Graphical abstract
Highlights
► Field trials used to compare four ivermectin treatment regimes in lambs. ► Targeting treatment to individuals provided benefits in maintenance of efficacy. ► Suppressive treatment selected most strongly for ivermectin resistance. ► Similar final bodyweights achieved in monthly, strategic and targeted groups. ► Reduced final bodyweights observed in metaphylactic/therapeutic treatment.
Keywords: Sheep, Gastrointestinal nematodes, Anthelmintic resistance, Refugia
Abstract
Refugia-based drenching regimes have been widely recommended to slow development of anthelmintic resistance but there are few comparisons between different treatment approaches in the UK. The impact of four ivermectin treatment regimes on drug efficacy, lamb body weight and nematode contamination during a 154 day grazing season were evaluated in a consecutive five year field study. Regimes were whole-flock treatment every 4 weeks (NST), targeted selective treatment (TST) based on individual performance, strategic whole-flock treatments at pre-determined times (SPT) or whole-flock treatment when clinical signs were apparent (MT). Mean numbers of ivermectin drenches administered per season were 4.0, 1.8, 2.0 and 1.4 for NST, TST, SPT and MT groups, respectively. The mean anthelmintic efficacy (AE) for each treatment group was based on faecal egg count reduction post-treatment employing a bootstrap sampling based algorithm. Mean AE was 95–98% for all groups in 2006 and mean AE (95% confidence limits) for NST declined to 62% (55%, 68%) in 2010. In comparison, AE for TST, SPT and MT in 2010 were 86% (81%, 92%), 86% (83%, 90%) and 83% (78%, 88%), respectively. Body weight in TST and SPT was similar to NST in all years (p > 0.05), however MT lambs were lighter than NST in 2006–2008 (p ⩽ 0.04). Tracer lamb worm burdens was lowest in NST but was not significantly different between other groups. Overall, both the TST and SPT regimes appeared to maintain animal performance and conserve anthelmintic efficacy compared with a neo-suppressive anthelmintic treatment regime.
1. Introduction
Anthelmintic resistance in ovine gastrointestinal nematodes is widespread throughout livestock production areas worldwide, and now threatens the sustainability of sheep production in many countries (Jackson and Coop, 2000; Kaplan, 2004; Pomroy, 2006; Molento et al., 2011). Although the effects of nematode infections, and the problem of anthelmintic resistance, have been known for some time, there is a limited number of studies that have investigated the effect of timing and frequency of drench administration throughout the season on the development of anthelmintic resistance and lamb weight gain (Waghorn et al., 2008, 2009; Leathwick et al., 2012). Prichard et al. (1980) advised that regular suppressive drenching strategies, with the same drug family, would select strongly for anthelmintic resistance. Furthermore, the same authors hypothesised that this approach would be more likely to select for resistance than a ‘strategic’ programme of 2–3 drenches per year. In contrast, other authors reported that treatment frequency alone may not be a suitable indicator for the rate of resistance development, especially if adult ewes were also drenched (Leathwick et al., 1995, 2006a).
Regardless of the anthelmintic treatment regime implemented, the importance of the provision of a nematode population in refugia has been highlighted as a major determinant of the speed at which anthelmintic resistance may develop (Prichard et al., 1980; Van Wyk, 2001). Consequently, anthelmintic treatment regimes that provide for a population in refugia can be anticipated to assist with slowing the development of resistance within a given population. Several studies have shown that either more effective targeting of anthelmintic use or part-flock drenching approaches (targeted selective treatment, TST), using a variety of indicators, can reduce the number of drenches administered with little or no negative effects on productivity (reviewed by Van Wyk et al., 2006; Besier, 2008; Kenyon et al., 2009) and provide benefits in terms of slowing the development of resistance in both the short (Besier, 2001; Gaba et al., 2010) and medium (Leathwick et al., 2006a) term. However, a logical consequence of maintaining a reservoir of susceptible nematodes in refugia may be an increase in nematode burden on pasture. This has been demonstrated in short-term field studies (Leathwick et al., 2006b; Gaba et al., 2010; Besier, 2012), and could potentially threaten the acceptability of a TST approach in the longer term.
Previously, the development of a decision support system, named the Happy Factor™, to identify individual lambs that are likely to benefit from receiving an anthelmintic drench as part of a TST regime in a fat lamb production system in South East Scotland has been reported (Greer et al., 2009). The system allows the prediction of individual lamb liveweight over a short period by taking into account the nutrition available to the animal, the lamb’s stage of development and environmental factors, such as temperature. Only those lambs that failed to reach the predicted weight gain target received anthelmintic. This approach was found to be able to sensitively identify those animals that were underperforming, with 88% of lambs with a Happy Factor lower than the pre-determined treatment threshold responding positively to anthelmintic treatment (Greer et al., 2009) and could thus provide a suitably relevant performance-based TST indicator.
The aim of this current study was to evaluate and compare the impact of different treatment approaches, including the TST approach described above, in a five year replicated field trial. This paper will discuss the impact of these various treatment regimes on the efficacy of ivermectin treatment, lamb body weight and nematode contamination of pasture.
2. Materials and methods
2.1. Experimental design
The replicated field trials were conducted over five consecutive years (2006–2010) at the Moredun Research Institute farm, Scotland, and were examined and approved by the Moredun Research Institute Experiments and Ethics Committee and were conducted under the legislation of a UK Home Office License (reference PPL 60/03899) in accordance with the Animals (Scientific Procedures) Act of 1986.
At the start of the experiment (2006), each of eight one hectare paddocks were randomly assigned to one of four anthelmintic treatment regimes (described in Section 2.3) with each treatment regime replicated twice. Each paddock received the same treatment for every consecutive year of the experiment, in an attempt to observe changes in the parasite population due to the application of different anthelmintic treatment regimes.
Pastures were naturally contaminated with Nematodirus battus predominating in spring and Teladorsagia circumcincta and Trichostongylus vitrinus being most prevalent in the summer.
2.2. Animals
At the beginning of each grazing season (May), twin lambs at 5–6 weeks of age, and their dams, were allocated to one of eight groups balanced for lamb weight and sex (Day 0). Each year lambs were fitted with electronic ear tags (Shearwell Ltd., Somerset, UK) prior to being randomly assigned by group to a paddock on which they grazed from May to October whilst exposed to natural infection. Dams were removed from the paddocks at weaning (on day 84 for 2006 and 2008, on day 98 for 2007 and on day 70 for 2009 and 2010), at the discretion of the farm manager.
The breed of sheep varied throughout the experiment as a result of a farm management decision to move from stocking Scottish Blackface to Texel breeds. In 2006, Scottish Blackface × Leicester lambs were used, but in subsequent years the breed changed to mule × Texel, with the influence of the Texel breed increasing each year. The change in breed also altered the recommended stocking density, beginning with 24 Scottish Blackface × Leicester lambs on each paddock in 2006/07, reducing to 20 Texel crosses in later years. In 2008, the number of lambs used was 16, as several lambs had to be removed from the study due to nematodirosis. In this year, four lambs were subsequently added to each paddock to ensure similar stocking densities as previous years, but data from these lambs were not included in later analysis.
Ewes were drenched with ivermectin (Oramec, Merial Animal Health Ltd., UK) upon entry to the paddocks each year (day 0) at the manufacturer’s recommended dose rate of 0.2 mg/kg liveweight (LW). All lambs were drenched with anthelmintic prior to the experimental period due to Nematodirus species infection. For 2006–2008, this consisted of one ivermectin drench, at the manufacturers recommended dose rate, on day 28 with the exception of the MT group (see Section 2.3) in 2006, which were not treated until day 56. In 2009, in response to increasing Nematodirus challenge, two drenches of ivermectin were given 3 weeks apart on day 0 and 21, and in 2010 one benzimidazole drench (Panacur, Intervet, at 5 mg/kg LW) was given on day 7 after turnout, followed by an ivermectin drench on day 28.
2.3. Anthelmintic treatment regimes
Cohorts of lambs were allocated to one of four anthelmintic treatment groups, with each regime being implemented after the drench to control Nematodirus spp. had been administered on day 28 (Section 2.2). All subsequent anthelmintic treatments consisted of ivermectin drench (Oramec). One regime consisted of neo-suppressive treatment (group NST) in which all lambs were drenched every 4 weeks throughout the experimental period. A second regime was a performance-based targeted selective treatment (group TST). For this, lambs were assessed on their ability to reach individualised target growth rates (described in Section 2.4) every two weeks, with those not achieving their set targets drenched. The third treatment regime was a strategic prophylactic treatment (group SPT) in which all lambs were drenched at strategically appropriate times – i.e. at weaning and 6 weeks post weaning. These times were determined by historical and epidemiological knowledge of the nematode species present on the property. The fourth was a metaphylactic/therapeutic regime (group MT) in which all lambs were drenched when individuals within the group exhibited clinical signs of parasitism, including high faecal egg count (FEC), scouring and weight loss. This MT group provided a comparison of the potential productivity losses that might occur in the absence of regular chemical intervention. These treatment regimes continued until the end of the grazing season at day 154 when all lambs were removed and the pastures spelled until the trial resumed with a fresh batch of lambs the following spring.
2.4. Determining performance targets for TST lambs
Target growth rates for each individual within the TST group were determined using the Happy Factor decision support model described by Greer et al. (2009). Briefly, this model predicts the expected growth rates once non-parasitological factors such as feed availability, climate and relative maturity of the animal are taken into account. The general principle is as follows: maximum likely energy intake is determined by the first limiting factor of either pasture availability or the animals’ physical capacity. The availability of energy for growth is determined once requirements for maintenance are subtracted and which are then divided by an estimate of the energy cost of the live weight gained (A.F.R.C., 1993) to calculate an efficiency of energy utilisation. Once corrected for the effects of temperature, this efficiency of energy utilisation can provide an estimate of how well an animal is growing in comparison to its theoretical maximum. A simplified approach was used for the 2006 grazing season, data from which were used to refine the model which was then used from 2007 to 2010 with an efficiency threshold for treatment of 0.66 (Greer et al., 2009). Feed availability was determined by measuring the mass of pasture by walking the paddocks in a W pattern using a grassmaster II probe (Novel Ways, New Zealand) and the mean temperature was obtained from the UK Meteorological Office using data from a nearby site in South Eastern Scotland.
2.5. Measurements and sampling
Live weights of all lambs were recorded every 2 weeks throughout the experimental period at which time a decision to treat TST animals based on their ability to reach live weight gain targets was made. In addition, at each measurement time, faecal samples were collected per rectum from each lamb. FEC were conducted following a modification of the salt-floatation method (Jackson, 1974), with a sensitivity of up to one egg per gram of faeces (epg). Briefly, faecal samples were homogenised in 10 ml water per gram of faeces and passed through a 1 mm sieve with 5 ml water to remove large debris. The filtrate was transferred to 15 ml polyallomer centrifuge tubes (Beckman, UK) and centrifuged at 304g for 2 min. The supernatant was removed and the faecal pellet resuspended in saturated sodium chloride solution (specific gravity of 1.2), inverted to mix, centrifuged at 304g for 2 min, the fluid near the meniscus (which contains the eggs) was isolated using artery clamps and transferred to a cuvette (Sigma, UK) for microscopic identification and enumeration of nematode eggs. Nematodirus spp. eggs were differentiated morphologically from other strongyle species. The number of nematode eggs present pre and post treatment, in the same animal, were used to calculate the percentage reduction in FEC to estimate the efficacy of ivermectin.
2.6. Estimation of numbers of nematodes present on pasture to determine changes in pasture contamination due to each treatment approach
Two nematode-naive ‘tracer’ lambs were grazed on each paddock for 28 days in May (early season) and September (late season) of each year to give an indication of the number and species of nematode present on pasture at the time of grazing. The animals were removed from pasture and housed for 2 weeks prior to being euthanized. Abomasa, small intestine and large intestine were collected and processed for the estimation of worm burden. Each of the organs were opened longitudinally, placed separately into 5 L of physiological saline (0.85% sodium chloride w/v) and incubated for 4 h at 37 °C. The mucosa was washed into the saline and the organ removed. From each organ, a 10% subsample was collected and immediately fixed in 2% formalin. The worms present in a 2% subsample for each organ were counted, with sex and stage of development recorded (MAFF, 1986). Approximately 25 male worms from each organ from each animal were used to determine species prevalence by morphological identification after clearing in lactophenol (MAFF, 1986).
2.7. Statistical analyses
The total number of anthelmintic drenches administered per lamb during the experimental period for each of NST, SPT and MT groups was the same in each year. Hence, only the data on the total number of drenches administered to lambs of the TST group across all years were analysed with a linear mixed model. The model included year and sex of lamb as fixed effects and paddock as a random effect. As twin lambs were used in the study, variability attributable to the ewe was taken into account by also including ewe within paddock as a random effect.
The data on the body weight of lambs at day 154 were analysed with a linear mixed model which included the paddock and ewe within paddock as random effects and the group (four levels: NST, TST, SPT and MT), sex of the lamb (two levels: male and female), year of experiment (five levels: 2006, 2007, 2008, 2009 and 2010) and all possible two and three-way interaction effects as fixed effects. The model also incorporated the initial (day 0 of the experiment) body weight of lamb (as a deviation from overall mean) as a covariate.
Parameters of the linear mixed models described above were estimated using the restricted maximum likelihood (REML) method and overall statistical significance of fixed effects was assessed from the corresponding p-value estimated from the conditional F-statistic (Pinheiro and Bates, 2000). If the conditional F-statistic was statistically significant (p ⩽ 0.05) for a fixed effect, then the mean differences between levels of that fixed effect were compared. To compare the differences in mean values for levels of fixed effects, two-sided probabilities for each comparison were obtained. These probabilities were then adjusted using a False Discovery Rate (FDR) approach (Benjamini and Hochberg, 1995) to take into account the multiple comparisons of means so that the overall false discovery rate was less than 5%. The FDR-adjusted p-value is the minimum FDR for which the observed difference and associated p-value would be accepted as statistically significant. This value, denoted in this paper as ‘pf’, therefore summarises the strength of evidence for there being a real difference, analogous to a standard p-value.
To estimate anthelmintic efficacy and corresponding 95% confidence intervals for each group in each year, a two-step approach was taken. The estimate of anthelmintic efficacy for each group is based on the reduction in FEC which constitutes the FEC data pre and post anthelmintic treatment. The data on post-anthelmintic FEC was assumed to follow a Binomial distribution conditional on the pre-anthelmintic FEC and a probability ‘p’ (where ‘p’ represents one minus the anthelmintic efficacy). To fulfil the properties of a binomial distribution, the pre-treatment FEC was assigned the same value as the post-treatment FEC, if the pre-treatment FEC was lower than the post-treatment FEC. Also, if the pre-treatment FEC was zero, then all data (both pre- and post-treatment FEC) were removed. A generalised linear mixed model was fitted including group, time and their interaction as fixed effects and lamb as a random effect. The model included the covariate time effect (week of the experiment) as a deviation from the overall mean time. The logit function of anthelmintic efficacy was modelled as a linear predictor of possible explanatory variables. An overdispersion parameter was estimated to take into account the extra-Binomial variability in the data. In this paper, we are primarily interested in obtaining an overall estimate of anthelmintic efficacy for each group. To achieve this, in the second step, a bootstrap sampling algorithm was employed to sample the data in each replicate. Within each replicate, the requisite number of lambs, with replacement, was drawn from those in each paddock in each year. The sampled data were fitted to the generalised linear mixed model described earlier and the fitted model was used to predict the anthelmintic efficacy in the sampled data. An estimate of mean anthelmintic efficacy (averaging over the estimates on all time points) was obtained for each treatment group within each bootstrap sample. A total of 1000 replicates were run for each year and the mean efficacy value and corresponding 95% confidence intervals were summarised for each group in each year from these replicates. A ratio of estimates of anthelmintic efficacy for two different groups and corresponding 95% confidence intervals were used to compare the efficacy between each pair of groups.
To determine the effect of the treatment decision for lambs in the TST group on subsequent (4 weeks later) treatment decisions, a generalised liner mixed model was fitted to the data on the incidence of subsequent drenching, assuming that the data followed a Bernoulli distribution. The logit function of the probability of incidence of drenching was modelled as a linear predictor of possible explanatory variables. The model included the most recent treatment decision, cumulative number of drenches, year, time and their interaction effects as fixed effects and lamb as a random effect. Data from the years 2007 to 2010 were used.
Finally, different variables on the worm burden data in tracer lambs were analysed using generalised linear mixed models assuming Poisson distribution and log link function. The model included paddock as a random effect and year, season and treatment along with their possible interaction as fixed effects. An over-dispersion parameter was estimated and fitted in the model to account for variability in excess of that expected from Poisson distributed data.
All statistical analyses were carried out using R software version 2.13.2 (R Development Core Team 2011).
3. Results
3.1. Anthelmintic drenches administered
The mean number of anthelmintic drenches administered per individual lamb during the experimental period for each treatment group and each year are given in Table 1. The NST group received four drenches in all years on days 56, 84, 112 and 140. SPT lambs consistently received two drenches per year as per the trial protocol. MT lambs received three drenches in 2006 on days 56, 112 and 140 and one treatment in subsequent years on day 140 in both 2007 and 2008 and on day 98 in both 2009 and 2010. For the TST group, there was no statistically significant increase in the mean number of drenches administered from 2006 to 2009 (ranged from 1.6 to 1.9). However, more treatments were administered on average in 2010 compared with 2006 only (p = 0.002). Overall, the reduction in the mean number of drenches administered to TST lambs compared with NST lambs varied from 60% in 2007 to 45% in 2010.
Table 1.
Mean number of drenches administered per lamb for each group per year.
| Group | Year |
||||
|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2010 | |
| NST | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| TST | 1.8 | 1.6 | 1.9 | 1.7 | 2.2 |
| SPT | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| MT | 3.0 | 1.0 | 1.0 | 1.0 | 1.0 |
As varying numbers of drenches were administered to lambs in the TST group, the data from this group was analysed with a linear mixed model to estimate the mean number of drenches, which showed that the corresponding 95% lower and upper confidence limits were ±0.2 drenches.
The mean percentage of TST lambs drenched at each sampling time was 25.9% (ranging from 5% to 68.7%). There was no obvious pattern for the timing of these drenches from year to year (data not shown). The frequency of the number of drenches administered to individuals within the TST group is shown in Table 2. Overall on average, 3.1%, 23.9%, 61.6%, 11.0% and 0.5% of lambs required 0, 1, 2, 3 and 4 treatments, respectively. Four drenches were only administered in 2009, when this was required by one lamb.
Table 2.
Percentage of TST lambs that required varying numbers of drenches for each year.
| Number of drenches administered | Year/percentage of lambs drenched |
||||
|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2010 | |
| 0 | 4.2 | 6.3 | 0 | 5 | 0 |
| 1 | 16.7 | 35.4 | 25 | 27.5 | 15.0 |
| 2 | 79.2 | 54.2 | 59.4 | 65.0 | 50.0 |
| 3 | 0 | 4.2 | 15.6 | 0 | 35.0 |
| 4 | 0 | 0 | 0 | 2.5 | 0 |
3.2. Efficacy of ivermectin treatment
The median FEC (minimum, maximum), at day 56 for the 5 years of the trial is shown in Table 3, to give an indication of the parasite challenge experienced by the lambs in each group when the TST approach was implemented. In the year 2010, the overall observed mean pre and post-treatment FEC for MT, NST, SPT and TST groups were (359, 45), (116, 33), (287, 42) and (231, 36) respectively suggesting an overall anthelmintic efficacy of 87%, 71%, 86% and 84% for those groups. However it should be noted that these estimates were purely based on the observed raw data across all time points without taking into account the underlying uncertainty and variability in the data. The predicted mean overall efficacy for each treatment regime for each year is given in Fig. 1 and henceforth the expression ‘mean efficacy’ in this paper indicates the predicted mean overall efficacy. The model used to estimate the mean efficacy value for each group does not take into account the full extent of the effects of variability and uncertainty on estimates of pre-treatment egg counts and hence the confidence intervals presented in Fig. 1 are likely to be conservative. Results showed a similar mean efficacy of 95–98% for all groups in 2006 followed by a decline in the mean efficacy in all groups which was more pronounced in the NST regime where mean efficacy was around 60% from 2009 onwards. The mean efficacy in the NST group was statistically significantly (p < 0.05) lower than the other three groups from 2007 onwards. Mean efficacy for the MT regime remained above 97% until 2010, when the mean efficacy declined to 83%, and was significantly higher (p < 0.05) than the NST, SPT and TST regimes from 2007 until 2009. For the SPT, the mean efficacy remained above 93% until a decline to 73% in 2009 followed by recovery to 86% in 2010. In comparison, the mean efficacy in the TST regime remained above 92% until 2008 and above 82% from 2009 onwards. There was no evidence that the mean efficacies in TST and SPT regimes were statistically different during the study.
Table 3.
The median FEC at day 56 (minimum and maximum), the beginning of the experimental period.
| Group | Year/FEC (minimum, maximum) |
||||
|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2010 | |
| NST | 58.5 (6.0, 369.0) |
63.0 (3.0, 702.0) |
30.0 (2.0, 144.0) |
36.0 (9.0, 135.0) |
81.0 (2.0, 234.0) |
| TST | 54.0 (3.0, 450.0) |
45.0 (0.0, 360.0) |
81.0 (2.0, 432.0) |
81.0 (23.0, 450.0) |
145.5 (4.0, 612.0) |
| SPT | 90.0 (18.0, 1035.0) |
171.0 (3.0, 792.0) |
73.5 (0.0, 270.0) |
54.0 (9.0, 180.0) |
67.5 (2.0, 468.0) |
| MT | 265.5 (24.0, 1512.0) |
27.0 (0.0, 621.0) |
54.0 (2.0, 261.0) |
78.0 (0.0, 369.0) |
94.5 (3.0, 666.0) |
Fig. 1.
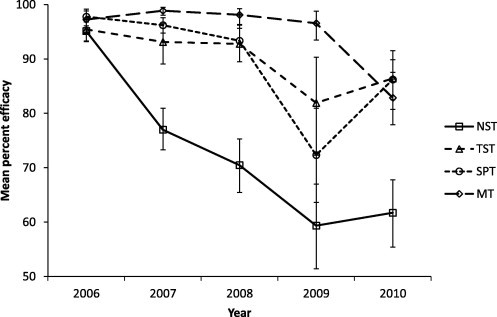
The mean efficacy of ivermectin treatment in each group, error bars show 95% confidence interval.
3.3. Body weight
The mean body weight of all lambs at day 0 was 12.64 ± 0.08 kg. The interaction effect of year and group was statistically significant (p = 0.032) on the mean body weight at day 154. On average, male lambs were 0.69 kg heavier than females at day 154 (p = 0.004). Table 4 presents the predicted mean body weight at day 154 for female lambs for each group in each year of the study. In all years, mean bodyweights of NST, TST and SPT at day 154 were similar (p > 0.05). However, body weights of MT lambs were lower than NST lambs in 2006 and 2007 (pf < 0.001, pf = 0.042, respectively) and lower than all other treatment groups in 2008 (pf = 0.042 for all) but not in 2009 and 2010. There was no statistically significant difference in mean bodyweight of all groups in 2009 and 2010.
Table 4.
The predicted mean bodyweight (kg) at experimental day 154 (lower and upper 95% confidence limits).
| Group | Year/bodyweight (kg) |
||||
|---|---|---|---|---|---|
| 2006 | 2007 | 2008 | 2009 | 2010 | |
| NST | 37.4 (35.5, 39.2) |
36.3 (34.5, 38.2) |
36.4 (34.4, 38.5) |
34.4 (32.5, 36.3) |
39.6 (37.4, 41.6) |
| TST | 34.4 (32.6, 36.3) |
35.6 (33.8, 37.4) |
36.3 (34.3, 38.4) |
35.3 (33.4, 37.2) |
38.5 (36.6, 40.4) |
| SPT | 34.9 (33.0, 36.8) |
34.8 (33.0, 36.7) |
36.3 (34.3, 38.3) |
34.3 (32.4, 36.2) |
38.9 (37.0, 40.8) |
| MT | 31.9 (30.1, 33.7) |
32.7 (30.9, 34.6) |
32.4 (30.4, 34.5) |
33.1 (31.2, 35.0) |
38.0 (36.1, 39.9) |
The figures quoted here are for female lambs, assuming the mean bodyweight on day 0 of the experiment was 12.64 kg. Mean bodyweight of male lambs were similar, but males weighed on average 0.69 kg more than females.
3.4. Effect of prior treatment decision on the incidence of subsequent drenches in the TST group
Results showed that the effect of recent drenching had a statistically significant effect (p < 0.001) on the probability of occurrence of subsequent drenching in lambs and that the incidence of drench administration to lambs in the TST group was 79% less likely to happen if the lamb was treated in the previous 4-week period (p < 0.001).
3.5. Nematode prevalence and species on pasture
The mean total worm burden recovered from tracer lambs grazing pastures in each of the four treatment groups is shown in Fig. 2. The mean worm burden was consistently lower in the NST group (overall mean of 12,538 worms) compared with all other groups where the overall mean number of worms was 26,222, 28,519 and 26,553 for the TST, SPT and MT groups respectively. There was no evidence of a statistically significant interaction effect of treatment group and year (p = 0.452) suggesting that within a treatment group, the mean worm burden did not change across years, or conversely, within a year, there was no difference in the mean worm burden among treatment groups.
Fig. 2.
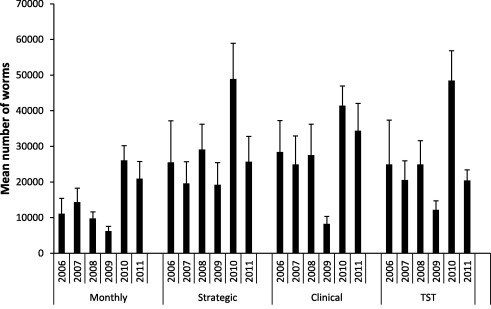
Mean total worm burden from tracer lambs, with error bars showing the standard error of the mean.
The percentages of the most common nematode species identified from each treatment group are shown in Table 5. Genera from the large intestine are not included as the number of nematodes recovered was always a very low percentage of the overall total (range 0.2–3%, mean 0.8%). Haemonchus contortus was not found. Like total worm burden, there was no evidence of a statistically significant interaction effect of treatment group and year on the mean number of the different worm species present (p values ranged from 0.084 to 0.892). The main effect of treatment group was statistically significant on the mean number of T. vitrinus worms (p = 0.009) where the NST group showed the lowest mean worm burden of this species.
Table 5.
The mean percentage prevalence of each of the major nematode species present in tracer lambs.
| Group | Year | % Teladorsagia circumcincta | % Nematodirus battus | % Nematodirus filicollis | % Trichostrongylus vitrinus | Others⁎ |
|---|---|---|---|---|---|---|
| NST | 2006 | 42.4 | 38.0 | 4.3 | 13.2 | 2.2 |
| 2007 | 12.4 | 74.6 | 4.0 | 3.9 | 5.2 | |
| 2008 | 18.2 | 81.5 | 0.0 | 0.4 | 0.0 | |
| 2009 | 28.0 | 62.8 | 6.0 | 0.9 | 2.3 | |
| 2010 | 26.0 | 66.5 | 3.0 | 0.0 | 4.4 | |
| TST | 2006 | 38.9 | 18.7 | 4.1 | 37.7 | 0.6 |
| 2007 | 19.4 | 46.1 | 4.6 | 28.7 | 1.2 | |
| 2008 | 12.2 | 73.5 | 5.3 | 8.9 | 0.0 | |
| 2009 | 24.9 | 52.8 | 5.8 | 13.4 | 3.1 | |
| 2010 | 20.8 | 51.3 | 2.1 | 21.1 | 4.7 | |
| SPT | 2006 | 31.1 | 23.9 | 3.3 | 36.9 | 4.9 |
| 2007 | 22.9 | 38.8 | 6.2 | 31.7 | 0.4 | |
| 2008 | 9.6 | 79.2 | 2.4 | 8.8 | 0.1 | |
| 2009 | 56.5 | 32.5 | 1.2 | 2.5 | 7.3 | |
| 2010 | 26.0 | 52.7 | 4.5 | 14.7 | 2.1 | |
| MT | 2006 | 25.2 | 47.7 | 5.8 | 18.5 | 2.9 |
| 2007 | 21.8 | 63.5 | 6.7 | 7.6 | 0.5 | |
| 2008 | 5.1 | 74.3 | 2.0 | 18.4 | 0.2 | |
| 2009 | 45.3 | 33.8 | 1.1 | 9.2 | 10.5 | |
| 2010 | 18.7 | 49.2 | 3.3 | 24.8 | 3.9 | |
Others are composed of Teladorsagia trifucata, Trichostrongylus axei, Nematodirus helvetianus, Trichostrongylus colubriformis and Cooperia spp.
4. Discussion
The primary objective of this study was to evaluate the impact of different anthelmintic treatment regimes on the efficacy of ivermectin in the medium term. As such, the trial clearly demonstrated that implementation of different treatment regimes affects the rate of change in efficacy of ivermectin, despite the relatively short time period of five years. This was achieved on a property with a nematode population that has a history of anthelmintic resistance (<95% efficacy) to both benzimidazoles and levamizole and where ivermectin was not fully effective, as indicated by the initial efficacy of 95–98% in the current study. The resistance status of the nematodes were relatively typical of farms in Scotland, where the prevalence of anthelmintic resistance has been reported to be 64% for the benzimidazole class (Bartley et al., 2003) and 35% for macrocyclic lactones (Bartley et al., 2006). The treatment regimes tested were also typical of the farming situation, as recent surveys of UK sheep farms have reported a mean of 3.3 drenches administered to lambs throughout the grazing season (Burgess et al., 2012). In contrast, the use of the same drug family for the duration of the study, which deviates from best practice advice, may have affected the rate of development of anthelmintic resistance. Although the presence of untreated ewes would have provided a source of refugia, which may lead the current study to underestimate the differences between treatment regimes, as it is common for untreated ewes to be present throughout the grazing season in the UK, the relative rates of change in ivermectin efficacy between treatment regimes can still be anticipated to be indicative of what may be expected to occur under normal UK commercial farming practice.
In this study, efficacy estimates are based on the change in the concentration of nematode eggs in faeces, rather than genetic analysis of resistant alleles, which are not currently available for macrocyclic lactones. However, it seems reasonable to suggest that sustained changes in efficacy are likely to be reflective of changes in the anthelmintic resistance status of the nematode population. We adopted a statistical approach that would give a conservative estimate of anthelmintic efficacy for each group backed up by bootstrapping to avoid over-reliance on model assumptions in calculating confidence intervals and significance tests.
One of the most striking results of the current study is the impact of treatment frequency on anthelmintic efficacy. In particular, the treatment regime where anthelmintic was administered most frequently (NST) produced the greatest decline in efficacy from 95.1% in 2006 to 61.7% in 2010. This decline was evident from the first year of the study and was consistent thereon, being significantly lower than all other groups from 2007. In comparison, the impact on ivermectin efficacy of the remaining treatment regimes, was much less; with efficacy reduced by only 9.0%, 11.6% and 14.4% between 2006 and 2010, for the TST, SPT and MT groups respectively. These observations are in agreement with the hypothesis of Prichard et al. (1980) that regular suppressive drenching would select strongly for anthelmintic resistance, and that this approach would select more strongly for resistance than a strategic programme of 2–3 drenches per year. Until now, there has been limited direct evidence for the importance of treatment frequency on the development of anthelmintic resistance, with most support relying on epidemiological simulation (Barnes et al., 1995) or short-term grazing studies (Waghorn et al., 2008; Gaba et al., 2010) with some authors suggesting that treatment frequency alone is not a good indicator of the rate of development of resistance (Leathwick et al., 2006a). However, it appears from the results of this study that, at least within the confines of the fat lamb production system used here, treatment frequency does provide an indicator of the likely speed of development of anthelmintic resistance.
Each of the three regimes designed to reduce anthelmintic use (TST, SPT and MT) appeared to be successful in slowing the development of anthelmintic resistance compared with the NST regime. Given that the proportion of the population in refugia is generally considered to be the main factor influencing the rate of development of anthelmintic resistance (Prichard et al., 1980; Van Wyk, 2001), the differences in the rate of change in efficacy observed between each treatment group presumably reflects their comparative ability to provide a population of nematodes unexposed to treatment. As such, it is likely that in the current study nematode populations unexposed to treatment in the TST, SPT and MT regimes were created by differing means. Both the MT and SPT regimes consisted of whole-flock treatments, thus it can be expected that the source of refugia was almost wholly restricted to the suprapopulation that existed on pasture at the time of drenching and which provided a source of re-infection. In comparison, the TST regime consisted of part-flock treatments with an average of 25.9% of individuals drenched at any time. Under these circumstances, both the suprapopulation and the infrapopulation residing in the untreated individuals have the potential to provide refugia. While the reductions in efficacy observed here for the TST group are comparable to those reported after 3 years of a TST regime in which the heaviest 10% of lambs were left untreated with ivermectin (Leathwick et al., 2012), the efficacies given in Fig. 1 relate only to the individuals within each group that were drenched. Consequently, the ability of the TST regime to maintain efficacy is likely to be understated here as efficacy was calculated from only the small proportion of lambs that were drenched and not the infrapopulation present in the group as a whole. It could also be expected that the effects of the TST approach on efficacy, when compared with the SPT or MT regimes, are likely to be magnified if environmental influences outside the host become unfavourable for parasite survival. In such situations the refugia existing at the time of whole flock treatments may be considerably reduced. Nevertheless, it is clear from the results of the current study that maintaining a nematode population unexposed to treatment, regardless of the means through which it was achieved, can provide an effective means of slowing the decline in anthelmintic efficacy, as found in a number of other studies (Leathwick et al., 2006b, 2012; Gaba et al., 2010).
In this study, lamb liveweight gain was recorded from each group to allow the comparison of productivity between the different anthelmintic treatment regimes, there was no attempt to maximise production. In this context, compared with the NST regime, mean lamb growth rates were reduced by 9% in the MT regime and were not significantly reduced for either TST or SPT (2%). The MT group was intended to provide an indication of the production costs associated with parasite challenge if treatment is withheld until clinical signs are evident. However, while it is apparent that both the TST and SPT regimes were successful at maintaining animal performance when compared with the NST group, the true effects of these regimes on performance cannot be estimated from the current study. On the one hand, failure to control parasite infections through a decline in efficacy to 60% has been reported to be associated with a 10–24% reduction in animal performance (Besier, 2008; Leathwick et al., 2008) while another study conducted with BZ (benzimidazole)-resistant nematodes reported a reduction in carcass value of 10–14% in lambs treated with an ‘ineffective’ BZ drench compared with an effective drench (Sutherland et al., 2010; Miller et al., 2011). But on the other hand, the body weight of NST animals did not appear to decline with time, as may be expected as efficacy declines. This may, in part, reflect the change in breed as the trial progressed, or, alternatively, this may reflect a comparatively low larval challenge to the NST animals due to a highly efficacious, suppressive regime in 2006. Support for this is provided by the consistently lower estimates of mean FEC, infective larval recovery from pasture (Kenyon et al., unpublished data) and worm burdens in tracer animals grazing the NST pastures (Fig. 2). The poorer performance of the MT group, which was evident even in 2006 when the number of drenches administered to this group exceeded that given to both TST and SPT groups may, at least in part, be attributable to the timing of drench administration, which allowed the production costs associated with a loss of appetite and nutrient utilisation (Coop et al., 1977) to be expressed in MT animals.
Both TST and SPT regimes appeared to be effective at maintaining an acceptable level of animal performance. By virtue of its nature, it may be expected that any TST regime based on animal performance would provide some protection from production loss. However, the effectiveness of such regimes is dependent on the parameters used to indicate the need for treatment. The lack of evidence for any difference in lamb weight gain in the TST regime, compared with NST and SPT treatments in the current study, suggests the threshold for anthelmintic treatment, at an efficiency value of 0.66, was adequately set. This is further supported by the fact that lambs identified as in need of treatment in the TST group were 79% less likely to require further drenching in the subsequent 4 week period which ultimately points to the unnecessary use of drench in regimes with a 4 week drenching interval (NST). The TST regime described here does require some commitment of effort as fortnightly weighing of lambs is needed. However, the advent of automatic weighing and drafting systems may help to reduce the labour requirements. It may also be possible to extend the weighing interval although the impact of this on performance is yet to be determined. By comparison, the similar productivity in SPT animals also suggests that this anthelmintic treatment regime was well suited to this environment. While clearly simpler in design and application than the TST regime, it is worth noting that the SPT regime used here was developed with considerable experience and knowledge of the parasite population on this property and its suitability for farming systems in different environments remains to be determined. In some circumstances, where regular weighing of animals is not possible this regime may serve to slow the development of anthelmintic resistance, although, SPT regimes inherently have a greater risk of production loss than performance-based TST regimes due to their inability to respond to both the individualised nature in which parasitism affects animals within a population (Greer et al., 2008) and to subtle temporal changes in parasite populations, which may be expected to occur when regimes designed to encourage a nematode population in refugia are used (Besier, 2012). Indeed, several studies have shown that leaving a proportion of animals untreated results in increased pasture contamination, compared with whole-flock anthelmintic treatment approaches, in short-term (1 year) field studies (Besier, 2001; Leathwick et al., 2006b; Waghorn et al., 2008; Gaba et al., 2010). In the current study, mean tracer worm burden was reduced in NST, compared with all other treatment approaches tested. However, there was no evidence that tracer worm burdens increased disproportionately across years in any of the regimes intended to provide refugia (TST, SPT or MT). With this in mind, the increase in the mean number of drenches administered in the TST group in 2010 to 2.2 drenches per lamb presumably reflects the increase in nematode challenge from pasture which was observed in all groups in 2010 (Fig. 2). It remains to be determined if this increase in treatment frequency has any impact on the parasite population in subsequent grazing seasons.
In conclusion, the comparison of four different anthelmintic treatment regimes on the efficacy of ivermectin has shown that regular monthly administration of anthelmintic to all lambs in a group does result in reduced drug efficacy. Further, the provision of refugia, regardless of how it is produced, does assist in slowing the decline in drug efficacy and anthelmintic regimes that provide an acceptable balance between the provision of refugia and animal performance can be achieved in a temperate grazing environment.
Acknowledgements
This study was funded by the Scottish Government and the EU FP6 PARASOL Project (FOOD-CT-2005-022851). A.G. received funding from AGMARDT, NZ. The authors would also like to thank the following for their help with practical aspects of the study: Scott Roger, Jim Rayburn, Moredun Bioservices, Heather McDougal, Rachael Baker, Ailie Robinson, Claire McArthur, Karoliene Penders, Lottie Gaunt, Hazel Wilkie, Mark Lutton, Heather Laurie, Danielle Gordon, Frank Turnbull, Glen Lauder and Lynsey Melville. Authors are also grateful to Iain McKendrick, Giles Innocent and David Elston for their advices on statistical analyses of the data.
References
- A.F.R.C., 1993. Energy and protein requirements of ruminants. In: An Advisory Manual Prepared by the Agriculture and Food Research Council technical committee on responses to nutrients.
- Barnes E.H., Dobson R.J., Barger I.A. Worm control and anthelmintic resistance: adventures with a model. Parasitol. Today. 1995;11:56–63. doi: 10.1016/0169-4758(95)80117-0. [DOI] [PubMed] [Google Scholar]
- Bartley D.J., Jackson E., Johnston K., Coop R.L., Mitchell G.B.B., Sales J., Jackson F. A survey of anthelmintic resistant nematode parasites in Scottish sheep flocks. Vet. Parasitol. 2003;117:61–71. doi: 10.1016/j.vetpar.2003.07.023. [DOI] [PubMed] [Google Scholar]
- Bartley D.J., Donnan A.A., Jackson E., Sargison N., Mitchell G.B., Jackson F. A small scale survey of ivermectin resistance in sheep nematodes using the faecal egg count reduction test on samples collected from Scottish sheep. Vet. Parasitol. 2006;137:112–118. doi: 10.1016/j.vetpar.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate – a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- Besier R.B. Rethinking the summer drenching program. J. Agric. West. Aust. 2001;42:6–9. [Google Scholar]
- Besier R.B. Targeted treatment strategies for sustainable worm control in small ruminants. Trop. Biomed. 2008;25:9–17. [PubMed] [Google Scholar]
- Besier R.B. Refugia-based strategies for sustainable worm control: factors affecting the acceptability to sheep and goat owners. Vet. Parasitol. 2012;186:2–9. doi: 10.1016/j.vetpar.2011.11.057. [DOI] [PubMed] [Google Scholar]
- Burgess C.G.S., Bartley Y., Redman E., Skuce P.J., Nath M., Whitelaw F., Tait A., Gilleard J.S., Jackson F. A survey of the trichostrongylid nematode species present on UK sheep farms and associated anthelmintic control practices. Vet. Parasitol. 2012;189:299–307. doi: 10.1016/j.vetpar.2012.04.009. [DOI] [PubMed] [Google Scholar]
- Coop R.L., Sykes A.R., Angus K.W. Effect of a daily intake of Ostertagia circumcincta larvae on body weight, food-intake and concentration of serum constituents in sheep. Res. Vet. Sci. 1977;23:76–83. [PubMed] [Google Scholar]
- Gaba S., Cabaret J., Sauve C., Cortet J., Silvestre A. Experimental and modeling approaches to evaluate different aspects of the efficacy of targeted selective treatment of anthelmintics against sheep parasite nematodes. Vet. Parasitol. 2010;171:254–262. doi: 10.1016/j.vetpar.2010.03.040. [DOI] [PubMed] [Google Scholar]
- Greer A.W., Huntley J.F., Mackellar A., McAnulty R.W., Jay N.P., Green R.S., Stankiewicz M., Sykes A.R. The effect of corticosteroid treatment on local immune responses, intake and performance in lambs infected with Teladorsagia circumcincta. Int. J. Parasitol. 2008;38:1717–1728. doi: 10.1016/j.ijpara.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Greer A.W., Kenyon F., Bartley D.J., Jackson E.B., Gordon Y., Donnan A.A., McBean D.W., Jackson F. Development and field evaluation of a decision support model for anthelmintic treatments as part of a targeted selective treatment (TST) regime in lambs. Vet. Parasitol. 2009;164:12–20. doi: 10.1016/j.vetpar.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Jackson F. New technique for obtaining nematode ova from sheep faeces. Lab. Pract. 1974;23:65–66. [PubMed] [Google Scholar]
- Jackson F., Coop R.L. The development of anthelmintic resistance in sheep nematodes. Parasitology. 2000;120:S95–S107. doi: 10.1017/s0031182099005740. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kenyon F., Greer A.W., Coles G.C., Cringoli G., Papadopoulos E., Cabaret J., Berrag B., Varady M., Van Wyk J.A., Thomas E., Vercruysse J., Jackson F. The role of targeted selective treatments in the development of refugia-based approaches to the control of gastrointestinal nematodes of small ruminants. Vet. Parasitol. 2009;164:3–11. doi: 10.1016/j.vetpar.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Vlassoff A., Barlow N.D. A model for nematodiasis in New Zealand lambs: the effect of drenching regime and grazing management on the development of anthelmintic resistance. Int. J. Parasitol. 1995;25:1479–1490. doi: 10.1016/0020-7519(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Miller C.M., Atkinson D.S., Haack N.A., Alexander R.A., Oliver A.M., Waghorn T.S., Potter J.F., Sutherland I.A. Drenching adult ewes: implications of anthelmintic treatments pre- and post-lambing on the development of anthelmintic resistance. N. Z. Vet. J. 2006;54:297–304. doi: 10.1080/00480169.2006.36714. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Waghorn T.S., Miller C.M., Atkinson D.S., Haack N.A., Oliver A.M. Selective and on-demand drenching of lambs: impact on parasite populations and performance of lambs. N. Z. Vet. J. 2006;54:305–312. doi: 10.1080/00480169.2006.36715. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Miller C.M., Atkinson D.S., Haack N.A., Waghorn T.S., Oliver A.M. Managing anthelmintic resistance: untreated adult ewes as a source of unselected parasites, and their role in reducing parasite populations. N. Z. Vet. J. 2008;56:184–195. doi: 10.1080/00480169.2008.36832. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Waghorn T.S., Miller C.M., Candy P.M., Oliver A.M. Managing anthemintic resistance – use of combination anthelmintic and leaving some lambs untreated to slow the development of resistance to ivermectin. Vet. Parasitol. 2012;187:285–294. doi: 10.1016/j.vetpar.2011.12.021. [DOI] [PubMed] [Google Scholar]
- MAFF, 1986. Ministry of Agriculture, Fisheries and Food, Manual of veterinary parasitological laboratory techniques, Reference Book 418. Third Edition.
- Miller C.M., Waghorn T.S., Leathwick D.M., Candy P.M., Oliver A.-M.B., Watson T.G. The production cost of anthelmintic resistance in lambs. Vet. Parasitol. 2011;186:376–381. doi: 10.1016/j.vetpar.2011.11.063. [DOI] [PubMed] [Google Scholar]
- Molento M.B., Fortes F.S., Pondelek D.A., Borges F.A., Chagas A.C., Torres-Acosta J.F., Geldhof P. Challenges of nematode control in ruminants: focus on Latin America. Vet. Parasitol. 2011;180:126–132. doi: 10.1016/j.vetpar.2011.05.033. [DOI] [PubMed] [Google Scholar]
- Pinheiro J.C., Bates D.M. Springer; New York: 2000. Mixed-Effects Models in S and S-Plus. [Google Scholar]
- Pomroy W.E. Anthelmintic resistance in New Zealand: a perspective on recent findings and options for the future. N. Z. Vet. J. 2006;54:265–270. doi: 10.1080/00480169.2006.36709. [DOI] [PubMed] [Google Scholar]
- Prichard R.K., Hall C.A., Kelly J.D., Martin I.C., Donald A.D. The problem of anthelmintic resistance in nematodes. Aust. Vet. J. 1980;56:239–251. doi: 10.1111/j.1751-0813.1980.tb15983.x. [DOI] [PubMed] [Google Scholar]
- Sutherland I.A., Shaw J., Shaw R.J. The production costs of anthelmintic resistance in sheep managed within a monthly preventative drench program. Vet. Parasitol. 2010;171:300–304. doi: 10.1016/j.vetpar.2010.03.035. [DOI] [PubMed] [Google Scholar]
- Van Wyk J.A. Refugia – overlooked as perhaps the most potent factor concerning the development of anthelmintic resistance. Onderstepoort J. Vet. Res. 2001;68:55–67. [PubMed] [Google Scholar]
- Van Wyk J.A., Hoste H., Kaplan R.M., Besier R.B. Targeted selective treatment for worm management – how do we sell rational programs to farmers? Vet. Parasitol. 2006;139:336–346. doi: 10.1016/j.vetpar.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Waghorn T.S., Leathwick D.M., Miller C.M., Atkinson D.S. Brave or gullible: testing the concept that leaving susceptible parasites in refugia will slow the development of anthelmintic resistance. N. Z. Vet. J. 2008;56:158–163. doi: 10.1080/00480169.2008.36828. [DOI] [PubMed] [Google Scholar]
- Waghorn T.S., Miller C.M., Oliver A.M., Leathwick D.M. Drench-and-shift is a high-risk practice in the absence of refugia. N. Z. Vet. J. 2009;57:359–363. doi: 10.1080/00480169.2009.64723. [DOI] [PubMed] [Google Scholar]


