Graphical abstract
Relationship between genotype, at positions 1183, 1188, 1308 and 1545 in the β-tubulin gene, and community ivermectin response phenotype in Onchocerca volvulus from individuals showing rapid repopulation of microfilariae in the skin.
Highlights
► Sequencing the 3696 bp β-tubulin gene of O. volvulus revealed 24 SNPs. ► 8 SNPs occurred at higher frequencies in poor response compared with other O. volvulus. ► Genotype (1183GG/1188CC/1308TT/1545GG) was associated with a poor response to IVM. ► This β-tubulin genotype may be a marker for IVM resistance in O. volvulus.
Keywords: Onchocerca volvulus, Ivermectin, Anthelmintic resistance, Genetic changes
Abstract
Ivermectin (IVM) has been in operational use for the control of onchocerciasis for two decades and remains the only drug of choice. To investigate the parasitological responses and genetic profile of Onchocerca volvulus, we carried out a 21 month epidemiological study to determine the response of the parasite to IVM in 10 Ghanaian endemic communities. Onchocerca nodules were surgically removed from patients in three IVM response categories (good, intermediate and poor) and one IVM naïve community. DNA from adult worms was analyzed to determine any association between genotype and IVM response phenotypic. Embryogramme analysis showed significantly higher reproductive activity in worms from poor response communities, which had up to 41% of females with live stretched microfilaria (mf) in utero, despite IVM treatment, compared with good response communities, which had no intra-uterine stretched mf.
β-tubulin isotype 1 gene has been shown to be linked to IVM selection in O. volvulus and also known to be associated with IVM resistance in veterinary nematodes. We have genotyped the full length genomic DNA sequence of the β-tubulin gene from 127 adult worms obtained from the four community categories. We found SNPs at 24 sites over the entire 3696 bp. Eight of the SNPs occurred at significantly higher (p < 0.05) frequencies in the poor response communities compared with the good response communities and the IVM naïve community. Phenotypic and genotypic analyses show that IVM resistance has been selected and the genotype (1183GG/1188CC/1308TT/1545GG) was strongly associated with the resistance phenotype. Since the region in the β-tubulin gene where these four SNPs occur is within 362 bp, it is feasible to develop a genetic marker for the early detection of IVM resistance.
1. Introduction
Over the past two decades, ivermectin (IVM) has been central to the control of human onchocerciasis through self-sustainable community-based treatment. IVM, taken as a single annual dose, markedly suppresses onchocercal disease, reduces skin microfilarial (mf) loads and maintains them at very low levels for 9–12 months (Awadzi et al., 1989; Brown and Neu, 1990; Kläger et al., 1996; Abiose, 1998; Brieger et al., 1998). This has the effect of reducing morbidity and suppressing transmission for most of the year. Though IVM does not kill significant numbers of the adult worm, it has been demonstrated that treatment with repeated standard doses (150 μg/kg) markedly impairs female worm fecundity and reduces the life span of adult worms (Duke et al., 1990; Chavasse et al., 1992; Plaisier et al., 1995). IVM resistance is a growing problem in the livestock industry. Until recently, there was no unequivocal evidence that resistance to IVM in Onchocerca volvulus populations existed (Prichard, 2005), but the possibility for IVM resistance to develop in O. volvulus was a concern due to the speed with which IVM resistance developed in other nematodes. For example, IVM was introduced to South Africa for the control of veterinary parasites in the middle 1980s and within 33 months, IVM resistance was detected (Shoop, 1993). However, it should be noted that treatment frequencies had been very high in this instance and the species of parasite was Haemonchus contortus.
Large numbers of individuals in Africa have taken IVM annually for up to 20 years, with millions of doses of IVM being administered (Basáñez et al., 2006). This is likely to impose enormous selection pressure on the parasite and the risk of drug resistance developing. Boussinesq and Gardon (1999) and Grant (2000) have suggested the possibility of parasitological unresponsiveness to IVM treatment or selection for drug resistance in O. volvulus. The latter author has suggested that IVM resistance in O. volvulus may be manifested in the adult parasites, leading to adult female worms regaining their fertility after IVM treatment sooner and repopulating the skin more rapidly than would occur in a susceptible population and that the consequences of this manifestation of resistance may be more serious than a loss in microfilaricidal effect. Recent clinico-parasitological studies have shown sub-optimal responses to IVM treatment in some onchocerciasis patients in Sudan and Ghana (Ali et al., 2002; Awadzi et al., 2004a,b). Ali et al. speculated that the sub-optimal responses, observed in Sudan, were possibly due to host immunocompetence. On the other hand, Awadzi et al. investigated host, pharmaceutical and parasite parameters and concluded that the sub-optimal responses, they observed in Ghana, were due to the development of tolerance or the selection for resistance to IVM in the adult female worms.
β-tubulin, a protein component of microtubules, has been linked to IVM selection and resistance in parasitic nematodes. Freeman et al. (2003) compared the amphid structures of IVM sensitive and resistant strains of H. contortus, and found a significant shortening and diffused morphology of the amphidial dendrites, most notably with a marked loss of microtubule bundles, in IVM resistant strains. Mottier and Prichard (2008), reported IVM selection on β-tubulin isotype 1 in a large number of H. contortus isolates resistant to IVM. They found two single nucleotide polymorphisms (SNPs) causing amino acid changes from phenylalanine to tyrosine, at codons 167 and 200, in β-tubulin isotype 1 gene that were associated with IVM selection and resistance in H. contortus. This selection was observed in strains that had not been subject to benzimidazole selection. Eng and Prichard (2005) and Eng et al. (2006) showed significant changes in allele frequencies of β-tubulin isotype 1 gene in O. volvulus exposed to repeated rounds of IVM treatment. Bourguinat et al. (2007) have also reported that IVM causes genetic selection on the β-tubulin gene in O. volvulus worms, with the most marked effect on selection being associated with a lower reproductive rate of the female parasites.
In veterinary parasites, anthelmintic resistance is usually first suspected when animals fail to respond to treatment. In O. volvulus, the response to treatment relies on the parasitological measures of IVM effectiveness, namely the rate of skin microfilarial clearance, repopulation of the skin by microfilariae after treatment, assessment of female worm fertility, via embryogrammes, and the presence of infective stages in blackfly vectors following years of IVM control. We have previously reported parasitological and epidemiological evidence of emerging IVM resistance in O. volvulus, expressed in adult populations (Osei-Atweneboana et al., 2007). A followed-up study was carried out to assess the adult worm response to IVM by performing nodulectomies and embryogramme analysis that showed further phenotypic evidence of emerging IVM resistance in O. volvulus (Osei-Atweneboana et al., 2011). Here we report a genetic analysis of the full length genomic DNA sequence of the β-tubulin isotype 1 gene, showing evidence of selection on this gene, consistent with the emergence of IVM resistance in O. volvulus.
2. Materials and methods
2.1. Epidemiological study
A 21 month epidemiological study was carried out in 10 onchocerciasis endemic communities located in three districts in Ghana; Kintampo and Atebubu districts in the Brong-Ahafo Region and Gonja East district in the Northern region. The full details of the study are described in Osei-Atweneboana et al. (2007) and Osei-Atweneboana et al. (2011). Briefly a total of 301 subjects who were mf positive at the start of the study were selected from nine IVM treated communities and one IVM naïve community. With the exception of the IVM naïve community, all subjects had received between 6 and 18 rounds of IVM treatments at the start of the study. Before the commencement of the study, pretreatment skin microfilaria profiles were assessed a week (day-7) before IVM treatment. Serial mf assessments were carried out on days 30, 90, 180 and 364 after IVM treatment for the characterization of parasitological responses, in terms of microfilarial clearance and skin mf repopulation rates. A second IVM treatment was administered 1 year after commencement of the study and nodulectomies carried out 90 days after this second study IVM treatment. Ethical approval was obtained from the Institutional Review Board of Noguchi Memorial Institute for Medical Research, Ghana and McGill University, Canada.
2.2. Nodulectomies and adult worm collection and embryogrammes
All subjects were examined for Onchocerca nodules by palpating the entire body, at day 90 after the second IVM treatment (i.e. day 445 after first study treatment). A total of 140 patients selected from 10 endemic communities were prepared for nodulectomies. Using local anesthesia, all palpable nodules were surgically removed from the patients. Nodules were digested using 0.5% collagenase in a shaking water bath for 10–24 h, adult worms were harvested and embryogrammes constructed on intact female worms as described (Schulz-Key, 1988). Worms were stored at −80°C until DNA extractions were carried out.
2.3. Classification of IVM response categories in the 10 communities
We had previously classified the 10 communities into three IVM response categories (poor IVM response category, intermediate IVM response category, good IVM response category) and the IVM naïve community (Osei-Atweneboana et al., 2011). The classification of the three IVM response categories was based on, (i) skin microfilaria repopulation rates at days 90 and 180 post-IVM treatment; (ii) skin mf recovery rate at 364 days post-IVM treatment; (iii) nodule/worm viability and worm density; (iv) reproductive activity (embryogrammes) of female worms; and (v) proportion of individual subjects showing good or poor overall IVM response. Four communities (Baaya, Beposo, Senyase and Hiampe) were classified as good IVM response communities, two communities (Asubende and Wiae) fell into the intermediate IVM response category, and three communities (Kyingakrom, New Longoro and Jagbenbendo) were classified as poor IVM response communities. The fourth group is the previously IVM naïve community (Begbomdo) which had no IVM treatment prior to the study and served as the baseline response community. The naïve community responded in a similar manner to the good response category.
The good IVM response category had virtually no mf repopulation at day 90 post-IVM treatment (Osei-Atweneboana et al., 2007). By day 180, mf repopulation rates were below 17% of pretreatment mf densities. The skin mf recovery rates at 364 days post-IVM treatment were less than the pre-IVM treatment mf densities (<80% of pre-treatment mf densities). Worm density per nodule was less than 0.5, embryogenesis had not resumed at 90 days after IVM (Osei-Atweneboana et al., 2011). The IVM naïve community was very similar to the good responders. However, very few of nodules and worms were calcified, individuals in this community had high worm densities per nodule, as a result of only two exposures to IVM, but skin mf recovery rate at 364 days after the first study IVM treatment was low (<42% of pre-treatment). For the intermediate IVM response category, mf repopulation rate at day 90 post-IVM treatment was very low (< 4% of pre-treatment mf density) or absent, at day 180 post-IVM treatment, mf repopulation rate was up to 22% of pretreatment mf densities. The skin mf recovery rates at 364 days post-IVM treatment was about 100% of pre-IVM treatment mf densities and about 50% of nodules were calcified and worm density per nodule was close to one. Embryogenesis had resumed at 90 days after treatment in some worms with up to 25% of worms producing intra-uterine mf. The poor IVM response category had mf repopulation rates of up to 21% of pre-treatment densities at 90 post-IVM treatment, and by day 180 post-IVM treatment, mf repopulation rates was up to 53.8% of pretreatment mf densities. Skin mf recovery rates at 364 days post-IVM treatment was above 110% of pre-IVM treatment mf densities, few nodules were calcified, worm density per nodule was more than one and embryogenesis had resumed 90 days after treatment with up to 40.6% of worms producing intra-uterine mf.
2.4. Onchocerca volvulus samples selected for genetic analysis
For each IVM response category, one or more communities were selected from which O. volvulus nodules were taken for genetic analysis of the adult worms. A total of 152 adult worms were collected for genetic analysis; 50 worms from Kyingakrom (poor IVM response), 44 worms from Begbombo (IVM naïve community), 26 worms from the Asubende (intermediate IVM response) and 32 worms from all communities in the good IVM response category (made up of Baaya, Beposo, Senyase and Hiampe). Only small numbers of worms were obtained from each of these four communities because most of the nodules and worms were calcified. Kyingakrom was selected for the poor IVM response communities because it showed the worse IVM response characteristics. Asubende was selected for the intermediate IVM response category because it is located close to the good response communities. In addition to classifying the worms in terms of community IVM response categories, we also classified the worms based on IVM response of individual subjects, regardless of community, into two groups: (i) worms from good responding individuals, (ii) worms from poorly responding individuals. The characteristics for classification according to the response of the individual host included, (i) mf repopulation rates of >6 microfilariae/skin snip (mf/s) at days 90 and >20 mf/s at day 180 post-IVM treatment, (ii) high mf recovery rates of greater than pre-treatment densities at day 364 post-IVM treatment. In addition to coming from an individual host showing a good or poor response according to these repopulation rates at different intervals after treatment, female worms had embryogrammes constructed and the reproductive activity and output determined (Osei-Atweneboana et al., 2011). Female worms, coming from individual hosts that showed rapid repopulation with mf and that were found to be reproductive active at 90 days post IVM treatment were considered to have responded poorly to treatment.
2.5. DNA extraction, PCR amplification and sequencing of full length genomic DNA of O. volvulus β-tubulin isotype 1 gene
DNA was extracted from adult worms using the genomic DNA isolation kit supplied by Qiagen Inc., Canada (DNeasy™ Tissue Kit). Extraction was done for individual male and female worms according to the manufacturer’s protocol.
The full length genomic DNA sequence of the β-tubulin isotype 1 gene of O. volvulus was amplified using nine sets of primer pairs (Table 1). The genomic sequence of O. volvulus β-tubulin isotype (GenBank Accession No. AF019886) has 3696 bp, consisting of 9 exons and 8 introns. Genomic DNA was extracted from each individual worm from the three IVM response categories and the previously IVM naïve community. For each sample the full length DNA of the β-tubulin gene was amplified. The general amplification conditions for the PCR were an initial denaturation at 95 °C for 3 min, followed by 35 cycles of 95 °C for 45 s, an annealing temperature of 55 °C for 45 s, initial extension temperature at 68 °C for 1 min and a final extension at 68 °C for 5 min. Modifications were made to the annealing temperature depending on the primer pairs and the amplicon length as shown in Table 1. Proof reading Taq polymerase was used to avoid errors during PCR amplification. The PCR products obtained from all nine primer pairs were examined on a 1% agarose (TAE) gel electrophoresis at 110 V and visualized using the Bio-Rad gel documentation system Gel Doc 2000, and fragment sizes of all nine PCR products were confirmed. Heat shock protein 60 (hsp60) (GenBank No. AF121264), which was known to be polymorphic from previous analyses (Eng et al., 2006; Bourguinat et al., 2007) was also partially sequenced in worms from the different communities and response categories. A fragment of 376 bp of hsp60 containing a known polymorphic site, was amplified by PCR from individual adult worms with the primers 5′-CAA TCA TGG GGA AGT CCA AAG-3′ and 5′-CTC AAA ACC TTC CTT TGC AAT-3′ at annealing temperature of 53 °C. A high fidelity Platinum Taq DNA polymerase (Invitrogen) was used. The amplicon included 100 bp of exonic, followed by 276 bp of intronic sequence. The A/G polymorphism was located in the intron region.
Table 1.
Primer pairs for amplification of full length genomic sequence of the β-tubulin gene in O. volvulus.
| Primer pairs | Annealing temperature (°C) | Region of genomic sequence amplified |
|---|---|---|
| ⁎5′-ATGAGAGAAATTGTTCATG-3′ 5′-GAAATTCGAATTAAGAACTTC-3′ | 50 | 2–747 |
| ⁎5′-CAAAGTTGGAACTTCGAAG-3′ 5′-CTCGAATGGAATCCATAGTA-3′ | 52 | 665–1493 |
| ⁎5′-ATATGTACCACGAGCAATC-3′ 5′-CGATCCGGATATTCCTCACGA-3′ | 51 | 1437–1856 |
| ⁎5′-CATTTCGTAAATAATTCAGT-3′ 5′-GTATCCACTCTAGACACTTG-3′ | 49 | 1721–1999 |
| ⁎5′-GTATGGGAACATTGTTGATC-3′ 5′-GATTGAGATCGCCATAAGT-3′ | 52 | 1809–2268 |
| ⁎5′-GACATCTGCTTCCGAAC-3′5′-CAGCGAAAATCTTGATAGT-3′ | 50 | 2214–2652 |
| ⁎5′-CTTCGCACGTCTCTTAGT-3′ 5′-GATGAATTCTTATTCTGCAC-3′ | 52 | 2596–3079 |
| ⁎5′-ATGATGCAAGTGCAGAATAAG-3′ 5′-CATAAAGTATATCAGATGACG -3′ | 51 | 3051–3350 |
| ⁎5′-GATAATGGATGAATGTGAT-3′ 5′-GCTTTTACTCTTCCTGTTC-3′ | 50 | 3288–3644 |
Sense primers.
All nine sets of PCR products of the full length genomic DNA sequence of β-tubulin were sequenced directly at the McGill University/Genome Quebec Innovation Centre sequencing platform. Sequences received were screened for sequencing errors by comparing the sequences with the accompanying chromatograms and also with the O. volvulus β-tubulin reference DNA sequence (GenBank No. AF019886). The sequences were analyzed and heterozygosity determined using Sequencher™ 4.7 software (Gene Code Corporation, Ann Arbor, MI 48108, USA). To avoid the effect of background noise on heterozygosity, we only considered secondary peaks as significant if they constituted at least 75% of the major peak on the chromatogram. All SNPs site were examined to determine whether they were homozygous or heterozygous. After the analysis of all the SNPs observed in the full length genomic DNA sequence of O. volvulus, the SNPs sites showing significant (p < 0.05) differences were further considered.
2.6. Statistical analysis
Pair-wise comparisons of SNP frequencies of worms from the four IVM responses groups were carried out to determine the differences in genetic changes in the β-tubulin gene. Chi-square test was used for these analyses. However, for small sample sizes, Fisher’s exact test was used. Differences in genotype and allele frequencies between the four IVM response groups were also determined. Also pair-wise comparisons of SNP frequencies were performed to determine the difference between worms from individual hosts showing good IVM responses and those showing poor IVM responses.
3. Results
The full length genomic DNA sequence (3696 bp) of β-tubulin isotype 1 gene of O. volvulus was successfully amplified (Fig. 1). Of 152 worms that were amplified and sequenced, 127 worms (65 males and 62 female) produced good quality sequences for all 9 amplicons of the full length β-tubulin gene. From all the samples sequenced and analyzed, we found 24 single nucleotide polymorphisms (SNPs) occurring at significant frequencies and some appeared to be associated with IVM selection. Seven SNPs occurred in exons (3 in exon 2; 2 in exon 3; 2 in exon 7) and 17 SNPs in introns or exon/intron junctions (5 in intron 1; 6 in intron 2; 2 in intron 3, with 1 at the exon/intron junction, 3 in intron 7 and 1 in intron 8). In our samples, only one SNP in an exon caused a coding change, at position 320, from arginine (AGA) to lysine (AAA). When the frequencies of the 24 SNPs were compared between the four IVM response communities, significant differences (p < 0.05) were found at eight SNP sites in the β-tubulin gene (Table 2). The amplicon from the control gene, hsp60, showed A/G polymorphism in an intron, but there were no significant differences between worms from the different IVM response communities. Further analyses were conducted on the 6 SNPs showing the highest frequency for the alternative nucleotide to that reported in the reference sequence (GenBank No. AF019886). These SNPs were at 194 (A–T), 340 (C–T), 1183 (T–G), 1188 (T–C), 1308 (C–T) and 1545 (A–G). The most striking result was the genetic change observed at SNP position 1545 (A–G), which occurred at a significantly higher frequency (p < 0.0001) in worms from the poor IVM response community compared with worms from the good IVM response communities and the IVM naïve community. Also at the same site, 1545G occurred more frequently (p < 0.05) in worms from the poor IVM response community than O. volvulus from the intermediate response community (Asubende), while the latter also had a significantly higher (p ⩽ 0.02) frequency than worms from both the good response communities and the IVM naïve community. This indicates that at these 6 SNPs sites, the changes to the alternative nucleotide showed a strong association with worms from the poor IVM response community.
Fig. 1.

Full length genomic sequence of β-tubulin isotype 1 gene of O. volvulus, showing exons (in bold and italics) and introns (regular). Nucleotides indicated in upper cases and underlined are positions where SNPs were observed. Nucleotide with superscript marks the end of an exon and the exon number.
Table 2.
Genetic analysis of full length genomic sequence of β-tubulin genotype isotype 1, showing frequencies at eight single nucleotide polymorphism (SNP) loci.
| Ivermectin response/treatment community category [no of worms analysed] | Frequencies of single nucleotide polymorphisms at genomic sequence positions of β-tubulin isotype 1 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 194, A/T% (n) |
340, C/T% (n) |
1183, T/G% (n) |
1188, T/C% (n) |
1308, C/T% (n) |
1545, A/G% (n) |
2913, G/A% (n) |
3226, A/T% (n) |
|||||||||
| A | T | C | T | T | G | T | C | C | T | A | G | G | A | A | T | |
| Previously naïve (Begbomdo) [42] | 70.2 (59) | 29.8 (25)b | 41.7 (35) | 58.3 (49)a | 82.1 (69) | 17.9 (15)d | 72.1 (69) | 17.9 (15)e | 72.1 (69) | 17.9 (15)d | 77.4 (65) | 22.6 (19)f | 98.7 (79) | 1.3 (1)c | 95.2 (82) | 4.8 (2)a |
| Good# [26] | 82.6 (43) | 17.3 (9)b | 23.1 (12) | 76.9 (40)a | 90.4 (47) | 9.6 (5)d | 88.5 (46) | 11.5 (6)e | 88.5 (46) | 11.5 (6)d | 84.6 (44) | 15.4 (8)f | 98.1 (51) | 1.9 (1)c | 96.2 (51) | 3.8 (1)a |
| Intermediate (Asubende) [16] | 71.9 (23) | 28.1 (9)b | 25.0 (8) | 75.0 (24)a | 65.6 (21) | 34.4 (11) | 68.7 (22) | 31.3 (10) | 73.6 (23) | 28.1 (9)d | 56.2 (18) | 43.8 (14)§f,a | 90.6 (29) | 9.4 (3) | 94.0 (28) | 6.0 (2) |
| Poor (Kyingakrom) [43] | 46.5 (40) | 53.5 (46)§b | 9.3 (8) | 90.7 (78)§a | 57.0 (47) | 43.0 (37)§d | 52.2 (44) | 48.8 (42)§e | 56.9 (49) | 43.1 (37)§d | 38.4 (36) | 61.6 (52)§f,§a | 76.5 (52) | 23.5 (16)§c | 78.2 (61) | 21.8 (17)§a |
#Good response communities were Baaya, Beposo, Hiampe and Senyase. At each SNP position, a pair-wise comparison of the allele frequencies was carried out between all of the four community categories. Significant levels: §ap < 0.05 compared with a, §bp < 0.01 compared with b, §cp < 0.005 compared with c, §dp < 0.001 compared with d, §ep < 0.0005 compared with e, §fp < 0.0001 compared withf.
The numbers in square parenthesis [X] indicate the number of worms analyzed, while the number in regular parentheses (X) indicate the number of each alternative nucleotides at each SNP site for worms in each community category.
When we analyzed the response profile of the 127 genotyped adult worms, obtained from the four IVM response groups, for individual host response to IVM treatment, 65.4% were obtained from individual hosts showing good responses while 34.6% were obtained from individual hosts showing poor responses. The genotypic analyses at the 6 SNP sites (194A/G, 340C/T, 1183T/G, 1188T/C, 1308C/T and 1545A/G) on worms obtained from individual hosts showing good responses to treatment (regardless of community) indicated that 22% of the worms had the same genotype at these 6 SNP sites as the reference genomic sequence of β-tubulin which had been cloned from worms not previously exposed to IVM (Geary et al., 1998) and, overall, showed a very high degree of sequence identity to the reference sequence. Several worms from individual hosts showing good responses, from the IVM naïve community and the good IVM response community, showed 100% homology to the reference sequence at all 6 SNP positions. For this study, we have considered the reference sequence and worms having the same homozygous genotype (194AA/340CC/1183TT/1188TT/1308CC/1545AA) as wild type (WT). However, for most of the worms from good responding individuals, we found that 194Tand 340T occurred at significantly higher (p < 0.01) frequencies, and 1183G and 1545G at moderate frequencies (p < 0.05). The SNP 194A/T occurred mainly as homozygous AA in 63.1% of worms, of which more than two thirds were from the IVM naïve and good IVM response communities. Also the SNP 340C/T occurred as homozygous TT or heterozygous CT in most worms from all response communities. The genotype combinations of worms from the good responding individuals show a total of 11 genotype configurations at the four loci (194A/T, 340C/T, 1183T/G, 1545A/G), termed WTA–WTK (Fig. 2). Thus the genetic profiles observed in worms obtained from good responding individuals showed that 22% of the worms had the genotype WTA (AA/CC/TT/AA), 10.6% the genotype WTB (AA/CT/TT/AA), 34.4% genotype WTC (AA/TT/TT/AA) and 16.7% of the worms shared the three genotypes, WTD, WTE, and WTF. The genotypes WTA, WTB and WTC occurred mainly in worms from good responding individuals in the IVM naïve community and good IVM response communities as well as the intermediate response community. All 11 genotypes were present in the IVM naïve community. However, three of these genotypes were absent in worms from the good IVM response communities while the moderate and poor IVM response communities did not have five of the genotypes found in the naïve community.
Fig. 2.
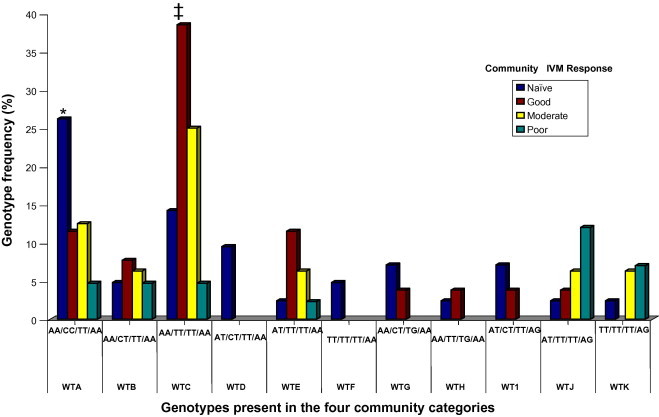
Genotype frequencies in the β-tubulin gene in worms from individuals showing good IVM response from the four community categories. Four SNPs, found at genomic positions 194 (A–T), 340 (C–T), 1183 (T–G) and 1545 (A–G), were analyzed. Eleven genotype configurations (WTA–WTK) were found in worms isolated from individuals showing a good response to IVM, with all being present in the naïve community. The most abundant genotype in the naïve community was the homozygote represented by WTA (AA/CC/TT/AA). WTA occurred at significantly higher (∗p < 0.02) frequency in the naïve group ( ) than the in the poor response community (
) than the in the poor response community ( ). Also WTC (AA/TT/TT/AA) was significantly higher (∗∗p < 0.02) in the good response communities (
). Also WTC (AA/TT/TT/AA) was significantly higher (∗∗p < 0.02) in the good response communities ( ) than the poor response and naïve groups. We observed loss of polymorphism for five genotypes: WTD, WTF, WTG, WTH, WTI, in both the poor and intermediate response communities (
) than the poor response and naïve groups. We observed loss of polymorphism for five genotypes: WTD, WTF, WTG, WTH, WTI, in both the poor and intermediate response communities ( ). For the good response communities, only the two genotypes, WTD and WTF were not found. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
). For the good response communities, only the two genotypes, WTD and WTF were not found. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The second set of worms analyzed were from the poor responding individual hosts in all four IVM response community groups, comprising 34.6% of all worms genotyped. A comparison of worms from the poorly responding individuals showed genetic changes at 4 SNPs sites 1183 T–G, 1188 T–C, 1308 C–T and 1545 A–G (see Table 2 and Fig. 3) which were at significantly higher frequencies (p < 0.001) in the poor IVM response community (Kyingakrom) than in the good IVM response communities and the IVM naïve community. Also the genetic change occurring at SNP site 1188 T/C was found to be usually linked with changes at SNP site 1183T/G, 1308C/T and 1545A/G occurring mostly as homozygotes. This suggested that the genetic changes occurring at these SNPs sites are strongly associated with poor IVM responses at both the community and individual host levels.
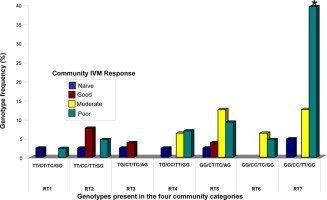
Fig. 3.
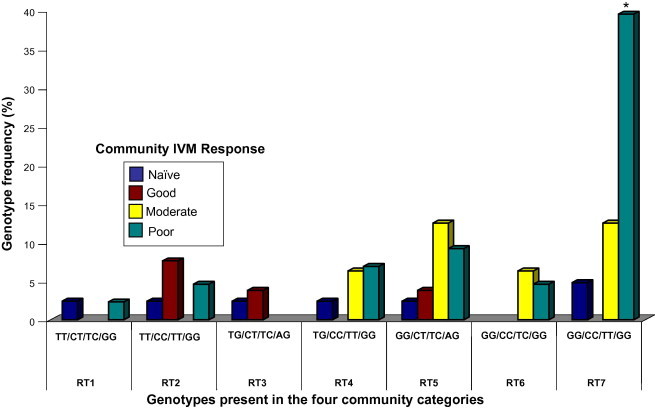
Genotype frequencies in the β-tubulin gene in worms from individuals showing poor IVM response from the four community categories. Four SNPs found at genomic positions 1183 (T–G), 1188 (T–C), 1308 (C–T) and 1545 (A–G) appeared to be linked with poor response in individual hosts. Only seven genotype configurations were found in worms from individuals showing a poor response to IVM, and are represent by RT1 (TT/CC/TT/GG) to RT7 (GG/CC/TT/GG). The homozygous RT7 occurred at significantly higher (∗p < 0.002) frequencies in the poor response community ( ) than the good (
) than the good ( ) and naïve communities (
) and naïve communities ( ), as well as intermediate response community (
), as well as intermediate response community ( ) (∗∗p < 0.05). There were no significant differences in the genotype frequencies of RT1 to RT6 between the different community categories. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
) (∗∗p < 0.05). There were no significant differences in the genotype frequencies of RT1 to RT6 between the different community categories. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Considering the possible combinations at these four loci, one could expect up to 48 possible configurations for the genotypes. However, because of possible linkage between some of the loci, we found only seven genotypes configurations in the worms from poorly responding individuals, which we have termed, RT1–RT7 (Fig. 3). Table 3 summarizes the proportion of worms showing genotype (wild type sequence) associated with good response (WTA–WTK), or genotype associated with poor response (RT1–RT7) in individuals hosts classified as showing good or poor phenotypic responses. We found different levels of genotype association with worms from poorly responding individuals in the four IVM response categories. The genotype configuration RT7, represented by GG/CC/TT/GG showed a strong association (p < 0.01) with nearly 40% of worms from the poor IVM response community (Kyingakrom) having this genotype. In the IVM naïve community, all the genotypes (RT1–RT7) occurred at low frequencies. In the good IVM response communities, RT7 was absent, but other genotypes RT2, RT3 and RT5 were present at low frequencies. Four genotypic configurations were found in the intermediate response community, with RT7 occurring at a frequency of 12.5%. The absence of some genotypes in the intermediate response community may possibly be due to the small sample size. Also worms from the poor IVM response community had six genotypes, five occurred at frequencies of 2.3–10%. Considering all the worms from poorly responding individuals in all four community categories, we found that almost half the worms from the poorly responding individuals had the genotype RT7, and out of these, 78% were from the poor IVM response community (Kyingakrom).
Table 3.
The association between worm β-tubulin genotypes and IVM response in individual hosts.
| IVM response category/community | % (n) of subjects showing poor response to IVM | No. of worms genotyped | % (n) of worms from individuals showing good response to IVM | % (n) of worms from individuals showing poor response to IVM | Genotypic analysis of worm from individuals showing good IVM response |
Genotypic analysis of worms from individuals showing poor IVM response |
Total% (n) of worms having genotype associated with poor response in host (RT1–RT7) | % (n) of worms having the most marked genotype associated with resistant phenotypes (RT7) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| % (n) of worms showing genotype (wild type sequence) associated with good response in host (WTA–WTK) | % (n) of worms showing genotype associated with poor response in host (RT1–RT7) | % (n) of worms showing genotype (wild type sequence) associated with good response in host (WTA–WTK) | % (n) of worms showing genotype associated with poor response in host (RT1–RT7) | |||||||
| Previously naive | 10.8 (4) | 42 | 85.7 (36) | 14.3 (6) | 78.6 (33)§b | 7.1 (3) | 4.8 (2) | 7.1 (4) | 16.7 (7)c | 4.8 (2)a |
| Good | 8.8 (5) | 26 | 84.6 (22) | 15.4 (4) | 84.6 (21)§b | 3.8 (1) | 7.7 (1) | 11.5 (3) | 15.4 (4)c | 0a |
| Moderate | 33.3 (4) | 16 | 62.5 (10) | 37.5 (6) | 44.0 (8) | 18.5 (2) | 12.5 (2) | 25.0 (4) | 37.5 (6) | 12.5 (2)a |
| Poor | 66.7 (18) | 43 | 34.8 (15) | 65.1 (28) | 27.9 (12)b | 6.9 (3) | 11.6 (2) | 53.5 (26) | 67.4 (29)§c | 39.5 (19)§a |
The four community categories were compared for the proportion of worms associated with a particular genotype (WT or RT). Significant levels: §ap < 0.05 compared with a, §bp < 0.02 compared with b, §cp < 0.01 compared with c.
To determine the correlation between the worm genotypes and phenotypes, comparisons were made between the worm genotypes RT1–RT7 and the phenotypic characteristics of worms obtained from the poorly responding individuals as well as the IVM response categories. The phenotypic characteristics included mf repopulation rates and female worm embryogrammes. For the genotypes, RT1–RT6, we did not find strong associations with the worm or host phenotypic response characteristics. However, the association between RT7 frequency and the poor response phenotype of the worm and host mf repopulation was very pronounced. Worms from individuals with mf repopulation at day 90 post IVM treatment, mf recovery rates of greater than pre-treatment densities at day 364 post-IVM treatment and female worms that were reproductively active 90 days after treatment had a strong association (p < 0.01) with the genotype RT7. Furthermore, there were other genetic changes at positions 1093 (C–T), 1298 (T–C), 2913 (G–A) and 3226 (A–T), but these occurred at relatively moderate frequencies and predominantly in poorly responding individuals. More than 87% of these changes were found in worms in the poor IVM response community having the genotype RT7. Another genetic change of importance occurred at genomic position 2754 corresponding to the amino acid at position 320 of the protein sequence. The SNP (AAA–AGA) resulted in a change from arginine to lysine. This change occurred at a frequency of 5% in worms from poorly responding individuals and interestingly, worms carrying 320Lys also had the RT7 genotype and mostly female worms with these genetic changes were reproductively active at day 90 post IVM treatment.
4. Discussion
Anthelmintic resistance is a heritable phenomenon resulting from changes in the genetic profile of a parasite population that subsequently leads to a phenotype of a reduced response to treatment. Drug resistance involves changes in genes and their proteins that can result in mechanisms of resistance. However, some genetic changes may not themselves be the cause of resistance mechanisms, but be linked to other genes whose products are associated with resistance mechanisms. In veterinary nematodes, genes for P-glycoprotein (Xu et al., 1998), β-tubulin (Mottier and Prichard, 2008), glutamate chloride channels (GluCls) (Njue et al., 2004) and gamma-amino butyric acid receptor subunits (Blackhall et al., 2003) have been reported to be associated or involved in IVM selection and resistance. In O. volvulus, genetic selection has been found in β-tubulin in populations under repeated IVM treatments (Eng and Prichard, 2005; Eng et al., 2006; Bourguinat et al., 2007). To further explore and elucidate the involvement and importance of the β-tubulin gene in genetic selection and resistance to IVM and also for possible development of DNA markers for resistance, we carried out genetic analysis on this gene in O. volvulus obtained during the epidemiological study by Osei-Atweneboana et al. (2007) and further study by Osei-Atweneboana et al. (2011). This study provided excellent material for this genetic analysis, because O. volvulus adult worms were obtained from hosts and parasites that were characterized in terms of the IVM response phenotype; this makes our study unique. Furthermore, in contrast to previous studies, the genetic analysis was carried out on the whole β-tubulin gene. Eight out of the 24 SNPs found in both the introns and exons show genetic changes that provided significant associations with the response phenotype. Worm genotypes from individuals showing good IVM responses were predominantly the wild type (WT) genotypes: WTA, WTB and WTC, which we have considered as susceptible genotypes. A similar but much stronger association was observed in worm genotypes from individuals showing poor IVM responses. We found seven genotypes (RT1–RT7) at four loci (1183T/G, 1188T/C, 1308C/T and 1545A/G). Two of the SNPs (1188T–C, and 1308 C–T) were strongly linked. In addition, they mostly occurred together with the two other SNPs (1183T/G and 1545A/G) found in the RT genotypes. These RT genotypes showed a significant association (p < 0.05) with worms obtained from individuals showing poor IVM responses. A further comparative analysis of the RT genotypes with worm phenotypes from individuals showing poor IVM responses revealed a stronger association with RT6 and RT7 genotypes. We consider RT6 and RT7 genotypes as resistance genotypes (RT) and the worms associated with these genotypes as resistance phenotype. The presence of a few resistance genotype worms in the IVM naïve community (Fig. 3) is not surprising, because it has been suggested that alleles conferring resistance are likely to be already present in an anthelmintic-naive populations, but at low frequencies (Kelly et al., 1978; Prichard et al., 1980; Jackson, 1993) and that IVM resistance probably occurs through the selection of existing alleles present in the population (Anderson et al., 1998).
The most interesting genotype, RT7 (GG/CC/TT/GG), was homozygous for all the alternate nucleotides and showed the strongest association (p < 0.002) with the resistance phenotype worms, at a frequency of 39.5% in the poor IVM response community (Kyinkagrom). It must be emphasized that RT7 was found predominantly in female worms that were reproductively active at day 90 post-IVM treatment and in male worms from the same individuals and also from individual hosts that had high mf repopulation rates at day 90 and/or high mf recovery rates at day 364 post-IVM treatment. We see the presence of RT7, together with the other genotypes (RT1–RT6) in the poor IVM response community as possible markers for the emergence of IVM resistance in O. volvulus.
The loss of five ‘susceptibility’ genotypes and reduction in frequencies of other genotypes observed in the poor IVM response community suggests that in these communities, IVM has selected against worms carrying ‘susceptibility’ genotypes and allowed worms carrying the ‘resistance-associated’ genotype RT7 to continue to reproduce, contributing their gene pool to the next generation (Prichard et al., 1980). Eng and Prichard (2005) have previously reported two β-tubulin alleles, and observed that IVM had selected for one of these β-tubulin alleles while the other allele predominated in IVM naïve populations. The treatment-associated allele was distinguished from allele predominant in the naïve population by having three amino acid changes (Met117Leu, Val120Ile and Val124Ala) in the coding region and a 24 bp intronic deletion. In another study, Eng et al. (2006) found the absence or very low frequency of the treatment-associated allele in O. volvulus samples obtained in 1989/90, before the widespread introduction of IVM for onchocerciasis, but moderate frequencies of the treatment-associated allele in samples obtained, in Ghana, 8 years after widespread use of IVM, with the frequency being highest in communities which had been under annual treatment for 3–8 years. They also observed a higher frequency of the ‘treatment-associated’ allele in samples of microfilaria obtained from people who had responded sub-optimally to IVM, and had proposed the possibility that the more IVM tolerant adult worms, which were presumable homozygous or heterozygous for the ‘treatment-associated’ allele would be responsible for passing on this allele to their microfilaria progeny and therefore the frequency of the treatment-associated allele could increase in the microfilariae with repeated rounds of treatment. Surprisingly, in our current study in Ghana, we observed in all communities, O. volvulus populations had these three amino acid changes (117Leu, 120Ile and 124Ala) at 100% frequency, suggesting that two decades of IVM treatment in Ghana had already exerted marked selection on the β-tubulin gene regardless of whether the response to treatment still appeared to be good or was poor. While the treatment naïve community had itself not been under IVM treatment prior to the study a great portion of the onchocerciasis endemic regions of Ghana, including districts close to the naïve community, have been under IVM treatment for many years. Nevertheless, it is perhaps difficult to explain this apparently rapid genetic transition in O. volvulus populations in Ghana, given the long life span of this parasite. However, it may be due to the fact that strong IVM selection pressure on O. volvulus has been ongoing for two decades leading to a relatively quick shift to the treatment-associated allele as a first step in the selection for a resistance genotype. These changes, coupled with the selection for genotypes such as RT7 may indicate that a resistance genotype has already emerged, and that the RT7 genotype can be used to monitor for resistance.
The recent reports of sub-optimal responses and emergence of IVM resistance in O. volvulus (Ali et al., 2002; Awadzi et al., 2004a,b; Osei-Atweneboana et al., 2007, 2011), coupled with the fact that IVM has been used for over two decades and remains the only drug available for treatment of onchocerciasis makes the need to monitor for possible IVM resistance in O. volvulus of great importance. Ali and co-workers reported that the subjects showing sub-optimal O. volvulus responses to IVM had reduced proliferative responses to Onchocerca antigens and speculated that the sub-optimal responses may be due to host immunocompetence. Credence for this hypothesis could be drawn from the recent observation that in Brugia malayi mf, a glutamate-gated chloride channel (GluCl) receptor was localized only to the excretory pore and that in the mf, IVM reduced the secretion of protein from the mf (Moreno et al., 2010). These authors speculated that the excretory pore is the main source of excretory/secretory protein from the mf and that components of this secreted protein may regulate the local immune response to the mf, so that a diminution in the level of secretion, caused by IVM action on the excretory pore GluCl, could render filarial mf susceptible to immune killing. A similar effect of IVM only on the excretory pore leading to an inability of the parasite to modulate the local immune response in the host has not been shown for adult worms. In the sub-optimal responses observed in subjects infected with O. volvulus in Ghana, killing of mf was not affected (Osei-Atweneboana et al., 2007). On the other hand, the production of mf by the adult worms and the return of fertility in the adult worms was altered (Osei-Atweneboana et al., 2011) in a manner similar to that observed earlier (Awadzi et al., 2004a). It should also be noted that other than the possibility of an immune involvement in the sub-optimal responses observed in Sudan (Ali et al., 2002), there are no other reports that the immune status in humans affects the response of O. volvulus to IVM, and in particular the repopulation rate of skin mf. In addition, an investigation of O. volvulus responses to IVM in HIV patients showed no diminution in the effect of treatment (Fischer et al., 1995).
As an alternative explanation for the high skin microfilarial repopulation rates, it has been postulated that there may have been repeated re-infections due to poor drug coverage in the study communities and in surrounding communities (Remme et al., 2007). A high proportion of young adult worms could indeed lead to high skin mf counts as was found in the pre-study IVM-naive community (21% of worms were young adults in this community; Osei-Atweneboana et al., 2011), where obviously coverage was non-existent prior to this study. However, all of the communities categorized as poor or intermediate responders had good records of treatment coverage. While treatment coverage in some communities in the East Gonja district (where the pre-study treatment naive community is found) may have been poor, coverage in surrounding communities in the Atebubu and Kintempo districts where the poorly responding Kyingakrom and New Longoro communities are located, was generally good (Osei-Atweneboana and Prichard, unpublished). The mechanism underlying the proposed hypothesis that poor coverage could explain the sub-optimal responses, is that recruitment of significant numbers of new adult worms would account for the higher than expected skin mf load. However, the embryogrammes from the multi-dosed communities showed a dearth of young adult worms at a modest level of reproductive activity. These findings do not support a hypothesis that the sub-optimal responses were due to poor treatment coverage, nor does the analysis of the annual transmission rate that would be required to account for the skin mf repopulation rates observed in the poor responder communities (Churcher et al., 2009). It is also of interest that in the example of poor coverage, in the treatment naive community, the recovery rate of skin mf count 364 days after IVM treatment was only 41.6% of pre-treatment mf count, in contrast to the situation seen in the poor responder communities where the recovery rates were all well in excess of 100% of pretreatment mf counts (Osei-Atweneboana et al., 2011). It also needs to be noted that for the 2 study IVM doses, treatment of each individual subject was observed by the study team, and elimination of mf 30 days after treatment, and a marked reduction in skin mf 90 days after treatment, were observed. Therefore, one can be certain that the subjects responding poorly to IVM treatment, in terms of rate of skin mf repopulation and embryogrammes, were treated twice in the study and that poor coverage cannot explain the sub-optimal responses.
Resistance in O. volvulus can be defined as either a reduction in the duration of the anti-fecundity effect of IVM on adult O. volvulus or a reduction in the microfilaricidal effect of IVM (Grant, 2000). The evidence of marked phenotypic and genotypic differences between the poorly responding communities/individuals and parasites, compared with the naïve and good responding communities/individuals and parasites lead us to a conclusion that selection for resistance is occurring in O. volvulus in Ghana and that, so far, the manifestation of resistance is in the shortening of the anti-fecundity effect of IVM. We concur with the suggestion of Grant (2000) that this manifestation of resistance could be more serious for the onchocerciasis control programs, because it enables resistant worms to breed and be transmitted for most of the year, than a reduction in the microfilaricidal effects alone.
Monitoring for resistance in O. volvulus should be undertaken as part of onchocerciasis control programs because once resistance reaches phenotypically-detectable levels, irreversible changes in the genetic structure of the worm population may have already occurred. Since there is still no alternative chemotherapeutic agent for mass treatment of onchocerciasis, it is highly desirable that IVM can continue to be used for a long time to control the disease. Sustaining the efficacy of the drug for as long as possible requires the development of sensitive molecular markers that can detect emerging resistance at its early stage. This then can be used for routine surveillance to monitor for resistance in order to prevent the spread of resistance genotypes. The identification of these specific genetic changes that are linked with IVM resistance phenotypes should be very useful. The study suggests that the genetic changes occurring at genomic positions 1183T/G, 1188T/C, 1308C/T and 1545A/G, and especially the genotype RT7 (GG/CC/TT/GG) make good candidate markers for resistance surveillance. Moreover, since these SNPs occur within a region of 362 bp, it will be entirely practical to use this region of β-tubulin gene to develop a DNA assay for early detection and monitoring for IVM resistance. Initial monitoring for possible IVM resistance, as part of IVM distribution programs, could be conducted using a genetic marker on the larval stages of the parasite found in vectors.
Acknowledgements
This study received financial support from CIHR, Canada, UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases and Health, and the Government of Ghana. Research at the Institute of Parasitology is supported by the Centre for Host-Parasite Interactions and FQRNT, Quebec.
References
- Abiose A. Onchocercal eye disease and the impact of Mectizan treatment. Ann. Trop. Med. Parasitol. 1998;92:S1–S22. doi: 10.1080/00034989859519. [DOI] [PubMed] [Google Scholar]
- Ali M.M.M., Mukhtar M.M., Baraka O.Z., Homeida M.M.A., Kheir M.M., Mackenzie C.D. Immunocompetence may be important in the effectiveness of Mectizan® (ivermectin) in the treatment of human onchocerciasis. Acta Trop. 2002;84:49–53. doi: 10.1016/s0001-706x(02)00117-1. [DOI] [PubMed] [Google Scholar]
- Anderson T.J.C., Blouin M.S., Beech R.N. Population biology of parasitic nematodes: application of genetic markers. Adv. Parasitol. 1998;41:219–283. doi: 10.1016/s0065-308x(08)60425-x. [DOI] [PubMed] [Google Scholar]
- Awadzi K., Dadzie K.Y., Kläger S., Gilles H.M. The chemotherapy of onchocerciasis. XIII. Studies with ivermectin in onchocerciasis, patients in northern Ghana, a region with long lasting vector control. Trop. Med. Parasitol. 1989;40:361–366. [PubMed] [Google Scholar]
- Awadzi K., Boakye D.A., Edwards G., Opoku N.O., Attah S.K., Osei-Atweneboana M.Y., Lazdins-Helds J.K., Ardrey A.E., Addy E.T., Quartey B.T., Ahmed K., Boatin B.A., Soumbey-Alley E.W. An investigation of persistent microfilaridermias despite multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann. Trop. Med. Parasitol. 2004;98:231–249. doi: 10.1179/000349804225003253. [DOI] [PubMed] [Google Scholar]
- Awadzi K., Attah S.K., Addy E.T., Opoku N.O., Quartey B.T., Lazdins-Helds J.K., Ahmed K., Boatin B.A., Boakye D.A., Edwards G. Thirty-month follow-up of sub-optimal responders to multiple treatments with ivermectin, in two onchocerciasis-endemic foci in Ghana. Ann. Trop. Med. Parasitol. 2004;98:359–370. doi: 10.1179/000349804225003442. [DOI] [PubMed] [Google Scholar]
- Basáñez M.G., Pion S.D.S., Churcher T.S., Breitling L.P., Little M.P., Boussinesq M. River blindness: a success story under threat? PLoS Med. 2006;3:e371. doi: 10.1371/journal.pmed.0030371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackhall W.J., Prichard R.K., Beech R.N. Selection at a γ-aminobutyric acid receptor gene in Haemonchus contortus resistant to avermectins/milbemycins. Mol. Biochem. Parasitol. 2003;131:137–145. doi: 10.1016/s0166-6851(03)00201-9. [DOI] [PubMed] [Google Scholar]
- Bourguinat C., Pion S.D., Kamgno J., Gardon J., Duke B.O., Boussinesq M., Prichard R.K. Genetic selection of low fertile Onchocerca volvulus by ivermectin treatment. PLoS Negl. Trop. Dis. 2007;1((1)e72):12–22. doi: 10.1371/journal.pntd.0000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussinesq M., Gardon J. Ivermectin-clinical trials and treatment schedules in onchocerciasis. Acta Leidesia. 1999;59:160–175. [PubMed] [Google Scholar]
- Brieger W.R., Awedoba A.K., Eneanya C.I., Hagan M., Ogbuagu K.F., Okello D.O., Ososanya O.O., Ovuga E.B., Noma M., kale O.O., Burnham G.M., Remme J.H. The effect of ivermectin on Onchocerca skin disease and severe itching: results of a multicenter trial. Trop. Med. Int. Health. 1998;3:951–961. doi: 10.1046/j.1365-3156.1998.00339.x. [DOI] [PubMed] [Google Scholar]
- Brown K.R., Neu D.C. Ivermectin-clinical trials and treatment schedules in onchocerciasis. Acta Leidensia. 1990;59:169–175. [PubMed] [Google Scholar]
- Chavasse D.C., Post R.J., Lemoh P.A., Whitworth J.A. The effect of repeated doses of ivermectin on adult female Onchocerca volvulus in Sierra Leone. Trop. Med. Parasitol. 1992;43:256–262. [PubMed] [Google Scholar]
- Churcher T.S., Pion S.D.S., Osei-Atweneboana M.Y., Prichard R.K., Awadzi K., Boussinesq M., Collins R.C., Beatriz Muñoz B., Whitworth J.A., Basáñez M.-G. Identifying sub-optimal responses to ivermectin in the treatment of river blindness. Proc. Natl. Acad. Sci. USA. 2009;106:16716–16726. doi: 10.1073/pnas.0906176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke B.O.L., Zea-Flores G., Castro J., Cupp E.W., Munoz B. Effect of multiple monthly doses of ivermectin on adult Onchocerca volvulus. Am. J. Trop. Med. Hyg. 1990;43:657–664. doi: 10.4269/ajtmh.1990.43.657. [DOI] [PubMed] [Google Scholar]
- Eng J.K., Prichard R.K. A comparison of genetic polymorphism in populations of Onchocerca volvulus from untreated- and ivermectin-treated patients. Mol. Biochem. Parasitol. 2005;142:193–202. doi: 10.1016/j.molbiopara.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Eng J.K., Blackhall W.J., Osei-Atweneboana M.Y., Bourguinat C., D.Beech R.N., Unnasch T.R., Awadzi K., Lubega G.W., Prichard R.K. Ivermectin selection on beta-tubulin: evidence in Onchocerca volvulus and Haemonchus contortus. Mol. Biochem. Parasitol. 2006;50:229–235. doi: 10.1016/j.molbiopara.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Fischer P., Kipp W., Kabwa P., Buttner D.W. Onchocerciasis and human immunodeficiency virus in Western Uganda: prevalences and treatment with ivermectin. Am. J. Trop. Med. Hyg. 1995;53:171–178. [PubMed] [Google Scholar]
- Freeman A., Nghiem C., Li J., Ashton F., Guerrero J., Shoop W., Schad G. Amphidial structure of ivermectin resistant and susceptible laboratory and field strains of Haemonchus contortus. Vet. Parasitol. 2003;110:217–226. doi: 10.1016/s0304-4017(02)00321-7. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Nulf S.C., Alexander-Bowman S.J., Mahmoud B.M., Prichard R.K., Klein R.D. Cloning and characterization of cDNAs encoding beta-tubulin from Dirofilaria immitis and Onchocerca volvulus. J. Parasitol. 1998;84:356–360. [PubMed] [Google Scholar]
- Grant W. What is the real target for ivermectin resistance in Onchocerca volvulus. Parasitol. Today. 2000;16:458–459. doi: 10.1016/s0169-4758(00)01804-4. [DOI] [PubMed] [Google Scholar]
- Jackson F. Anthelmintic resistance – the state of play. Brit. Vet. J. 1993;149:123–138. doi: 10.1016/S0007-1935(05)80083-1. [DOI] [PubMed] [Google Scholar]
- Kelly J.D., Whitlock H.V., Thompson H.G., Hall C.A., Martin I.C., Le Jambre L.F. Physiological characteristics of free-living and parasitic stages of strains of Haemonchus contortus, susceptible or resistant to benzimidazole anthelmintics. Res. Vet. Sci. 1978;25:376–385. [PubMed] [Google Scholar]
- Kläger S.L., Whitworth J.A.G., Downham M.D. Viability and fertility of adult Onchocerca volvulus after 6 years of treatment with ivermectin. Trop. Med. Int. Health. 1996;1:581–589. doi: 10.1111/j.1365-3156.1996.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Moreno Y., Nabhan J.F., Solomon J., Mackenzie C.D., Geary T.G. Ivermectin disrupts the function of the excretory-secretory apparatus in microfilariae of Brugia malayi. Proc. Natl. Acad. Sci. USA. 2010;107:20120–20125. doi: 10.1073/pnas.1011983107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottier M.L., Prichard R.K. Genetic analysis of a relationship between macrocyclic lactone and benzimidazole anthelmintic selection on Haemonchus contortus. Pharmacogen. Genomics. 2008;18:129–140. doi: 10.1097/FPC.0b013e3282f4711d. [DOI] [PubMed] [Google Scholar]
- Njue A., Hayashi J., Kinne L., Feng X.P., Prichard R. Mutations in the extracellular domains of glutamate-gated chloride channel a3 and b subunits from ivermectin-resistant Cooperia oncophora affect agonist sensitivity. J. Neurochem. 2004;89:1137–1147. doi: 10.1111/j.1471-4159.2004.02379.x. [DOI] [PubMed] [Google Scholar]
- Osei-Atweneboana M.Y., Eng J.K., Boakye D.A., Gyapong J.O., Prichard R.K. Prevalence and intensity of Onchocerca volvulus infection and efficacy of ivermectin in endemic communities in Ghana: a two-phase epidemiological study. Lancet. 2007;369:2021–2029. doi: 10.1016/S0140-6736(07)60942-8. [DOI] [PubMed] [Google Scholar]
- Osei-Atweneboana M.Y., Awadzi K., Atta S.K., Boakye D.A., Gyapong J.O., Prichard R.K. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. Plos Negl. Trop. Dis. 2011;5(3):e998. doi: 10.1371/journal.pntd.0000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaisier A.P., Alley E.S., Boatin B.A., van Oortmarssen G.J., Remme H., de Vlas S.J., Bonneux L., Habbema J.D. Irreversible effects of ivermectin on adult parasites in onchocerciasis patients in the onchocerciasis control program in West-Africa. J. Infect. Dis. 1995;172:204–210. doi: 10.1093/infdis/172.1.204. [DOI] [PubMed] [Google Scholar]
- Prichard R.K. Is anthelmintic resistance a concern for heartworm control? What can we learn from the human filariasis control programs? Vet. Parasitol. 2005;133:243–253. doi: 10.1016/j.vetpar.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Prichard R.K., Hall C.A., Kelly J.D., Martin I.C., Donald A.D. The problem of anthelmintic resistance in nematodes. Aust. Vet. J. 1980;56:239–251. doi: 10.1111/j.1751-0813.1980.tb15983.x. [DOI] [PubMed] [Google Scholar]
- Remme J.H.F., Amazigo U., Engels D., Barryson A., Yameogo L. Efficacy of ivermectin against Onchocerca volvulus in Ghana. Lancet. 2007;370:1123–1124. doi: 10.1016/S0140-6736(07)61503-7. [DOI] [PubMed] [Google Scholar]
- Schulz-Key H. The collagenase technique: how to isolate and examine adult Onchocerca volvulus for the evaluation of drug trials. Trop. Med. Parasitol. 1988;39:423–440. [PubMed] [Google Scholar]
- Shoop W.L. Ivermectin resistance. Parasitol. Today. 1993;9:154–159. doi: 10.1016/0169-4758(93)90136-4. [DOI] [PubMed] [Google Scholar]
- Xu M., Molento M., Blackhall W., Ribeiro P., Beech R., Prichard R. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol. Biochem. Parasitol. 1998;91:327–335. doi: 10.1016/s0166-6851(97)00215-6. [DOI] [PubMed] [Google Scholar]


