Graphical abstract
Highlights
► Study on efficacy of drugs and natural course to eradicate Dientamoeba fragilis. ► Paromomycin treatment eradicated Dientamoeba fragilis in 60/61 patients (98%). ► D. fragilis infections were spontaneously cleared in 9/22 untreated patients (41%).
Keywords: Dientamoeba fragilis, Metronidazole, Parasite, Diarrhea
Abstract
Dientamoeba fragilis is a debated protozoan parasite that is often detected in stools of patients with chronic gastro-intestinal complaints. A retrospective follow-up study of a large cohort of patients was performed to better understand the natural course of the infection and possible treatment options. D. fragilis was spontaneously cleared in 41% of untreated cases. With an eradication rate of 98%, treatment with paromomycin appeared more effective than treatment with clioquinol (83%) or metronidazole (57%).
1. Introduction
Dientamoeba (D.) fragilis is one of the most common protozoan organisms detected in human stools, with reported frequencies up to 30% (Johnson et al., 2004; Stensvold et al., 2007; Schuster and Jackson, 2009; Barratt et al., 2011). D. fragilis was originally described as an a-pathogenic protozoan organism, but over the years many reports have provided indications for the pathogenic potential of D. fragilis (Johnson et al., 2004; Barratt et al., 2011). Abdominal pain and diarrhea are commonly reported in patients infected with D. fragilis in the absence of other bowel pathogens (Barratt et al., 2011). Furthermore, eradication of D. fragilis by treatment is frequently associated with clinical improvement of symptoms (Girginkardesler et al., 2003; Bosman et al., 2004; Johnson et al., 2004; Vandenberg et al., 2007). For these reasons, persisting D. fragilis infestations are often treated with the intention to eradicate this protozoon from the gut.
Although D. fragilis infections are frequently detected and treated, there is no consensus as to the best treatment for dientamoebiasis (Johnson et al., 2004; Barratt et al., 2011). Until now only 11 studies on the efficacy of drugs to eradicate D. fragilis have been published (see Table 1 for an overview). These 11 studies investigated the efficacy of 12 distinct drugs, of which eight drugs were investigated by a single study only. So far, only a single randomized study has been performed in which the treatment efficacy of metronidazole was compared to that of ornidazole (Kurt et al., 2008) and all other studies had a retrospective study design. These studies reported eradication efficacies that were either low (50% for erythromycin (Preiss et al., 1991), 75% for doxycycline (Preiss et al., 1991), and 67–89% for metronidazole (Vandenberg et al., 2006; Stark et al., 2010), or the result of very small retrospective cohort studies involving maximal 27, 12, 9 and 15 patients for clioquinol (81–100% (Bosman et al., 2004; Stark et al., 2010)), iodoquinol (83–100% (Spencer et al., 1982; Millet et al., 1983), diphetarsone (100% (Keystone et al., 1983)) and paromomycin (80–100% (Vandenberg et al., 2007; Stark et al., 2010)), respectively. Two compounds, secnidazol and ornidazol, were reported by single studies to have a high eradication efficacy for D. fragilis (93% and 97%, respectively) in 56 and 34 patients (Girginkardesler et al., 2003; Kurt et al., 2008). Finally, it is unclear to what extent D. fragilis infections are spontaneously cleared, which may further hamper the interpretation of data regarding the eradication rates associated with some drugs. Therefore, the current information on the efficacy of drugs for eradication of D. fragilis is surprisingly small and hampers the formulation of unambiguous guidelines for treatment of dientamoebiasis, which is for instance demonstrated by the listing of four recommended drugs for treatment of dientamoebiasis in the latest Medical Letter (2010).
Table 1.
Reported parasitological cure rate for Dientamoeba fragilis.
| Drug | Efficacy (%) | Patients included (n) | Ref. |
|---|---|---|---|
| Clioquinol | 100 | 3 | Bosman et al. (2004) |
| 81 | 27 | Stark et al. (2010) | |
| Diphetarsone | 100 | 9 | Keystone et al. (1983) |
| Doxycycline | 75 | 4 | Preiss et al. (1991) |
| Erythromycin | 50 | 6 | Preiss et al. (1991) |
| Hydroxychinoline | 20 | 5 | Preiss et al. (1991) |
| Iodoquinol | 83 | 12 | Millet et al. (1983) |
| 100 | 3 | Spencer et al. (1982) | |
| Metronidazole | 84 | 41 | Banik et al. (2011) |
| 89 | 28 | Stark et al. (2010) | |
| 70 | 56 | Kurt et al. (2008) | |
| 67 | 12 | Vandenberg et al. (2006) | |
| 69 | 10 | Bosman et al. (2004) | |
| 70 | 91 | Preiss et al. (1991) | |
| Ornidazole | 93 | 56 | Kurt et al. (2008) |
| Oxytetracycline | 90 | 9 | Preiss et al. (1991) |
| Paromomycin | 100 | 5 | Stark et al. (2010) |
| 80 | 15 | Vandenberg et al. (2007) | |
| 100 | 4 | Vandenberg et al. (2006) | |
| Secnidazole | 97 | 34 | Girginkardesler et al. (2003) |
In order to better understand the natural course of the disease and the efficacy of potential treatment options, we analyzed the results of a retrospective follow-up study of a relatively large cohort of 93 patients with a documented D. fragilis infection.
2. Materials and methods
This retrospective cohort study was performed at the Institute for Tropical Diseases of the Harbor Hospital in Rotterdam, The Netherlands, which is a national referral center for adult patients who recently visited tropical countries. Patients were included when D. fragilis was demonstrated in a stool sample analyzed in the period 2004–2010 by Triple Feces Test (TFT), an all-round and sensitive method for microscopic detection of ‘helminth ova and protozoa, among which trophozoites of D. fragilis (van Gool et al., 2003). In addition, a follow-up stool sample had to be available for evaluation of an empirical treatment or for evaluation of the natural course of the infestation in case of untreated patients. The majority of the included patients had gastro-intestinal symptoms and all were diagnosed with D. fragilis after exclusion of viral, bacterial and parasitological causes of gastro-intestinal illness by thorough investigations including fecal cultures for pathogenic bacteria (Salmonella, Shigella and Campylobacter) and PCR analysis for Rotavirus, Norovirus and Adenoviruses. In the majority of the patients the investigations also included upper and lower GI endoscopy, histological examination of duodenal and colonic biopsy specimens, anti-trans Tissue Glutamate antibodies determination as well as hydrogen breath tests for exclusion of lactose intolerance. Empirical treatment with either paromomycin (3 daily doses of 500 mg for 7–10 days), clioquinol (3 daily doses of 250 mg for 7 days) or metronidazole (3 daily doses of 500 mg for 7–10 days) was prescribed off-label on doctor’s declaration and after informed consent of the patient. The patient records of all included patients were retrospectively examined for demographic data, therapy and clinical follow-up data. Patients with an age of less than 18 years and those patients who were treated for another protozoal or helminth infection were excluded. The time interval between two TFT analyses was truncated at 180 days. Parasitological eradication was defined as complete clearance of parasites in the subsequent stool examination.
3. Results and discussion
In the study period 5491 outpatients were examined for gastro-intestinal parasites by TFT and D. fragilis was demonstrated in stools of 451 patients (8%) of which 93 patients fulfilled the inclusion criteria. General demographic and clinical characteristics of the included patients with D. fragilis at presentation are shown in Table 2. Diarrhea (42%), abdominal pain (37%) and malaise (51%) were frequently observed. The laboratory parameters C-reactive protein, leukocyte and eosinophil counts were not elevated (Table 2). Patients with D. fragilis frequently had co-infestations with Blastocystis sp. (74%) and a-pathogenic protozoa (<10% for individual species) (Table 2).
Table 2.
Characteristics of patients at presentation with a Dientamoeba fragilis infestation.
| Demographic data | n | % |
|---|---|---|
| Patients | 93 | |
| Age (median, IQR) | 41 (29, 49) | |
| Male gender | 27 | 29 |
| Female gender | 66 | 71 |
| Visit to the (sub) tropics (n = 78)a | 72 | 92 |
| Clinical symptoms | n | % |
| Duration of complaints (n = 70)a | ||
| 0–1 month | 7 | 10 |
| 1–3 months | 6 | 9 |
| 3–6 months | 11 | 16 |
| More than 6 months | 46 | 66 |
| Type of complaints (n = 83)a | Present (n) | Present (%) |
| Abdominal pain | 31 | 37 |
| Diarrhea | 35 | 42 |
| Malaise | 42 | 51 |
| Weight loss | 17 | 20 |
| Laboratory findings | Median (IQR) | N |
| Blood leukocyte count, ×109/L | 6.5 (5.7, 7.5) | 77 |
| Blood eosinophil count, ×109/L | 0.1 (0.1, 0.2) | 42 |
| C-reactive protein, mg/L | 2 (1, 5) | 75 |
| Erythrocyte sedimentation rate, mm/h | 7 (3, 11) | 72 |
| Stool examination | n | % |
| Blastocystis hominis | 69 | 74 |
| Endolimax nana | 8 | 9 |
| Entamoeba coli | 8 | 9 |
| Entamoeba hartmanni | 1 | 1 |
| Iodamoeba butschlii | 2 | 2 |
Specified data on travel history, duration of complaints and type of complaints was available in the medical records for 78, 70 and 83 patients, respectively.
The 93 included patients received a total of 102 treatments. Three patients received two treatments (first no treatment followed by paromomycin treatment) and another three patients received three subsequent treatments (one received no treatment first, then metronidazole treatment and finally paromomycin treatment, another received paromomycin treatment first, followed by two treatments with metronidazole, and the third patient received paromomycin treatment first, then no treatment and then paromomycin treatment combined with doxycycline). Therefore, results could be examined of 102 treatments with either paromomycin (n = 61), clioquinol (n = 12), metronidazole (n = 7) or no treatment (n = 22). This ‘wait and see’ policy was considered to represent the natural course of D. fragilis infestation. In 30 treatment cases (paromomycin n = 27, clioquinol n = 2, metronidazole n = 1) treatment was combined with doxycycline. Mean time between analysis of subsequent stool samples was 62–76 days and did not differ significantly between treatment regimens. Because treatment was not started directly after laboratory diagnosis of the initial stool sample but after the subsequent outpatient clinic visit, the second stool sample was collected shortly after completion of treatment, which minimizes reinfection as a cause of persisting D. fragilis infections.
D. fragilis was spontaneously cleared in 41% of the untreated cases. Treatment with paromomycin, clioquinol and metronidazole was associated with eradication rates of 98%, 83% and 57%, respectively (Fig. 1). Although patient numbers in each treatment group are uneven, statistical analysis showed that paromomycin treatment had superior eradication rates compared to both metronidazole and no treatment (P < 0.005) and a trend towards superiority over clioquinol (P = 0.06) (Fig. 1). Treatment with clioquinol was significantly more effective when compared to “wait and see” (P < 0.05). Metronidazole treatment was not significantly more effective than a ‘wait and see’ policy (P = 0.667). Statistical analysis showed that the eradication rates of paromomycin, clioquinol or metronidazole were not affected by a Blastocystis sp. co-infestation or a combined treatment including doxycycline. Furthermore, the time interval between the first and subsequent follow-up stool examination did not significantly differ between the patient groups.
Fig. 1.
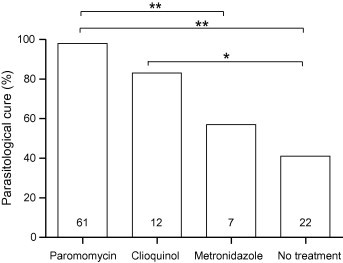
Eradication efficacy of treatment for Dientamoeba fragilis. Parasitological cure efficacy was defined as clearance of parasites in the subsequent stool examination. Numbers in the bars represent number of included treatments. Pair wise statistical significant differences are indicated, with ∗ and ∗∗ representing P-values of <0.03 and <0.003, respectively.
The retrospective design of this study may hamper the generalizability of the findings of this study, because (1) the various treatment modes were not assigned randomly, (2) an all-round microscopic detection method was used instead of a more sensitive D. fragilis PCR method (Bruijnesteijn van Coppenraet et al., 2009), and (3) a placebo-control was lacking. Since in this study gastro-intestinal complaints were not systematically recorded nor evaluated, this study focused on eradication of D. fragilis as a hard end-point in relation to various treatment modes but not on resolution of gastro-intestinal complaints or evaluation of the pathogenic potential of D. fragilis. However, even within this framework of limitations, the following clinical relevant observations could be made. First, the natural course of D. fragilis infestation in untreated patients is characterized by a spontaneous clearance in 41% of cases. Second, this study suggests the superior efficacy of paromomycin for eradication of D. fragilis in adults (98%) as compared to treatment with metronidazole and possibly also to clioquinol. In this study the eradication efficacy of metronidazole was low (57%), but the relatively small number of included patients treated with metronidazole (n = 7) prohibits firm conclusions on the eradication efficacy of this drug. Although the eradication efficacies might be lower when a more sensitive PCR method had been used, the observed relative differences in drug efficacy are not dependent on the sensitivity of the detection method.
The high efficacy of paromomycin was suggested by previous reports for small numbers of patients (maximal 15) comprising predominantly children (Vandenberg et al., 2006, 2007; Stark et al., 2010). This study now showed that paromomycin is a highly effective drug for treatment of D. fragilis in a large group of adult patients, since 60/61 (98%) patients cleared D. fragilis after paromomycin treatment. Our observations seem to justify validation of the treatment efficacy of paromomycin for eradication of D. fragilis in properly designed placebo-controlled trials in adults and may be of help in evaluating the pathogenic potential of D. fragilis in the etiology of persistent gastro-intestinal complaints.
Acknowledgment
The Port of Rotterdam is acknowledged for their financial support for this study.
Appendix A. Supplementary data
References
- Anonymous Drugs for Parasitic Infections. The Medical Letter Inc. 2010 [Google Scholar]
- Banik G.R., Barratt J.L., Marriott D., Harkness J., Ellis J.T., Stark D. A case-controlled study of Dientamoeba fragilis infections in children. Parasitology. 2011:1–5. doi: 10.1017/S0031182011000448. [DOI] [PubMed] [Google Scholar]
- Barratt J.L., Harkness J., Marriott D., Ellis J.T., Stark D. A review of Dientamoeba fragilis carriage in humans: several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes. 2011;2:3–12. doi: 10.4161/gmic.2.1.14755. [DOI] [PubMed] [Google Scholar]
- Bosman D.K., Benninga M.A., van de Berg P., Kooijman G.C., van Gool T. Dientamoeba fragilis: possibly an important cause of persistent abdominal pain in children. Ned. Tijdschr. Geneeskd. 2004;148:575–579. [PubMed] [Google Scholar]
- Bruijnesteijn van Coppenraet L.E., Wallinga J.A., Ruijs G.J., Bruins M.J., Verweij J.J. Parasitological diagnosis combining an internally controlled real-time PCR assay for the detection of four protozoa in stool samples with a testing algorithm for microscopy. Clin. Microbiol. Infect. 2009;15:869–874. doi: 10.1111/j.1469-0691.2009.02894.x. [DOI] [PubMed] [Google Scholar]
- Girginkardesler N., Coskun S., Cuneyt Balcioglu I., Ertan P., Ok U.Z. Dientamoeba fragilis, a neglected cause of diarrhea, successfully treated with secnidazole. Clin. Microbiol. Infect. 2003;9:110–113. doi: 10.1046/j.1469-0691.2003.00504.x. [DOI] [PubMed] [Google Scholar]
- Johnson E.H., Windsor J.J., Clark C.G. Emerging from obscurity: biological, clinical, and diagnostic aspects of Dientamoeba fragilis. Clin. Microbiol. Rev. 2004;17:553–570. doi: 10.1128/CMR.17.3.553-570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keystone J.S., Proctor E., Glenn C., McIntyre L. Safety and efficacy of diphetarsone in the treatment of amoebiasis, non-pathogenic amoebiasis and trichuriasis. Trans. R. Soc. Trop. Med. Hyg. 1983;77:84–86. doi: 10.1016/0035-9203(83)90022-6. [DOI] [PubMed] [Google Scholar]
- Kurt O., Girginkardesler N., Balcioglu I.C., Ozbilgin A., Ok U.Z. A comparison of metronidazole and single-dose ornidazole for the treatment of dientamoebiasis. Clin. Microbiol. Infect. 2008;14:601–604. doi: 10.1111/j.1469-0691.2008.02002.x. [DOI] [PubMed] [Google Scholar]
- Millet V., Spencer M.J., Chapin M., Stewart M., Yatabe J.A., Brewer T., Garcia L.S. Dientamoeba fragilis, a protozoan parasite in adult members of a semicommunal group. Dig. Dis. Sci. 1983;28:335–339. doi: 10.1007/BF01324950. [DOI] [PubMed] [Google Scholar]
- Preiss U., Ockert G., Broemme S., Otto A. On the clinical importance of Dientamoeba fragilis infections in childhood. J. Hyg. Epidemiol. Microbiol. Immunol. 1991;35:27–34. [PubMed] [Google Scholar]
- Schuster H., Jackson R.S. Prevalence of Dientamoeba fragilis among patients consulting complementary medicine practitioners in the British Isles. J. Clin. Pathol. 2009;62:182–184. doi: 10.1136/jcp.2008.059659. [DOI] [PubMed] [Google Scholar]
- Spencer M.J., Chapin M.R., Garcia L.S. Dientamoeba fragilis: a gastrointestinal protozoan infection in adults. Am. J. Gastroenterol. 1982;77:565–569. [PubMed] [Google Scholar]
- Stark D., Barratt J., Roberts T., Marriott D., Harkness J., Ellis J. A review of the clinical presentation of dientamoebiasis. Am. J. Trop. Med. Hyg. 2010;82:614–619. doi: 10.4269/ajtmh.2010.09-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensvold C.R., Arendrup M.C., Molbak K., Nielsen H.V. The prevalence of Dientamoeba fragilis in patients with suspected enteroparasitic disease in a metropolitan area in Denmark. Clin. Microbiol. Infect. 2007;13:839–842. doi: 10.1111/j.1469-0691.2007.01760.x. [DOI] [PubMed] [Google Scholar]
- van Gool T., Weijts R., Lommerse E., Mank T.G. Triple Faeces Test: an effective tool for detection of intestinal parasites in routine clinical practice. Eur. J. Clin. Microbiol. Infect. Dis. 2003;22:284–290. doi: 10.1007/s10096-003-0919-1. [DOI] [PubMed] [Google Scholar]
- Vandenberg O., Peek R., Souayah H., Dediste A., Buset M., Scheen R., Retore P., Zissis G., van Gool T. Clinical and microbiological features of dientamoebiasis in patients suspected of suffering from a parasitic gastrointestinal illness: a comparison of Dientamoeba fragilis and Giardia lamblia infections. Int. J. Infect. Dis. 2006;10:255–261. doi: 10.1016/j.ijid.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Vandenberg O., Souayah H., Mouchet F., Dediste A., van Gool T. Treatment of Dientamoeba fragilis infection with paromomycin. Pediatr. Infect. Dis. J. 2007;26:88–90. doi: 10.1097/01.inf.0000247139.89191.91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


