Graphical abstract
Highlights
► Avermectins and moxidectin have a macrocyclic lactone ring but different substituents. ► They differ in terms of physicochemical properties, pharmacodynamics and kinetics. ► They act on ligand-gated anion channels of invertebrates but with binding differences. ► They interact differently with ABC transporters affecting toxicity. ► In nematodes, this may explain different abilities to induce anthelmintic resistance.
Keywords: Avermectin, Ivermectin, Moxidectin, Nematode, Toxicity, ATP binding cassette transporter, Ligand-gated ion channels
Abstract
The avermectins and milbemycins contain a common macrocyclic lactone (ML) ring, but are fermentation products of different organisms. The principal structural difference is that avermectins have sugar groups at C13 of the macrocyclic ring, whereas the milbemycins are protonated at C13. Moxidectin (MOX), belonging to the milbemycin family, has other differences, including a methoxime at C23. The avermectins and MOX have broad-spectrum activity against nematodes and arthropods. They have similar but not identical, spectral ranges of activity and some avermectins and MOX have diverse formulations for great user flexibility. The longer half-life of MOX and its safety profile, allow MOX to be used in long-acting formulations. Some important differences between MOX and avermectins in interaction with various invertebrate ligand-gated ion channels are known and could be the basis of different efficacy and safety profiles. Modelling of IVM interaction with glutamate-gated ion channels suggest different interactions will occur with MOX. Similarly, profound differences between MOX and the avermectins are seen in interactions with ABC transporters in mammals and nematodes. These differences are important for pharmacokinetics, toxicity in animals with defective transporter expression, and probable mechanisms of resistance. Resistance to the avermectins has become widespread in parasites of some hosts and MOX resistance also exists and is increasing. There is some degree of cross-resistance between the avermectins and MOX, but avermectin resistance and MOX resistance are not identical. In many cases when resistance to avermectins is noticed, MOX produces a higher efficacy and quite often is fully effective at recommended dose rates. These similarities and differences should be appreciated for optimal decisions about parasite control, delaying, managing or reversing resistances, and also for appropriate anthelmintic combination.
1. Introduction
The anthelmintic macrocyclic lactones (MLs) endectocides are the cornerstone of modern parasite control. They belong to a large family of hydrophobic, structurally related compounds widely used in animals and humans and also for pest control on crops. This review will focus on the comparison of moxidectin (MOX), a member of the milbemycin subfamily of MLs, and the avermectin subfamily of MLs used in animals and humans. Since their development in the early 1980s, the success of the MLs has relied on their remarkable broad-spectrum activity, safety profile and ease of administration (McKellar and Benchaoui, 1996; Molyneux et al., 2003; Kita et al., 2007). Furthermore, the MLs are effective against benzimidazole-, levamisole- and pyrantel-resistant strains of nematodes, whose emergence severely restricted the fight against parasites in the late 1970s (McKellar and Scott, 1990). The avermectins are derived from a soil bacterium Streptomyces avermitilis, selected on the basis of insecticidal and anthelmintic activity. Ivermectin (IVM) and abamectin (ABM) were the first MLs developed in the early 1980s for use in animals and IVM, in particular, revolutionized parasite control in production animals, heartworm disease prevention in companion animals and antifilarial treatment in humans. Subsequently, a number of avermectins, including doramectin (DRM), eprinomectin (EPM) and selamectin (SLM) were developed. The other subfamily of MLs, called milbemycins, was isolated, before the avermectins, initially from fermentation of a distinct soil bacterium, Streptomyces hygroscopicus in 1967 and subsequently from Streptomyces cyaneogriseus in 1983. MOX was derived from the latter, as nemadectin (F-29249α), chemically modified and subsequently commercialised. The avermectins and milbemycins, also referred to as endectocides because of their activity against endoparasites and ectoparasites, received considerable interest in the agricultural chemical industry because of their extremely high activity against arachnoid and nematode pests, low toxicity to mammals, generally benign environmental characteristics, and unique mode of action.
The milbemycins and avermectins have a common pharmacophore: the 16-member macrocyclic lactone ring fused with both benzofurane and spiroketal functions in a three-dimensional arrangement, which is recognised by specific chloride ion channel receptors. The high affinity binding of IVM and other MLs to these receptors is responsible for the mode of action of this class of drugs. However, they also display structural differences related to the presence or absence of several substituents. Although it has been claimed that these drugs have identical modes of action, many differences in terms of pharmacokinetics, pharmacodynamics and toxicity have been reported between MOX and the avermectins. Because MLs have systemic actions and must cross the tissues of the host organism before reaching the target parasite, the drug disposition in the host, e.g., concentration and half-life, are determinant for drug efficacy and utility. Thus, any factor that modulates the amount of active drug that reaches the target and the duration of its effects is important. The physicochemical properties modulate the rate of exchange between the tissues and the blood stream. MLs are metabolized, to a small and variable extent, both in the host (Chiu et al., 1987; Alvinerie et al., 2001) and nematode parasites (Alvinerie et al., 2001). Biotransformation plays only a minor role in the in vivo elimination of MLs. Rather, the ability of MLs to be actively transported by efflux proteins present in mammalian and parasite cell membranes is a major pathway for drug elimination (Lespine et al., 2008). Molecular studies indicate that these drugs interact strongly not only with ligand-gated ion channels, the mode-of action receptors of nematodes and arthropods, but also with efflux ABC transporters. Mammalian efflux ABC transporters are involved in the efflux of a broad range of xenobiotics and play a major role in the toxico- and pharmacokinetics of MLs in the host. These interactions clearly differ between MOX and avermectins and the interaction of MOX with mammalian P-gp is much weaker compared with IVM (Lespine et al., 2006). Furthermore, the overexpression of these multidrug ABC transporters, such as P-glycoprotein (P-gp) and multidrug-resistance associated protein (MRPs), appears to be part of the mechanism of IVM resistance in nematodes. MOX has markedly different and reduced effects on causing overexpression of both P-gps and MRPs in nematodes (Prichard and Roulet, 2007; Ardelli and Prichard, 2008). These differences suggest different pathways for the control of MOX disposition and resistance selection, compared with IVM, and may also be the basis for different toxicity. Research continues to elucidate the similarities and differences in IVM and MOX resistance.
An intention of this review is to raise similarities and differences in some depth so that optimal use can be made of the different MLs. We will consider the similarities and differences between avermectins and MOX in their chemistry, formulation and use, spectrum of activity, pharmacodynamics, pharmacokinetics, resistance and safety. Differences in the substituents and physicochemical properties may impact on efficacy, resistance selection, safety and flexibility in formulation and use. Where possible we attempt to explain the differences observed in these properties by the inferred molecular interactions with the receptors of interest.
2. Chemistry and physico-chemical properties
2.1. Avermectins
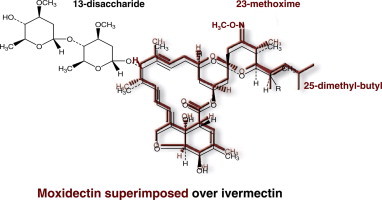
Avermectin are members of a group of pentacyclic 16-membered lactone compounds with endectocide activity (Campbell, 1989). Abamectin (ABM) first arose from the isolation, in the laboratories of the Kitasato Institute, of a soil-dwelling bacterium, S. avermitilis, and its transmittal in 1974 to the laboratories of Merck & Co., Inc. where its activities against nematodes and many ectoparasites were discovered (Stapley and Woodruff, 1982). ABM is the mixture of avermectin B1a (>90%) and avermectin B1b (<10%). A-series compounds are methoxylated at the 5-position, whereas the B-series have an underivatized hydroxyl group at that position. The 1-subset compounds have an olefinic bond between C22 and C23; the 2-subset compounds possess a hydroxyl group at position 23 due to the hydration of the double bonds (see Supplementary Fig. S1). They are considered to have very similar biological activities and toxicological properties. Overall avermectins are characterised among other MLs by the presence of a sugar substituent on the 13-position and of secondary butyl or isopropyl in the 25-position.
Ivermectin (IVM), the most commonly used avermectin, is a chemically reduced 22,23-dihydro derivative of ABM, and is a mixture of 22,23-dihydroavermectin B1a (>90%) and 22,23-dihydroavermectin B1b (<10%), differing from the components of abamectin by a single methylene group at the 26 position (Campbell, 1989). The filiations between the major MLs are shown in Fig. 1.
Fig. 1.
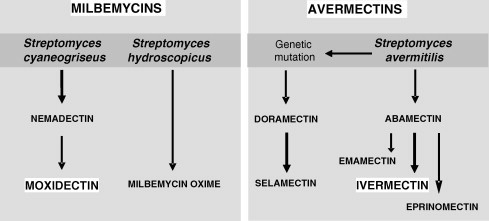
Schematic filiations of macrocyclic lactones: from the soil bacteria to the therapeutic products.
Eprinomectin (EPM) is the amino-avermectin derived from avermectin B1 with modified terminal oleandrose moiety called 4″-epiacetylamino-4″-deoxy-avermectin B1. It was found, from IVM analogues synthesised by Merck, to have a favourable milk residue profile (Shoop et al., 1996).
Doramectin (DRM) is prepared by mutational biosynthesis and it has a closer structural similarity to ABM than to IVM (Goudie et al., 1993). There is a different substituent at the 25 position without the dihydro modification at the 22–23 position. It differs from IVM by having a cyclohexyl group in the C25 position of the avermectin ring. Selamectin (SLM) is a semisynthetic monosaccharide oxime derivative of DRM. This drug has been selected for its efficacy against heartworms and for providing utility against fleas at a dose that is safe for use in dogs and cats (Banks et al., 2000). In terms of chemical structure and because of its monosaccharide, it is an interesting intermediate between the disaccharide avermectins and milbemycins.
2.2. Moxidectin and other milbemycins
A fermentation product with very high acaricide activity was isolated in 1967 from S. hygroscopicus. In 1972, the 16-membered macrocyclic lactones structure of the active compound was elucidated and identified as milbemycin and from this first discovery, the anthelmintic milbemycin oxime (MO) was derived (Takiguchi et al., 1983). Subsequently, an active fermentation milbemycin product nemadectin was isolated from S. cyaneogriseus in 1983 and moxidectin (MOX) was later chemically derived from this compound by the addition of a methoxime moiety at C-23 (Fig. 1).
Chemically, when compared to avermectins, milbemycins are unglycosylated, lacking a bisoleandrosyl moiety in the 13-position (Supplementary Fig. S1). The milbemycins not only differ from the avermectins in lacking sugar groups at position-13, they also differ from the avermectin aglycones by being protonated at this position in contrast to the avermectin aglycones which are hydroxylated at this position. They also have an ethyl or a methyl at the 25-position. They can differ from one to another milbemycin by their substituant in the 5- and 25-position. MOX has a substituted olefinic side chain at the 25-position and methoxime moity at the 23-position which are both characteristics specific to this drug and not found in other commercial milbemycins or avermectins.
3. Formulations and mode of use
Because most of the avermectins are used at relatively low dose rates, have high lipophilicity, stability and safety, there is a great degree of flexibility in their formulation. For example, IVM is formulated as a tablet for human use (Mectizan®) or as chewables for dogs (e.g., Heartgard®); as an oral liquid for humans (Stromectol®) and drench for small ruminants (e.g., Ivomec® Drench); injectable (e.g., Ivomec® Injection for cattle and pigs), including long-acting injectable formulations in some markets (e.g., Ivomec Gold®), pour-on (Ivomec® Pour-on), and long-acting boluses in some markets for cattle; and pastes for horses (e.g., Eqvalan®). There are many other formulations for the various avermectins, including formulations which combine the avermectin with another pharmacologically active ingredient to broaden the spectrum of action.
MOX is similarly a very versatile, stable and safe molecule. Because of its exceptionally high potency and safety, it is used in dose rates varying between 3 μg/kg (e.g., ProHeart® for dogs) to 2.5 mg/kg (e.g., Advantage Multi® for dogs) and comes in tablets (e.g., ProHeart®), oral drench for sheep, oral gel/paste for horses (e.g., Quest®), injectable (e.g., Cydectin®) and pour-on (e.g., Cydectin Pour-on®) for cattle, and topical solution for dogs and cats (e.g., Advocate®, Advantage Multi®). Because of its remarkable potency against nematodes and long half-life due to its lipophilicity, low susceptibility to transport by ABC transporters (see discussion below) and in vivo stability, MOX is particularly suitable for long-acting formulations ranging from Cydectin LA®, given as an injection at 1 mg/kg for cattle which has activity for up to 150 days, and ProHeart®6 providing dogs with heartworm disease prevention for 6 months, and in some countries, ProHeart®12 which provides dogs with protection against heartworm disease for 1 year.
This is a far from exhaustive list of formulations and modes of administration for these remarkable pharmaceuticals. While the avermectins and MOX show many similarities in their ability to be formulated and are used in a variety of ways, the characteristics and use are not identical in all respects; most notably in the ability of MOX to maintain activity over very long period of time in different long-acting formulations. In addition to the inherent half-life of the ML, the vehicle also plays an important role in achieving a long period of activity.
4. Spectrum of activity
The activity of the avermectins and MOX against nematode and ectoparasites of veterinary importance has been comprehensively reviewed in Vercruysse and Rew (2002), and in this review section only differences between the MLs or between formulations will be discussed.
4.1. Cattle
IVM, ABM, DRM, EPM and MOX are all used to control gastrointestinal and lung nematodes and ectoparasites of cattle. Subcutaneous injection is the most efficient route for ML administration in terms of drug bioavailability in cattle and other species, when compared to oral and topical administration (Gayrard et al., 1999; Laffont et al., 2001; Lespine et al., 2003). The usual dose rate for all of the MLs in cattle is 0.2 mg/kg for injectable and 0.5 mg/kg for pour-on formulations. The higher dose rate for pour-on formulations reflects the fact that the active ingredient is less well absorbed through the skin than from the injection site or the gastrointestinal tract. However, cattle will lick each other after pour-on application of anthelmintic and a variable portion of the pour-on dose may be orally ingested, and will lead to unexpected levels of drug in the organisms and efficacy (Bousquet-Melou et al., 2011). It is well known that IVM and MOX have different plasma and tissue kinetics (see paragraph 6.1) which affect the duration of activity. For a given method of administration, the avermectins have activity for 14–28 days, while MOX has the longest period of activity (from 14 to 42 days, depending on the target species) when given as a single dose (non long-acting formulation). This is in accordance with the longer persitance of MOX efficacy in the host organism. Generally, IVM and MOX should not be used in dairy cattle that are producing milk for human consumption, unless milk withholding periods are observed. Because very little is excreted in the milk, EPM was developed for dairy animals (see discussion below). However, in some jurisdictions, MOX pour-on is registered for use in lactating dairy cattle with a zero milk withdrawal, based on the lower toxicity of MOX compared with IVM.
Route of administration affects, to some extent, the efficacy of MLs against ectoparasites. For example oral administration is less effective than injectable or pour-on administration against mange mites (Benz et al., 1989), and injection may be less efficacious than pour-on administration against some biting lice, such as Bovicola bovis (Chick et al., 1993). As single therapeutic (not long-acting) treatment, the MLs have variable activity against the single host tick, Rhipicephalus (Boophilus) microplus, with IVM pour-on (0.5 mg/kg) or injectable (0.2 mg/kg) showing 50% and 80% efficacy, respectively (Cramer et al., 1988). Higher and more persistent activity (up to 28 days) was achieved with DRM (Muniz et al., 1995) and MOX (up to 21 days) (Guglielmone et al., 2000). The MOX long-acting injectable formulation (Cydectin LA®, at 1 mg/kg) was found to provide 50 days of protection against B. microplus (Davey et al., 2011).
4.2. Sheep and goats
Registration of different ML formulations for sheep and goats varies in different jurisdictions and largely reflects economic factors. In general, oral administration is preferred for small ruminants. Although there are few jurisdictions where EPM has been registered for use in small dairy ruminants, the cattle formulations are sometimes used because of low residues in milk. All of the MLs show high efficacy against nematode endoparasites and some ectoparasites of small ruminants, provided resistance has not developed. Unfortunately, resistance to the avermectins has become widespread in nematode parasites of small ruminants. Usually when avermectin resistance first develops, it affects the efficacy of all of the avermectins. However, MOX is usually still highly effective at its recommended dose rate against the avermectin-resistant parasites (see below). However, MOX resistance can occur with ongoing selection pressure from MOX use.
4.3. Equines
IVM (Eqvalan® and other proprietary names) and MOX (Quest® and other proprietary names) are registered for use in horses. They are usually given orally as a paste, although a liquid oral formulation of IVM for horses is also available. IVM and MOX are highly effective against most of the important nematode parasites of horses as well as bots. They show a very similar spectrum, except that MOX is more efficacious against encysted small strongyle larvae. As small strongyles are considered one of the most pathogenic worm parasites of horses, removal of encysted larvae can be advantageous. With other equine anthelmintics, adult small strongyles, which have been removed by treatment, may be rapidly replaced from developing larval stages which remain in the equine after treatment. Removal of the encysted larval stages by MOX means that new infective larvae must be ingested and develop to adults before the risk of morbidity from small strongyles can become important again.
4.4. Dogs and cats
The avermectins (IVM and SLM) and the milbemycins (MO and MOX) are used for heartworm disease prevention in dogs and cats. SLM, MO and the Advantage Multi® (or Advocate®) formulation of MOX also have activity against immature and adult stages of roundworms and hookworms in dogs and cats, whipworms and lungworms of dogs, ear mites (Otodectes cynotis), sarcoptic mange mites and demodex mites. Advantage Multi® (Advocate®) contains imidacloprid in combination with MOX, to extend efficacy against fleas and lice. SLM also has activity against fleas, as well as lice and sarcoptes mange mites. Normally, the heartworm preventatives are administered every 30 days during the heartworm transmission season. However, the long-acting MOX injectable preparations ProHeart®6 (and ProHeart®12) provide 6 months (and 12 months) of protection against heartworm disease by Dirofilaria immitis. IVM (at 50 μg/kg) and MO are also known to kill microfilariae of D. immitis and IVM has been used in a “slow kill” regime over 18 to 36 months to remove all stages of D. immitis, including the adult worms (McCall, 2005). However, the recent reports of apparent resistance to some of the ML heartworm preventatives (see below) has led to the suggestion that the use of ML heartworm preventatives in “slow kill” regimes should be discouraged.
4.5. Humans
IVM, as an oral tablet (Mectizan®), is the only ML currently registered for use in humans. It was developed for use, at 150 μg/kg, as a microfilaricide against Onchocerca volvulus, which causes river blindness in Africa, the Arabian Peninsula and Central and South America. In addition to its high efficacy against O. volvulus microfilariae, IVM also inhibits reproduction by adult O. volvulus for 6 months or more (WHO, 1995) and the combination of the anti-fecundity and microfilaricidal effects reduce morbidity and parasite transmission so that control and elimination has been achieved in some locations with repeated annual or 6-monthly treatments. Recently, MOX has been under evaluation for used against human onchocerciasis (http://www.apps.who.int/tdr/svc/news-events/news/phase3-trial-moxidectin). Based on animal studies, the possibility that MOX may provide greater effectiveness than IVM against onchocerciasis is being evaluated (Etya’ale, 2001). IVM is also used in Sub-Saharan Africa for the control of lymphatic filariasis. For this indication it is usually administered, at 200 μg/kg, in combination with albendazole. IVM exerts similar effects on Wuchereria bancrofti, the causative agent of lymphatic filariasis in Africa, as it does on O. volvulus, killing microfilariae and inhibiting reproduction of adult filarial worms for several months. Although IVM is not registered for the treatment of soil transmitted helminths (STH) in humans, it is known that the combination of IVM with albendazole is more efficacious against Trichuris trichiura than albendazole alone (Belizario et al., 2003) and that IVM also has activity against Ascaris lumbricoides (Stepek et al., 2006). IVM has also proved efficacious for the treatment of strongyloidiasis and scabies in humans (Stepek et al., 2006; Sharma and Singal, 2011).
4.6. Other target species
IVM, DRM and MOX are formulated for use in pigs, as oral feed pre-mix or as injectable preparations, while IVM, EPM and MOX are formulated as pour-ons for deer (http://www.omafra.gov.on.ca/english/livestock/alternat/facts/info_paras.htm#anthel).
5. Pharmacodynamics
5.1. Glutamate-gated chloride channels
Ivermectin was shown to bind irreversibly to a Caenorhabditis elegans glutamate-gated chloride channel (GluCl) subunit, expressed in Xenopus oocytes, and to open the channel with much higher affinity (EC50 of 140 nM) than the physiological ligand glutamate (EC50 of 380 μM) and it has been shown that the two ligands share an allosteric interaction (Cully et al., 1994). The binding characteristics of IVM and MOX to Hco-GLC-5, a subunit of a glutamate receptor of Haemonchus contortus not found in C. elegans, were similar in the absence of glutamate (Forrester et al., 2002). However, 10 μM glutamate resulted in a 7-fold increase in IVM affinity, but only a 1.5-fold increase in MOX affinity, suggesting that while both MOX and IVM bind the same receptor site, the interaction with the receptor is different between MOX and IVM in the presence of the natural ligand. In another study, the effects of IVM and MOX on opening the Cooperia oncophora GluClα3 (a homologue of Cel-AVR-14) homomeric channel, expressed in Xenopus oocytes, were compared, after wash out of glutamate, with IVM having an EC50 of 0.5 μM, while MOX had an EC50 of 0.2 μM. In an allele of this receptor isolated from IVM resistant C. oncophora, and containing the L256F single nucleotide polymorphism (SNP), a similar ratio, of MOX being approximately 2.5 times more sensitive on the receptor than IVM, was observed, even though the 256F SNP caused an approximate 2.5-fold decrease in sensitivity to both IVM and MOX (Njue et al., 2004).
Recently, Hibbs and Gouaux (2011), based on the crystal structure, have proposed a model for the IVM binding site and atomic interactions with amino acids in a homopentameric C. elegans receptor composed of the GluCl subunit, GLC-1. Considering the structural differences between IVM (as a representative avermectin) and MOX, i.e., absence of the disaccharide moiety (or -OH) on the C-13 of the macrocycle, a methoxime moiety at C-23 and an olefinic side chain at C-25, it can be postulated that the interaction of MOX will be different from that of the avermectins. In Fig. 2, the structure of MOX is superimposed on the proposed IVM-GluCl interaction model (Hibbs and Gouaux, 2011). It can be seen that some of the interaction sites (4 sites) involved with IVM binding to the GluCl will be retained by MOX binding, but 3 of the proposed IVM interaction sites are either not present or will be blocked when MOX is fitted to the same model. The methoxime on the spiroskeletal ring of MOX may prevent H-binding to a M3 loop and may cause some molecular displacement of this loop, while the absence of the disaccharide substituent should result in MOX lacking two van der Waal binding sites (to M2–M3 loops) to the nematode GluCl. This suggests that MOX may interact, in some respects, differently with GluCls. However, firm conclusions on this must await modelling with MOX.
Fig. 2.
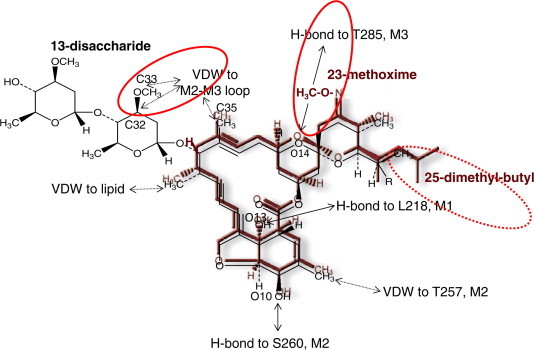
Interaction of ivermectin (IVM) with a glutamate-gated chloride channel (GluCl) as proposed by Hibbs and Gouaux (2011), showing moxidectin (MOX; maroon) superimposed over IVM (black). Note that while some of the interaction sites are shared in common between MOX and IVM (O10, O13, C18, C35 and C48), other interaction sites of IVM with the GluCl are either absent (C32, C33; both forming van der Waal (VDW) interactions with the GluCl in the case of IVM) due to the absence of any saccharide group in MOX, or are blocked/altered (O14; H-bond in the case of IVM) by the C23 methoxime group of MOX. The sites where interactions will be different for MOX compared with IVM are highlighted by a solid red circle. The 25-dimethly-butyl of MOX may also affect interaction with the GluCl (dashed red circle). IVM is a mixture of C25 ethyl (B1a; ∼10%) and C25 methyl (B1b; ∼90%). Against nematodes the B1b component is usually more potent than the B1a component, showing that the change from methyl to ethyl at this position affects potency. Thus it is likely that the 25-dimethyl-butyl group of MOX will also affect the interaction with a GluCl. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Nematodes have considerable diversity in GluCl subunits that are expressed, with both C. elegans and H. contortus known to have 6 GluCl genes each and, with truncations, at least 8 different GluCl subunit variants are expressed in each of these nematodes. However, not all of the H. contortus GluCls are homologues of those found in C. elegans (Glendinning et al., 2011). Furthermore, in both C. elegans and H. contortus only 4 GluCl genes, in each case, are known to code for subunits that are sensitive to IVM. The diversity in other parasitic nematodes is also likely to vary considerably. In most cases, we do not yet know the specificity of different MLs for different GluCls, or indeed for other amino-gated chloride channels found in nematodes.
Studies have been conducted on the effects of IVM and MOX on pharyngeal pumping and motility in adult C. elegans and on larval development (Ardelli et al., 2009). IVM and MOX do not exert the same effects, at similar concentrations, on this nematode. For example, pharyngeal pumping is paralysed at approximately 5 nM by IVM, but a similar paralysis requires 80 nM MOX. In terms of worm motility, IVM causes an initial activation of C. elegans which is not seen after MOX exposure. Larval development is very sensitive to IVM, being inhibited by 0.6 nM, whereas with MOX a 64-fold higher concentration is required to inhibit development. In other experiments with adult H. contortus that were IVM-sensitive, pharyngeal pumping in the presence of glutamate was also found to be significantly more sensitive to IVM than to MOX. In an IVM-resistant strain of H. contortus, IVM effects on pharyngeal pumping were significantly decreased, while the responses to MOX did not change in the IVM-resistant worms (Paiement et al., 1999).
Laboratory and field studies have shown that IVM is potently active against Anopheles spp. mosquitoes at concentrations present in human blood after standard drug administration. Using an in vitro blood feeding assay, IVM and EPM were shown to be efficient at killing the mosquitoes at similar concentrations (LC50 of 22.4 and 23.6 ng/ml, respectively), against the African malaria vector Anopheles gambiae, while MOX was 125-fold less effective (LC50 of 2789 ng/ml) (Butters et al., 2012). It is believed that the three drugs are agonists of the insect glutamate-gated chloride ion channels. Nevertheless, it can be speculated that the differential activity between the avermectins and MOX may originate from a lower affinity of MOX for the receptor or lower drug concentration arriving at the target.
These marked differences between responses to IVM and MOX suggest differences at the level of GluCl interaction with avermectins and MOX, or the differential involvement of other amino-gated chloride channels with the actions of avermectins and MOX.
5.2. Other amino-gated chloride channels in nematodes
Ivermectin is known to open other ligand-gated chloride channels, in addition to glutamate-gated chloride channels and GABA-gated chloride channels (Ludmerer et al., 2002). For example, it activates histamine-gated chloride channels in Drosophila melanogaster (Zheng et al., 2002) and IVM and MOX select on a dopamine-gated chloride channel in H. contortus (Rao et al., 2009). Furthermore, there are tyrosine-gated chloride channels (Rao et al., 2010) and serotonin-gated chloride channels (Ringstad et al., 2009), which could be sensitive to some MLs.
GABA receptors from nematodes appear as a secondary target of MLs (Feng et al., 2002; Beech et al., 2010) but at higher concentrations than are required to open GluCl channels. According to recent studies performed on 2 subunits of a GABA-gated chloride channel, Hco-UNC-49B and C, both IVM and MOX were able to enhance the response to GABA of the homomeric and dimeric channels, but by different amounts (Brown et al., 2012). There has been a perception that MOX and IVM act on the same receptors, but that MOX is a more potent ligand. However, this is clearly too simplistic because of observed differences between MOX and IVM in different ligand-gated ion channels so far investigated. In some receptors IVM was more potent than MOX, while in other receptors, MOX was more potent than IVM. Thus, more investigations are needed to elucidate the different interaction of avermectins and MOX with the large number of ligand-gated chloride channels present in nematodes and arthropods and to establish the full range of similarities and differences between MOX and the avermectins on these receptors.
6. Pharmacokinetics
6.1. Plasma and tissue distribution
As with any other drug with systemic action, the concentrations and the length of residence time of the MLs in the host tissues contribute to the efficacy of the drug against the target parasites. Compared with IVM, MOX is characterised by a larger volume of distribution, a remarkably long mean residence time in the host organism and both drugs have an extensive elimination in milk during lactation (Hennessy and Alvinerie, 2002). This has been evident in several species including humans where two independent clinical trials have been performed in healthy volunteers to evaluate the safety, tolerability and pharmacokinetics of single doses of IVM (Guzzo et al., 2002) and of MOX (Cotreau et al., 2003). Besides a higher concentration and area under the plasma concentration versus time curve (AUC), MOX when given orally to humans at a dose rate averaging 0.5 mg/kg had a longer half-life and larger volume of distribution, when compared with IVM at a similar dose rate (Table 1). Similarly in pigs, MOX kinetics were very different from those of IVM and showed a greater apparent volume of distribution, larger distribution, longer elimination half-lives and slower clearance rate than IVM. Subsequently, MOX was detectable in plasma for over 40 days compared with only 8–10 days for IVM. Altering body composition in these animals had no detectable influence on the kinetic disposition of IVM, but MOX was distributed within and eliminated from the lean animals more rapidly than from the fat animals (Craven et al., 2001, 2002). In horses, MOX has a pharmacokinetic profile which profoundly differs from that of IVM and the mean residence time is fourfold higher for MOX (18.4 days) than for IVM (4.8 days) following oral administration (Perez et al., 1999). In dogs, after oral administration, MOX has a longer elimination half-life and larger volume of distribution than IVM (Al-Azzam et al., 2007).
Table 1.
Comparative pharmacokinetic parameters of MLs in different species. This table show example of MLs with different kinetic parameters when administered to the same species, with same dose rate and route of administration.
| Administration route (dose) | AUC (ng day/ml) | T1/2 (or MRT) (days) | Vd(/F) (l/kg) | Reference | |
|---|---|---|---|---|---|
| Humans | Oral (0.5 mg/kg) | ||||
| Ivermectin | 190 ± 79 | 0.8 | – | Cotreau et al. (2003) | |
| Moxidectin | 451 ± 48 | 20.2 | 1.2 | Guzzo et al. (2002) | |
| Pig | i.v. (0.2 mg/kg) | ||||
| Ivermectin | 75 | 1.16 | 5.3 | Craven et al. (2002) | |
| Moxidectin | 271 | 14 | 17.9 | ||
| Cattle | Sc (0.2 mg/kg) | ||||
| Ivermectin | 459 ± 47 | 5.5 | 0.45 | Lanusse et al. (1997) | |
| Moxidectin | 217 ± 15 | 8.9 | 0.94 | ||
| Eprinomectin | 306 ± 77 | 6.8 | Baoliang et al. (2006) | ||
| i.v. (0.07 mg/kg) | |||||
| Ivermectin | 211 ± 48 | 7.8 ± 1.6 | 2.72 | Bousquet-Melou et al. (2004) | |
| Moxidectin | 115 ± 26 | 21.5 ± 1.5 | 14.9 | ||
| Sheep | Sc (0.2 mg/kg) | ||||
| Ivermectin | 230 | 3.7 | – | Marriner et al. (1987) | |
| Moxidectin | 161 | 16 | – | Hennessy et al. (2000) | |
| Goat | Sc (0.2 mg/kg) | ||||
| Ivermectin | 56 | – | Alvinerie et al. (1993) | ||
| Moxidectin | 137 | (12.4) | – | Escudero et al. (1999) | |
| Eprinomectin | 63 ± 23 | (6.6 ± 1.3) | – | Lespine et al. (2003) | |
| Rabbit | Sc (0.3 mg/kg) | ||||
| Ivermectin | 191 | 2.7 | Gokbulut et al. (2010) | ||
| Moxidectin | 83 | 8.2 | |||
| Dog | Oral (0.25 mg/kg) | ||||
| Ivermectin | 233 ± 75 | 3.3 | 1.20 | Al-Azzam et al. (2007) | |
| Moxidectin | 491 ± 96 | 25.9 | 0.53 | ||
AUC, area under the concentration time curve; MRT, mean resistance time; T1/2, elimination half-life; Vd(/F), apparent volume of distribution; Sc, subcutaneous.
In cattle, major differences exist between IVM and MOX with a larger volume of distribution and a faster (plasma) clearance for MOX (Lanusse et al., 1997; Bousquet-Melou et al., 2004), presumably due to a more rapid partition into adipose tissue. The mean residence time of MOX was much longer than for IVM (24 versus 7 days, respectively) after IV injection (Bousquet-Melou et al., 2004). In these species, the concentration of MOX in fat, 28 days after treatment, was ninety-fold higher than in plasma and the half-life of MOX in adipose tissue was 14 days versus 7 days for IVM (Chiu et al., 1990).
Such differences between IVM and MOX have also been reported in goats, sheep and rabbits (Marriner et al., 1987; Alvinerie et al., 1993; Escudero et al., 1999; Hennessy and Alvinerie, 2002; Gokbulut et al., 2010) (Table 1). It is noteworthy that EPM presents pharmacokinetics close to IVM in cattle and in goats (Lespine et al., 2003; Baoliang et al., 2006) (Table 1), in agreement with the structural similarities of the two drugs.
A close correlation between the drug disposition in the plasma and in tissues where parasites live, such as the gastrointestinal tract, skin or lung has been established for MOX in cattle (Zulalian et al., 1994; Lifschitz et al., 1999; Sallovitz et al., 2003) and for IVM in other species (Chiu et al., 1990; Lespine et al., 2003) and therefore plasma drug concentration is considered as a good predictor of anthelmintic efficacy for MLs. The longer persistence of MOX concentration in these tissues when compared with IVM is in accordance with the longer persistence of MOX activity.
MLs can be formulated differently and the formulation can affect the initial absorption process. But when focusing on the late elimination phase, reflected in the half-life of elimination, these differences are likely to be related to the lipophilicity and efflux potential, via ABC transporters. Indeed, fat is known to act as a reservoir for lipophilic compounds and the higher lipophilicity of MOX compared with IVM or EPM (log PMOX = 6; log PIVM = 4.8; log PEPM = 4.0) favours MOX retention in fatty tissue and longer elimination half-life. In addition, given the higher capacity of IVM to be actively efflux out of the organisms compared with MOX (see paragraph 6), ABC transporters also contribute to the different kinetics in the host observed between the two MLs.
6.2. Elimination in milk
The mammary gland epithelium, like other biologic membranes, acts as a lipid barrier, and the high lipophilicity of the MLs is in favour of partitioning into milk. Milk/plasma concentration ratios close to unity have been reported for IVM in many species (Table 2). However, one of the studies reported a higher milk/plasma ratio of 2.5 ± 0.4 in sheep (Imperiale et al., 2004). The amount of lipid in sheep milk is twice the amount found in other species which may explain the higher rate of IVM transfer into milk in this species. The partitioning of MOX into milk is higher than that of IVM with milk/plasma concentration ratios ranging from 4.2 to 18.5 in different animals, and with a huge MOX elimination occurring in sheep milk (Imperiale et al., 2004). This is consistent with the higher lipophilicity of MOX compared with IVM, and with the high fat content of sheep milk. As expected, EPM has a lower rate of milk partitioning in the cow (Alvinerie et al., 1999c) and in the goat (Alvinerie et al., 1999a), allowing for its use in lactating dairy cattle. However, in other species, the milk/plasma partitioning of EPM is close to that of IVM (Bengoumi et al., 2007; Dupuy et al., 2008; Hodoscek et al., 2008), suggesting that factors other than drug lipophilicity and milk fat composition are involved in the partitioning of this anthelmintic. In humans, IVM (Ogbuokiri et al., 1993) and MOX (Korth-Bradley et al., 2011) have been evaluated in breast milk and although the partitioning of MOX remains higher than that of IVM, it is lower than in other species.
Table 2.
Comparative ratio of area under the concentration time curves AUC milk/AUC plasma of MLs in different species.
| Humans | Cattle | Buffaloes | Sheep | Goat | Camel | |
|---|---|---|---|---|---|---|
| Ivermectin | 0.6a | 0.8 (Toutain et al., 1988) | – | 1.0 (Bogan and McKellar, 1988) 2.5 ± 0.4 (Imperiale et al., 2004) |
1.1 ± 0.2 (Alvinerie et al., 1993) | 1.2 ± 0.4 (Oukessou et al., 1999) |
| Eprinomectin | – | 0.1b (Alvinerie et al., 1999a,b,c) | 0.8 ± 0.2 (Dupuy et al., 2008) | 0.8 (Hodoscek et al., 2008) | 0.1–0.2 (Dupuy et al., 2001) | 2.9 ± 1.5 (Bengoumi et al., 2007) |
| Moxidectin | 1.8 (Korth-Bradley et al., 2011) | – | 5.0 ± 2.1 (Dupuy et al., 2008) | 18.5 ± 1.2 (Imperiale et al., 2004) | 7.5 (Carceles et al., 2001)c | 4.2 ± 0.7 (Oukessou et al., 1999) |
–: Not determined.
Extrapolation from data of Guzzo et al. (2002) and Ogbuokiri et al. (1993).
0.094 in zebu gobra (Bengone-Ndong et al., 2006).
Estimation.
6.3. Biotransformation
Enzymes can metabolise MLs in mammals (Chiu et al., 1987) and in nematodes (Alvinerie et al., 2001), contributing to drug elimination and modifying drug disposition. On the basis of radioactivity recovered in faeces after 3H-IVM administration, it was shown that the major component excreted was the parental compound. Nevertheless, a substantial amount of radioactivity was accounted for in the metabolites (Hennessy and Alvinerie, 2002). IVM was metabolized in rat microsomes and the main metabolite detected was 24-hydroxy-IVM. In pigs and goats, the main metabolite detected was O-desmethyl-IVM. In humans, the major metabolites formed by liver microsomes were the 24-monohydroxylated and 3-O-demethyl metabolites. Cytochrome P450 3A4 was identified as the predominant enzyme involved in IVM metabolism (Zeng et al., 1998).
MOX produces essentially C29-30- and C14-mono-hydroxy-methyl derivatives as metabolic products (Zulalian et al., 1994), which are formed by the cytochrome P450 3A (Dupuy et al., 2001) and cytochrome P450 2B (Dupuy et al., 2001). In cattle, the fractions metabolized were found to be around 8% for IVM and 13% for MOX. The higher clearance of MOX in cattle after IV administration (Lanusse et al., 1997; Bousquet-Melou et al., 2004) could be due to higher metabolism or larger distribution compared with IVM. However, metabolism is considered to contribute only to a small extent to ML elimination compared with elimination of parental compound via efflux transporters.
7. Interaction of avermectins and moxidectin with mammalian multidrug ABC transporters
Efflux of MLs via active transport from mammalian and parasite cells are especially important. The transporters involved strongly influence the pharmaco- and toxicokinetics of these drugs. Their presence at the intestinal level contributes extensively to the elimination of these drugs in the faeces. In addition, being located in the blood–brain barrier, they limit the net entrance of the drug into the brain which explains the low toxicity of the MLs in most mammals (Lespine et al., 2009; Kiki-Mvouaka et al., 2010).
ATP-binding cassette (ABC) transporters include P-glycoprotein (P-gp or MDR1 of the ABCB family), the Multidrug Resistance associated Proteins (MRPs of the ABCC family) and the ‘half-transporter’ subfamily, Breast Cancer Resistance Protein (BCRP or ABCG2) (Doyle and Ross, 2003). These transporters are found on barrier epithelia such as intestine, plancenta, mammary gland and the blood–brain barrier. The complexity and also the performance of the system lies in the ubiquitous presence of different transporters expressed at various levels and presenting considerable overlap in their substrate specificities. By their action, these transporters provide an efficient barrier of protection for the organism from the toxicity of most contaminants and they limit the entrance of many drugs, restricting their efficacy. They also play a clinically relevant role in drug interactions when drugs are combined (Sarkadi et al., 2006). Many ABC transporters are also found in nematodes, protozoa, fungi and bacteria and some of them are involved in drug resistance in these organisms. Indeed, drug resistance in pathogens is very commonly associated with alterations in drug transport, resulting in lower drug concentrations at the site of the relevant drug receptor(s) than in susceptible strains.
7.1. Molecular interaction of avermectins and moxidectin with P-glycoprotein
Among the MLs only IVM has, so far, been reported to be a substrate of P-gp and is also known to be an inhibitor of P-gp-mediated transport (Didier and Loor, 1996). This has been shown by different approaches. Firstly, by measuring the capacity of IVM to inhibit the efflux of rhodamine 123, a fluorescent substrate of P-gp, it has been shown that IVM is one of the most potent known inhibitors of P-gp (Eneroth et al., 2001). Secondly, the ATPase activity of the transporter is inhibited by IVM (Lespine et al., 2007). This suggests that the molecular basis for inhibition of P-gp by IVM lies in the fact that this drug and certainly other avermectins are slowly transported by P-gp blocking the binding site for other compounds.
Later, by studying the molecular interactions of several MLs with P-gp, it became evident that there are major differences between MOX and avermectins which can account for the differences in their kinetics. All avermectins interfered with P-gp transport activity with a similar potency as IVM. By contrast, MOX was found to be a poor inhibitor of the P-gp-mediated rhodamine123 transport compared with IVM, SLM, EPM, ABM or DRM. Another approach showed a differential effect of the avermectins and MOX with P-gp ATPase activity (Lespine et al., 2007). Based on the capacity of different MLs to inhibit verapamil ATPase activation, it is possible to localise their association on the drug binding site of the P-gp and to determine their inhibitory constant (Ki). This confirmed that MOX has a weaker affinity than the avermectins (Table 3). From these data, it appears that the integrity of the disaccharide moiety present on avermectins (excluding selamectin which has only one sugar) plays a determining role in the affinity for P-gp (Lespine et al., 2007). The presence or absence of the somewhat lipophilic sugar groups changes the physico-chemical properties of the drug. Indeed, the hydrophobicity of the molecule dictates its partitioning within the lipid membrane, which is an obligatory first step for the interaction of a substrate with P-gp. The presence of the sugar moieties influences the degree of hydrophobicity of the molecule, and a relationship has been proposed between the octanol/water partition coefficient (log P) of the MLs and the affinity for P-gp. The log P, calculated by using atomic parameters (HyperChem 7.0, HyperCube Inc. (Vellarkad et al., 1989)) are 6 and 6.3 respectively for MOX and SEL, while the other MLs have lower partition coefficients (4.8, 4.4, 5.3 and 5.6 for IVM, EPM, ABM and DRM, respectively).
Table 3.
Relation between the ability of MLs to inhibit the P-gp transport activity, to modulate ATPase activity and their lipophilicity.
| In LLCPK1-mdr1a cells | ATPase activity in P-gp overexpressing vesicles | ML lipophilicity | |
|---|---|---|---|
| IC50 (μM) | Calculated Ki (μM) | log Pa | |
| Reference P-gp inhibitors | |||
| Valspodar | 0.11 | nd | |
| Cyclodsporin | nd | 0.015b | |
| Verapamil | 3.2 | nd | |
| Macrocyclic lactones | |||
| Ivermectin | 0.44 | 0.05 | 4.8 |
| Eprinomectin | 0.50 | 0.02 | 4.4 |
| Abamectin | 0.11 | 0.02 | 5.3 |
| Doramectin | 0.31 | 0.03 | 5.6 |
| Selamectin | 0.60 | 1.0 | 6.3 |
| Moxidectin | 4.4 | 0.5 | 6 |
HyperChem 7.0, HyperCube Inc.
The presence or absence of the sugar part of the drug also changes the steric hindrance of the drug which can impact on the binding of the drug to the transporter. Data on the interaction with P-gp, obtained with DRM and SLM are very informative because the core of the chemical structure of these two avermectins is similar and they mainly differ by the presence of one sugar for SLM and 2 sugars for DRM. On the basis of the different ability of DRM and SLM to compete with verapamil on P-gp, it can be suggested that the second sugar unit plays an important role in the interaction with P-gp. These observations are consistent with the role of the sugar groups in governing the ability of MLs to interact with the P-gp drug binding sites (Lespine et al., 2007) but it cannot be excluded that other functions that characterise MOX are also important such as the olefinic side chain at the 25-position and the methoxime moiety at the 23-position. The P-gp substrate drug cyclosporin A has been extensively studied as a strong P-gp inhibitor. Comparison between cyclosporin and IVM shows both compounds are strong P-gp inhibitors that are rich in hydrogen bonds inducing a slow transport (Saeki et al., 1993; Pouliot et al., 1997), possibly as a result of lower dissociation rates from the protein (Seelig and Gerebtzoff, 2006). Both interfere with calcein-acetoxymethyl ester transport in CaCo-2 cells with similar efficacy (Eneroth et al., 2001) and show similar competition with P-gp specific antibodies (Nagy et al., 2004). Cyclosporin A also competes with IVM on membranes from drug-resistant cells overexpressing P-gp (Pouliot et al., 1997). In membrane vesicles overexpressing P-gp, cyclosporin A competed with verapamil displaying a Ki for P-gp of 0.015 μM (Garrigues et al., 2002), which is comparable to the one calculated for the avermectins (0.02–0.05 μM) (Lespine et al., 2006). Altogether, these results support the view that cyclosporin and avermectins share a P-gp binding site also common to verapamil. Based on an integrated approach using enzymatic assays and in silico molecular modelling of some P-gp substrates, a two pharmacophore model has been proposed (Garrigues et al., 2002), with at least two close hydrophobic pockets within P-gp able to bind specific compounds. One has been shown to bind verapamil and the other tentoxine. The similar competitive effect of cyclosporin A and IVM on verapamil and on various other P-gp substrates, such as calcein or tentoxine, is the consequence of the large molecular size of the drugs leading to overlapping binding on the two P-gp recognition sites. With regard to the interactive function, it is likely that for IVM, the macrolactone and the hexahydrobenzofuran unit, which constitute the core of the ML, participate in its binding to P-gp while the disaccharide moiety is probably involved at an additional binding site, further increasing affinity. On the basis of structural analogy and on biochemical studies showing competition with verapamil (Lespine et al., 2006) and tentoxine (Lespine, unpublished data), MOX may bind similarly to the two binding sites of verapamil and tentoxine on P-gp. However, with MOX, the absence of the disaccharide prevents the binding to the third putative IVM binding site and leads to weaker binding. Such an approach to study and compare the interactions of drugs with the binding sites of the transporter is of interest in order to predict drug-drug interactions or to design effective inhibitors of the transporter.
7.2. P-gp contributes to the pharmacokinetics of avermectins and moxidectin
P-gp is also located on the apical surface of the enterocytes and hepatocytes, and thus expels drugs into the intestinal lumen and the bile. A strong accumulation of IVM (10-fold) occurred in gall bladder cells of P-gp deficient mice (Schinkel et al., 1994) and biliary secretion was originally thought to be the major route of ML excretion (Hennessy and Alvinerie, 2002). Nevertheless, P-gp-mediated intestinal secretion plays a greater role than biliary secretion in the overall elimination of IVM in rodents (Laffont et al., 2002; Ballent et al., 2006; Kiki-Mvouaka et al., 2010). These results support the extensive elimination of avermectins by the faecal route and the main consequence of P-gp action is to limit the systemic level of IVM and the duration of animal drug exposure and subsequently the drug efficacy.
A recent study comparing the kinetics of MLs in P-gp deficient mice revealed major differences in the contribution of P-gp to the disposition of MOX compared with avermectins. EPM is efficiently effluxed by P-gp which is consistent with its higher affinity for the transporter when compared with IVM and MOX. The plasma kinetics of MOX are not influenced by the lack of P-gp in mice, while pharmacokinetic parameters for IVM are strongly affected by the presence or absence of P-gp, and even more so for EPM. The AUC was more than doubled for IVM and EPM and was unchanged for MOX, in P-gp deficient mice compared with wild-type. Therefore it can be concluded that IVM and EPM are actively excreted from the intestine via an active P-gp-dependent pathway, while MOX is mostly excreted via a P-gp-independent pathway at the intestinal barrier (Kiki-Mvouaka et al., 2010). Similarly, IVM is actively transported via P-gp into the apical compartment in polarised CaCo-2 cells and MOX is not (Griffin et al., 2005). Surprisingly, there are several other data showing that the behaviour of MOX in the host organism is controlled by P-gp or related transporters. In vivo, MOX is effluxed out of the brain at the blood–brain barrier level as testified by the low concentration of the drug in the brain of control mice versus the higher concentration of MOX in the brain of P-gp deficient mice. Because in the same study, P-gp did not appear to contribute to MOX efflux at the intestinal level (Kiki-Mvouaka et al., 2010), these results suggest tissue specificity in the P-gp efflux capacity of MOX. Also, the accumulation of MOX into rat hepatocytes was increased by inhibitors of P-gp or of other ABC transporters showing their possible involvement in MOX efflux out of these cells (Dupuy et al., 2006). In addition, in vivo studies based on co-administration of P-gp inhibitors with MLs suggested that MOX kinetics was somewhat dependent on P-gp or another ABC transporter. Loperamide, known to be a potent P-gp inhibitor, induced changes in the pharmacokinetic behaviour of both IVM and MOX in cattle. When associated with loperamide, both drugs showed increased AUC, but for IVM the absorption was increased while for MOX the elimination rate was decreased. This may reflect differences in the process of P-gp-mediated transport for the two drugs (Lifschitz et al., 2010). Similarly, the co-administration of ketoconazole was able to increase IVM AUC in sheep (Alvinerie et al., 2008) and also that of MOX (Dupuy et al., 2003). However, in another study where verapamil was co-administered with MOX or with IVM, only the IVM kinetics were impacted whereas the MOX kinetics were unchanged (Molento et al., 2004).
Because MLs are P-gp substrates and also lipophilic drugs, their behaviour inside the body is expected to be the resultant of two main driving forces: the P-gp efflux and their affinity for adipose tissue. The balanced of the two actions is related to the log P of each ML, and inversely related to the drug affinity for P-gp. The higher the affinity of the ML for P-gp, the more efficiently the drug is effluxed via the transporter out of the tissue or the organism. Given that IVM and EPM display higher affinity for P-gp than MOX does (Lespine et al., 2007), it is reasonable to assume that the elimination of EPM is mainly due to its high affinity for P-gp and its lower hydrophobicity (Table 3, log P = 4; Ki = 0.02 μM) which results in a lower attraction to the adipose tissue, in accordance with its lower persistence in the plasma (MRT) and the lower entrance into the brain. In contrast, the higher lipophilicity and lower affinity for P-gp of MOX (log P = 6; Ki = 0.5 μM), is consistent with fat accumulation, to the detriment of P-gp efflux. Therefore, MOX behaviour in the host appears to be governed mainly by lipophilicity resulting in a relative delay in drug elimination and to a prolonged MRT. Finally, IVM having intermediate lipophilicity and affinity for P-gp (log P = 4.8, Ki = 0.05 μM), displays an intermediate behaviour between EPM and MOX and is equally governed by both factors.
7.3. Interaction of avermectins and moxidectin with other efflux transporters
Since BCRP (ABCG2) is co-localised with P-gp on the major epithelial barriers such as the intestine and the blood–brain barrier, it may contribute significantly to the transport of a number of substrates. This has therapeutic and physiological implications in humans and in animals (Mealey, 2011). Both IVM and MOX interact with BCRP (ABCG2), and thus we cannot exclude the possibility that this transporter also plays a role in the pharmacokinetics of both avermectins and MOX. IVM efficiently inhibits human (Muenster et al., 2008; Jani et al., 2010) and sheep BCRP (Real et al., 2011). No data are available on the direct transport of IVM by BCRP. Nevertheless, MOX was identified as a BCRP substrate because of its Bcrp1-mediated secretion into breast milk and the involvement of Bcrp1 in intestinal and bile secretion of MOX (Perez et al., 2009). However, the interactions of MOX and avermectins with BCRP have not yet been parameterized. Given the role of BCRP in the efflux of substrates into the milk (Jonker et al., 2005), these interactions of avermectins and MOX with BCRP will have pharmacokinetic, toxicological and milk residue consequences. Indeed, the low elimination of EPM in milk compared with other MLs could be due to a lower transport of this drug by BCRP.
IVM also interacts with MRPs (ABCCs). Data showing the lower accumulation of IVM in cells overexpressing MRP1 together with the polycyclic structure and lipophilic nature of IVM strongly suggest that IVM could also be a MRP transport substrate, even though no transport was shown in polarised cells overexpressing MRP1 (Brayden and Griffin, 2008). IVM inhibits the calcein transport mediated by MRP1 and to a lesser extent that by MRP2 and MRP3 (Lespine et al., 2006). However, the inhibitory potency of IVM with MRPs is weak compared with its interaction with P-gp, and data on interaction of MOX with MRPs is lacking.
7.4. Avermectins and moxidectin modulate the expression of mammalian efflux transporters
Another possible interaction between MLs and MDR efflux transporters concerns the ability of the drug to modulate the expression of transporter genes. Considerable evidence has accumulated to indicate that the expression of drug transporter genes can be rapidly and transiently induced following treatment by chemotherapeutic agents (Chin et al., 1990). The subsequent change in the activity of the transporter can have a major impact on metabolism, toxicity, drug-drug interactions and the efficacy of drugs. Indeed, even a moderate increase of P-gp expression was shown to be associated with resistance to the anticancer drug doxorubicin (Pajic et al., 2009).
IVM was shown to induce the expression of the mdr1 gene encoding for P-gp in mouse hepatocyte cell lines, which was associated with increased efflux activity of P-gp (Menez et al., 2012). In another study using adriamycin-resistant cancer cells overexpressing P-gp, a 24 h exposure to DRM or nemadectin led to a decrease in the expression of the P-gp gene (Gao et al., 2010). The different responses may be due to the differences in cell models; the second study being performed in cells considerably overexpressing P-gp, while the first one used hepatocytes with relatively low constitutive expression of the P-gp gene. We can expect that the regulatory mechanism in the two models is different. The IVM P-gp induction occurred through increasing the mRNA half-life (Menez et al., 2012), while the mechanisms by which nemadectin and DRM decreased the P-gp gene expression, have not been investigated. Nevertheless, in the study using drug resistant cancer cells, the P-gp gene down-regulation was more pronounced in the presence of DRM than with nemadectin suggesting differences in the potency of DRM and the milbemycin in regulating P-gp gene expression.
Deciphering the respective contribution of avermectins and MOX to the regulation of the detoxification network controlling gene expression of transporters will help in the understanding of the differences in the occurrence of resistance under avermectin or MOX pressure.
7.5. Avermectins and moxidectin as multidrug resistance reversing agents in cancer cells
A common mechanism that confers simultaneous resistance to different drugs, based on drug efflux from cells or organisms mediated by multidrug ABC transporters, represents a critical phenomenon hampering anticancer, antibacterial, antifungal and antiparasitic chemotherapy. Because the affinity of avermectins for P-gp is among the highest described to date, they compete with a large number of P-gp substrates (Lespine et al., 2008). In addition, they also inhibit MRP1 and BCRP which makes their action not restricted to P-gp and, at least theoretically, makes it possible to design an efficient broad-spectrum MDR reversal agent, active whatever the relevant MDR transporter involved.
The high efficiency of MDR reversal by avermectins was demonstrated in cultured tumour cells overexpressing P-gp. IVM was able to increase vinblastine efficacy in vinblastine-resistant human lymphoma cells (Pouliot et al., 1997). In vincristine-resistant human lympholeukemic cells P388 and in taxol-resistant larynx tumour Hep-2 cells, IVM was more effective than the traditional MDR transport inhibitor cyclosporin A (Korystov et al., 2004). Some avermectin aglycone compounds were recently shown to be also potent MDR reversing agents in adriamycin-resistant cancer cells overexpressing P-gp (Gao et al., 2011). However, DRM was more potent in reversing MDR than the milbemycin, nemadectin (Gao et al., 2011). Because nemadectin, the parent compound used for MOX production, is structurally closely related to MOX and also lacks a disaccharide moiety, its lower capacity to reverse MDR is probably linked to its lower ability to interact with P-gp (Gao et al., 2010). This is in agreement with the weaker interaction of MOX with P-gp and the relationship between structure and function in terms of the interaction of MLs with P-gp (Lespine et al., 2007).
Owing to the strong interaction of avermectins with the MDR transporters, they have been proposed as possible reversal agents to potentiate chemotherapy based on MDR transporter substrates. However, such a possibility was rejected because of the potential neurotoxicity of avermectins which dictates caution for their use in humans, in particular when co-administered with another MDR substrate and potential MDR inhibitor in clinical situations. In this respect, it would also be of major interest to know the ability of different MLs to bind to mammalian GABA receptors implicated in their mammalian neurotoxicity.
8. Toxicology
8.1. Receptor selectivity and the toxicology of the avermectins and moxidectin
As discussed in the section on pharmacodynamics, the primary receptors for ML anthelmintics appear to be glutamate-gated chloride channels which occur in nematodes and some arthropods, but do not occur in mammals. Thus the lack of these receptors in mammalian hosts provides some measure of safety from ML toxicity in mammals. Nevertheless, MLs also act on GABA-gated chloride channels, although in nematodes these GABA channels appear to be less sensitive than some GluCl receptors. GABA receptors are expressed in the central nervous system (CNS) of mammals. Thus, there is the potential for mammalian toxicity if moderately high levels of some MLs reach the brain. Fortunately in mammals, the ABC transporters located at the blood–brain barrier normally exclude most MLs, such as IVM, from reaching GABA receptors in the CNS at concentrations that would induce toxicity. Thus the interplay between receptor sensitivity and integrity of the exclusion function of the blood–brain barrier are vitally important in safety and toxicity of MLs and the interaction of different MLs with the blood–brain barrier ABC transporters and the brain’s GABA receptors will influence their relative toxicity.
8.2. Contribution of P-glycoprotein to toxicity of avermectins and moxidectin in dogs
Since the link between IVM and P-gp was found, a considerable body of research has been carried out in order to throw light on the role of P-gp in IVM toxicology and pharmacology. Indeed, IVM was the first ML designated as a P-gp substrate when genetically engineered mice lacking the gene coding for P-gp (mdr1a−/−) were treated with a standard antiparasitic procedure in the animal facility. The treated P-gp gene-mutated mice showed signs of toxicity, and nearly all those homozygous for the mutation died; they were found to have a 100-fold higher IVM concentration in their brain than the wild type mice (Schinkel et al., 1994). This is consistent with the property of avermectins to bind to GABA receptors in the CNS of mammals.
P-gp is thus clearly responsible for preventing IVM from entering the brain tissue, and its role is extended to controlling the tissue distribution of the drug in the whole organism (Schinkel et al., 1994). IVM hypersensitivity with neurotoxicity is also observed in a subpopulation of CF1 mice that are mutated and do not produce P-gp (Kwei et al., 1999). The identification of subpopulations of Collie dogs and other herding breeds that display a similar sensitivity, to IVM, as P-gp knockout mice (Paul et al., 1987), has considerably broadened clinical interest of P-gp in the canine veterinary clinic. As expected, P-gp deficient dogs are also sensitive to other drugs which are substrates of P-gp and develop a neurotoxic effect with compound such as loperamide (Hugnet et al., 1996; Sartor et al., 2004) and vincristine (Krugman et al., 2011).
A deletion mutation of the gene encoding P-gp exists in IVM-sensitive Collies and produces a frame shift that generates a premature stop codon in the MDR1 gene resulting in a severely truncated, nonfunctional protein (Mealey et al., 2001; Roulet et al., 2003). The P-gp gene mutation in Collies appears to be widely distributed in the population with 35% of Collie dogs homozygous for the mutant allele and 42% heterozygous (Mealey et al., 2002; Hugnet et al., 2004; Neff et al., 2004; Geyer et al., 2005a; Geyer and Janko, 2011). Several other herding breeds including the Shetland Sheepdog, Australian Shepherd, Old English Sheepdog, Border Collie, Longhaired Whippet, Silken Windhound, McNab, English Shepherd, and Swiss White Shepherd breeds have also been identified to carry the mutation. Anecdotal reports of IVM toxicity in cats after standard doses have been published, but whether or not the underlying cause is a result of altered P-gp expression or function is not currently known (Pritchard, 2011). Apart from SLM, avermectins are rarely used in cats, and so less information is available regarding the toxicity of different avermectins, and the functioning of P-gp at the blood–brain barrier in cats. However, SLM as Revolution®, and MOX as Advantage Multi® topical solutions, as well as MO as Milbemax® tablets, are widely used in cats and have excellent safety.
Collie dogs, homozygous for the deletion on P-gp gene, experience adverse neurologic effects after a single dose of IVM (120 μg/kg) and are also sensitive to toxic effects of some other avermectins. Different MLs have different toxicity, not only in their target parasites but also in mammals, and ABM is known to be more toxic, and MOX less toxic when compared with IVM. However, although several studies have addressed the toxicity of the different MLs in the host, it is sometimes difficult to extract clear information.
CF1 mice lacking P-gp are sensitive to the neurotoxicity induced by IVM and are also sensitive to ABM and the toxic effects are visible at 0.2 mg/kg for IVM and 0.075 mg/kg for ABM, supporting the higher toxicity of ABM compared with IVM. It has been suggested that these differences may be due to differences in accumulation of these compounds in the brain (Lankas et al., 1997, 1998) and may also be caused by different interactions with brain GABA receptors.
In parallel with these studies, clinical observations have shown that the two avermectins, IVM and DRM, at doses of 0.2 and 0.2–1.0 mg/kg, respectively, provoke severe signs of neurotoxicosis in P-gp deficient dogs, including apparent depression, ataxia, somnolence, mydriasis, salivation, and tremor (Paul et al., 1987; Hopper et al., 2002; Yas-Natan et al., 2003; Geyer et al., 2007). On the other hand, SLM produced no severe signs of toxicosis at a topical dose rate of 40 mg/kg, or an oral dose rate of 15 mg/kg, which are three to seven times the recommended dose rates (Bishop et al., 2000; Novotny et al., 2000). However, IVM sensitive Collies have been shown to be also sensitive to the effects of MO administered at 10 mg/kg (20 times the recommended dose rate); a similar margin of safety as IVM. Adverse reaction (neurological toxicity) was observed after treatment with milbemycin 5-oxime with an oral dose ranging from 1.0 to 2.2 mg/kg per day (Barbet et al., 2009). However, milbemycin 5-oxime at lower doses, up to 5 and 10 times the maximum label dose rate, and combined with spinosad, appears to be safe in P-gp deficient dogs and no signs of toxicosis were observed in dogs with the P-gp gene mutation (Sherman et al., 2010).
MOX administered to P-gp deficient dogs, sensitive to a dose of IVM of 120 μg/kg that corresponds to 20 times the recommended dose for heartworm prevention, produced no signs of toxicosis in any dog receiving MOX orally at 30, 60, or 90 μg/kg (up to 30-fold the monthly heartworm prophylaxis oral dose rate), revealing that the MOX heartworm monthly prophylaxis formulation used in the study has a wider margin of safety than IVM in avermectin-sensitive Collies (Paul et al., 2000). However, there is a report of one Australian Shepherd dog, which was homozygous for the mdr1 defect, and which received 0.4 mg/kg MOX orally, showing neurotoxicity (Geyer et al., 2005b). Surprisingly, the same dog had been treated daily for 7 days with 0.1 mg/kg of MOX orally without any signs of toxicosis. After showing signs of toxicosis following treatment at 0.4 mg/kg, the dog received diazepam (IV) and fully recovered by 48 h after high dose MOX administration. When similar avermectin-sensitive Collie dogs having toxicosis signs at 120 μg/kg of IVM, were subsequently exposed to up to 32.5 mg/kg moxidectin (270 times the IVM dose rate to which they were sensitive) they showed no signs of toxicity to MOX (Paul et al., 2004). In this study, MOX (and imidacloprid in combination) was applied topically which administration route is well known to be associated with a relatively low systemic drug concentration (for a given dose rate) compared with oral administration, and the MOX was co-administered with 26–130 mg/kg imidacloprid. Considered together, these studies suggest that MOX is much safer than IVM in the P-gp deficient dogs. However, in another case report, three dogs which were not defective in the mdr1 gene (normals), accidentally orally ingested the topical endectocide formulation. They consumed between 1.89–2.85 mg/kg of MOX and 7.54–11.4 mg/kg of imidacloprid and showed intoxication with signs of ataxia, generalised muscle tremors, paresis, hypersalivation and disorientation (See et al., 2009). All three dogs, after gastrointestinal decontamination, fluid and benzodiazepine therapy made complete recoveries within 48 h of ingestion. As imidacloprid is a nicotinic agonist and the dogs ingested a topical formulation designed to maximise dermal absorption, it is not clear from these cases how much of the observed toxicity was due to MOX and/or the likely very rapid rate of absorption. In chronic toxicity studies Beagle dogs (normal) receiving MOX orally at between 2 and 4 mg/kg/day for 5 days (10–20 mg/kg total dose over 5 days) showed clinical, but non-lethal signs similar to those that were observed in the case report on the three intoxicated dogs (WHO, 1996). The relatively similar low toxicity response to MOX in mdr1 defective and normal dogs suggests that a fully functional blood–brain barrier may be less important for MOX safety than is the case with IVM and other avermectins in which a fully functional blood–brain barrier is critically important to achieving safety in mammals.
As mentioned above, differences between the toxicity of different MLs could be related to the drug penetration into the brain in the absence of P-gp, the dose and the interaction of different MLs with GABA receptors in the mammalian brain. It is of special interest in such studies to monitor the drug concentrations in the animals together with any toxicity signs in order to understand the risk of toxicity. To address this question, studies have been performed in P-gp-deficient mice by comparing MLs administered at similar dose rates and route of administration and by measuring drug concentrations in the brain. It was shown that IVM, EPM and MOX levels were very low in the brain of wild-type mice while the three drugs accumulate in the brain of P-gp-deficient mice. These data reveal that P-gp at the blood–brain barrier effluxes out not only IVM but also EPM, and to a lesser extent MOX. MLs appeared to have different abilities to be expelled out of the brain via a P-gp-dependent pathway. Indeed, the data showed that P-gp contributes considerably to preventing IVM and EPM penetration into the brain, while this transporter has a relatively lower contribution in controlling the entrance of MOX (Kiki-Mvouaka et al., 2010). Other work showed that SLM is efficiently expelled out of the brain by P-gp but accumulates to a lesser degree than IVM in the brain of P-gp-deficient mice (Geyer et al., 2009). In this study, IVM was used at a standard therapeutic dose rate of 0.2 mg/kg, and SLM was applied at the maximum therapeutic dose rate for the body weight range of 12 mg/kg. Based on the 60 times higher dose rate of SLM used, compared with IVM, these data suggest that SLM has a higher safety margin in P-gp-deficient dogs than IVM, as has been described above. The lower CNS toxicity provoked by MOX and SLM compared with IVM, ABM, DRM and EPM suggests also that the two drugs may interact differently with GABA receptors. Indeed, MOX and SLM have lower affinity for mammalian P-gp than do IVM, ABM, DRM and EPM (see Table 3 for Ki values) and this difference has been related to their structures (Lespine et al., 2007). Although no data are available on MOX and SLM interaction with GABA receptors, we cannot exclude that the ML structures determine different interaction with GABA receptors.
8.3. Toxicity of ivermectin in humans: severe adverse events
It has been known for some time that severe adverse events (SAEs) can occur, rapidly after IVM treatment, in some individual humans carrying high burdens of the filarial nematode Loa loa (Gardon et al., 1997; Boussinesq et al., 1998; Pion et al., 2006), including death. Over 130 human SAEs, including mortalities due to IVM, have been recorded. A high L. loa microfilaraemia (e.g. 30,000 microfilariae/ml blood) seems to be a pre-condition for these SAEs. However, some individuals with high L. loa microfilaraemia do not show SAEs (Mackenzie et al., 2007), suggesting that, in addition to L. loa, some other factor(s) may predispose some individuals to IVM SAEs. Men tend to have higher burdens of L. loa and are more predisposed to IVM SAEs than women and this is thought to be genetic/physiological rather than environmental. A recent study on a small number of IVM SAE patients indicated that a genetic change in the mdr1 gene may be associated with a predisposition to IVM SAEs (Bourguinat et al., 2010). Mdr1 polymorphism has been associated with altered distribution and behaviour of a number of pharmaceuticals which are ligands for P-gp efflux, but studies on more SAE patients are required to confirm whether an association between mdr1 polymorphism, altered levels of IVM reaching the brain and L. loa microfilarial burdens in the brain are involved in IVM SAEs in humans.
Moxidectin has undergone phase I and phase II evaluation in humans and is slated for phase III evaluation (Bockarie and Deb, 2010). There have been no reports of severe adverse events after MOX treatment in humans (http://www.apps.who.int/tdr/svc/publications/tdr-research-publications/moxidectin). However, care has been taken to not expose individuals with loaiasis to MOX in these studies. Based on the lower CNS toxicity in dogs and mice following MOX exposure, compared with IVM toxicity, and different effects of mdr1ab knockout in mice on accumulation of MOX in the brain compared with IVM, MOX could be less likely to precipitate SAEs in people under treatment. Nevertheless, despite these theoretical possibilities, this will not be confirmed until more experience has been gained with using MOX in humans and until a better understanding of IVM SAEs is realised. However, if further studies confirm an association between mdr1 polymorphism, burden of L. loa microfilaraemia and IVM SAEs, MOX may prove safer for use in these humans than IVM because it is less dependent on the integrity of blood–brain barrier P-gp for maintaining non-toxic levels in the brain and thus for CNS safety.
8.4. Ecotoxicology of avermectins and moxidectin
The world wide use of MLs and their broad-spectrum of activity, including against some arthropods, have raised the issue of the environmental consequences of ML-based treatments for non-target organisms. The problem of ecotoxicology of MLs relies on the primarily excretion of MLs, in the faeces, with noticeable insecticidal activity on non-target organisms. Thus, dung beetles which are essential for recycling of dung are exposed to significant amounts of drug that may impair their positive contribution to the ecosystem. Alarm was raised when a field trial reported that faeces of calves fitted with intra-ruminal slow-release IVM boluses failed to degrade in the normal way and this failure was associated with the absence of dung-degrading insects (Wall and Strong, 1987). Since it releases drug over 135 days, the intra-ruminal slow-release IVM bolus formulation has been successfully marketed to treat cattle and ensure protection against reinfestation in the field. Based on the almost complete excretion of the parental IVM in the faeces, and the high dose provided, a large amount of IVM is found in dung during and following the period of protection (Alvinerie et al., 1999b). The dramatic impact on dung fauna resulted in this long-acting IVM formulation being retired from some markets in 2004.
Since then, several exhaustive reviews summarised the impact of anthelmintics on non-target fauna (Lumaret and Errouissi, 2002) with the most recent review reporting the influence of MLs on the terrestrial and aquatic environment and their living organisms (Lumaret et al., 2011). This section draws extensively on these reports.
Information on the impact on the environment of IVM and other avermectins has been compiled for drug registration. Avermectin residues in animal faeces, at amounts generated by administration of a standard dose, affect arthropod development by causing mortality and many sublethal effects on adults and larvae of several species of Diptera and Coleoptera (Steel and Wardhaugh, 2002). The potential ecotoxicity of MOX has also been studied. From many studies comparing the effects of different MLs on dung fauna, it appears clearly that MOX is much less toxic that the avermectins against different developmental stages and adult arthropods. Several studies showed a reduction in the number of flies and other organisms in dung after treatment of cattle with MOX without any delay in the degradation of dung (Lumaret and Errouissi, 2002). It was concluded that MOX was ecologically safer than IVM (Herd, 1995). In cattle, a dose rate of 0.2 mg/kg IVM is toxic to dung beetles feeding on the faeces, while MOX had no adverse effects on adult dung beetles (Fincher and Wang, 1992). Similar conclusions have been reported with treated sheep (Wardhaugh et al., 1993) and horses (Lumaret and Errouissi, 2002). More specifically, major differences between IVM and MOX effects have been shown in experimental tests with the dung beetle, Aphodius constans. IVM was the more toxic substance against larvae with a LC50 of 0.88–0.98 mg per kg of dung dry weight versus a MOX LC50 of 4–5 mg per kg of dung dry weight (Hempel et al., 2006). Another study performed in Argentina followed the survival of dung beetles after DRM or MOX administration in cattle, at therapeutic dose rates of 0.2 mg/kg (Suarez et al., 2009). The number of living insects recovered at days 3, 11 and 21 post-treatment from the control pats was significantly higher than those from the treated-animal pats, confirming the insecticidal activity of both MLs. However, a lower adverse effect was observed for MOX as more insects were recovered in the dung of animals receiving MOX treatments than those receiving DRM. Faecal residues of both drugs presented a similar pattern of chemical degradation after environmental exposure of the dung, revealing that the differential non-target toxicity effects between the two drugs was not due to different drug degradation kinetics.
This lower toxicity of MOX against dung fauna when compared with IVM and other avermectins is in full agreement with the lower efficacy of MOX reported against other insects such as the Anopheles mosquito (Butters et al., 2012) (see Section 5). The mechanism underlying these differences remains to be clarified. It may be linked to a lower affinity of MOX for the insect ligand-gated ion channel receptors or to the avermectins and MOX not targeting the same subset of receptors. In the same vein, these data may parallel those of Ardelli et al. (2009) who showed that IVM inhibited pharyngeal pumping in C. elegans with a 64-fold greater potency than did MOX (see Pharmacodynamics section). These observations suggest that the use of MOX rather than avermectins in long-acting formulations, that release drug for a long period of time, would be less ecotoxic.
9. Interaction of avermectins and moxidectin with ABC transporters in nematodes
9.1. Nematode ABC transporters
IVM and other MLs are known to select P-gp and other ABC transporter genes in nematodes such as H. contortus and O. volvulus (see discussions below on modulation of expression and resistance mechanisms). A recent study performed on H. contortus eggs to study nematode P-gp (or other multidrug ABC transporters) has reported effects of different MLs (Kerboeuf and Guegnard, 2011) on the inhibition of rhodamine 123 transport. In this model, no direct transport of the anthelmintics was shown, but rhodamine 123 was clearly actively transported. Given that rhodamine 123 is a reference substrate for mammalian P-gp and other multidrug ABC transporters, this suggests the presence of functional ABC transporters in H. contortus eggs. MLs, which inhibit P-gp-mediated efflux in mammals, activated the efflux of rhodamine 123 in this nematode model. The inducing activities, reflected by the EC50 values, differed significantly between the MLs. IVM had no significant effect, followed by ABM, MOX, EPM, DRM, EMM and SLM (greatest effect). The presence of a double bond at C22–23 correlated with higher inducing activity (ABM > IVM), as did the presence of the cyclohexyl group at C25 (SLM > all other MLs; DRM > ABM). This work suggests that the mechanism of transport, mediated through MDR transporters, may differ between nematodes and mammals. But it also provides information on the differential interactions of the various MLs with nematode transporters which can be related to their different structures.
More recently, preliminary data on the direct interaction of a nematode transporter with xenobiotics were determined using a model of heterologous expression of H. contortus P-gp 2 (Hco-Pgp 2) in mammalian cells. This transporter was able to efflux rhodamine 123 and this efflux was inhibited by valspodar, IVM and MOX (Godoy and Prichard, personal communication). As observed with mammalian P-gp, MOX was markedly less potent in inhibiting P-gp transport activity which confirms that the structure of the drug is also important for its interaction with the nematode P-gp. However, it is clear that the nematode P-gp differs significantly from mammalian MDR1 (P-gp) in the nature of the MOX inhibition of rhodamine transport and further investigations need to be performed in order to describe the precise interaction of different avermectins and MOX with nematode P-gps, MRPs and half-transporters.
9.2. Avermectins and moxidectin modulate the expression of efflux transporters in nematodes and invertebrates
Modulation of P-gp expression and that of other related multidrug transporters has been reported under ML pressure in many organisms including nematodes and it is considered to be a molecular mechanism for avermectin-resistance. In parasitic nematodes, multidrug ABC transporter homologue genes are selected under IVM pressure (Prichard, 2007; Lespine et al., 2012) and several were shown to be overexpressed in IVM-resistant worms such as H. contortus (Xu et al., 1998) and C. elegans (James and Davey, 2009). Recently, Dicker et al. (2011) and Williamson and Wolstenholme (2012) have shown significant constitutive upregulation of some P-gps in IVM-resistant H. contortus and T. circumcincta, while the feeding of multidrug resistance reversing chemicals to IVM-resistant larvae of H. contortus and T. circumcincta in vitro, reversed the resistance (Bartley et al., 2009). Prichard and Roulet (2007) reported that IVM, and to a lesser extent MOX, upregulated the expression of several P-gps in H. contortus. In the cattle tick Rhipicephalus (Boophilus) microplus (Acari Ixodide), a gene sequence identified as an ABC transporter was significantly up-regulated in IVM-resistant females suggesting that such overexpression contributes to IVM detoxification in this arthropod (Pohl et al., 2011). It is noteworthy to mention that such induction was also reported after exposure to the avermectin emamectin in the marine parasitic sea lice Lepeophtheirus salmoni (Tribble et al., 2007).
10. Resistance
10.1. Resistance to avermectins and to moxidectin in the field
Resistance to IVM was first reported within 3 years of its commercial launch (Carmichael et al., 1987). Resistance to the avermectins has developed in many nematode parasites of animals, while resistance to MOX is increasing (Sutherland and Leathwick, 2011; Kaplan, 2004; Wolstenholme et al., 2004; Craig, 2006; Pomroy, 2006; Prichard, 2007; Geary et al., 2011; Sargison, 2011). IVM resistance has also been found in human O. volvulus (Osei-Atweneboana et al., 2011). In this review, we have not attempted to catalogue all of the reports of avermectin resistance or MOX resistance. A summary of all of the known literature in which a comparison has been made between an avermectin (usually IVM) and MOX in the same isolate or the same study, as well as the result, location of the isolate/study and the reference may be found in Supplementary Table S1. More details on each individual study can be found in the references to that table. At recommended dose rates, resistance to avermectins in H. contortus is very widespread, and resistance to MOX occurs, but is less widespread. Avermectin resistance is also increasing alarmingly in T. circumcincta, particularly in New Zealand and UK. MOX resistance in T. circumcincta occurs, but is still relatively rare. Avermectin resistance in T. colubriformis is still relatively rare, but it has been reported in Australia (Le Jambre et al., 2005), New Zealand, Italy and Brazil (see Supplementary Table S1), and MOX resistance, still less common, has been found in this species in Australia (Le Jambre et al., 2005) and Brazil (Almeida et al., 2010). Resistance to avermectins and to MOX is also becoming common in Cooperia spp., especially C. oncophora in cattle around the world, although usually MOX remains more effective than the avermectins once resistance is detected. Avermectin resistance also occurs in H. placei (and H. contortus) in cattle. There are few reports of MOX resistance in Haemonchus spp. in cattle. IVM resistance has been found in O. ostertagi L4 larvae, but not so far to MOX. There is preliminary evidence of possible resistance to a number of avermectins, and to MOX in lungworm in cattle in Brazil (Molento et al., 2006). In horses, resistance to both IVM and MOX was first detected in Parascaris equorum (Boersema et al., 2002). IVM resistance has arisen only recently in small strongyles in horses, while MOX still seems to be effective against these species (Milillo et al., 2009; Traversa et al., 2009) (Supplementary Table S1). IVM and MOX resistances have been detected in nematode parasites of llama and alpaca (Gillespie et al., 2010). Recently, there has been growing evidence that resistance to heartworm preventatives is developing in USA (see Geary et al., 2011). In a recent study comparing all of the heartworm preventative ML molecules at their recommended dose rates against one strain of D. immitis, low numbers of infective larvae were able to establish in dogs receiving IVM, SLM or milbemycin oxime, but MOX remained 100% effective (Blagburn et al., 2011). Another study (Snyder et al., 2010) also found that IVM and MO did not completely prevent D. immitis establishment with the same strain of heartworm. Resistance to avermectins is known in ectoparasites of animals and arthropod pests of crops (Clark et al., 1995; Humeres and Morse, 2005) and IVM resistance in human Sarcoptes scabiei has been reported (Currie et al., 2004).
A full understanding of the mechanisms and genetics of avermectin and MOX resistances is still lacking, however, these types of resistance appear to be multigenic (Prichard, 2001; McCavera et al., 2007). Some early studies suggested that avermectin and MOX resistances may be the same (Conder et al., 1993; Shoop, 1993; Shoop et al., 1993, 1995). These authors conducted assays against IVM resistant H. contortus in model host systems, at dose rates below those recommended for livestock, and found shifts in responses to both IVM and MOX compared with IVM susceptible laboratory strains. The expression of resistance is affected by pharmaceutical dose rate and low dose rates can be used to search for possible developing resistance which can foreshadow future resistance to the normal use dose rate. However, both the early and more recent experience with avermectin resistance and MOX resistance, and the measured efficacy in the field, indicate that while there is some degree of shared resistance between the avermectins and MOX, at its recommended dose rate MOX is more effective, often still highly effective, than an avermectin assayed at the same time against the same isolates of nematodes in sheep, goats, cattle, horses, and dogs (Supplementary Table S1). Le Jambre et al. (2005) found that the mode of inheritance of resistance to MOX and ABM, in H. contortus, was different and concluded that “it should be made clear whether it is resistance to the avermectins (IVM resistance) or resistance to the milbemycins (MOX resistance) that is being described.”
A report by Rendell et al. (2006) suggested that the use of MOX led to a higher frequency of ML resistance in T. circumcincta than did use of IVM (or ABM) in sheep farms in South-eastern Australia. The conclusions were based on records of the previous 5 years, and in some cases 10 years, of anthelmintic treatments and an assessment of ML resistance based on FECRT results following treatment with a half-dose rate of IVM (0.1 mg/kg) and a cut-off of <95% efficacy for a declaration of ML resistance. A careful analysis of the paper makes it difficult to conclude that either IVM or MOX repeated treatments more rapidly selects for avermectin (or MOX) resistance for the following reasons. Although the study looked at a 5–10 year history of anthelmintic use, in fact only 6 properties had used exclusively MOX in the 5 years prior to the study and of these 4 had resistance to the half-dose IVM (none were reported as resistant to MOX), and 2 were still susceptible to IVM. Meanwhile, there were only 2 properties that had used an avermectin exclusively for the previous 5 years and both were resistant to the half-dose IVM. Of the 103 properties investigated 49.5% had T. circumcincta that were resistant to the half-dose IVM. There was no evidence presented, or any claim, of any MOX resistance on any property, even though the study spoke of ML-resistance based on FECRT to the half-dose IVM. Finally, properties using MOX are quite likely to have changed from using an avermectin because IVM resistance had resulted in treatments with avermectins not producing the level of parasite control that was desired. This would of course be hard to assess, but information on farmer perceptions of possible resistance was not presented from the questionnaire survey that was conducted. Thus, the study was possibly biased by the properties that used MOX having an existing level of avermectin resistance. As 49.5% of the properties were found to have a measure of IVM resistance and no MOX resistance was reported, despite its use over several years, the study does suggest that avermectin resistance may be easier to select, using avermectins, or possibly MOX, than actual MOX resistance. This conclusion is supported by the controlled selection study of Ranjan et al. (2002) who subjected an anthelmintic susceptible isolate of H. contortus to selection by either IVM or MOX, 28 days after infection of sheep, at dose rates that produced 85–95% reduction in faecal egg count, over 22 generations. After the 22 generations of selection, the H. contortus selected by either IVM or MOX were resistant to 0.2 mg/kg IVM (IVM efficacies of selected strains of 70% and 88%, respectively), whereas following selection with either IVM or MOX, both strains were highly susceptible to MOX, even at a reduced dose rate of 0.05 mg/kg (>99% efficacy in both strains). The data in this study support a conclusion that there is some degree of selection by both IVM and MOX for avermectin resistance, but also is consistent with a conclusion that additional factors (genes/mechanisms) are required before MOX resistance shows to its recommended dose rate, compared with avermectin resistance. In this review, we have compared what is known about the avermectins (usually IVM), and MOX as a specific type of ML. What is known about the mechanisms of avermectin and MOX resistances and possible reasons why avermectin resistance and MOX resistance share some similarities, but are not identical, are briefly discussed below.
10.2. Resistance mechanisms for avermectin and moxidectin resistances
Evidence for the involvement of chloride channel receptors in avermectin-resistance in parasitic nematodes is limited, although extensive investigations have been conducted to look for such an explanation of avermectin-resistance. In C. elegans, the deletion of the three GluCl genes avr-14, avr-15 and glc-1 (Dent et al., 2000) causes a marked decrease in sensitivity of this nematode to IVM, showing that the products of these GluCl genes are involved with the sensitivity of C. elegans to IVM. The gene GluClα, an avr-14 homologue, has been shown to be under selection pressure in IVM resistant strains of H. contortus that had been under IVM or MOX selection (Blackhall et al., 1998), but no specific resistance-associated sequence changes were identified. The most convincing evidence that a sequence change in a GluCl may cause IVM resistance comes from studies with the cattle parasite, C. oncophora. Njue and Prichard (2004) showed that an avr-14 homologue was under selection in an IVM-resistant field isolate and subsequently, Njue et al. (2004) showed that a L256F change in the GluClα3 caused a threefold loss of sensitivity to IVM and MOX. Using site-directed mutagenesis to introduce the L256F mutation into the H. contortus GluClα3B (avr-14B homologue) subunit, McCavera et al. (2007) found the mutation reduced IVM affinity by 6.5-fold. However, to date the L256F SNP has only been found naturally in one field isolate of C. oncophora despite investigations of many IVM resistant isolates. Recently, a decrease in polymorphism and in level of expression of the avr-14B homologue has been reported in IVM resistant C. oncophora and O. ostertagi, suggesting a possible link of this gene with IVM resistance (El-Abdellati et al., 2011). These authors and Williamson et al. (2012) found a slight reduction in expression of two other GluCls, Hco-glc-3 and Hco-glc-5 in an isolate of IVM resistant H. contortus. It is of interest that a naturally avermectin resistant strain of C. elegans, CB4856, showed significant genetic diversity in the glc-1 gene, compared with the wild-type N2 Bristol strain. This GluCl based resistance appeared to be recessive and was associated with a general fitness deficit, which appeared to be maintained by a balanced selection between general fitness and resistance to pathogenic bacteria producing avermectins (Ghosh et al., 2012).
A possible involvement in IVM resistance of the putative GABA-gated chloride channel subunit HG1 has been reported in H. contortus (Blackhall et al., 2003). It was co-expressed with the C. elegans GAB-1 subunit in Xenopus oocytes to form a functional IVM-sensitive GABA-gated chloride channel. When two different alleles identified from the Blackhall study (A: wild type and E: IVM selected) were co-expressed with GAB-1, 10 μM IVM, when co-applied with 10 μM GABA, potentiated the current of the HG1A/GAB-1 (sensitive) receptor, but attenuated the GABA response of the HG1E/GAB-1 (resistant) receptor (Feng et al., 2002). However, more data are needed on whether the effects are likely to occur at pharmacologically relevant IVM concentrations in vivo to conclude an involvement with IVM resistance. The large diversity of ligand-gated chloride channels in nematodes and the different effects that IVM and MOX have on nematodes and on expression of ligand-gated chloride channels, raise the strong possibility that while some ligand-gated chloride channels may be affected to a similar extent by both avermectins and MOX, not all of the ligand-gated chloride channels will respond similarly to both avermectins and MOX and be under the same selection in nematodes that are avermectin resistant compared to those that are selected with MOX.
There is considerable body of evidence that IVM and other MLs select on nematode P-gps and other ABC transporters (Lespine et al., 2012), suggesting their involvement in avermectin resistance mechanisms. Nematodes have a large and diverse number of ABC transporters which may be involved with either avermectin or MOX transport and the same specific transporters may not be involved to the same extent with each type of ML. In addition to possible ligand-gated chloride channel receptors and ABC transporters, other, yet to be identified genes and mechanisms may be important for either avermectin resistance or MOX resistance. The complexity of the putative mechanisms and number of possible genes involved in avermectin or MOX resistance leave very large possibilities which could explain the differences in the expression of resistance between the avermectins and MOX. Evidence for the involvement of P-gps, or other MDR transporters, in ML resistance in nematodes is indicated by the capacity of typical P-gp inhibitors to increase the efficacy of IVM in susceptible worms or to reverse resistance in resistant strains. Indeed, the in vitro susceptibility to IVM of both sensitive and resistant isolates of T. circumcincta and H. contortus was increased (Bartley et al., 2009) by valspodar, verapamil and pluronic acid, a surfactant polyol; compounds which have been shown to inhibit mammalian P-gp and to reverse multidrug resistance in mammalian cells. Similarly, studies performed on IVM-resistant C. elegans strains showed that multidrug resistance reversing agents, verapamil and PSC833 (valspodar), were able to restore IVM sensitivity even in a highly resistant strain. Similarly, the MRP inhibitors, MK571 and buthionine sulfoxamine also reversed resistance. Thus, ABC transport proteins appear to be involved in the development of IVM resistance in C. elegans which is in agreement with the increased expression of P-gp and MRP genes in IVM-resistant strains (James and Davey, 2009). In addition, differential response was observed with MOX when compared with IVM in one of the two C. elegans strains which had been selected under IVM pressure. The worms resistant to 6 ng/ml IVM (>6-fold resistance) showed low cross-resistance to MOX (1.5-fold). The strain which was resistant to 10 ng/ml IVM (19-fold) was highly resistant to MOX (35-fold), levamisole (5.2-fold), and pyrantel (4.9-fold). These data suggest an involvement of ABC transporters in IVM resistance and that those transporter-based resistance mechanisms can lead to multidrug resistance.
11. Future prospects and conclusions
These findings have important biological consequences, considering that if the differences in structure lead to different pharmacokinetic and pharmacodynamic behaviour, they could also have an impact on their toxicity, efficacy, the emergence of resistance, and the extent of cross-resistance. A better understanding of the interaction between avermectins, or MOX, with transporters and ligand-gated ion channels may help to improve the current ML-based chemotherapy. This is desirable in order to sustain the efficacy of this broad-spectrum class of endectocides in the context of worsening resistance that threatens to severely limit current parasite control strategies.
Where a major component of a multigenic resistance is due to altered expression or activity of ABC transporters in resistant isolates, it is feasible to consider pharmacologically reversing that resistance with MDR reversing agents and so remove the advantage that genetically resistant strains enjoy in the presence of the anthelmintic to which they are resistant. Furthermore, when efflux based mechanisms are key components of resistance, it may be possible to screen for new ML entities which are not, or are less susceptible to transporter based resistance. Similarly, a better understanding of the similarities and differences in the interaction of different avermectins and milbemycins with ligand-gated ion channels in parasites would be useful for optimising parasiticide-receptor interactions and possibly extending the spectra and effectiveness of the MLs, or help in the discovery of other chemical structures with antiparasitic activity.
In the face of increasing anthelmintic resistance, combinations of MLs with other anthelmintics are increasingly being advocated (Leathwick and Hosking, 2009). Improving our knowledge of the differences and similarities of MOX and the avermectins can be important, for numerous reasons, when pharmaceutical combinations are being considered. As discussed above, because MOX and the avermectins have different effects on multidrug ABC transporters, a combination of a non-ML anthelmintic with an avermectin or MOX can bring different advantages or disadvantages to efforts to delay the selection for resistance. Furthermore, because a potent ABC transport ligand, such as IVM, can alter the pharmacokinetics, in the host and/or in the parasite of another anthelmintic, consideration of the pharmacological consequences of combination, in terms of anti-parasite efficacy and potential host toxicity, should be considered when different anthelmintics are combined to broaden the spectrum of activity and convenience of administration.
Better understandings of the factors which can lead to host toxicity are also important. The available data with IVM-sensitive dogs and P-gp deficient mice indicate that MOX is much less dependent on normally functioning blood–brain barrier transporters to regulate drug entry into/efflux from the CNS than are the avermectins. Further investigations into these differential effects will be important for improving antiparasite safety in dogs and probably other host species. In fact, this could also be critically important for avoiding possible severe adverse events in humans which have led to mortalities in some individuals heavily infected with L. loa, and which has led to a failure to implement onchocerciasis and lymphatic filariasis control in some regions of Africa.
There are other potential benefits from obtaining a better understanding of the similarities and differences between the avermectins and MOX, including possible decisions about which MLs are most suitable and safest, from the point of view of ecotoxicity and resistance selection, for development in long-acting formulations or high dose rate broad-spectrum formulations. The avermectins and milbemycins are remarkable pharmaceuticals and a better understanding of their specific properties in biological systems is likely to reveal new uses and new products from these families in the future.
Acknowledgements
Research of R.K.P. is currently supported by NSERC (Canada), MAPAQ (Quebec), WHO-TDR, Wellcome Trust, FQRNT (Quebec), Bayer, Novartis and McGill University. Research of A.L. and C.M. is currently supported by INRA, ANR (France) and Bayer. The contents of the review are entirely the work of the authors and research sponsors influenced no control over the contents of the review.
Appendix A. Supplementary data
Comparison of chemical structures of avermectins (A) and milbemycins (B).
Comparative efficacy of avermectins (usually ivermectin) and moxidectin against field isolates of nematode parasites in different hosts, in which some ML resistance was suspected.
References
- Al-Azzam S.I., Fleckenstein L., Cheng K.J., Dzimianski M.T., McCall J.W. Comparison of the pharmacokinetics of moxidectin and ivermectin after oral administration to beagle dogs. Biopharm. Drug Dispos. 2007;28:431–438. doi: 10.1002/bdd.572. [DOI] [PubMed] [Google Scholar]
- Almeida F.A., Garcia K.C., Torgerson P.R., Amarante A.F. Multiple resistance to anthelmintics by Haemonchus contortus and Trichostrongylus colubriformis in sheep in Brazil. Parasitol. Int. 2010;59:622–625. doi: 10.1016/j.parint.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Alvinerie M., Sutra J.F., Galtier P. Ivermectin in goat plasma and milk after subcutaneous injection. Vet. Res. 1993;24:417–421. [PubMed] [Google Scholar]
- Alvinerie M., Lacoste E., Sutra J.F., Chartier C. Some pharmacokinetic parameters of eprinomectin in goats following pour-on administration. Vet. Res. Commun. 1999;23:449–455. doi: 10.1023/a:1006373609314. [DOI] [PubMed] [Google Scholar]
- Alvinerie M., Sutra J.F., Galtier P., Lifschitz A., Virkel G., Sallovitz J., Lanusse C. Persistence of ivermectin in plasma and faeces following administration of a sustained-release bolus to cattle. Res. Vet. Sci. 1999;66:57–61. doi: 10.1053/rvsc.1998.0240. [DOI] [PubMed] [Google Scholar]
- Alvinerie M., Sutra J.F., Galtier P., Mage C. Pharmacokinetics of eprinomectin in plasma and milk following topical administration to lactating dairy cattle. Res. Vet. Sci. 1999;67:229–232. doi: 10.1053/rvsc.1999.0312. [DOI] [PubMed] [Google Scholar]
- Alvinerie M., Dupuy J., Eeckhoutte C., Sutra J.F., Kerboeuf D. In vitro metabolism of moxidectin in Haemonchus contortus adult stages. Parasitol. Res. 2001;87:702–704. doi: 10.1007/s004360100408. [DOI] [PubMed] [Google Scholar]
- Alvinerie M., Dupuy J., Kiki-Mvouaka S., Sutra J.F., Lespine A. Ketoconazole increases the plasma levels of ivermectin in sheep. Vet. Parasitol. 2008;157:117–122. doi: 10.1016/j.vetpar.2008.06.017. [DOI] [PubMed] [Google Scholar]
- Ardelli B.F., Prichard R.K. Effects of ivermectin and moxidectin on the transcription of genes coding for multidrug resistance associated proteins and behaviour in Caenorhabditis elegans. J. Nemato. 2008;40:290–298. [Google Scholar]
- Ardelli B.F., Stitt L.E., Tompkins J.B., Prichard R.K. A comparison of the effects of ivermectin and moxidectin on the nematode Caenorhabditis elegans. Vet. Parasitol. 2009;165:96–108. doi: 10.1016/j.vetpar.2009.06.043. [DOI] [PubMed] [Google Scholar]
- Ballent M., Lifschitz A., Virkel G., Sallovitz J., Lanusse C. Modulation of the P-glycoprotein-mediated intestinal secretion of ivermectin: in vitro and in vivo assessments. Drug Metab. Dispos. 2006;34:457–463. doi: 10.1124/dmd.105.007757. [DOI] [PubMed] [Google Scholar]
- Banks B.J., Bishop B.F., Evans N.A., Gibson S.P., Goudie A.C., Gration K.A., Pacey M.S., Perry D.A., Witty M.J. Avermectins and flea control: structure-activity relationships and the selection of selamectin for development as an endectocide for companion animals. Bioorg. Med. Chem. 2000;8:2017–2025. doi: 10.1016/s0968-0896(00)00120-6. [DOI] [PubMed] [Google Scholar]
- Baoliang P., Yuwan W., Zhende P., Lifschitz A.L., Ming W. Pharmacokinetics of eprinomectin in plasma and milk following subcutaneous administration to lactating dairy cattle. Vet. Res. Commun. 2006;30:263–270. doi: 10.1007/s11259-006-3230-7. [DOI] [PubMed] [Google Scholar]
- Barbet J.L., Snook T., Gay J.M., Mealey K.L. ABCB1-1 Delta (MDR1-1 Delta) genotype is associated with adverse reactions in dogs treated with milbemycin oxime for generalized demodicosis. Vet. Dermatol. 2009;20:111–114. doi: 10.1111/j.1365-3164.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- Bartley D.J., McAllister H., Bartley Y., Dupuy J., Menez C., Alvinerie M., Jackson F., Lespine A. P-glycoprotein interfering agents potentiate ivermectin susceptibility in ivermectin sensitive and resistant isolates of Teladorsagia circumcincta and Haemonchus contortus. Parasitology. 2009;136:1081–1088. doi: 10.1017/S0031182009990345. [DOI] [PubMed] [Google Scholar]
- Beech R., Levitt N., Cambos M., Zhou S., Forrester S.G. Association of ion-channel genotype and macrocyclic lactone sensitivity traits in Haemonchus contortus. Mol. Biochem. Parasitol. 2010;171:74–80. doi: 10.1016/j.molbiopara.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Belizario V.Y., Amarillo M.E., de Leon W.U., de los Reyes A.E., Bugayong M.G., Macatangay B.J. A comparison of the efficacy of single doses of albendazole, ivermectin, and diethylcarbamazine alone or in combinations against Ascaris and Trichuris spp. Bull. World Health Organ. 2003;81:35–42. [PMC free article] [PubMed] [Google Scholar]
- Bengone-Ndong T., Ba M.A., Kane Y., Sané I., Sutra J.F., Alvinerie M. Eprinomectin in dairy zebu gobra cattle (Bos indicus): plasma kinetics and excretion in milk. Parasitol. Res. 2006;98:501–506. doi: 10.1007/s00436-005-0103-x. [DOI] [PubMed] [Google Scholar]
- Bengoumi M., Hidane K., Bengone N., Van-Gool F., Alvinerie M. Pharmacokinetics of eprinomectin in plasma and milk in lactating camels (Camelus dromedarius) Vet. Res. Commun. 2007;31:317–322. doi: 10.1007/s11259-006-3284-6. [DOI] [PubMed] [Google Scholar]
- Benz G.W., Roncalli R.A., Gross S.J. Use of iveremectin in cattle, sheep, goats, and swine. In: Campbell W.C., editor. Ivermectin and Abamectin. Springer-Verlag; New York: 1989. pp. 215–229. [Google Scholar]
- Bishop B.F., Bruce C.I., Evans N.A., Goudie A.C., Gration K.A., Gibson S.P., Pacey M.S., Perry D.A., Walshe N.D., Witty M.J. Selamectin: a novel broad-spectrum endectocide for dogs and cats. Vet. Parasitol. 2000;91:163–176. doi: 10.1016/s0304-4017(00)00289-2. [DOI] [PubMed] [Google Scholar]
- Blackhall W.J., Pouliot J.F., Prichard R.K., Beech R.N. Haemonchus contortus: selection at a glutamate-gated chloride channel gene in ivermectin- and moxidectin-selected strains. Exp. Parasitol. 1998;90:42–48. doi: 10.1006/expr.1998.4316. [DOI] [PubMed] [Google Scholar]
- Blackhall W.J., Prichard R.K., Beech R.N. Selection at a gamma-aminobutyric acid receptor gene in Haemonchus contortus resistant to avermectins/milbemycins. Mol. Biochem. Parasitol. 2003;131:137–145. doi: 10.1016/s0166-6851(03)00201-9. [DOI] [PubMed] [Google Scholar]
- Blagburn B.L., Dillon A.R., Arther R.G., Butler J.M., Newton J.C. Comparative efficacy of four commercially available heartworm preventive products against the MP3 laboratory strain of Dirofilaria immitis. Vet. Parasitol. 2011;176:189–194. doi: 10.1016/j.vetpar.2010.12.049. [DOI] [PubMed] [Google Scholar]
- Bockarie M.J., Deb R.M. Elimination of lymphatic filariasis: do we have the drugs to complete the job? Curr Opin Infect Dis. 2010;23:617–620. doi: 10.1097/QCO.0b013e32833fdee5. [DOI] [PubMed] [Google Scholar]
- Boersema J.H., Eysker M., Nas J.W. Apparent resistance of Parascaris equorum to macrocylic lactones. Vet. Rec. 2002;150:279–281. doi: 10.1136/vr.150.9.279. [DOI] [PubMed] [Google Scholar]
- Bogan J.A., McKellar Q.A. The pharmacodynamics of ivermectin in sheep and cattle. J. Vet. Pharmacol. Ther. 1988;11:260–268. doi: 10.1111/j.1365-2885.1988.tb00151.x. [DOI] [PubMed] [Google Scholar]
- Bourguinat C., Kamgno J., Boussinesq M., Mackenzie C.D., Prichard R.K., Geary T.G. Analysis of the mdr-1 gene in patients co-infected with Onchocerca volvulus and Loa loa who experienced a post-ivermectin serious adverse event. Am. J. Trop. Med. Hyg. 2010;83:28–32. doi: 10.4269/ajtmh.2010.09-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousquet-Melou A., Mercadier S., Alvinerie M., Toutain P.L. Endectocide exchanges between grazing cattle after pour-on administration of doramectin, ivermectin and moxidectin. Int. J. Parasitol. 2004;34:1299–1307. doi: 10.1016/j.ijpara.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Bousquet-Melou A., Jacquiet P., Hoste H., Clement J., Bergeaud J.P., Alvinerie M., Toutain P.L. Licking behaviour induces partial anthelmintic efficacy of ivermectin pour-on formulation in untreated cattle. Int. J. Parasitol. 2011;41:563–569. doi: 10.1016/j.ijpara.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Boussinesq M., Gardon J., Gardon-Wendel N., Kamgno J., Ngoumou P., Chippaux J.P. Three probable cases of Loa loa encephalopathy following ivermectin treatment for onchocerciasis. Am. J. Trop. Med. Hyg. 1998;58:461–469. doi: 10.4269/ajtmh.1998.58.461. [DOI] [PubMed] [Google Scholar]
- Brayden D.J., Griffin J. Avermectin transepithelial transport in MDR1- and MRP-transfected canine kidney monolayers. Vet. Res. Commun. 2008;32:93–106. doi: 10.1007/s11259-007-9007-9. [DOI] [PubMed] [Google Scholar]
- Brown D.D., Siddiqui S.Z., Kaji M.D., Forrester S.G. Pharmacological characterization of theHaemonchus contortus GABA-gated chloride channel, Hco-UNC-49: modulation by macrocyclic lactone anthelmintics and a receptor for piperazine. Vet. Parasitol. 2012;185:201–209. doi: 10.1016/j.vetpar.2011.10.006. [DOI] [PubMed] [Google Scholar]
- Butters M.P., Kobylinski K.C., Deus K.M., da Silva I.M., Gray M., Sylla M., Foy B.D. Comparative evaluation of systemic drugs for their effects against Anopheles gambiae. Acta Trop. 2012;121:34–43. doi: 10.1016/j.actatropica.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W., editor. Ivermectin and Abamectin. Springer-Verlag; New York: 1989. [Google Scholar]
- Carceles C.M., Diaz M.S., Vicente M.S., Sutra J.F., Alvinerie M., Escudero E. Milk kinetics of moxidectin and doramectin in goats. Res. Vet. Sci. 2001;70:227–231. doi: 10.1053/rvsc.2001.0463. [DOI] [PubMed] [Google Scholar]
- Carmichael I., Visser R., Schneider D., Soll M. Haemonchus contortus resistance to ivermectin. J. S. Afr. Vet. Assoc. 1987;58:93. [PubMed] [Google Scholar]
- Chick B.F., McDonald D., Cobb R., Kieran P.J., Wood I. The efficacy of injectable and pour-on formulations of moxidectin against lice on cattle. Aust. Vet. J. 1993;70:212–213. doi: 10.1111/j.1751-0813.1993.tb03306.x. [DOI] [PubMed] [Google Scholar]
- Chin K.V., Chauhan S.S., Pastan I., Gottesman M.M. Regulation of mdr RNA levels in response to cytotoxic drugs in rodent cells. Cell Growth Differ. 1990;1:361–365. [PubMed] [Google Scholar]
- Chiu S.H., Taub R., Sestokas E., Lu A.Y., Jacob T.A. Comparative in vivo and in vitro metabolism of ivermectin in steers, sheep, swine, and rat. Drug Metab. Rev. 1987;18:289–302. doi: 10.3109/03602538708998309. [DOI] [PubMed] [Google Scholar]
- Chiu S.H., Green M.L., Baylis F.P., Eline D., Rosegay A., Meriwether H., Jacob T.A. Absorption, tissue distribution, and excretion of tritium-labeled ivermectin in cattle, sheep, and rat. J. Agric. Food Chem. 1990;38:2072–2078. [Google Scholar]
- Clark J.M., Scott J.G., Campos F., Bloomquist J.R. Resistance to avermectins: extent, mechanisms, and management implications. Annu. Rev. Entomol. 1995;40:1–30. doi: 10.1146/annurev.en.40.010195.000245. [DOI] [PubMed] [Google Scholar]
- Conder G.A., Thompson D.P., Johnson S.S. Demonstration of co-resistance of Haemonchus contortus to ivermectin and moxidectin. Vet. Rec. 1993;132:651–652. doi: 10.1136/vr.132.26.651. [DOI] [PubMed] [Google Scholar]
- Cotreau M.M., Warren S., Ryan J.L., Fleckenstein L., Vanapalli S.R., Brown K.R., Rock D., Chen C.Y., Schwertschlag U.S. The antiparasitic moxidectin: safety, tolerability, and pharmacokinetics in humans. J. Clin. Pharmacol. 2003;43:1108–1115. doi: 10.1177/0091270003257456. [DOI] [PubMed] [Google Scholar]
- Craig T.M. Anthelmintic resistance and alternative control methods. Vet. Clin. N. Am. Food Anim. Pract. 2006;22:567–581. doi: 10.1016/j.cvfa.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Cramer L.G., Carvalho L.A., Bridi A.A., Amaral N.K., Barrick R.A. Efficacy of topically applied ivermectin against Boophilus microplus (Canestrini, 1887) in cattle. Vet. Parasitol. 1988;29:341–349. doi: 10.1016/0304-4017(88)90151-3. [DOI] [PubMed] [Google Scholar]
- Craven J., Bjorn H., Hennessy D., Friis C., Nansen P. Pharmacokinetics of moxidectin and ivermectin following intravenous injection in pigs with different body compositions. J. Vet. Pharmacol. Ther. 2001;24:99–104. doi: 10.1046/j.1365-2885.2001.00309.x. [DOI] [PubMed] [Google Scholar]
- Craven J., Bjorn H., Hennessy D.R., Friis C. The effects of body composition on the pharmacokinetics of subcutaneously injected ivermectin and moxidectin in pigs. J. Vet. Pharmacol. Ther. 2002;25:227–232. doi: 10.1046/j.1365-2885.2002.00400.x. [DOI] [PubMed] [Google Scholar]
- Cully D.F., Vassilatis D.K., Liu K.K., Paress P.S., Van der Ploeg L.H., Schaeffer J.M., Arena J.P. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- Currie B.J., Harumal P., McKinnon M., Walton S.F. First documentation of in vivo and in vitro ivermectin resistance in Sarcoptes scabiei. Clin. Infect. Dis. 2004;39:e8–12. doi: 10.1086/421776. [DOI] [PubMed] [Google Scholar]
- Davey R.B., Pound J.M., Klavons J.A., Lohmeyer K.H., Freeman J.M., Perez de Leon A.A., Miller R.J. Efficacy and blood sera analysis of a long-acting formulation of moxidectin against Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) on treated cattle. J. Med. Entomol. 2011;48:314–321. doi: 10.1603/me10154. [DOI] [PubMed] [Google Scholar]
- Dent J.A., Smith M.M., Vassilatis D.K., Avery L. The genetics of ivermectin resistance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2000;97:2674–2679. doi: 10.1073/pnas.97.6.2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker A.J., Nisbet A.J., Skuce P.J. Gene expression changes in a P-glycoprotein (Tci-pgp-9) putatively associated with ivermectin resistance in Teladorsagia circumcincta. Int. J. Parasitol. 2011;41:935–942. doi: 10.1016/j.ijpara.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Didier A., Loor F. The abamectin derivative ivermectin is a potent P-glycoprotein inhibitor. Anticancer Drugs. 1996;7:745–751. doi: 10.1097/00001813-199609000-00005. [DOI] [PubMed] [Google Scholar]
- Doyle L.A., Ross D.D. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2) Oncogene. 2003;22:7340–7358. doi: 10.1038/sj.onc.1206938. [DOI] [PubMed] [Google Scholar]
- Dupuy J., Escudero E., Eeckhoutte C., Sutra J.F., Galtier P., Alvinerie M. In vitro metabolism of 14C-moxidectin by hepatic microsomes from various species. Vet. Res. Commun. 2001;25:345–354. doi: 10.1023/a:1010686508307. [DOI] [PubMed] [Google Scholar]
- Dupuy J., Larrieu G., Sutra J.F., Lespine A., Alvinerie M. Enhancement of moxidectin bioavailability in lamb by a natural flavonoid: quercetin. Vet. Parasitol. 2003;112:337–347. doi: 10.1016/s0304-4017(03)00008-6. [DOI] [PubMed] [Google Scholar]
- Dupuy J., Lespine A., Sutra J.F., Alvinerie M. The interaction between moxidectin and MDR transporters in primary cultures of rat hepatocytes. J. Vet. Pharmacol. Ther. 2006;29:107–111. doi: 10.1111/j.1365-2885.2006.00721.x. [DOI] [PubMed] [Google Scholar]
- Dupuy J., Sutra J.F., Alvinerie M., Rinaldi L., Veneziano V., Mezzino L., Pennacchio S., Cringoli G. Plasma and milk kinetic of eprinomectin and moxidectin in lactating water buffaloes (Bubalus bubalis) Vet. Parasitol. 2008;157:284–290. doi: 10.1016/j.vetpar.2008.07.027. [DOI] [PubMed] [Google Scholar]
- El-Abdellati A., De Graef J., Van Zeveren A., Donnan A., Skuce P., Walsh T., Wolstenholme A., Tait A., Vercruysse J., Claerebout E., Geldhof P. Altered avr-14B gene transcription patterns in ivermectin-resistant isolates of the cattle parasites, Cooperia oncophora and Ostertagia ostertagi. Int. J. Parasitol. 2011;41:951–957. doi: 10.1016/j.ijpara.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Eneroth A., Astrom E., Hoogstraate J., Schrenk D., Conrad S., Kauffmann H.M., Gjellan K. Evaluation of a vincristine resistant Caco-2 cell line for use in a calcein AM extrusion screening assay for P-glycoprotein interaction. Eur. J. Pharm. Sci. 2001;12:205–214. doi: 10.1016/s0928-0987(00)00117-2. [DOI] [PubMed] [Google Scholar]
- Escudero E., Carceles C.M., Diaz M.S., Sutra J.F., Galtier P., Alvinerie M. Pharmacokinetics of moxidectin and doramectin in goats. Res. Vet. Sci. 1999;67:177–181. doi: 10.1053/rvsc.1998.0304. [DOI] [PubMed] [Google Scholar]
- Etya’ale D. Vision 2020: update on onchocerciasis. Commun. Eye Health. 2001;14:19–21. [PMC free article] [PubMed] [Google Scholar]
- Feng X.P., Hayashi J., Beech R.N., Prichard R.K. Study of the nematode putative GABA type-A receptor subunits: evidence for modulation by ivermectin. J. Neurochem. 2002;83:870–878. doi: 10.1046/j.1471-4159.2002.01199.x. [DOI] [PubMed] [Google Scholar]
- Fincher G.T., Wang G.T. Injectable ivermectin for cattle: effects on some dung-inhabiting insects. Environ. Entomol. 1992;21:871–876. [Google Scholar]
- Forrester S.G., Prichard R.K., Beech R.N. A glutamate-gated chloride channel subunit from Haemonchus contortus: expression in a mammalian cell line, ligand binding, and modulation of anthelmintic binding by glutamate. Biochem. Pharmacol. 2002;63:1061–1068. doi: 10.1016/s0006-2952(02)00852-3. [DOI] [PubMed] [Google Scholar]
- Gao A., Wang X., Xiang W., Liang H., Gao J., Yan Y. Reversal of P-glycoprotein-mediated multidrug resistance in vitro by doramectin and nemadectin. J. Pharm. Pharmacol. 2010;62:393–399. doi: 10.1211/jpp.62.03.0016. [DOI] [PubMed] [Google Scholar]
- Gao A., Liang H., Wang X., Zhang X., Jing M., Zhang J., Yan Y., Xiang W. Reversal effects of two new milbemycin compounds on multidrug resistance in MCF-7/adr cells in vitro. Eur. J. Pharmacol. 2011;659:108–113. doi: 10.1016/j.ejphar.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Gardon J., Gardon-Wendel N., Demanga N., Kamgno J., Chippaux J.P., Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet. 1997;350:18–22. doi: 10.1016/S0140-6736(96)11094-1. [DOI] [PubMed] [Google Scholar]
- Garrigues A., Loiseau N., Delaforge M., Ferte J., Garrigos M., Andre F., Orlowski S. Characterization of two pharmacophores on the multidrug transporter P-glycoprotein. Mol. Pharmacol. 2002;62:1288–1298. doi: 10.1124/mol.62.6.1288. [DOI] [PubMed] [Google Scholar]
- Gayrard V., Alvinerie M., Toutain P.L. Comparison of pharmacokinetic profiles of doramectin and ivermectin pour-on formulations in cattle. Vet. Parasitol. 1999;81:47–55. doi: 10.1016/s0304-4017(98)00236-2. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Bourguinat C., Prichard R.K. Evidence for macrocyclic lactone anthelmintic resistance in Dirofilaria immitis. Top. Companion Anim. Med. 2011;26:186–192. doi: 10.1053/j.tcam.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Geyer J., Doring B., Godoy J.R., Leidolf R., Moritz A., Petzinger E. Frequency of the nt230 (del4) MDR1 mutation in Collies and related dog breeds in Germany. J. Vet. Pharmacol. Ther. 2005;28:545–551. doi: 10.1111/j.1365-2885.2005.00692.x. [DOI] [PubMed] [Google Scholar]
- Geyer J., Doring B., Godoy J.R., Moritz A., Petzinger E. Development of a PCR-based diagnostic test detecting a nt230(del4) MDR1 mutation in dogs: verification in a moxidectin-sensitive Australian Shepherd. J. Vet. Pharmacol. Ther. 2005;28:95–99. doi: 10.1111/j.1365-2885.2004.00625.x. [DOI] [PubMed] [Google Scholar]
- Geyer J., Klintzsch S., Meerkamp K., Wohlke A., Distl O., Moritz A., Petzinger E. Detection of the nt230(del4) MDR1 mutation in White Swiss Shepherd dogs: case reports of doramectin toxicosis, breed predisposition, and microsatellite analysis. J. Vet. Pharmacol. Ther. 2007;30:482–485. doi: 10.1111/j.1365-2885.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- Geyer J., Gavrilova O., Petzinger E. Brain penetration of ivermectin and selamectin in mdr1a, b P-glycoprotein- and bcrp-deficient knockout mice. J. Vet. Pharmacol. Ther. 2009;32:87–96. doi: 10.1111/j.1365-2885.2008.01007.x. [DOI] [PubMed] [Google Scholar]
- Geyer, J., Janko, C., 2011. Treatment of MDR1 mutant dogs with macrocyclic lactones. Curr. Pharm. Biotechnol. PMID: 22039792. [DOI] [PMC free article] [PubMed]
- Ghosh R., Andersen E.C., Shiparo J.A., Gerke J.P., Kruglyak L. Natural variation in a chloride channel subunit confers avermectin resistance in C. elegans. Science. 2012;335:574–578. doi: 10.1126/science.1214318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie R.A., Williamson L.H., Terrill T.H., Kaplan R.M. Efficacy of anthelmintics on South American camelid (llama and alpaca) farms in Georgia. Vet. Parasitol. 2010;172:168–171. doi: 10.1016/j.vetpar.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Glendinning S.K., Buckingham S.D., Sattelle D.B., Wonnacott S., Wolstenholme A.J. Glutamate-gated chloride channels of Haemonchus contortus restore drug sensitivity to ivermectin resistant Caenorhabditis elegans. PLoS ONE. 2011;6:e22390. doi: 10.1371/journal.pone.0022390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokbulut C., Biligili A., Kart A., Turgut C. Plasma dispositions of ivermectin, doramectin and moxidectin following subcutaneous administration in rabbits. Lab. Anim. 2010;44:138–142. doi: 10.1258/la.2009.009053. [DOI] [PubMed] [Google Scholar]
- Goudie A.C., Evans N.A., Gration K.A., Bishop B.F., Gibson S.P., Holdom K.S., Kaye B., Wicks S.R., Lewis D., Weatherley A.J. Doramectin-a potent novel endectocide. Vet. Parasitol. 1993;49:5–15. doi: 10.1016/0304-4017(93)90218-c. [DOI] [PubMed] [Google Scholar]
- Griffin J., Fletcher N., Clemence R., Blanchflower S., Brayden D.J. Selamectin is a potent substrate and inhibitor of human and canine P-glycoprotein. J. Vet. Pharmacol. Ther. 2005;28:257–265. doi: 10.1111/j.1365-2885.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- Guglielmone A.A., Mangold A.J., Munoz Cobenas M.E., Scherling N., Garcia Posse F., Anziani O.S., Ioppolo M. Moxidectin pour-on for control of natural populations of the cattle tick Boophilus microplus (Acarina: Ixodidae) Vet. Parasitol. 2000;87:237–241. doi: 10.1016/s0304-4017(99)00173-9. [DOI] [PubMed] [Google Scholar]
- Guzzo C.A., Furtek C.I., Porras A.G., Chen C., Tipping R., Clineschmidt C.M., Sciberras D.G., Hsieh J.Y., Lasseter K.C. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J. Clin. Pharmacol. 2002;42:1122–1133. doi: 10.1177/009127002401382731. [DOI] [PubMed] [Google Scholar]
- Hempel H., Scheffczyk A., Schallnass H.J., Lumaret J.P., Alvinerie M., Rombke J. Toxicity of four veterinary parasiticides on larvae of the dung beetle Aphodius constans in the laboratory. Environ. Toxicol. Chem. 2006;25:3155–3163. doi: 10.1897/06-022r2.1. [DOI] [PubMed] [Google Scholar]
- Hennessy D.R., Alvinerie M.R. Pharmacocinetics of the macrocyclic lactones: conventional wisdom and new paradigms. In: Vercruysse J., Rew R.S., editors. Macrocyclic Lactones and Antiparasitic Therapy. CAB International; New York: 2002. pp. 97–123. [Google Scholar]
- Hennessy D.R., Page S.W., Gottschall D. The behaviour of doramectin in the gastrointestinal tract, its secretion in bile and pharmacokinetic disposition in the peripheral circulation after oral and intravenous administration to sheep. J. Vet. Pharmacol. Ther. 2000;23:203–213. doi: 10.1046/j.1365-2885.2000.t01-2-00286.x. [DOI] [PubMed] [Google Scholar]
- Herd, R., 1995. Endectocidal drugs: ecological risks and counter-measures. Int. J. Parasitol. 25, 875–885. [DOI] [PubMed]
- Hibbs R.E., Gouaux E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodoscek L., Grabnar I., Milcinski L., Sussinger A., Erzen N.K., Zadnik T., Pogacnik M., Cerkvenik-Flajs V. Linearity of eprinomectin pharmacokinetics in lactating dairy sheep following pour-on administration: excretion in milk and exposure of suckling lambs. Vet. Parasitol. 2008;154:129–136. doi: 10.1016/j.vetpar.2008.02.032. [DOI] [PubMed] [Google Scholar]
- Hopper K., Aldrich J., Haskins S.C. Ivermectin toxicity in 17 collies. J. Vet. Intern. Med. 2002;16:89–94. doi: 10.1892/0891-6640(2002)016<0089:itic>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Hugnet C., Cadore J.L., Buronfosse F., Pineau X., Mathet T., Berny P.J. Loperamide poisoning in the dog. Vet. Hum. Toxicol. 1996;38:31–33. [PubMed] [Google Scholar]
- Hugnet C., Bentjen S.A., Mealey K.L. Frequency of the mutant MDR1 allele associated with multidrug sensitivity in a sample of collies from France. J. Vet. Pharmacol. Ther. 2004;27:227–229. doi: 10.1111/j.1365-2885.2004.00585.x. [DOI] [PubMed] [Google Scholar]
- Humeres E.C., Morse J.G. Baseline susceptibility of persea mite (Acari: Tetranychidae) to abamectin and milbemectin in avocado groves in Southern California. Exp. Appl. Acarol. 2005;36:51–59. doi: 10.1007/s10493-005-1545-7. [DOI] [PubMed] [Google Scholar]
- Imperiale F.A., Busetti M.R., Suarez V.H., Lanusse C.E. Milk excretion of ivermectin and moxidectin in dairy sheep: assessment of drug residues during cheese elaboration and ripening period. J. Agric. Food Chem. 2004;52:6205–6211. doi: 10.1021/jf049117n. [DOI] [PubMed] [Google Scholar]
- James C.E., Davey M.W. Increased expression of ABC transport proteins is associated with ivermectin resistance in the model nematode Caenorhabditis elegans. Int. J. Parasitol. 2009;39:213–220. doi: 10.1016/j.ijpara.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Jani M., Makai I., Kis E., Szabo P., Nagy T., Krajcsi P., Lespine A. Ivermectin interacts with human ABCG2. J. Pharm. Sci. 2010;100:94–97. doi: 10.1002/jps.22262. [DOI] [PubMed] [Google Scholar]
- Jonker J.W., Merino G., Musters S., van Herwaarden A.E., Bolscher E., Wagenaar E., Mesman E., Dale T.C., Schinkel A.H. The breast cancer resistance protein BCRP (ABCG2) concentrates drugs and carcinogenic xenotoxins into milk. Nat. Med. 2005;11:127–129. doi: 10.1038/nm1186. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kerboeuf D., Guegnard F. Anthelmintics are substrates and activators of nematode P glycoprotein. Antimicrob. Agents Chemother. 2011;55:2224–2232. doi: 10.1128/AAC.01477-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiki-Mvouaka S., Menez C., Borin C., Lyazrhi F., Foucaud-Vignault M., Dupuy J., Collet X., Alvinerie M., Lespine A. Role of P-glycoprotein in the disposition of macrocyclic lactones: a comparison between ivermectin, eprinomectin, and moxidectin in mice. Drug Metab. Dispos. 2010;38:573–580. doi: 10.1124/dmd.109.030700. [DOI] [PubMed] [Google Scholar]
- Kita K., Shiomi K., Omura S. Advances in drug discovery and biochemical studies. Trends Parasitol. 2007;23:223–229. doi: 10.1016/j.pt.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Korth-Bradley J.M., Parks V., Chalon S., Gourley I., Matschke K., Gossart S., Bryson P., Fleckenstein L. Excretion of moxidectin into breast milk and pharmacokinetics in healthy lactating women. Antimicrob. Agents Chemother. 2011;55:5200–5204. doi: 10.1128/AAC.00311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korystov Y.N., Ermakova N.V., Kublik L.N., Levitman M., Shaposhnikova V.V., Mosin V.A., Drinyaev V.A., Kruglyak E.B., Novik T.S., Sterlina T.S. Avermectins inhibit multidrug resistance of tumor cells. Eur. J. Pharmacol. 2004;493:57–64. doi: 10.1016/j.ejphar.2004.03.067. [DOI] [PubMed] [Google Scholar]
- Krugman L., Bryan J.N., Mealey K.L., Chen A. Vincristine-induced central neurotoxicity in a collie homozygous for the ABCB1Delta mutation. J. Small Anim. Pract. 2012;53:185–187. doi: 10.1111/j.1748-5827.2011.01155.x. [DOI] [PubMed] [Google Scholar]
- Kwei G.Y., Alvaro R.F., Chen Q., Jenkins H.J., Hop C.E., Keohane C.A., Ly V.T., Strauss J.R., Wang R.W., Wang Z., Pippert T.R., Umbenhauer D.R. Disposition of ivermectin and cyclosporin A in CF-1 mice deficient in mdr1a P-glycoprotein. Drug Metab. Dispos. 1999;27:581–587. [PubMed] [Google Scholar]
- Laffont C.M., Alvinerie M., Bousquet-Mélou A., Toutain P.L. Licking behaviour and environmental contamination arising from pour-on ivermectin for cattle. Int. J. Parasitol. 2001;31:1687–1692. doi: 10.1016/s0020-7519(01)00285-5. [DOI] [PubMed] [Google Scholar]
- Laffont C.M., Toutain P.L., Alvinerie M., Bousquet-Melou A. Intestinal secretion is a major route for parent ivermectin elimination in the rat. Drug Metab. Dispos. 2002;30:626–630. doi: 10.1124/dmd.30.6.626. [DOI] [PubMed] [Google Scholar]
- Lankas G.R., Cartwright M.E., Umbenhauer D. P-glycoprotein deficiency in a subpopulation of CF-1 mice enhances avermectin-induced neurotoxicity. Toxicol. Appl. Pharmacol. 1997;143:357–365. doi: 10.1006/taap.1996.8086. [DOI] [PubMed] [Google Scholar]
- Lankas G.R., Wise L.D., Cartwright M.E., Pippert T., Umbenhauer D.R. Placental P-glycoprotein deficiency enhances susceptibility to chemically induced birth defects in mice. Reprod. Toxicol. 1998;12:457–463. doi: 10.1016/s0890-6238(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Lanusse C., Lifschitz A., Virkel G., Alvarez L., Sanchez S., Sutra J.F., Galtier P., Alvinerie M. Comparative plasma disposition kinetics of ivermectin, moxidectin and doramectin in cattle. J. Vet. Pharmacol. Ther. 1997;20:91–99. doi: 10.1046/j.1365-2885.1997.00825.x. [DOI] [PubMed] [Google Scholar]
- Le Jambre L.F., Geoghegan J., Lyndal-Murphy M. Characterization of moxidectin resistant Trichostrongylus colubriformis and Haemonchus contortus. Vet. Parasitol. 2005;128:83–90. doi: 10.1016/j.vetpar.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Leathwick D.M., Hosking B.C. Managing anthelmintic resistance: modelling strategic use of a new anthelmintic class to slow the development of resistance to existing classes. N.Z. Vet. J. 2009;57:203–207. doi: 10.1080/00480169.2009.36902. [DOI] [PubMed] [Google Scholar]
- Lespine A., Sutra J.F., Dupuy J., Alvinerie M. Eprinomectin in goat: assessment of subcutaneous administration. Parasitol. Res. 2003;89:120–122. doi: 10.1007/s00436-002-0727-z. [DOI] [PubMed] [Google Scholar]
- Lespine A., Dupuy J., Orlowski S., Nagy T., Glavinas H., Krajcsi P., Alvinerie M. Interaction of ivermectin with multidrug resistance proteins (MRP1, 2 and 3) Chem. Biol. Interact. 2006;159:169–179. doi: 10.1016/j.cbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Lespine A., Martin S., Dupuy J., Roulet A., Pineau T., Orlowski S., Alvinerie M. Interaction of macrocyclic lactones with P-glycoprotein: structure–affinity relationship. Eur. J. Pharm. Sci. 2007;30:84–94. doi: 10.1016/j.ejps.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Lespine A., Alvinerie M., Vercruysse J., Prichard R.K., Geldhof P. ABC transporter modulation: a strategy to enhance the activity of macrocyclic lactone anthelmintics. Trends Parasitol. 2008;24:293–298. doi: 10.1016/j.pt.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Lespine A., Dupuy J., Alvinerie M., Comera C., Nagy T., Krajcsi P., Orlowski S. Interaction of macrocyclic lactones with the multidrug transporters: the bases of the pharmacokinetics of lipid-like drugs. Curr. Drug Metab. 2009;10:272–288. doi: 10.2174/138920009787846297. [DOI] [PubMed] [Google Scholar]
- Lespine A., Ménez C., Bourguinat C., Prichard R.K. P-glycoproteins and other ABC transporters in the pharmacology of anthelmintics: prospects for reversing transport-dependent anthelmintic resistance. Int. J. Parasitol. Drugs Drug Res. 2011;2:58–75. doi: 10.1016/j.ijpddr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschitz A., Virkel G., Imperiale F., Sutra J.F., Galtier P., Lanusse C., Alvinerie M. Moxidectin in cattle: correlation between plasma and target tissues disposition. J. Vet. Pharmacol. Ther. 1999;22:266–273. doi: 10.1046/j.1365-2885.1999.00222.x. [DOI] [PubMed] [Google Scholar]
- Lifschitz A., Suarez V.H., Sallovitz J., Cristel S.L., Imperiale F., Ahoussou S., Schiavi C., Lanusse C. Cattle nematodes resistant to macrocyclic lactones: comparative effects of P-glycoprotein modulation on the efficacy and disposition kinetics of ivermectin and moxidectin. Exp. Parasitol. 2010;125:172–178. doi: 10.1016/j.exppara.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Ludmerer S.W., Warren V.A., Williams B.S., Zheng Y., Hunt D.C., Ayer M.B., Wallace M.A., Chaudhary A.G., Egan M.A., Meinke P.T., Dean D.C., Garcia M.L., Cully D.F., Smith M.M. Ivermectin and nodulisporic acid receptors in Drosophila melanogaster contain both gamma-aminobutyric acid-gated Rdl and glutamate-gated GluCl alpha chloride channel subunits. Biochemistry. 2002;41:6548–6560. doi: 10.1021/bi015920o. [DOI] [PubMed] [Google Scholar]
- Lumaret J.P., Errouissi F. Use of anthelmintics in herbivores and evaluation of risks for the non target fauna of pastures. Vet. Res. 2002;33:547–562. doi: 10.1051/vetres:2002038. [DOI] [PubMed] [Google Scholar]
- Lumaret, J.P., Errouissi, F., Floate, K., Rmbke, J., Wardhaugh, K., 2011. A review on the toxicity and non-target effects of macrocyclic lactones in terrestrial and aquatic environment. Curr. Pharm. Biotechnol. PMID: 22039795. [DOI] [PMC free article] [PubMed]
- Mackenzie C., Geary T., Prichard R., Boussinesq M. Where next with Loa loa encephalopathy? Data are badly needed. Trends Parasitol. 2007;23:237–238. doi: 10.1016/j.pt.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Marriner S.E., McKinnon I., Bogan J.A. The pharmacokinetics of ivermectin after oral and subcutaneous administration to sheep and horses. J. Vet. Pharmacol. Ther. 1987;10:175–179. doi: 10.1111/j.1365-2885.1987.tb00097.x. [DOI] [PubMed] [Google Scholar]
- McCall J.W. The safety-net story about macrocyclic lactone heartworm preventives: a review, an update, and recommendations. Vet. Parasitol. 2005;133:197–206. doi: 10.1016/j.vetpar.2005.04.005. [DOI] [PubMed] [Google Scholar]
- McCavera S., Walsh T.K., Wolstenholme A.J. Nematode ligand-gated chloride channels: an appraisal of their involvement in macrocyclic lactone resistance and prospects for developing molecular markers. Parasitology. 2007;134:1111–1121. doi: 10.1017/S0031182007000042. [DOI] [PubMed] [Google Scholar]
- McKellar Q.A., Scott E.W. The benzimidazole anthelmintic agents—a review. J. Vet. Pharmacol. Ther. 1990;13:223–247. doi: 10.1111/j.1365-2885.1990.tb00773.x. [DOI] [PubMed] [Google Scholar]
- McKellar Q.A., Benchaoui H.A. Avermectins and milbemycins. J. Vet. Pharmacol. Ther. 1996;19:331–351. doi: 10.1111/j.1365-2885.1996.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Mealey K.L., Bentjen S.A., Gay J.M., Cantor G.H. Ivermectin sensitivity in collies is associated with a deletion mutation of the mdr1 gene. Pharmacogenetics. 2001;11:727–733. doi: 10.1097/00008571-200111000-00012. [DOI] [PubMed] [Google Scholar]
- Mealey K.L., Bentjen S.A., Waiting D.K. Frequency of the mutant MDR1 allele associated with ivermectin sensitivity in a sample population of collies from the northwestern United States. Am. J. Vet. Res. 2002;63:479–481. doi: 10.2460/ajvr.2002.63.479. [DOI] [PubMed] [Google Scholar]
- Mealey K.L. ABCG2 transporter: therapeutic and physiologic implications in veterinary species. J. Vet. Pharmacol. Ther.. 2011 doi: 10.1111/j.1365-2885.2011.01313.x. [DOI] [PubMed] [Google Scholar]
- Menez C., Mselli-Lakhal L., Foucaud-Vignault M., Balaguer P., Alvinerie M., Lespine A. Ivermectin induces P-glycoprotein expression and function through mRNA stabilization in murine hepatocyte cell line. Biochem. Pharmacol. 2012;83:269–278. doi: 10.1016/j.bcp.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Milillo, P., Boeckh, A., Cobb, R., Otranto, D., Lia, R., Perrucci, S., di Regalbono, A.F., Beraldo, P., von Samson-Himmelstjerna, G., Demeler, J., Bartolini, R., Traversa, D., 2009. Faecal cyathostomin egg count distribution and efficacy of anthelmintics against cyathostomins in Italy: a matter of geography? Parasit. Vectors 2, S4. [DOI] [PMC free article] [PubMed]
- Molento M.B., Lifschitz A., Sallovitz J., Lanusse C., Prichard R. Influence of verapamil on the pharmacokinetics of the antiparasitic drugs ivermectin and moxidectin in sheep. Parasitol. Res. 2004;92:121–127. doi: 10.1007/s00436-003-1022-3. [DOI] [PubMed] [Google Scholar]
- Molento M.B., Depner R.A., Mello M.H. Suppressive treatment of abamectin against Dictyocaulus viviparus and the occurrence of resistance in first-grazing-season calves. Vet. Parasitol. 2006;141:373–376. doi: 10.1016/j.vetpar.2006.01.061. [DOI] [PubMed] [Google Scholar]
- Molyneux D.H., Bradley M., Hoerauf A., Kyelem D., Taylor M.J. Mass drug treatment for lymphatic filariasis and onchocerciasis. Trends Parasitol. 2003;19:516–522. doi: 10.1016/j.pt.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Muenster U., Grieshop B., Ickenroth K., Gnoth M.J. Characterization of substrates and inhibitors for the in vitro assessment of Bcrp mediated drug-drug interactions. Pharm. Res. 2008;25:2320–2326. doi: 10.1007/s11095-008-9632-1. [DOI] [PubMed] [Google Scholar]
- Muniz R.A., Hernandez F., Lombardero O., Leite R.C., Moreno J., Errecalde J., Goncalves L.C. Efficacy of injectable doramectin against natural Boophilus microplus infestations in cattle. Am. J. Vet. Res. 1995;56:460–463. [PubMed] [Google Scholar]
- Nagy H., Goda K., Fenyvesi F., Bacso Z., Szilasi M., Kappelmayer J., Lustyik G., Cianfriglia M., Szabo G., Jr. Distinct groups of multidrug resistance modulating agents are distinguished by competition of P-glycoprotein-specific antibodies. Biochem. Biophys. Res. Commun. 2004;315:942–949. doi: 10.1016/j.bbrc.2004.01.156. [DOI] [PubMed] [Google Scholar]
- Neff M.W., Robertson K.R., Wong A.K., Safra N., Broman K.W., Slatkin M., Mealey K.L., Pedersen N.C. Breed distribution and history of canine mdr1-1Delta, a pharmacogenetic mutation that marks the emergence of breeds from the collie lineage. Proc. Natl. Acad. Sci. USA. 2004;101:11725–11730. doi: 10.1073/pnas.0402374101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njue A.I., Hayashi J., Kinne L., Feng X.P., Prichard R.K. Mutations in the extracellular domains of glutamate-gated chloride channel alpha3 and beta subunits from ivermectin-resistant Cooperia oncophora affect agonist sensitivity. J. Neurochem. 2004;89:1137–1147. doi: 10.1111/j.1471-4159.2004.02379.x. [DOI] [PubMed] [Google Scholar]
- Njue A.I., Prichard R.K. Genetic variability of glutamate-gated chloride channel genes in ivermectin-susceptible and -resistant strains of Cooperia oncophora. Parasitology. 2004;129:741–751. doi: 10.1017/s0031182004006183. [DOI] [PubMed] [Google Scholar]
- Novotny M.J., Krautmann M.J., Ehrhart J.C., Godin C.S., Evans E.I., McCall J.W., Sun F., Rowan T.G., Jernigan A.D. Safety of selamectin in dogs. Vet. Parasitol. 2000;91:377–391. doi: 10.1016/s0304-4017(00)00306-x. [DOI] [PubMed] [Google Scholar]
- Ogbuokiri J.E., Ozumba B.C., Okonkwo P.O. Ivermectin levels in human breastmilk. Eur. J. Clin. Pharmacol. 1993;45:389–390. doi: 10.1007/BF00265962. [DOI] [PubMed] [Google Scholar]
- Osei-Atweneboana M.Y., Awadzi K., Attah S.K., Boakye D.A., Gyapong J.O., Prichard R.K. Phenotypic evidence of emerging ivermectin resistance in Onchocerca volvulus. PLoS Negl. Trop. Dis. 2011;5:e998. doi: 10.1371/journal.pntd.0000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oukessou M., Berrag B., Alvinerie M. A comparative kinetic study of ivermectin and moxidectin in lactating camels (Camelus dromedarius) Vet. Parasitol. 1999;83:151–159. doi: 10.1016/s0304-4017(99)00045-x. [DOI] [PubMed] [Google Scholar]
- Paiement J.P., Leger C., Ribeiro P., Prichard R.K. Haemonchus contortus: effects of glutamate, ivermectin, and moxidectin on inulin uptake activity in unselected and ivermectin-selected adults. Exp. Parasitol. 1999;92:193–198. doi: 10.1006/expr.1999.4413. [DOI] [PubMed] [Google Scholar]
- Pajic M., Iyer J.K., Kersbergen A., van der Burg E., Nygren A.O., Jonkers J., Borst P., Rottenberg S. Moderate increase in Mdr1a/1b expression causes in vivo resistance to doxorubicin in a mouse model for hereditary breast cancer. Cancer Res. 2009;69:6396–6404. doi: 10.1158/0008-5472.CAN-09-0041. [DOI] [PubMed] [Google Scholar]
- Paul A.J., Tranquilli W.J., Seward R.L., Todd K.S., Jr., DiPietro J.A. Clinical observations in collies given ivermectin orally. Am. J. Vet. Res. 1987;48:684–685. [PubMed] [Google Scholar]
- Paul A.J., Tranquilli W.J., Hutchens D.E. Safety of moxidectin in avermectin-sensitive collies. Am. J. Vet. Res. 2000;61:482–483. doi: 10.2460/ajvr.2000.61.482. [DOI] [PubMed] [Google Scholar]
- Paul A.J., Hutchens D.E., Firkins L.D., Borgstrom M. Dermal safety study with imidacloprid/moxidectin topical solution in the ivermectin-sensitive collie. Vet. Parasitol. 2004;121:285–291. doi: 10.1016/j.vetpar.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Perez M., Blazquez A.G., Real R., Mendoza G., Prieto J.G., Merino G., Alvarez A.I. In vitro and in vivo interaction of moxidectin with BCRP/ABCG2. Chem. Biol. Interact. 2009;180:106–112. doi: 10.1016/j.cbi.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Pérez R., Cabezas I., García M., Rubilar L., Sutra J.F., Galtier P., Alvinerie M. Comparison of the pharmacokinetics of moxidectin (Equest) and ivermectin (Eqvalan) in horses. J. Vet. Pharmacol. Ther. 1999;22:174–180. doi: 10.1046/j.1365-2885.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- Pion S.D., Filipe J.A., Kamgno J., Gardon J., Basanez M.G., Boussinesq M. Microfilarial distribution of Loa loa in the human host: population dynamics and epidemiological implications. Parasitology. 2006;133:101–109. doi: 10.1017/S0031182006000035. [DOI] [PubMed] [Google Scholar]
- Pohl P.C., Klafke G.M., Carvalho D.D., Martins J.R., Daffre S., da Silva Vaz I., Jr., Masuda A. ABC transporter efflux pumps: a defense mechanism against ivermectin in Rhipicephalus (Boophilus) microplus. Int. J. Parasitol. 2011;41:1323–1333. doi: 10.1016/j.ijpara.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Pomroy W.E. Anthelmintic resistance in New Zealand: a perspective on recent findings and options for the future. N.Z. Vet. J. 2006;54:265–270. doi: 10.1080/00480169.2006.36709. [DOI] [PubMed] [Google Scholar]
- Pouliot J.F., L’Heureux F., Liu Z., Prichard R.K., Georges E. Reversal of P-glycoprotein-associated multidrug resistance by ivermectin. Biochem. Pharmacol. 1997;53:17–25. doi: 10.1016/s0006-2952(96)00656-9. [DOI] [PubMed] [Google Scholar]
- Prichard R. Genetic variability following selection of Haemonchus contortus with anthelmintics. Trends Parasitol. 2001;17:445–453. doi: 10.1016/s1471-4922(01)01983-3. [DOI] [PubMed] [Google Scholar]
- Prichard R. Ivermectin resistance and overview of the consortium for anthelmintic resistance. Expert Opin. Drug Discov. 2007;2:41–52. doi: 10.1517/17460441.2.S1.S41. [DOI] [PubMed] [Google Scholar]
- Prichard R.K., Roulet A. ABC transporters and beta-tubulin in macrocyclic lactone resistance: prospects for marker development. Parasitology. 2007;134:1123–1132. doi: 10.1017/S0031182007000091. [DOI] [PubMed] [Google Scholar]
- Pritchard J. Treating ivermectin toxicity in cats. Vet. Rec. 2011;166:766. doi: 10.1136/vr.c3056. [DOI] [PubMed] [Google Scholar]
- Ranjan S., Wang G.T., Hirschlein C., Simkins K.L. Selection for resistance to macrocyclic lactones by Haemonchus contortus in sheep. Vet. Parasitol. 2002;103:109–117. doi: 10.1016/s0304-4017(01)00551-9. [DOI] [PubMed] [Google Scholar]
- Rao V.T., Siddiqui S.Z., Prichard R.K., Forrester S.G. A dopamine-gated ion channel (HcGGR3∗) from Haemonchus contortus is expressed in the cervical papillae and is associated with macrocyclic lactone resistance. Mol. Biochem. Parasitol. 2009;166:54–61. doi: 10.1016/j.molbiopara.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Rao V.T., Accardi M.V., Siddiqui S.Z., Beech R.N., Prichard R.K., Forrester S.G. Characterization of a novel tyramine-gated chloride channel from Haemonchus contortus. Mol. Biochem. Parasitol. 2010;173:64–68. doi: 10.1016/j.molbiopara.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Real R., Egido E., Perez M., Gonzalez-Lobato L., Barrera B., Prieto J.G., Alvarez A.I., Merino G. Involvement of breast cancer resistance protein (BCRP/ABCG2) in the secretion of danofloxacin into milk: interaction with ivermectin. J. Vet. Pharmacol. Ther. 2011;34:313–321. doi: 10.1111/j.1365-2885.2010.01241.x. [DOI] [PubMed] [Google Scholar]
- Rendell D.K., Rentsch T.E., Smith J.M., Chandler D.S., Callinan A.P. Evidence that moxidectin is a greater risk factor than ivermectin in the development of resistance to macrocyclic lactones by Ostertagia spp. in sheep in south eastern Australia. N.Z. Vet. J. 2006;54:313–317. doi: 10.1080/00480169.2006.36716. [DOI] [PubMed] [Google Scholar]
- Ringstad N., Abe N., Horvitz H.R. Ligand-gated chloride channels are receptors for biogenic amines in C. elegans. Science. 2009;325:96–100. doi: 10.1126/science.1169243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roulet A., Puel O., Gesta S., Lepage J.F., Drag M., Soll M., Alvinerie M., Pineau T. MDR1-deficient genotype in Collie dogs hypersensitive to the P-glycoprotein substrate ivermectin. Eur. J. Pharmacol. 2003;460:85–91. doi: 10.1016/s0014-2999(02)02955-2. [DOI] [PubMed] [Google Scholar]
- Saeki T., Ueda K., Tanigawara Y., Hori R., Komano T. Human P-glycoprotein transports cyclosporin A and FK506. J. Biol. Chem. 1993;268:6077–6080. [PubMed] [Google Scholar]
- Sallovitz J.M., Lifschitz A., Imperiale F., Virkel G., Lanusse C. A detailed assessment of the pattern of moxidectin tissue distribution after pour-on treatment in calves. J. Vet. Pharmacol. Ther. 2003;26:397–404. doi: 10.1046/j.0140-7783.2003.00537.x. [DOI] [PubMed] [Google Scholar]
- Sargison N.D. Pharmaceutical control of endoparasitic helminth infections in sheep. Vet. Clin. N. Am. Food Anim. Pract. 2011;27:139–156. doi: 10.1016/j.cvfa.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Homolya L., Szakacs G., Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol. Rev. 2006;86:1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- Sartor L.L., Bentjen S.A., Trepanier L., Mealey K.L. Loperamide toxicity in a collie with the MDR1 mutation associated with ivermectin sensitivity. J. Vet. Intern. Med. 2004;18:117–118. doi: 10.1892/0891-6640(2004)18<117:ltiacw>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Schinkel A.H., Smit J.J., van Tellingen O., Beijnen J.H., Wagenaar E., van Deemter L., Mol C.A., van der Valk M.A., Robanus-Maandag E.C., te Riele H.P. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell. 1994;77:491–502. doi: 10.1016/0092-8674(94)90212-7. [DOI] [PubMed] [Google Scholar]
- See A.M., McGill S.E., Raisis A.L., Swindells K.L. Toxicity in three dogs from accidental oral administration of a topical endectocide containing moxidectin and imidacloprid. Aust. Vet. J. 2009;87:334–337. doi: 10.1111/j.1751-0813.2009.00448.x. [DOI] [PubMed] [Google Scholar]
- Seelig A., Gerebtzoff G. Enhancement of drug absorption by noncharged detergents through membrane and P-glycoprotein binding. Expert Opin. Drug Metab. Toxicol. 2006;2:733–752. doi: 10.1517/17425255.2.5.733. [DOI] [PubMed] [Google Scholar]
- Sharma R., Singal A. Topical permethrin and oral ivermectin in the management of scabies: a prospective, randomized, double blind, controlled study. Indian J. Dermatol. Venereol. Leprol. 2011;77:581–586. doi: 10.4103/0378-6323.84063. [DOI] [PubMed] [Google Scholar]
- Sherman J.G., Paul A.J., Firkins L.D. Evaluation of the safety of spinosad and milbemycin 5-oxime orally administered to Collies with the MDR1 gene mutation. Am. J. Vet. Res. 2010;71:115–119. doi: 10.2460/ajvr.71.1.115. [DOI] [PubMed] [Google Scholar]
- Shoop W.L. Ivermectin resistance. Parasitol. Today. 1993;9:154–159. doi: 10.1016/0169-4758(93)90136-4. [DOI] [PubMed] [Google Scholar]
- Shoop W.L., Haines H.W., Michael B.F., Eary C.H. Mutual resistance to avermectins and milbemycins: oral activity of ivermectin and moxidectin against ivermectin-resistant and susceptible nematodes. Vet. Rec. 1993;133:445–447. doi: 10.1136/vr.133.18.445. [DOI] [PubMed] [Google Scholar]
- Shoop W.L., Mrozik H., Fisher M.H. Structure and activity of avermectins and milbemycins in animal health. Vet. Parasitol. 1995;59:139–156. doi: 10.1016/0304-4017(94)00743-v. [DOI] [PubMed] [Google Scholar]
- Shoop W.L., Egerton J.R., Eary C.H., Haines H.W., Michael B.F., Mrozik H., Eskola P., Fisher M.H., Slayton L., Ostlind D.A., Skelly B.J., Fulton R.K., Barth D., Costa S., Gregory L.M., Campbell W.C., Seward R.L., Turner M.J. Eprinomectin: a novel avermectin for use as a topical endectocide for cattle. Int. J. Parasitol. 1996;26:1237–1242. doi: 10.1016/s0020-7519(96)00123-3. [DOI] [PubMed] [Google Scholar]
- Snyder D.E., Wiseman S., Cruthers L.R., Slone R.L. Ivermectin and milbemycin oxime in experimental adult heartworm (Dirofilaria immitis) infection of dogs. J. Vet. Intern. Med. 2010;25:61–64. doi: 10.1111/j.1939-1676.2010.0657.x. [DOI] [PubMed] [Google Scholar]
- Stapley, E.O., Woodruff, H.B., 1982. avermectin, antiparasitic lactones produced by Streptomyces avermitilis isolated from a soil in Japan. In: Umezawa, H., Deamin, A.L., Hata, T., Hutchinson, C.R. (Eds.), Trends in Antibiotic Research. Japan Antibiotics Research Association, Tokyo, pp. 154–170.
- Steel J.W., Wardhaugh K.G. Ecological impact of macrocyclic lactones on fauna dung. In: Vercruysse J., Rew R.S., editors. Macrocyclic Lactones and Antiparasitic Therapy. CAB International; New York: 2002. pp. 141–162. [Google Scholar]
- Stepek G., Buttle D.J., Duce I.R., Behnke J.M. Human gastrointestinal nematode infections: are new control methods required? Int. J. Exp. Pathol. 2006;87:325–341. doi: 10.1111/j.1365-2613.2006.00495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez V.H., Lifschitz A.L., Sallovitz J.M., Lanusse C.E. Effects of faecal residues of moxidectin and doramectin on the activity of arthropods in cattle dung. Ecotoxicol. Environ. Saf. 2009;72:1551–1558. doi: 10.1016/j.ecoenv.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Sutherland I.A., Leathwick D.M. Anthelmintic resistance in nematode parasites of cattle: a global issue? Trends Parasitol. 2011;27:176–181. doi: 10.1016/j.pt.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Takiguchi Y., Ono M., Muramatsu S., Ide J., Mishima H., Terao M. Milbemycins, a new family of macrolide antibiotics. Fermentation, isolation and physico-chemical properties of milbemycins D, E, F, G, and H. J. Antibiot. (Tokyo) 1983;36:502–508. doi: 10.7164/antibiotics.36.502. [DOI] [PubMed] [Google Scholar]
- Traversa D., von Samson-Himmelstjerna G., Demeler J., Milillo P., Schurmann S., Barnes H., Otranto D., Perrucci S., di Regalbono A.F., Beraldo P., Boeckh A., Cobb R. Anthelmintic resistance in cyathostomin populations from horse yards in Italy, United Kingdom and Germany. Parasit. Vectors. 2009;2(Suppl. 2):S2. doi: 10.1186/1756-3305-2-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutain P.L., Campan M., Galtier P., Alvinerie M. Kinetic and insecticidal properties of ivermectin residues in the milk of dairy cows. J. Vet. Pharmacol. Ther. 1988;11:288–291. doi: 10.1111/j.1365-2885.1988.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Tribble N.D., Burka J.F., Kibenge F.S. Evidence for changes in the transcription levels of two putative P-glycoprotein genes in sea lice (Lepeophtheirus salmonis) in response to emamectin benzoate exposure. Mol. Biochem. Parasitol. 2007;153:59–65. doi: 10.1016/j.molbiopara.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Vellarkad N., Viswanadhan A.K., Ghose A.K., Revankar G.R., Robins R.K. Atomic physicochemical parameters for three dimensional structure directed quantitative structure–activity relationships. Additional parameters for hydrophobic and dispersive interactions and their application for an automated superposition of certain naturally occurring nucleoside antibiotics. J. Chem. Inf. Comp. Sci. 1989;29:163–172. [Google Scholar]
- Vercruysse J., Rew R.S. CABI Publishing; Wallingford, UK: 2002. Macrocyclic Lactones in Antiparasitic Therapy. [Google Scholar]
- Wall R., Strong L. Environmental consequences of treating cattle with the antiparasitic drug ivermectin. Nature. 1987;327:418–421. doi: 10.1038/327418a0. [DOI] [PubMed] [Google Scholar]
- Wardhaugh K.G., Mahon R.J., Axelsen A., Rowland M.W., Wanjura W. Effects of ivermectin residues in sheep dung on the development and survival of the bushfly, Musca vetustissima Walker and a scarabaeine dung beetle, Euoniticellus fulvus Goeze. Vet. Parasitol. 1993;48:139–157. doi: 10.1016/0304-4017(93)90151-c. [DOI] [PubMed] [Google Scholar]
- WHO, 1995. Onchocerciasis and its control: report of a WHO Expert Committee on onchocerciasis control. In: World Health Organization Technical Report Series No. 852. WHO, Geneva. [PubMed]
- WHO, 1996. Toxicological evaluations: moxidectin. Evaluation of certain drug residues in food. In: WHO Expert Committee on Food Additives. World Health Organization, Geneva.
- Williamson S.M., Wolstenholme A.J. P-glycoproteins of Haemonchus contortus: development of real-time PCR assays for gene expression studies. J. Helminthol. 2011;1:1–7. doi: 10.1017/S0022149X11000216. [DOI] [PubMed] [Google Scholar]
- Williamson S.M., Storey B., Howell S., Harper K.M., Kaplan R.M., Wolstenholme A.J. Candidate anthelmintic resistance-associated gene expression and sequence polymorphisms in a triple-resistant field isolate of Haemonchus contortus. Mol. Biochem. Parasitol. 2012;180:99–105. doi: 10.1016/j.molbiopara.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Wolstenholme A.J., Fairweather I., Prichard R., von Samson-Himmelstjerna G., Sangster N.C. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Xu M., Molento M., Blackhall W., Ribeiro P., Beech R., Prichard R. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol. Biochem. Parasitol. 1998;91:327–335. doi: 10.1016/s0166-6851(97)00215-6. [DOI] [PubMed] [Google Scholar]
- Yas-Natan E., Shamir M., Kleinbart S., Aroch I. Doramectin toxicity in a collie. Vet. Rec. 2003;153:718–720. [PubMed] [Google Scholar]
- Zeng Z., Andrew N.W., Arison B.H., Luffer-Atlas D., Wang R.W. Identification of cytochrome P4503A4 as the major enzyme responsible for the metabolism of ivermectin by human liver microsomes. Xenobiotica. 1998;28:313–321. doi: 10.1080/004982598239597. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Hirschberg B., Yuan J., Wang A.P., Hunt D.C., Ludmerer S.W., Schmatz D.M., Cully D.F. Identification of two novel Drosophila melanogaster histamine-gated chloride channel subunits expressed in the eye. J. Biol. Chem. 2002;277:2000–2005. doi: 10.1074/jbc.M107635200. [DOI] [PubMed] [Google Scholar]
- Zulalian J., Stout S.J., daCunha A.R., Garces T., Miller P. Absorption, tissue distribution, metabolism, and excretion of moxidetin in cattle. J. Agric. Food Chem. 1994;42:381–387. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of chemical structures of avermectins (A) and milbemycins (B).
Comparative efficacy of avermectins (usually ivermectin) and moxidectin against field isolates of nematode parasites in different hosts, in which some ML resistance was suspected.


