrogen, Carlsbad, CA), 100 mg/ml streptomycin (Gibco-Invitrogen).
2.3.2. DMSO concentration
The LMIA was performed with the Missouri strain of D. immitis using a 48 h incubation period and 2 h migration period. Fifty larvae per well were cultured in a 24-well plate in RPMI-1640 with antibiotics, as described above, with DMSO concentrations of 0%, 1%, and 2%. Assay results were scored as described for the optimized LMIA. Each concentration was tested in triplicate in three separate experiments.
2.3.3. Migration period
Migration tubes were prepared using a method modified from Wagland et al. (1992) and set in 400 μl RPMI-1640 plus antibiotics in the wells of a 48-well culture plate. A square of 20 μm mesh was secured over the bottom of each glass migration tube and held in place by a polymer collar fit around the tube. Tubes were suspended 2 mm above the bottom of the plate by a rubber o-ring fit around each collar. In a 100 μl volume, 50 B. pahangi L3 were introduced to each of three migration wells. Larvae were allowed to migrate while incubating at 37 °C and 5% CO2. For the first 3:45 h, the migration tubes were transferred to new wells in the plate every 15 min, and the tubes were finally removed 24 h after the beginning of the experiment. The number of L3 to migrate over each time interval was then determined by counting the larvae in each well.
2.3.4. Incubation period
The LMIA was performed with the Missouri strain of D. immitis using either a 48 or 72 h incubation period and 2 h migration period. Fifty larvae per well were cultured in a 24-well plate in RPMI-1640 medium with 1% DMSO. Four independent experiments were performed, with each group tested in triplicate.
2.3.5. Mesh size
The LMIA was performed with the Missouri strain of D. immitis using a 48 h incubation period and 2 h migration period. Fifty larvae per well were cultured in a 24-well plate in RPMI-1640 medium with eprinomectin (0.625–50 μM) in 1% DMSO or in a DMSO-only control. Larvae were allowed to migrate through nylon mesh of either 25 or 30 μm pore size. Three independent experiments were performed, with each group tested in triplicate.
To determine the effect of larval diameter on migration, 25 D. immitis L3 per well were cultured in a 24-well plate as above. Larvae that successfully migrated in the LMIA (n = 12) and those that failed to migrate (n = 12) were measured using an eyepiece micrometer on an inverted compound microscope (IX51; Olympus, Center Valley, PA) at 400× magnification.
2.4. Optimized protocol for larval migration inhibition assay
After collection from mosquitoes, D. immitis L3 were washed in RPMI-1640 and added to individual wells of a 24-well tissue culture plate (30 larvae per well) in 1 ml RPMI-1640 containing antibiotics and drug. Assay plates were then incubated at 37 °C and 5% CO2 for 48 h. Following this incubation period, acrylic migration tubes were placed into a new 24-well migration plate (Fig. 1); migration tubes were set in rows of six on a horizontal acrylic bar, as described by Demeler et al. (2010), and bottomed with 25 μm nylon mesh screens (Sefar, Inc., Heiden, Switzerland). With the migration tubes in place, 1.4 ml of culture medium warmed to 37 °C was added over the tube in each well; drug concentrations in the media added to each well corresponded to those in the incubation plate. Using a dissecting microscope, all 30 larvae in each well of the incubation plate were collected and gently transferred in a volume of 200 μl to the corresponding migration tube. Once all the L3 were transferred to migration tubes, they were placed back into the incubator at 37 °C and 5% CO2 for a 2 h migration period during which motile larvae may pass through the mesh screen (Supplementary video 1). After 2 h, the migration tubes were gently removed and the larvae that had migrated into each well of the migration plate were enumerated during examination with an inverted compound microscope. Three experiments were performed for each drug (ivermectin and eprinomectin) with concentrations tested in triplicate for each experiment.
Fig. 1.
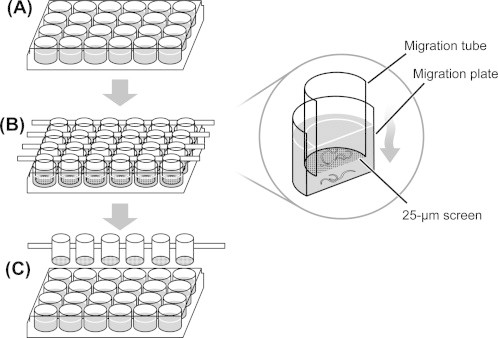
Larval migration inhibition assay procedure. (A) Larvae are incubated in media with or without drug for 48 h in the 24-well incubation plate. (B) Larvae are then transferred into migration tubes, which are suspended over the migration plate. The larvae are then allowed to migrate for 2 h through a 25 μm mesh and are collected in the migration plate. (C) Migration tubes are gently removed from the migration plate and the numbers of larvae that successfully migrated are enumerated.
2.5. Statistics
Differences in migration following incubation for 48 and 72 h (without drug) and differences in migration through meshes of different size were calculated using a one-tailed unpaired t-test. Correlation between larval diameter and the ability to migrate was calculated as a point-biserial correlation coefficient, with significance assessed by application of a two-tailed t-test.
Percent inhibition of migration was calculated for each well based on the number of larvae failing to migrate corrected for the percent of larvae that did not migrate in the no-drug control wells. Sigmoidal dose–response curves were calculated in GraphPad Prism® version 5 (GraphPad Software, Inc., San Diego, CA) using a variable slope nonlinear regression model. A four-parameter logistic equation was applied using global curve-fitting, with the bottom of the curves constrained to zero. For each treatment, the IC50 and IC95 (defined as the concentration effecting inhibition halfway between zero and the maximal response, and 95% of the maximal response, respectively) were calculated. R2 values and 95% confidence intervals were calculated by GraphPad Prism®. Differences between the IC50 values of IVM and EPR-treated groups were calculated using the extra sum-of-squares F test.
3. Results
3.1. Migration period
B. pahangi, a filarioid parasite closely related to D. immitis was used in some preliminary experiments with the LMIA, since this parasite is easier to cycle and was readily available through the FR3. In these early experiments, we observed that almost all larvae that successfully migrated over 24 h did so within the first 2 h of incubation (mean ± SD = 99.2 ± 6.56%; Fig. 2). To ensure a convenient yet sufficiently long migration period, the migration rate of D. immitis L3 over 1 h was determined, followed immediately by an additional 1 h in a separate migration plate. Significantly more larvae migrated over the combined 2 h (83%) period than 1 h (77%; p = 0.037), therefore a 2 h migration period was used for this LMIA.
Fig. 2.
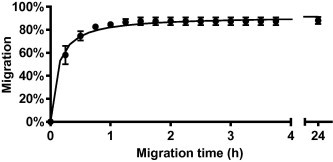
Rate of filarial larval migration. Fifty untreated Brugia pahangi L3 were allowed to migrate across a 20 μm mesh in each migration tube (n = 3). Tubes were transferred to new wells every 15 min for the first 3:45 h and finally removed 24 h after the beginning of the experiment.
3.2. Media and DMSO content
To determine the optimal culture medium for use in the D. immitis LMIA, RPMI- and NI-based media with added antibiotics were compared. Six groups of 50 L3 were incubated in each medium with no difference observed in migration rates (p = 0.81; data not shown). The RPMI-based medium was chosen for future assays because of its ease of preparation and convenience.
To solubilize macrocyclic lactone anthelmintics in an aqueous media it is necessary to use DMSO, but this solvent is known to negatively affect nematode viability. To determine an appropriate solvent concentration, larvae were incubated in media containing 1%, 2%, or no DMSO and their migration rates assessed. While no decrease from control migration rates was observed in the 1% DMSO group (p = 0.39), a significant decrease was noted when larvae were exposed to 2% DMSO (p = 0.018; data not shown). Based on these data, 1% DMSO was used in the LMIA culture medium for all further experiments.
3.3. Incubation period
To allow sufficient exposure of the larvae to the drug without inducing an excessive reduction in migration of the untreated control larvae, it was necessary to optimize the incubation period. Comparison of the LMIA performed using 48 h or 72 h incubation periods showed that migration rates were significantly lower following the longer incubation (p = 0.011; Fig. 3). Furthermore, the logIC50 for each assay was not significantly different (p = 0.51); therefore, a 48 h incubation period was selected for use in all future assays.
Fig. 3.
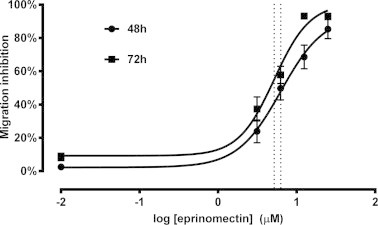
Effect of drug incubation period on larval migration. The LMIA was performed using 48 and 72 h incubation periods with eprinomectin. To more accurately compare the effect of incubation time on migration, data are not corrected for control inhibition. Dotted lines indicate logIC50 values. Each data point represents mean ± SD, n ⩾ 12 from four independent assays.
3.4. Larval diameter and mesh size
The pore size of the mesh sieve is a discriminating parameter for parasite migration in the LMIA. We observed that the diameter of L3 varied somewhat, so we wanted to confirm that this variable would not affect migration rates in the LMIA. Larvae that had either migrated or failed to migrate through a 25 μm mesh were measured using an ocular micrometer in an inverted compound microscope (Fig. 4). The largest D. immitis L3 diameter measured was 25 μm (mean ± SEM = 20.6 ± 0.268 μm). No correlation between migration and larval diameter was observed (p = 0.95), providing evidence that migration rates were not a function of variation in larval diameter.
Fig. 4.
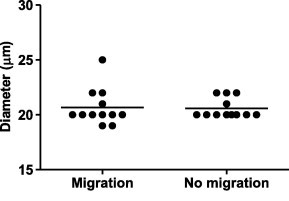
Effect of larval diameter on migration through 25 and 30 μm mesh sieves. The diameters of larvae that successfully migrated in the LMIA and those that failed to migrate were measured. Horizontal line indicates mean, n = 12.
Mesh size was selected so that it was no smaller than the largest larval diameter, yet remained fine enough to restrict the passage of immotile larvae. Given the commercial availability of mesh with a variety of pore sizes, migration rates through 25 and 30 μm mesh sieves were compared. No reduction in migration was observed for the smaller mesh size compared to the larger (three independent experiments, p = 0.38) for worms incubated without drug. However, migration of drug-treated larvae at the highest concentration tested was significantly reduced with the 25 μm mesh (p = 0.034; data not shown). A small percentage of larvae were observed to migrate through the 25 μm mesh at the highest drug concentrations tested, but it was confirmed visually that this was due to larval motility, and not the incidental passage of immotile larvae. In contrast, with the 30 μm mesh, small numbers of immotile larvae were found to pass through the mesh. Given these data, the 25 μm mesh size was selected as being optimal for use in this assay.
3.5. Optimized larval migration inhibition assay
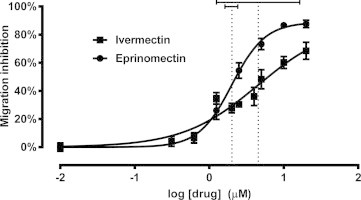
The mean migration inhibition rates in the control wells were consistently less than 10% (mean ± SD = 7.78 ± 7.75%) in most assays, indicating that D. immitis L3 retain high levels of motility after 48 h of incubation in RPMI media containing 1% DMSO. However, mean inhibition at the highest drug concentrations tested infrequently exceeded 90% (mean ± SD = 80.7 ± 13.9%). Dose–response curves were plotted for the ML-susceptible Missouri heartworm strain treated with either ivermectin or eprinomectin (Fig. 5) at concentrations ranging from 0.312 to 20 μM. The IC50 values for D. immitis L3 exposed to ivermectin and eprinomectin were 4.56 and 2.02 μM, respectively, and these values were significantly different from each other (p = 0.0428). The calculated IC95 values were less reliable, characterized by very large 95% confidence intervals, especially for ivermectin (Table 1). The fit of each curve to the global nonlinear regression model was expressed as an R2 value, and higher values were consistently found with eprinomectin, suggesting that eprinomectin is a better choice for use in this in vitro bioassay.
Fig. 5.
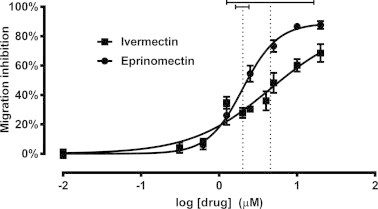
Larval migration inhibition assay dose–response curves. Data from the ML-susceptible Missouri (2005) D. immitis strain are plotted for both ivermectin (squares) and eprinomectin (circles). Dotted lines indicate mean logIC50 values, with solid horizontal bars representing the 95% confidence interval of each. Each data point represents the mean ± SD for three independent assays.
Table 1.
Larval migration inhibition assay results. IC50 and IC95 values of the ML-susceptible Missouri (2005) D. immitis strain are shown with their 95% confidence intervals (CI) for both ivermectin and eprinomectin. The R2 values are presented as a measure of how well dose–response data fit the nonlinear regression model used in this study. Three independent assays were performed for each group.
| Treatment |
||
|---|---|---|
| Ivermectin | Eprinomectin | |
| IC50 (μM) | 4.56 | 2.02 |
| 95% CI | 1.26–16.4 | 1.68–2.42 |
| IC95 (μM) | 142 | 9.20 |
| 95% CI | 7.02–2890 | 5.28–16.0 |
| R2 | 0.71 | 0.90 |
4. Discussion
The emergence of D. immitis that are resistant to macrocyclic lactone anthelmintics would represent a serious threat to canine health. However, there still is no consensus on the issue of whether ML resistance has emerged or if LOE cases are sufficiently explained by other factors. The major impediment to resolving this issue is the absence of a validated diagnostic test for measuring resistance in D. immitis. Here we report the development and optimization of an in vitro bioassay for measuring the in vitro susceptibility of D. immitis L3 to ML anthelmintics. Using an established ML-susceptible laboratory isolate, we demonstrated the ability to reproducibly measure the in vitro dose–response characteristics of D. immitis L3 to ivermectin and eprinomectin.
Considering the biology and epidemiology of D. immitis and its extreme sensitivity to ML drugs, the emergence of resistance in this parasite has historically been considered unlikely (Prichard, 2005). However, increasingly common LOE reports have raised concerns that ML resistance is developing in heartworms (Hampshire, 2005). In addition, several recent studies have reported genetic structural differences and/or sequence changes in some heartworm isolates leading these authors to conclude that resistance is present (Bourguinat et al., 2011a,b). Single nucleotide polymorphisms (SNPs) have been reported to occur in a gene encoding a P-glycoprotein, a class of proteins implicated in reduced ML efficacy against the filarioid Onchocerca volvulus (Prichard and Roulet, 2007), and associated with ML-resistance in Haemonchus contortus (Xu et al., 1998). Examining multiple D. immitis isolates, Bourguinat et al. (2011b) reported a correlation between the frequency of a pair of SNPs and reduced in vitro ML efficacy on microfilariae in an isolate-specific manner. In one case, a dog originating from Louisiana demonstrated persistent microfilaremia following multiple high-dose ML treatments (Bourguinat et al., 2011a). A high frequency of the aforementioned genotype was observed in this population of microfilariae, prompting the authors to cite ML resistance as the cause of this anomaly. However, even after numerous high-level doses of ML drugs, only 45% of the recovered microfilariae carried the SNPs suspected to be associated with resistance. Therefore, the significance of these SNPs as markers for resistance remains unclear. Though serious concerns of ML-resistance in D. immitis have existed for several years, no definitive studies that demonstrate this resistance under controlled conditions have yet been reported, and no assays for the detection of resistance have been validated.
The LMIA developed in this study was modified from those applied to gastrointestinal nematodes of ruminants for assessing drug-resistance status. Several other in vitro bioassays also exist for diagnosing ML resistance in gastrointestinal nematodes, the most commonly used being the larval development assay (LDA). However, the dissimilar biology of the filarioids prevents the application of this assay, and thus the LMIA was selected as the most viable and applicable technique. Extensive modification of the assay was required for optimal performance with filarial nematodes, which are, by comparison, highly sensitive to environmental exposure. In particular, four aspects of the D. immitis LMIA were the focus of optimization: (1) incubation time was adjusted to allow sufficient ML exposure while maintaining control parasite viability; (2) the medium and antibiotics were optimized for filarioid culture conditions; (3) the mesh size (25 μm) was selected for optimal discrimination of motile vs. non-motile D. immitis L3; and (4) individual L3 were counted and placed in wells of the assay plate rather than estimated numbers being aliquoted in known volumes of liquid, as is typically done in protocols for ruminant nematodes. This was done to reduce well-to-well variability given the relatively small numbers of D. immitis L3 that could be readily obtained for each experiment.
The optimized assay reported here permitted a consistent high-level migration in untreated control parasites and yielded consistent, reproducible dose–response data. This permitted us to calculate accurate IC50 values for each drug examined. However, even at the highest ML concentrations that could be solubilized, complete inhibition of larval migration was rarely observed; the mean inhibition at 20 μM was 88.8% (SD = 6.89%) for eprinomectin and 70.0% (SD = 14.0%) for ivermectin. Therefore, it was not possible to accurately measure IC95 values, leading to very wide 95% confidence intervals. Higher ML concentrations were not used owing to their poor solubility at the solvent level used (1% DMSO). Increasing the solvent level permits the use of higher ML concentrations, but this adversely affects parasite viability. Since it is critical that the untreated control parasites maintain high levels of viability throughout the entire experiment, this was not feasible. Any reduction in parasite viability in the no-drug control wells will increase variability and non-specific error, decreasing the consistency and accuracy of the data. The IC50 usually is the most robust and accurate measure of the overall susceptibility of a population because data is collected at concentrations both above and below this value. This was also true of our data, thus the IC50 was selected as the parameter of choice. Furthermore, with regard to the planned use of this assay, if an LOE case is not due to compliance failure, then all parasites in that dog would have established in the presence of regular ML prophylaxis. Consequently, all worms infecting the dog would be resistant, leading to reduced heterogeneity in the dose–response of the F2 generation. Thus, the IC50 is the most logical and appropriate parameter for evaluating the susceptibility of any suspected-resistant D. immitis isolate with an in vitro assay. In contrast, there is usually high variability at the upper plateau of the dose–response, especially when 100% inhibition is not achievable. This tends to make the IC95 a highly unstable parameter that is prone to variability-induced errors of interpretation.
Macrocyclic lactone resistance in H. contortus and Cooperia oncophora is well-established, and the LMIA has proven effective in discriminating resistant from susceptible isolates (Kotze et al., 2006; Demeler et al., 2010). Measuring these differences is also dependent upon how well dose–response data fit the statistical model being used, and in this study we found noticeably higher R2 values and smaller confidence intervals for IC50 in assays with eprinomectin than with ivermectin. This disparity is not unusual, as in vitro responses in the LMIA have been reported to vary with the ML compound used (Kotze et al., 2006). This group also found that eprinomectin yielded superior results as compared to ivermectin in the LMIA. Gill and Lacey (1998) first reported that the optimal ML compound for use in an in vitro assay varies among assay type and parasite species, and thus needs to be determined empirically by experimentation for each assay-species combination. This finding demonstrates that the drug used in a particular in vitro bioassay has broad relevance and applicability for the entire anthelmintic class it represents. For example, in the LDA, the analog that provides the greatest discrimination between ML-susceptible and ML-resistant isolates of H. contortus and Trichostrongylus spp. is avermectin aglycone (Gill and Lacey, 1998; Kaplan et al., 2007), a drug that has poor efficacy in vivo and has not been developed as an anthelmintic product. Consequently, it is unimportant that eprinomectin is not used clinically for the prevention of heartworm infection. Future work will involve performing the LMIA with suspected-resistant D. immitis isolates, and the testing of additional ML compounds to determine whether eprinomectin or another analog is the best choice for discriminating heartworm populations of differing drug susceptibilities.
The suitability of the LMIA for assessing ML susceptibility in filarioids must also be considered further. The LMIA is a motility-based assay and the ML anthelmintics are known to cause paralysis in gastrointestinal nematodes of ruminants (Boisvenue et al., 1983; Folz et al., 1987). This rationale was the basis of the assay’s development and validation as a diagnostic test for ML-resistance in ruminant nematodes. Given the extremely high ML sensitivity of D. immitis L3 and L4 stages, for which motility and migration are critical in both the initial infection of the canine host and subsequent tissue migration, we assumed that the LMIA would be the ideal assay for detecting ML resistance in this parasite. However, we observed that the ML concentrations required to induce an in vitro paralytic response were quite high. The preventive dose of ivermectin (6 μg/kg), which yields 100% clinical efficacy, produces peak plasma levels in the dog of approximately 3 ng/ml (Daurio et al., 1992). In contrast, the present study demonstrated an IC50 value of 4.56 μM (3.99 μg/ml) for ivermectin, and even at 20 μM concentrations (17.5 μg/ml ivermectin; 18.3 μg/ml eprinomectin) we failed to effect total migration inhibition. In vitro concentrations more than 6000 times higher than those achieved in vivo could not come close to reaching the level of 100% inhibition. The disparity in drug level and the limited achievable efficacy challenge our assumption that the in vitro and in vivo responses of D. immitis to MLs are comparable and lead us to believe that the drug may be acting through at least two different mechanisms. Indeed, the host immune response may be integral to ML activity in D. immitis as indicated by research in other filarioids. In vitro treatment of Brugia malayi microfilariae with ivermectin was reported to interfere with protein secretion, which may unmask this parasite to the host immune system and allow its clearance (Moreno et al., 2010). Furthermore, studies with Acanthocheilonema viteae microfilariae reported that, while this filarioid is unaffected by nanomolar concentrations of ivermectin in vitro, it is subject to cell-mediated cytotoxicity when treated in the presence of host serum (Rao et al., 1987). Similar observations were reported for Litomosoides carinii, though for this parasite, treatment with ivermectin before exposure to immune cells appeared to elicit a cytotoxic response when worms were later cultured in the absence of drug (Zahner and Schmidtchen, 1994). These observations, both recent and old, suggest that the ML mechanism of action in filarioid parasites (clade III) may differ from that in the phylogenetically distinct gastrointestinal nematodes (clade V). If this is true, then it follows that in vitro bioassays developed for detecting ML resistance in gastrointestinal nematodes will not be effective in filarioids like D. immitis. Further research will investigate these issues, aiming to improve our understanding of the in vivo action of the MLs. We hope that further elucidation of the mechanism by which ML drugs kill these parasites will give us new insight into the effective measurement of ML susceptibility and, by extension, the diagnosis of resistance.
Acknowledgements
We gratefully acknowledge Drs. J. Carmichael and R. Schenker for their support and Novartis Animal Health for funding. We also thank Drs. M.T. Dzimianski and P. Supakorndej for their instruction in the techniques described herein.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary data
Migration of larvae across a membrane. Dirofilaria immitis L3 are placed over a 25 μm mesh filter, across which they are seen to be actively migrating.
References
- Boisvenue R.J., Brandt M.C., Galloway R.B., Hendrix J.C. In vitro activity of various anthelmintic compounds against Haemonchus contortus larvae. Vet. Parasitol. 1983;13:341–347. doi: 10.1016/0304-4017(83)90050-x. [DOI] [PubMed] [Google Scholar]
- Bourguinat C., Keller K., Bhan A., Peregrine A., Geary T., Prichard R. Macrocyclic lactone resistance in Dirofilaria immitis. Vet. Parasitol. 2011;181:388–392. doi: 10.1016/j.vetpar.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Bourguinat C., Keller K., Blagburn B., Schenker R., Geary T.G., Prichard R.K. Correlation between loss of efficacy of macrocyclic lactone heartworm anthelmintics and P-glycoprotein genotype. Vet. Parasitol. 2011;176:374–381. doi: 10.1016/j.vetpar.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Bowman D.D., Atkins C.E. Heartworm biology, treatment, and control. Vet. Clin. North America: Small Anim. Practice. 2009;39:1127–1158. doi: 10.1016/j.cvsm.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Campbell W.C. Use of ivermectin in dogs and cats. In: Campbell W.C., editor. Ivermectin and Abamectin. Springer-Verlag New York Inc.; New York: 1989. pp. 245–259. [Google Scholar]
- Daurio C.P., Cheung E.N., Jeffcoat A.R., Skelly B.J. Biovailability of ivermectin administered orally to dogs. Vet. Res. Commun. 1992;16:125–130. doi: 10.1007/BF01839009. [DOI] [PubMed] [Google Scholar]
- Demeler J., Küttler U., El-Abdellati A., Stafford K., Rydzik A., Varady M., Kenyon F., Coles G., Höglund J., Jackson F., Vercruysse J., von Samson-Himmelstjerna G. Standardization of the larval migration inhibition test for the detection of resistance to ivermectin in gastro intestinal nematodes of ruminants. Vet. Parasitol. 2010;174:58–64. doi: 10.1016/j.vetpar.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Folz S.D., Pax R.A., Thomas E.M., Bennett J.L., Lee B.L., Conder G.A. Development and validation of an in vitro Trichostrongylus colubriformis motility assay. Int. J. Parasitol. 1987;17:1441–1444. doi: 10.1016/0020-7519(87)90080-4. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Bourguinat C., Prichard R.K. Evidence for macrocyclic lactone anthelmintic resistance in Dirofilaria immitis. Top. Companion Anim. Med. 2011;26:186–192. doi: 10.1053/j.tcam.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Gill J.H., Lacey E. Avermectin/milbemycin resistance in trichostrongyloid nematodes. Int. J. Parasitol. 1998;28:863–877. doi: 10.1016/s0020-7519(98)00068-x. [DOI] [PubMed] [Google Scholar]
- Hampshire V.A. Evaluation of efficacy of heartworm preventive products at the FDA. Vet. Parasitol. 2005;133:191–195. doi: 10.1016/j.vetpar.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Kaplan R. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., Vidyashankar A.N. An inconvenient truth: global worming and anthelmintic resistance. Vet. Parasitol. 2012;186:70–78. doi: 10.1016/j.vetpar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., Vidyashankar A.N., Howell S.B., Neiss J.M., Williamson L.H., Terrill T.H. A novel approach for combining the use of in vitro and in vivo data to measure and detect emerging moxidectin resistance in gastrointestinal nematodes of goats. Int. J. Parasitol. 2007;37:795–804. doi: 10.1016/j.ijpara.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Kotani T., Powers K.G. Developmental stages of Dirofilaria immitis in the dog. Am. J. Vet. Res. 1982;43:2199–2206. [PubMed] [Google Scholar]
- Kotze A., Le Jambre L., Ogrady J. A modified larval migration assay for detection of resistance to macrocyclic lactones in Haemonchus contortus, and drug screening with Trichostrongylidae parasites. Vet. Parasitol. 2006;137:294–305. doi: 10.1016/j.vetpar.2006.01.017. [DOI] [PubMed] [Google Scholar]
- McCall J.W. The role of arthropods in the development of animal models for filariasis research. J. Georgia Entomol. Soc. 1981;16:283–293. [Google Scholar]
- Moreno Y., Nabhan J.F., Solomon J., Mackenzie C.D., Geary T.G. Ivermectin disrupts the function of the excretory–secretory apparatus in microfilariae of Brugia malayi. Proc. Natl. Acad. Sci. USA. 2010;107:20120–20125. doi: 10.1073/pnas.1011983107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard R.K. Is anthelmintic resistance a concern for heartworm control?: what can we learn from the human filariasis control programs? Vet. Parasitol. 2005;133:243–253. doi: 10.1016/j.vetpar.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Prichard R.K., Roulet A. ABC transporters and beta-tubulin in macrocyclic lactone resistance: prospects for marker development. Parasitology. 2007;134:1123–1132. doi: 10.1017/S0031182007000091. [DOI] [PubMed] [Google Scholar]
- Rao U.R., Chandrashekar R., Subrahmanyam D. Effect of ivermectin on serum dependent cellular interactions to Dipetalonema viteae microfilariae. Trop. Med. Parasitol. 1987;38:123–127. [PubMed] [Google Scholar]
- Taylor M.A., Hunt K.R., Goodyear K.L. Anthelmintic resistance detection methods. Vet. Parasitol. 2002;103:183–194. doi: 10.1016/s0304-4017(01)00604-5. [DOI] [PubMed] [Google Scholar]
- Wagland B.M., Jones W.O., Hribar L., Bendixsen T., Emery D.L. A new simplified assay for larval migration inhibition. Int. J. Parasitol. 1992;22:1183–1185. doi: 10.1016/0020-7519(92)90040-r. [DOI] [PubMed] [Google Scholar]
- Wolstenholme A.J., Rogers A.T. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology. 2005;131(Suppl.):S85–S95. doi: 10.1017/S0031182005008218. [DOI] [PubMed] [Google Scholar]
- Xu M., Molento M., Blackhall W., Ribeiro P., Beech R., Prichard R. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol. Biochem. Parasitol. 1998;91:327–335. doi: 10.1016/s0166-6851(97)00215-6. [DOI] [PubMed] [Google Scholar]
- Zahner H., Schmidtchen D. Ivermectin-induced cell-dependent lethal effects on Litomosoides carinii microfilariae in vitro. Trop. Med. Parasitol. 1994;45:336–340. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Migration of larvae across a membrane. Dirofilaria immitis L3 are placed over a 25 μm mesh filter, across which they are seen to be actively migrating.


