Graphical abstract

Keywords: Anthelmintic resistance, Trichostrongyloid nematodes, Sheep, In vitro assay
Highlights
-
•
Ivermectin and its two components contribute to action and resistance.
-
•
Moxidectin tended to have lower resistance ratios than ivermectin in the LDA.
-
•
Moxidectin was the most potent inhibitor of migration in susceptible H. contortus.
-
•
LMIA performs better in detecting resistance to MOX than LDA.
Abstract
Anthelmintic resistance has emerged as an important problem in animal industries. Understanding resistance mechanisms, especially against macrocyclic lactones (MLs), is the first step in developing better diagnostic tools. Effects of several MLs including ivermectins and milbemycins were tested using two well established in vitro assays: the larval development assay (LDA) and the larval migration inhibition assay (LMIA). These were performed on free-living stages of susceptible and ML-resistant isolates of three trichostrongyloid nematode species of sheep. In general, dose response curves shifted to the right in the resistant isolates. Data showed that resistance was present to ivermectin and its two components suggesting that both components contribute to action and resistance. There were no consistent patterns of potency and resistance of the tested substances for the different isolates in the LDA except that moxidectin (MOX) tended to have lower resistance ratios than ivermectin (IVM). MOX was the most potent inhibitor in the LMIA in susceptible Haemonchus contortus while being less potent in Trichostrongylus colubriformis and particularly in Ostertagia circumcincta. MOX showed high resistance ratios in the LMIA in all three species. Based on these results, resistance to MOX has unique characteristics and the LMIA may perform better in detecting resistance to MOX in these parasite species.
1. Introduction
The macrocyclic lactone anthelmintics (MLs), typified by ivermectin (IVM) have become the mainstay of control of internal parasites of sheep and other production animals. However, the development of anthelmintic resistance to MLs in several nematode species limits the continued use of these anthelmintics. Resistance to IVM associated with reduced treatment efficacy in infected animals in the field has been reported for Haemonchus contortus, Trichostrongylus colubriformis and Ostertagia circumcincta (syn. Teladorsagia circumcincta) in several countries worldwide (Jackson and Coop, 2000; Le Jambre et al., 2000; Leathwick et al., 2001; Love, 2002; Kaplan, 2004). ML anthelmintics fall into two broad classes, the avermectins, including IVM and abamectin (ABA), and the milbemycins, including moxidectin (MOX). They differ in potency and, possibly, in their resistance profile and pharmacology (Sutherland et al., 1999; Ardelli et al., 2009; Demeler et al., 2010). MOX in particular has prolonged clearance kinetics in vivo which also contributes to its efficacy (see Prichard et al., 2012 for summary). IVM itself is composed of approximately 80% of the IVM B1a component and 20% IVM B1b.
In vitro assays have been used to measure the characteristics of resistance in parasite populations. Such assays are cheap, relatively quick, obviate host influences and, as they can be performed over a concentration range, provide reproducible parameters with which to measure resistance. Furthermore, they can be used to investigate the mode of action of anthelmintics, the pharmacology of anthelmintics and the basis of resistance in worms. In vitro tests also have utility as field diagnostic tools and some test systems have been validated where parasite populations resistant to IVM treatment in vivo have been found to be less sensitive to IVM in larval development and larval motility assays (Gill et al., 1991, 1995).
IVM is known to inhibit development and motility in larval stages of gastrointestinal nematodes (Gill et al., 1995). The pharynx of a number of nematodes has been found to be extremely sensitive to this drug (Geary et al., 1993; Brownlee et al., 1997; Dent et al., 1997; Kotze, 1998; Sheriff et al., 2002) and it has been suggested as a major site of action (Geary et al., 1993). The concentrations of IVM required to inhibit pharyngeal pumping in adults (Sheriff et al., 2002) are similar to the concentrations that inhibit larval development in vitro such as in the larval development assay (LDA) (Gill et al., 1995). Somatic muscle has also been identified as a site of action of the ML class. IVM has inhibitory effects on larvae (Gill et al., 1995; Kotze et al., 2006) and adult worms (Geary et al., 1993; Kotze et al., 2012). The potency at this site ranges between 0.015 and 100 nM depending on the method used to detect motility. Therefore, it can be assumed that the larval migration inhibition assay (LMIA) can be used to detect effects on somatic muscle and related neuromuscular sites (Sangster et al., 1988). On the other hand, the LDA is not specific for a particular organ and may involve pharyngeal and/or somatic muscle.
The aim of the present study was to characterise responses to MLs in resistant and susceptible isolates of three species of sheep parasitic nematodes and to evaluate possible differences between several chemical variants of both classes that comprise the anthelmintic MLs. In particular, we compared results from the two in vitro assays. Additional aspects of these experiments were to evaluate these in vitro test systems as indicators of the in vivo drug resistance pattern and therefore as diagnostic tools.
2. Materials and methods
2.1. Parasites
The H. contortus McMaster isolate (H.c. McM), the T. colubriformis McMaster (T.c. McM) and the O. circumcincta McMaster isolate (O.c. McM) were obtained from the McMaster laboratories, CSIRO (Commonwealth Scientific and Industrial Research Organisation, Armidale, Australia). They are all drug susceptible isolates with no history of exposure to MLs or other anthelmintics.
The H. contortus CAVR (Chiswick-AVermectin-Resistant) is highly resistant against IVM and avermectins and showed slightly decreased sensitivity to MOX in vivo (Le Jambre et al., 1995). The MOXidectin-Resistant isolate of T. colubriformis (T.c. MOX-R) was originally isolated from a goat farm on the North Coast, NSW, Australia, where goats had been treated with MOX eight times per year after IVM failed to remove the parasites. In a controlled test T.c. MOX-R were found to be resistant to a recommended dose of MOX (41% efficacy) and ABA (14% efficacy) (Le Jambre et al., 2005). Both isolates were also obtained from CSIRO. The resistant isolate O. circumcincta WAMIRO (Western-Australia-Moxidectin-Resistant Ostertagia) was obtained from Dr. Brown Besier (WA Department of Agriculture). This MOX-resistant isolate was originally isolated from sheep at Murdoch University, Australia, and was cultured without further selection. Use of FECRTs and slaughter trials showed it to be MOX-resistant (5% efficacy) and highly IVM-resistant (0% efficacy) (Besier, pers. comm.).
Each isolate was individually passaged every 2 months in 3–9 month old merino-cross wethers. Sheep carrying the resistant isolate of H. contortus were treated with IVM (0.2 mg/kg), sheep carrying the resistant isolates of T. colubriformis or O. circumcincta were treated with MOX (0.2 mg/kg) on each passage, 3 weeks after infection. Eggs and larvae obtained from at least three different passages of each isolate were used in the assays. No differences in response to the tested substances in either assay were observed for different parasitic stages obtained from different passages.
2.2. Materials
IVM was obtained from Sigma (Australia). IVM B1a, IVM B1b, milbemycin (Mil) A3 and A4 were supplied by the Microbial Screening Technologies (MST, Smithfield, Australia), MOX was supplied by Fort Dodge Animal Health (Sydney) as a 95% pure preparation. Stock dilutions of each of the six drugs (10−2 M = 10 mM) were prepared in 100% dimethyl sulfoxide (DMSO). For the LDA these were serially diluted 1:2 with DMSO to give 12 concentrations. Each were then diluted 1:5 with water (20% DMSO). Aliquots (10 μl) of each drug concentration were dispensed across the 12 wells of a 96-well-plate (Falcon). Each of the six compounds occupied one row and the others contained water with 0.5% DMSO (row A) or 20% DMSO (row H) as controls. 190 μl of molten agar (2%) was added to each well (0.5% final DMSO concentration) and the following ranges of final drug concentrations: IVM, its components and MOX: 250–0.122 nM; milbemycins: 500–0.244 nM.
For the LMIA, the stock solutions were diluted in distiled water to give five final concentrations each in 1% DMSO. For the resistant isolates, the concentration range was between 3 μM and 30 nM and for the susceptible isolates between 1 μM and 10 nM. Appropriate concentration ranges for each isolate were determined in preliminary studies.
2.3. Larval development assay (LDA)
The sensitivity of the isolates was determined in a LDA as described by Gill et al. (1995). It measures the potency of anthelmintics as inhibitors of the development of trichostrongyloid nematodes from eggs though to infective third stage larvae (L3).
Briefly, nematode eggs (∼80–100/10 μl) recovered from faeces were added to the surface of an agar matrix in each well of a 96-well plate. Eggs in these wells were supplemented with nutrient medium and incubated for 6–8 days at 25 °C. A susceptible and a resistant isolate of the same species were always run in quadruplicate at the same time and a total of three separate assays were performed for each isolate.
The proportion of undeveloped stages [(L1 and L2) + eggs] to the total (all larvae + eggs) present in each well was calculated and expressed in percent. Data were corrected for P0, the mean number of larvae not developing in the control wells. A four parameter logistic model (GraphPad Prism® software) was used to fit sigmoidal (logistic) curves to the dose–response data and to compare the curves statistically (using TOP, BOTTOM, EC50, HILLSLOPE as parameters). A sigmoidal dose response model with variable slope was chosen to allow fitting of the Hill slope. Positive and negative control values were defined as 0 and 100 by normalising the control data to allow exact calculation of the effective concentration for 50% effect (EC50). Data points from different experiments with the same drug and isolate were co-analysed and the EC50 and 95% confidence limits were calculated for each isolate. Each EC50 was derived from 288 data points.
For each parasite species, the sensitivity of the resistant isolate to a given drug was expressed as a resistance ratio (RR) (EC50 for resistant isolate/EC50 for susceptible isolate). P values, reflecting statistically highly significant differences between EC50 values and the Hill slopes for the resistant and susceptible isolates of each nematode species for each drug were determined by GraphPad Prism®.
2.4. Larval migration inhibition assay (LMIA)
This assay was carried out similarly to the method described previously (Sangster et al., 1988; Demeler et al., 2010, 2012); with some modifications. Briefly, L3 were incubated in different concentrations of drugs and then left to migrate through precision woven nylon sieves with 25 μm apertures. This size mesh allows active larvae but not dead larvae to pass through the sieve. Assays were conducted in 24-well tissue culture plates (Falcon).
L3 were incubated in the drug solutions (1.5 ml) for 24 h in the dark at 28 °C in the plates. A second set of plates (migration plates) were prepared with 400 μl agar (2%) in each well. The agar was added to assist counting by keeping the larvae in view under the microscope. Larvae were then transferred to the 25 μm aperture sieves in the second plate and allowed to migrate for 24 h at 28 °C.
For each concentration, migrated and non-migrated larvae were counted under a microscope and the percentage of (non-migrated larvae/total larvae) × 100 was calculated. Additionally, postures and movement characteristics of larvae were noted. The data were corrected for the average of all control wells from the same plate. Each assay was carried out in duplicate (on the same plate) and a total of 2 or 3 assays per isolate and drug were performed.
The data were then analysed using GraphPad Prism® software as for the larval development assay using four to six data points for each drug concentration. Different experiments with the same drug and isolate were co-analysed and the EC50, 95% confidence limits and P values for each isolate were calculated. Each EC50 was derived from 120 data points. The sensitivity of an isolate to a given drug was expressed as RR.
3. Results
3.1. Larval development assay
In the control wells more than 95% of the eggs hatched and about 95% of the hatched larvae developed through to L3. The goodness of fit (R2) for all curves was between 0.95 and 0.99.
For H. contortus, the dose–response-curve for all tested components (Fig. 1) showed a significant shift to the right (higher EC50) for the resistant compared with the susceptible isolate. All EC50 values including the confidence limits and the RR values, reflecting significant differences in the EC50 are shown in Table 1. IVM and the two milbemycins (Mil A3 and Mil A4) were the most potent (EC50 = 0.364–0.752 nM) for H. contortus. Interestingly, the two components of IVM, IVM B1a and IVM B1b, were less potent (∼0.950 nM) than the mixture. MOX was similar in potency to IVM B1a and IVM B1b. MOX had the lowest ranked RR (2.4), followed by IVM B1a, IVM B1b, IVM and the other two milbemycins.
Fig. 1.
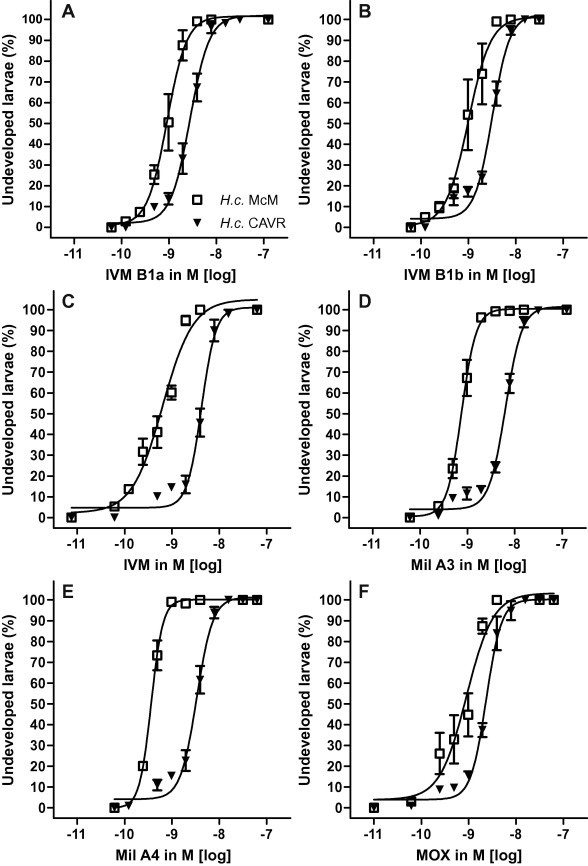
Activity of tested drugs (A: IVM B1a, B: IVM B1b; C: IVM; D: Mil A3; E: Mil A4; F: MOX) as inhibitors of larval development of susceptible versus resistant Haemonchus contortus isolates (H.c. McM, H.c. CAVR) in the larval development assay (LDA).
Table 1.
EC50 (effective concentration for 50% inhibition) with 95% confidence limits (CL) in nM and the resistance ratios (RR) for the tested macrocyclic lactones in the LDA for susceptible and resistant isolates of Haemonchus contortus (H.c. McM, H.c. CAVR), Trichostrongylus colubriformis (T.c. McM, T.c. MOX-R) and Ostertagia circumcincta (O.c. McM, O.c. WAMIRO).
| Drugs | H.c. McM | H.c. CAVR | RR | p | T.c. McM | T.c. MOX-R | RR | p | O.c. McM | O.c. WAMIRO | RR | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50 (CL) | EC50 (CL) | EC50 (CL) | EC50 (CL) | EC50 (CL) | EC50 (CL) | |||||||
| B1a | 0.913 (0.75–1.11) | 2.83 (2.42–3.31) | 3.10 | ⁎ | 3.54 (3.09–4.04) | 56.1 (47.42–66.31) | 15.85 | ⁎ | 3.59 (3.47–4.23) | 15.1 (10.31–22.23) | 4.21 | ⁎ |
| B1b | 0.996 (0.73–1.36) | 3.35 (3.01–3.73) | 3.38 | ⁎ | 6.76 (6.25–7.31) | 82.5 (64.14–106.1) | 12.20 | ⁎ | 3.62 (2.92–4.49) | 8.96 (7.577–10.59) | 2.48 | ⁎ |
| IVM | 0.635 (0.49–0.83) | 4.21 (3.74–4.73) | 6.63 | ⁎ | 5.57 (4.99–6.22) | 52.5 (44.64–61.64) | 9.43 | ⁎ | 5.58 (5.06–6.16) | 14.3 (10.87–18.85) | 2.56 | ⁎ |
| MOX | 0.965 (0.62–1.49) | 2.33 (2.07–2.62) | 2.42 | ⁎ | 7.00 (5.70–8.59) | 50.8 (41.81–61.60) | 7.26 | ⁎ | 5.04 (4.69–5.40) | 5.71 (4.67–6.99) | 1.13 | |
| Mil A3 | 0.752 (0.68–0.83) | 6.50 (5.96–7.10) | 8.64 | ⁎ | 8.45 (5.94–12.02) | 271 (215.9–339.4) | 32.07 | ⁎ | 3.56 (2.71–4.68) | 14.7 (12.68–16.3) | 4.13 | ⁎ |
| Mil A4 | 0.364 (0.34–0.39) | 3.40 (3.00–3.85) | 9.34 | ⁎ | 4.25 (3.58–5.06) | 123 (108.4–139.4) | 28.94 | ⁎ | 1.77 (1.33–2.36) | 7.09 (5.54–9.07) | 4.01 | ⁎ |
p < 0.0001.
Compared with H. contortus, the drugs were about 10-fold less potent (i.e. EC50 was 10-fold higher) in T. colubriformis. With T. colubriformis, there was little difference between the potency of drugs for the susceptible isolate (EC50 = 3.5–8.5 nM). The most potent drug was IVM B1a (EC50 = 3.54 nM), the least potent Mil A3 (EC50 = 8.45 nM). IVM B1b was less potent than IVM B1a and the potency of IVM was close to the mean of its two components (Table 1). The shifts in the dose–response curves of resistant isolates (Fig. 2) showed statistically significant increases (p < 0.0001). The milbemycins had the highest ranked RRs (32 for Mil A3, 29 for Mil A4). In contrast to H. contortus, both components of IVM showed higher RRs (IVM B1a 15.85 and IVM B1b 12.20) than IVM itself (9.43). The drug with the lowest ranked RR was again MOX (RR = 7.26).
Fig. 2.
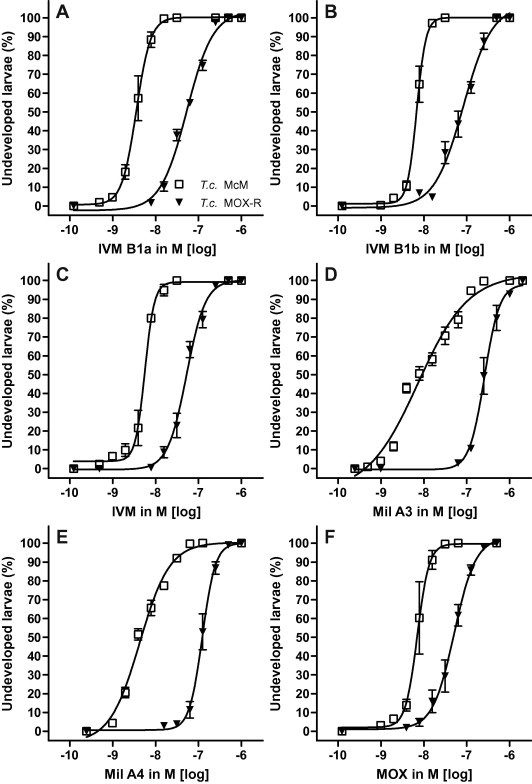
Activity of tested drugs (A: IVM B1a, B: IVM B1b; C: IVM; D: Mil A3; E: Mil A4; F: MOX) as inhibitors of larval development of susceptible versus resistant Trichostrongylus colubriformis isolates (T.c. McM, T.c. MOXR) in the larval development assay (LDA).
For O. circumcincta, the drugs were of similar potency as for T. colubriformis with EC50 values within the range 1.5–6 nM (Table 1) (Fig. 3). The most potent drug was Mil A4 followed, in rank order, by Mil A3, IVM B1a and IVM B1b. IVM itself was less potent than each of its components. MOX had similar potency as IVM. The RR’s for Mil A3, IVM B1a and Mil A4 exceeded 4, for IVM B1b and IVM were ∼2.5 and MOX was again ranked the lowest with RR of 1.13 which was not significant.
Fig. 3.
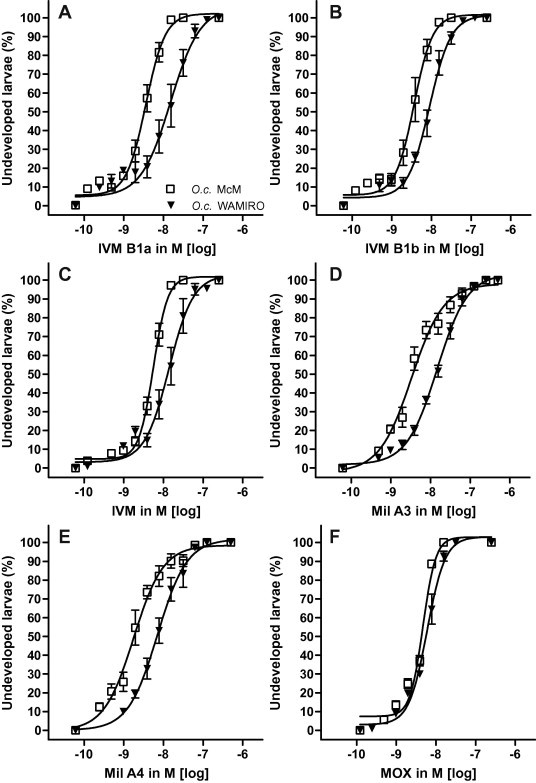
Activity of tested drugs (A: IVM B1a, B: IVM B1b; C: IVM; D: Mil A3; E: Mil A4; F: MOX) as inhibitors of larval development of susceptible versus resistant Ostertagia circumcincta isolates (O.c. McM, O.c. WAMIRO) in the larval development assay (LDA).
3.2. Larval migration inhibition assay
During incubation, larvae underwent transient paralysis, abatement of paralysis and, by 18 h, irreversible paralysis. As a result, reproducible migration performance was obtained for all species following 24 h of migration. Selected dose response curves highlighting the differences between IVM and MOX for H. contortus and O. circumcincta are shown in Fig. 4 and the data in Table 2. For H. contortus, the most potent drug was MOX with an EC50 of 371 nM. The three ivermectins ranged between 502 and 676 nM, followed by Mil A4 and Mil A3. The two components of IVM both ranked more highly than IVM itself and both milbemycins were less potent again (Table 2).
Fig. 4.
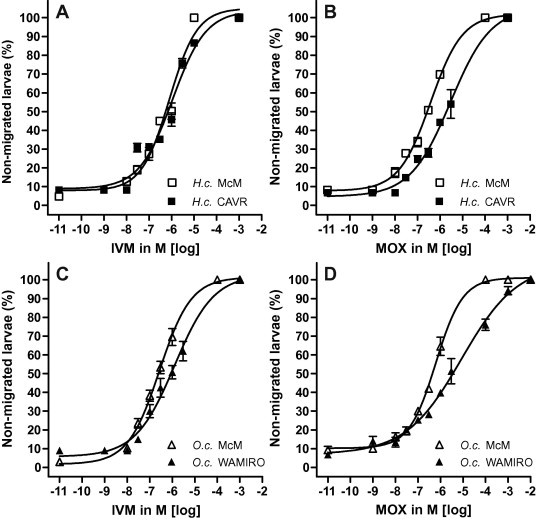
Activity of IVM and MOX as inhibitors of larval migration of susceptible versus resistant isolates of Haemonchus contortus (H.c. McM, H.c. CAVR) and Ostertagia circumcincta (O.c. McM, O.c. WAMIRO) in the larval migration inhibition assay (LMIA).
Table 2.
EC50 (effective concentration for 50% inhibition) with 95% confidence limits (CL) in nM and the resistance ratios (RR) for the tested macrocyclic lactones in the LMIA for susceptible and resistant isolates of Haemonchus contortus (H.c. McM, H.c. CAVR), Trichostrongylus colubriformis (T.c. McM, T.c. MOX-R) and Ostertagia circumcincta (O.c. McM, O.c. WAMIRO.
| Drugs | H.c. McM | H.c. CAVR | RR | p | T.c. McM | T.c. MOX-R | RR | p | O.c. McM | O.c. WAMIRO | RR | p |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50 (CL) | EC50 (CL) | EC50 (CL) | EC50 (CL) | EC50 (CL) | EC50 (CL) | |||||||
| B1a | 502 (360–699) | 621 (425.5–906.7) | 1.24 | 595 (407–868.6) | 1070 (715.8–1602) | 1.79 | ⁎ | 472 (288.5–773.4) | 1820 (1280–2600) | 3.86 | ⁎⁎ | |
| B1b | 652 (387.2–1098) | 873 (636.6–1198) | 1.34 | 535 (351.6–814.9) | 3780 (2429–5878) | 7.07 | ⁎⁎ | 514 (337.6–783.6) | 833 (643.1–1095) | 1.62 | ⁎ | |
| IVM | 676 (402.3–1136) | 755 (338.4–1683) | 1.12 | 447 (309.8–643.7) | 1420 (933.0–2152) | 3.18 | ⁎⁎ | 266 (197.7–357.4) | 1130 (744.3–1724) | 4.25 | ⁎⁎ | |
| MOX | 371 (301.6–456.4) | 2500 (1530–4070) | 6.74 | ⁎⁎ | 471 (326.8–678.4) | 3430 (3323–5332) | 7.28 | ⁎⁎ | 618 (485.2–788.2) | 13900 (6112–31590) | 22.45 | ⁎⁎ |
| Mil A3 | 1970 (1199–3225) | 670 (461.1–973.6) | n.c. | 803 (507.7–1271) | 2550 (1857–3498) | 3.18 | ⁎⁎ | 1240 (921.3–1667) | 7870 (4003–15480) | 6.35 | ⁎⁎ | |
| Mil A4 | 827 (522.8–1308) | 792 (498.7–1257) | n.c. | 1200 (753.4–1909) | 9870 (6254–15560) | 8.23 | ⁎⁎ | 1410 (817–2441) | 2510 (1519–4136) | 1.78 |
n.c. = not calculated.
p < 0.0001;
p < 0.05.
For H. contortus, MOX was the only drug for which a significant difference (RR = 6.74) was obtained. For Mil A3 and A4 RRs were not calculated due to lack of fit. Selected dose response curves for T. colubriformis highlighting the differences between IVM and its two components are shown in Fig. 5. Here, the most potent drugs were IVM and MOX, followed by IVM B1b and IVM B1a and then the two milbemycins. In contrast to H. contortus, both components of IVM were less potent than IVM itself (Fig. 5; Table 2). For T. colubriformis, shifts in the dose response curves and significant RRs were observed for all drugs tested. MOX and IVM B1b furnished similar RRs.
Fig. 5.
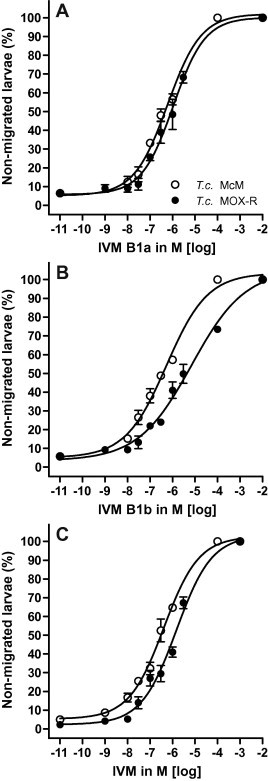
Activity of IVM and its components IVM B1a and IVM B1b as inhibitors of larval migration of susceptible versus resistant isolates of Trichostrongylus colubriformis (T.c. McM, T.c. MOXR) in the larval migration inhibition assay (LMIA).
For O. circumcincta, IVM (EC50 = 266 nM) was ranked as the most potent drug although both of its components were relatively less potent (472–514 nM) (Table 2). Of the three milbemycins, MOX was the highest ranked (EC50 = 618 nM). For RRs, significant differences in EC50 values between susceptible and resistant isolates were obtained for all but Mil A4 and the RR for MOX was ranked highest at 22.45.
4. Discussion
4.1. Parasite isolates and assays
Using a matrix of chemicals, assays and species provided valuable data on resistance pharmacology. Some differences that were observed between the species and their isolates may reflect histories of selection for resistance as well as inherent species differences in drug action and resistance. For example, while all three resistant isolates were IVM-resistant, only the T. colubriformis and O. circumcincta isolates were also MOX-resistant. Although the isolates used here may not be representative of field isolates, their high levels of resistance meant they were good tools for investigating the pharmacology of ML resistance because they provided clear contrasts in the assay results.
While the study of free-living stages has many costs, logistic and ethical advantages over in vivo tests, such in vitro assays are surrogates for adult parasites which are the target of anthelmintic control. Eggs and larval stages will likely differ from adults in many ways, such as expressing different biochemical sites, different (or no) resistance mechanisms and possible physicochemical differences in drug exposure. Nevertheless, these assays have proved useful in measuring ML resistance and mirror aspects of the resistance pharmacology seen in adult worms while removing the influence of drug pharmacokinetics in vivo. Further, in vitro assays are amenable to fitting concentration/response curves and thus statistical analysis. The two assays chosen for this study have the advantage that they are widely used and comparable to other studies. While other assays such as the larval feeding inhibition assay (Bartley et al., 2009) may have revealed activity specifically at the pharyngeal site, this assay has not furnished significant differences between ML susceptible and resistant isolates and it was therefore not used.
Avermectins and milbemycins are potent inhibitors of development of larvae in the low nM range for H. contortus, T. colubriformis and O. circumcincta (Table 1). The present data show similar EC50 values to previous studies using IVM in development assays (Giordano et al., 1988; Lacey et al., 1990; Taylor, 1990; Hubert and Kerboeuf, 1992; Gill et al., 1995). The effects of MOX have not been measured previously. The RRs in the present study clearly discriminate susceptible from resistant isolates and all but one of the drug/species comparisons are significant (Table 1).
Like previous studies on contraction and motility (Gill and Lacey, 1993; Kotze et al., 2006) our data show effects in the LMIA in the uM range. This assay relies on the ability of MLs to inhibit body muscle contraction and worm movement required for larvae to negotiate the sieve or move under observation. This was confirmed by observations that migrating worms were motile and those not migrating moved slowly or not at all or assumed angular postures and performed jerky movements consistent with the observations of larvae (Gill and Lacey, 1993) and adult H. contortus (Geary et al., 1993). In contrast, IVM and MOX inhibited locomotion but not velocity in Caenorhabditis elegans (Ardelli et al., 2009).
Dose response curves were obtained for all drugs in all three species (although two could not be fitted for statistical comparisons, Table 2). In general, the milbemycins (A3, A4) had poor potency (EC50s near 1 μM) while the related drug MOX was the most potent and also generated the highest RRs in H. contortus and O. circumcincta Larvae of H. contortus were also studied by Kotze et al. (2006). Although that work differed from ours in using an agar overlay on the migration sieve, results were similar, with the one point of direct comparison with that study being our EC50 value of 755 nM IVM with the published value of 1.87 μg/ml (2150 nM) (Kotze et al., 2006). While Geary et al. (1993) reported that IVM inhibited motility in adult H. contortus at >10nM, Kotze et al. (2012), using observational techniques, reported inhibition by ABA at 0.015 nM. The relevance of subtle motility effects on parasite expulsion are not known, but the data do argue for the muscle site as a potent site of action and resistance of the MLs.
4.2. Effects on parasite species
In both assays, the potency differences between IVM and MOX are least marked for T. colubriformis and the RR values also accord (7.26 versus 7.28). These data, together with the in vivo phenotype of IVM and MOX resistance for T.c. MOX-R, suggest that the drug receptors present in the two assays are similar and that resistance occurs by a similar mechanism. The ∼100-fold differences in potency between the assays may relate to the sensitivity of detection used in the assay or in the tissue-specific site of action relevant to the assay. Why ML-resistance in T. colubriformis has different characteristics to the other two species is not known. ML-resistance is relatively rare in T. colubriformis compared with the other two species suggesting that a different resistance mechanism may be operating compared with the other species. Because this isolate has a high level of resistance and a simple resistance pharmacology, it provides a useful model for exploring what might be a single mechanism.
For H. contortus, IVM and MOX have similar potency in each assay and values are 100–1000-fold less sensitive in the LMIA compared with the LDA. IVM has a marginally higher RR in the LDA (2-fold) but the RR for MOX is 6-fold higher in the LMIA. These data suggest that the two drugs share receptors in Haemonchus but the RRs suggest that IVM-resistance occurs at the sites present in the LDA and MOX resistance occurs at sites in the LMIA. For O. circumcincta, IVM and MOX are equipotent in the LDA, both with low RRs. In contrast, MOX is significantly less potent in the LMIA, but has a large RR. Research performed by Sutherland et al. (2002) suggests a genetic basis for differences between resistance to IVM and MOX in an isolate of O. circumcincta in vivo. These workers claim that IVM resistance is dominant and MOX resistance is semi-dominant. Le Jambre et al. (2005) draw similar conclusions for a H. contortus isolate. Together, these data suggest that a different or additional genetic mechanism is at play in MOX resistance compared with IVM resistance. In terms of diagnosis, these observations suggest that the LMIA may be a superior tool for field monitoring of resistance to MOX especially for detecting emerging MOX resistance. If this is true, then further selection of the H. contortus CAVR isolate will lead to MOX resistance.
4.3. Effects of analogues
One component of this study was to investigate if the two chemical components of IVM could account for the action of the mixture. In the LDA, for each species, IVM potency was of a similar order to that of its components showing that they share a common pharmacology in this assay. In the LMIA, IVM and its components show complex patterns, and the components do not suitably account for the activity of IVM. Only for T. colubriformis, where detailed comparisons are shown in Fig. 5, do the responses towards the two components achieve an equivalent effect to IVM. In this case, resistance appears to be predominantly to IVM B1b, but together the data do not suggest that the components differ in receptor site specificity. As a group, the milbemycins have heterogeneous effects. In the LDA, Mil A4 has significantly superior potency and the RRs for Mil A3 and Mil A4 are 3-fold higher than that for MOX. In the LMIA, the reverse is apparent for H. contortus and O. circumcincta where MOX is most potent and has high RRs (not statistically tested). Because Mil A3, Mil A4 and IVM appear to have equivalent effects in both assays and these are distinct from the effects of MOX it is likely that MOX binds to the same binding site(s) with different kinetics and/or has additional sites of action and/or resistance. This is an important finding in terms of IVM and MOX resistance. In silico models of a putative H. contortus receptor predict that IVM and MOX bind to common domains but that their various side chains affect binding kinetics. If MOX behaves differently to the other MLs, including other milbemycins and IVM, then it follows that the saccharide group, which is a feature of the ivermectins only, does not account for the unique properties of MOX. Rather, attention should be focused on the unique structural components of MOX such as at the C23 and the C25-associated side chains (see Prichard et al., 2012).
4.4. Sites of action and resistance
In a diagnostic setting, the LDA has the advantage that it can detect the resistance status across several species and anthelmintic classes. Benzimidazoles and levamisole are known to affect egg development and larval hatch respectively and their effects are likely to be independent of the pharynx. The inhibition of development by the MLs is claimed to be due to interruption to feeding (Gill and Lacey, 1998). The evidence cited for this is the equipotency of the compounds to inhibit pharyngeal activity and development. A site of action for IVM and ABA in the pharynx is strongly supported by previous studies in larvae, adults (Geary et al., 1993; Sheriff et al., 2002) and C. elegans, (Dent et al., 2000). Similarly, development of L1 of C. elegans is affected by IVM and MOX concentrations of <1 nM and 39 nM, respectively, which are similar to their effects on pharyngeal pumping (4.9 and 78 nM) (Ardelli et al., 2009). Although these data are suggestive, it is not possible to ascribe a site of action for the MLs in this assay, however, paralysis of the pharynx is likely to be one component contributing to responses in the LDA. It is also clear from the results of the present study that the LDA and the LMIA measure different effects presumably at distinct and/or overlapping sites of action.
It has been reported, that isolates resistant to IVM show decreased susceptibility to MOX in vivo and in vitro (Conder et al., 1993; Shoop, 1993; Gill et al., 1995). For some IVM-resistant parasites MOX has higher potency in vivo and retains therapeutic efficacy. (Craig et al., 1992; Kieran, 1994; Bartley et al., 2004, 2005). However, the superior potency of MOX against the CAVR isolate is not reflected in the present results on larvae in vitro where IVM and MOX are roughly equipotent. This suggests that the drugs act at similar sites and with similar affinity. Indeed, the LMIA may be a strong discriminator of MOX resistance that is diminished by the pharmacokinetic effect in vivo. An explanation for the increased potency of MOX in vivo thus probably resides in its pharmacokinetics where it has extended clearance kinetics and prolonged interaction with parasites (Hennessy and Alvinerie, 2002). In order to confirm this and the importance of apparent genetic differences, the potencies of IVM and MOX need to be compared in adult parasites in vitro, including the use of muscle–specific assays.
Knowledge of the mechanisms of ML resistance remains elusive and differences in IVM and MOX resistance are difficult to explain. They may reflect differences in their interactions with glutamate-gated chloride channel receptors (Glu Cl−) of nematodes which are the target of the MLs. For example, biochemical data reveal quantitative and qualitative differences between IVM and MOX binding to these receptors (Hejmadi et al., 2000; Wolstenholme and Rogers, 2005). In addition, putative resistance mechanisms may be associated with a multiplicity of different genes (Gilleard, 2006). For example, it has been suggested that MLs select parasite P-glycoproteins and that IVM and MOX interact in different ways with mammalian Pgps (Prichard et al., 2012). If such differences are borne out in studies on parasites, this might lead to an understanding of mechanisms that modulate ML resistance.
Acknowledgements
Dr. Brown Besier is acknowledged for supplying the moxidectin resistant Trichostrongylus isolate. The authors also would like to acknowledge E. Lacey for supplying the chemicals IVM B1a, IVM B1b, Mil A3, Mil A4 and Fort Dodge Animal Health for supplying MOX respectively.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Ardelli B.F., Stitt L.E., Tompkins J.B., Prichard R.K. A comparison of the effects of ivermectin and moxidectin on the nematode Caenorhabditis elegans. Vet. Parasitol. 2009;165:96–108. doi: 10.1016/j.vetpar.2009.06.043. [DOI] [PubMed] [Google Scholar]
- Bartley D.J., Jackson F., Jackson E., Sargison N. Characterisation of two triple resistant field isolates of Teladorsagia from Scottish lowland sheep farms. Vet. Parasitol. 2004;123:189–199. doi: 10.1016/j.vetpar.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Bartley D.J., Jackson E., Sargison N., Jackson F. Further characterisation of a triple resistant field isolate of Teladorsagia from a Scottish lowland sheep farm. Vet. Parasitol. 2005;134:261–266. doi: 10.1016/j.vetpar.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Bartley D.J., McAllister H., Bartley Y., Dupuy J., Menez C., Alvinerie M., Jackson F., Lespine A. P-glycoprotein interfering agents potentiate ivermectin susceptibility in ivermectin sensitive and resistant isolates of Teladorsagia circumcincta and Haemonchus contortus. Parasitology. 2009;136:1081–1088. doi: 10.1017/S0031182009990345. [DOI] [PubMed] [Google Scholar]
- Brownlee D.J.A., Holden-Dye L., Walker R.J. Actions of the anthelmintic ivermectin on the pharyngeal muscle of the parasitic nematode, Ascaris suum. Parasitology. 1997;115:553–561. doi: 10.1017/s0031182097001601. [DOI] [PubMed] [Google Scholar]
- Conder G.A., Thompson D.P., Johnson S.S. Demonstration of co-resistance of Haemonchus contortus to ivermectin and moxidectin. Vet. Rec. 1993;132:651–652. doi: 10.1136/vr.132.26.651. [DOI] [PubMed] [Google Scholar]
- Craig T.M., Hatfield T.A., Pankavich J.A., Wang G.T. Efficacy of moxidectin against an ivermectin-resistant strain of Haemonchus contortus in sheep. Vet. Parasitol. 1992;41:329–333. doi: 10.1016/0304-4017(92)90090-v. [DOI] [PubMed] [Google Scholar]
- Demeler J., Kuttler U., von Samson-Himmelstjerna G. Adaptation and evaluation of three different in vitro tests for the detection of resistance to anthelmintics in gastro intestinal nematodes of cattle. Vet. Parasitol. 2010;170:61–70. doi: 10.1016/j.vetpar.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Demeler J., Kleinschmidt N., Kuttler U., Koopmann R., von Samson-Himmelstjerna G. Evaluation of the egg hatch assay and the larval migration inhibition assay to detect anthelmintic resistance in cattle parasitic nematodes on farms. Par. Int. 2012;61(4):614–618. doi: 10.1016/j.parint.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Dent J.A., Davis M.W., Avery L. Avr-15 encodes a chloride channel subunit that mediates inhibitory glutamatergic neurotransmission and ivermectin sensitivity in Caenorhabditis elegans. EMBO J. 1997;16:5867–5879. doi: 10.1093/emboj/16.19.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent J.A., Smith M.M., Vassilatis D.K., Avery L. The genetics of ivermectin resistance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2674–2679. doi: 10.1073/pnas.97.6.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary T.G., Sims S.M., Thomas E.M., Vanover L., Davis J.P., Winterrowd C.A., Klein R.D., Ho N.F.H., Thompson D.P. Haemonchus contortus: ivermectin-induced paralysis of the pharynx. Exp. Parasitol. 1993;77:88–96. doi: 10.1006/expr.1993.1064. [DOI] [PubMed] [Google Scholar]
- Gill J.H., Lacey E. In vitro activity of paraherquamide against the free-living stages of Haemonchus contortus, Trichostrongylus colubriformis and Ostertagia circumcincta. Int. J. Parasitol. 1993;23:375–381. doi: 10.1016/0020-7519(93)90013-o. [DOI] [PubMed] [Google Scholar]
- Gill J.H., Lacey E. Avermectin/milbemycin resistance in trichostrongyloid nematodes. Int. J. Parasitol. 1998;28:863–877. doi: 10.1016/s0020-7519(98)00068-x. [DOI] [PubMed] [Google Scholar]
- Gill J.H., Redwin J.M., van Wyk J.A., Lacey E. Detection of resistance to ivermectin in Haemonchus contortus. Int. J. Parasitol. 1991;21:771–776. doi: 10.1016/0020-7519(91)90144-v. [DOI] [PubMed] [Google Scholar]
- Gill J.H., Redwin J.M., Van Wyk J.A., Lacey E. Avermectin inhibition of larval development in Haemonchus contortus – effects of ivermectin resistance. Int. J. Parasitol. 1995;25:463–470. doi: 10.1016/0020-7519(94)00087-5. [DOI] [PubMed] [Google Scholar]
- Gilleard J.S. Understanding anthelmintic resistance: the need for genomics and genetics. Int. J. Parasitol. 2006;36:1227–1239. doi: 10.1016/j.ijpara.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Giordano D.J., Tritschler J.P., Coles G.C. Selection of ivermectin-resistant Trichostrongylus colubriformis in lambs. Vet. Parasitol. 1988;30:139–148. doi: 10.1016/0304-4017(88)90161-6. [DOI] [PubMed] [Google Scholar]
- Hejmadi M.V., Jagannathan S., Delany N.S., Coles G.C., Wolstenholme A.J. l-Glutamate binding sites for parasitic nematodes: an association with ivermectin resistance? Parasitology. 2000;120:535–545. doi: 10.1017/s0031182099005843. [DOI] [PubMed] [Google Scholar]
- Hennessy D.R., Alvinerie M.R. Pharmacokinetics of the macrocyclic lactones: conventional wisdom and new paradigms. In: Vercruysse J., Rew R.S., editors. Macrocyclic Lactones in Antiparasitic Therapy. CABI Publishing; Wallingford: 2002. pp. 97–124. [Google Scholar]
- Hubert J., Kerboeuf D. A microlarval development assay for the detection of anthelmintic resistance in sheep nematodes. Vet. Rec. 1992;130:442–446. doi: 10.1136/vr.130.20.442. [DOI] [PubMed] [Google Scholar]
- Jackson F., Coop R.L. The development of anthelmintic resistance in sheep nematodes. Parasitology. 2000;120:95–107. doi: 10.1017/s0031182099005740. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20 doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kieran P.J. Moxidectin against ivermectin-resistant nematodes – a global view. Aust. Vet. J. 1994;71:18–20. doi: 10.1111/j.1751-0813.1994.tb00895.x. [DOI] [PubMed] [Google Scholar]
- Kotze A.C. Effects of macrocyclic lactones on ingestion in susceptible and resistant Haemonchus contortus larvae. J. Parasitol. 1998;84:631–635. [PubMed] [Google Scholar]
- Kotze A.C., Le Jambre L.F., O’Grady J. A modified larval migration assay for detection of resistance to macrocyclic lactones in Haemonchus contortus, and drug screening with Trichostrongylidae parasites. Vet. Parasitol. 2006;137:294–305. doi: 10.1016/j.vetpar.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Kotze A.C., Hines B.M., Ruffell A.P. A reappraisal of the relative sensitivity of nematode pharyngeal and somatic musculature to macrocyclic lactone drugs. Int. J. Parasitol. 2012;2:29–35. doi: 10.1016/j.ijpddr.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey E., Redwin J.M., Gil l.J.H., Demargherit i.V.M., Waller P.J. A larval development assay for the simultaneous detection of broad spectrum anthelmintic resistance. In: Boray J.C., Martin P.J., Roush R.T., editors. Resistance of Parasites to Antiparasitic Drugs. MSD Agvet; New Jersey: 1990. pp. 177–184. [Google Scholar]
- Le Jambre L.F., Gill J.H., Lenane I.J., Lacey E. Characterisation of an avermectin resistant strain of Australian Haemonchus contortus. Int. J. Parasitol. 1995;25:691–698. doi: 10.1016/0020-7519(94)00200-8. [DOI] [PubMed] [Google Scholar]
- Le Jambre L.F., Gill J.H., Lenane I.J., Baker P. Inheritance of avermectin resistance in Haemonchus contortus. Int. J. Parasitol. 2000;30:105–111. doi: 10.1016/s0020-7519(99)00172-1. [DOI] [PubMed] [Google Scholar]
- Le Jambre L., Geoghegan J., Lyndal-Murphy M. Characterization of moxidectin resistant Trichostrongylus colubriformis and Haemonchus contortus. Vet. Parasitol. 2005;128:83–90. doi: 10.1016/j.vetpar.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Pomroy W.E., Heath A.C.G. Anthelmintic resistance in New Zealand. N.Z. Vet. J. 2001;49:227–235. doi: 10.1080/00480169.2001.36237. [DOI] [PubMed] [Google Scholar]
- Love, S. (2002). Sheep worm control and drench resistance – no worries? Agnote, NSW Agriculture Australia DAI/87, 1–4.
- Prichard R., Ménez C., Lespine A. Moxidectin and the avermectins: consanguinity but not identity. Int. J. Parasitol. Drugs Drug Resist. 2012;2:134–153. doi: 10.1016/j.ijpddr.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangster N.C., Riley F.L., Collins G.H. Investigation of the mechanism of levamisole resistance in trichostrongylid nematodes of sheep. Int. J. Parasitol. 1988;18:813–818. doi: 10.1016/0020-7519(88)90123-3. [DOI] [PubMed] [Google Scholar]
- Sheriff J.C., Kotze A.C., Sangster N.C., Martin R.J. Effects of macrocyclic lactone anthelmintics on feeding and pharyngeal pumping in Trichostrongylus colubriformis in vitro. Parasitology. 2002;125:477–484. doi: 10.1017/s0031182002002251. [DOI] [PubMed] [Google Scholar]
- Shoop W.L. Ivermectin resistance. Parasitol. Today. 1993;9:154–159. doi: 10.1016/0169-4758(93)90136-4. [DOI] [PubMed] [Google Scholar]
- Sutherland I.A., Leathwick D.M., Brown A.E. Moxidectin:persistence and efficacy against drug-resistant Ostertagia circumcinta. J. Vet. Pharmacol. Ther. 1999;22:2–5. doi: 10.1046/j.1365-2885.1999.00188.x. [DOI] [PubMed] [Google Scholar]
- Sutherland I.A., Leathwick D.M., Moen I.C., Bisset S.A. Resistance to therapeutic treatment with macrocyclic lactone anthemintics in Ostertagia circumcincta. Vet. Parasitol. 2002;109:91–99. doi: 10.1016/s0304-4017(02)00247-9. [DOI] [PubMed] [Google Scholar]
- Taylor M.A. A larval development test for the detection of anthelmintic resistance in nematodes of sheep. Res. Vet. Sci. 1990;49:198–202. [PubMed] [Google Scholar]
- Wolstenholme A.J., Rogers A.T. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology. 2005;131(Suppl):S85–S95. doi: 10.1017/S0031182005008218. [DOI] [PubMed] [Google Scholar]


