Graphical abstract
Highlights
► Review current knowledge of neuropeptide signalling in Nematoda and Platyhelminthes. ► Discuss pros/cons of peptidergic signalling system as source of anthelmintic targets. ► Identify proteins yielding deleterious phenotypes in helminth reverse genetic screens.
Keywords: Anthelmintic, Nervous system, Neuromuscular, Receptor, Signalling
Abstract
The rationale for identifying drug targets within helminth neuromuscular signalling systems is based on the premise that adequate nerve and muscle function is essential for many of the key behavioural determinants of helminth parasitism, including sensory perception/host location, invasion, locomotion/orientation, attachment, feeding and reproduction. This premise is validated by the tendency of current anthelmintics to act on classical neurotransmitter-gated ion channels present on helminth nerve and/or muscle, yielding therapeutic endpoints associated with paralysis and/or death. Supplementary to classical neurotransmitters, helminth nervous systems are peptide-rich and encompass associated biosynthetic and signal transduction components – putative drug targets that remain to be exploited by anthelmintic chemotherapy. At this time, no neuropeptide system-targeting lead compounds have been reported, and given that our basic knowledge of neuropeptide biology in parasitic helminths remains inadequate, the short-term prospects for such drugs remain poor. Here, we review current knowledge of neuropeptide signalling in Nematoda and Platyhelminthes, and highlight a suite of 19 protein families that yield deleterious phenotypes in helminth reverse genetics screens. We suggest that orthologues of some of these peptidergic signalling components represent appealing therapeutic targets in parasitic helminths.
1. Introduction
Parasitic helminths (worms classified in phyla Nematoda and Platyhelminthes) remain an insidious threat to human health, social development and economic progress worldwide. Infections by parasitic nematodes and flatworms have direct effects on human health and well being throughout the developing world and are responsible for a large proportion of the so-called ‘Neglected Tropical Diseases’ (Hotez et al., 2008). Additionally, helminths maintain an economic grip on the worldwide agricultural economy, a situation compounded by the spread of anthelmintic-resistance. For example, nematodes displaying multidrug-resistance to all of the well-established therapeutic compounds threaten the sustainability of livestock farming in some areas of South America, South Africa, Malaysia, New Zealand and the USA (Kaplan, 2004; Sutherland and Leathwick, 2011). Ruminant farming worldwide is threatened further by Fasciola spp. liver flukes, which have displayed incidences of resistance to triclabendazole, the single most effective fasciolicide on the market (Keiser and Utzinger, 2007). The latter issue illustrates a salient point in anthelmintic therapy – we have only a limited arsenal of drugs with which to combat pathogenic helminths and, particularly in human infections, many of the drugs in use are sub-optimal and suffer from our poor understanding of their pharmacology (Geary et al., 2010). Clearly, new anthelmintic treatment regimens will be essential in the short to medium term.
The development of resistance to chemotherapeutics is inevitable. However, while novel non-chemical control alternatives have been suggested (Hoste and Torres-Acosta, 2011), and given the limited availability of helminth vaccines (Hotez et al., 2010), the intelligent and sustainable use of available anthelmintics remains our best short term option for helminth control (Kaplan, 2004). While a few new drug classes active against nematodes (cyclo-octadepsipeptide/emodepside, Harder et al. (2003); paraherquamide, Lee et al. (2002); aminoacetonitrile derivatives [AADs], Ducray et al. (2008)) are showing promise and progress has been reported for some new trematocidal drugs (Sayed et al., 2008; Keiser et al., 2010), to rely on these novel compounds/leads to the detriment of continued anthelmintic discovery would be to ignore the lessons of the past.
Modern drug discovery paradigms rely largely on mechanism-based screening of compounds against heterologously-expressed targets, which have been selected on the basis of biology, distinct host/pathogen pharmacology and ‘druggability’ (i.e. that the protein family in question is already targeted by existing drugs) (Geary et al., 1999). The quality of evidence required to support this target selection has increased as a factor of the economic and socio-political pressures experienced by the pharmaceutical industry (Knowles and Gromo, 2002; Geary et al., 2004), which attempts to discover new drugs for which profitability is a primary consideration. As such, the rationale for selecting a target must be strongly supported by evidence from basic research.
Our own interests in parasite neuropeptides evolved through the pioneering work of David W. Halton and Chris Shaw in the mid 1980s (see Halton et al., 1992, 1994) and progressed through neuropeptide localisation (Halton, 2004), characterisation (Maule et al., 1991, 1993; Marks et al., 1999) and physiology (Maule et al., 1995, 1996a,b, 2002) phases, prior to a slowing of impetus as classic approaches went out of vogue and genomic/post-genomic technologies captured attention. Recently, parasite neuropeptide research has been reinvigorated by two developments: the accumulation of transcriptomic/genomic resources for parasites (McVeigh et al., 2005a, 2008, 2009; see reviews by McVeigh et al., 2006; Marks and Maule, 2010; Mousley et al., 2010); and, the potential of RNA interference (RNAi) to facilitate gene function studies in parasites (Hussein et al., 2002; Urwin et al., 2002; Boyle et al., 2003; Skelly et al., 2003; Kimber et al., 2007; McGonigle et al., 2008; Pierson et al., 2010; Dalzell et al., 2011).
Whilst neuropeptide research continues to seed our understanding of parasite neurobiology, it also facilitates the identification and validation of candidate anthelmintic targets (e.g. Mair et al., 2004; Atkinson et al., 2010; Dalzell et al, 2010; Novozhilova et al., 2010; McVeigh et al., 2011). Targets originating from helminth neuropeptidergic signalling systems have generated intermittent interest from some of the major players in animal health drug discovery since the early 1990s. The target potential of helminth neuropeptidergic systems was recognised in particular by the successive Upjohn/Pharmacia and Pharmacia/Pfizer companies, where the animal health drug discovery research teams, initially led by Timothy G. Geary and David P. Thompson, generated significant amounts of valuable data on the basic structure, function and pharmacology of helminth neuropeptides and receptors (for reviews see Geary et al., 1992, 1995; Thompson et al., 1996; Greenwood et al., 2005; Geary and Kubiak, 2005). Since then, industry efforts in these areas appear to have waned. Here, we review current knowledge of helminth neuropeptide signalling mechanisms, and examine the evidence for drug target candidates within these pathways, including the receptors responsible for neuropeptide biological activity, and the enzymes involved in peptide synthesis, signal transduction and signal termination. We consider evidence from reverse genetics and RNAi experiments in parasites and model organisms, and whether existing inhibitors or drugs against each protein family are already in use. Also, we have attempted to illustrate those areas where further study is warranted.
2. Criteria for drug target selection and validation
It is well known that a large proportion of the anthelmintics used to control parasitic worms act on targets associated with the modulation of nerve and/or muscle systems and that these systems are pivotal to motor and sensory capabilities (Martin and Robertson, 2010). This is most evident amongst the compounds used for nematode parasite control and encompasses both long established drugs including dichlorovos, levamisole, morantel, piperazine, pyrantel and the macrocyclic lactones, and more recent additions to the anthelmintic arsenal such as the cyclooctadepsipeptides, paraherquamides and AADs. The empirical nature of anthelmintic discovery, at least to this point, largely dispels the potential of experimental prejudice having selected for neuro/myo-modulatory drugs, leaving us to ponder the preferential selection of parasite nerve/muscle targets by empirical drug screening processes. Although these anti-parasite drug screens were of empirical design, we cannot discount the possibility of bias inherent to the chemical libraries screened, and the obvious appeal of paralysis (flaccid or spastic) as a phenotypic endpoint, combining to skew the selection towards nerve/muscle targets. Nevertheless, it seems very unlikely that these alone could explain the propensity of frontline anthelmintics to act on targets associated with parasite nerve/muscle – especially as this marked bias is not as apparent amongst the platyhelminth-targeting anthelmintics. Therefore, a clear take home message from these observations is that parasite neuromuscular systems are lucrative for drug target discovery with much evidence that multiple targets from this resource are druggable. With front-line anthelmintics, much lauded for their efficacy and spectrum, facing the constant threat of resistance, we must consider sources of new targets that could facilitate the continued discovery of new drugs and so safeguard the future of helminth parasite chemotherapy.
The evolving strategies and changing pressures/expectations within the pharmaceutical sector demands mechanism-based approaches to drug discovery and strong, current market drivers, i.e. there needs to be resistance (or strong evidence of imposing threat) in key host species and geographical regions to justify investment being channelled to anthelmintic discovery/development programmes. The mechanism-based approaches to drug discovery dictate that we mine for new targets and initiate their validation prior to screen implementation. Although helminth parasites have been late entrants to the genomics/transcriptomics era, they have well and truly arrived such that we have the luxury of rapidly expanding resources from which to select targets for validation. This late deluge of resources has exposed the absence of functional genomics tools for many/most parasites. Although RNA interference (RNAi) has facilitated reverse genetic studies in distinct parasite species (Hussein et al., 2002; Urwin et al., 2002; Boyle et al., 2003; Skelly et al., 2003; McGonigle et al., 2008; Pierson et al., 2010), it is still a developing platform and not yet robust enough to drive post-genomic screens that would validate targets in the absence of volume filters that focus efforts on small sub-sets of parasite genes. Therefore, before the initiation of validation processes we are thrust back to the position of having to make selections based on such criteria as target function/importance and ‘druggability’ in other (model) species, criteria that can be provided to support a huge array of parasite gene sets by scientists’ enthusiastic about their individual merits.
Whilst pre-experimental validation processes claim rigour, they often rely on the assumption of functional conservation in target parasite and model species. Further, by their design, they unavoidably bias selections to those targets present in free-living, model species, leading to the relative neglect of molecules involved in host-parasite interplay. Weaknesses aside, initial target selections are founded on both biological and theoretical justifications prior to functional validation. At this time, the latter is constrained by the lack of available and rigorous functional validation tools.
There are a number of broad criteria that are commonly used to drive target selection processes, although the relative importance of each can vary with need and is open to debate. Below is a list of the most common criteria used to provide preliminary and experimental validations for parasite drug targets.
2.1. Criteria for anthelmintic target candidature
-
(i)Druggability of drug target candidate
-
(a)Existing drugs act on homologous targets in other phyla, i.e. target family is already known to be ‘druggable’ in other systems. This may facilitate pre-selection of drug libraries/chemistries to be screened, diminishing size of initial screening efforts.
-
(b)Drug target candidate is known to be amenable to heterologous expression; an obvious route to medium/high throughput screening is desirable.
-
(a)
-
(ii)Expression/occurrence of drug target candidate
-
(c)Drug target candidate is expressed in key parasite species for which control issues are a current or potential concern.
-
(d)Drug target candidate is expressed in key life stage(s) of parasite that are amenable to chemotherapy.
-
(c)
-
(iii)Resistance, spectrum and toxicity of drug target candidate
-
(e)Drug target candidate is new, i.e. it is not known to interact with available anthelmintics.
-
(f)Drug target candidate is unique to the parasite and does not occur in the host; in the case of orthologues occurring in both parasite and host, the candidate drug target must display structural differences from host protein(s).
-
(g)Evidence that drug target candidate is well conserved across multiple parasite species, potentially facilitating broad spectrum action of drug leads. A non-conserved drug target candidate may warrant selection where target species merit investment in selective drugs.
-
(e)
-
(iv)Function of drug target candidate
-
(h)Experimental evidence of functional importance to parasite, i.e. deleterious effects of either over- or under-expression of target protein; in the absence of functional data from parasite, evidence of functional importance in other (preferably related/model) species. It may be acceptable to move forward with strong theoretical support for functional importance. Obviously, evidence of critical function in parasite is needed at some stage.
-
(h)
With these criteria in mind, we now consider the selection of neuropeptide signalling processes as a key resource for parasite drug targets.
3. Do helminth neuropeptidergic signalling proteins represent good drug targets?
Firstly, and with respect to the first broad selection criterion of druggability, we know that nerve/muscle function has provided many of the most useful targets for broad spectrum anti-parasite chemotherapeutics. This observation extends beyond anthelmintics to encompass some of the leading nematicides, such as the carbamate aldicarb (Baron, 1994), used to control plant parasitic nematodes through the inhibition of acetylcholinesterase (AChE), the enzyme responsible for the hydrolysis of the classical neurotransmitter acetylcholine (ACh). Although most evidence supporting nerve/muscle function as a target for parasite control derives from nematodes, praziquantel, used to control schistosomes and many tapeworms, provides evidence that this target system has validity in flatworm parasites (Pax et al., 1978). Therefore, taken in toto, the parasite neuromuscular system is eminently ‘druggable’. However, this does not mean that all proteins associated with signalling mechanisms within parasite neuromusculature have equal merit and/or are equally druggable – so whilst this can be used to support the selection of these tissue types as sources of potential drug targets, it cannot be used to vindicate the selection of individual proteins in the absence of additional justification.
Accepting the broad notion that parasite neuromuscular systems could continue to provide opportunities for novel drug target discovery, what are the targets we know of that have additional attributes which promote their selection? Since most of the current anthelmintic targets drawn from this system are ion channels that are gated by classical neurotransmitters such as ACh, γ-aminobutyric acid (GABA) and glutamate, then other ion channels associated with classical transmitter signalling would seem obvious as putative drug targets that await individual validation. Whilst past success would place ligand-gated ion channels firmly at the head of the queue of putative targets for progression to validatory screens, there are some impediments to their selection as drug target candidates. For example, performing biological studies on these channels is a major challenge and often requires highly skilled physiologists to work through single cell or cell patch-based electrophysiology experiments to derive data on protein functionality (Robertson et al., 2008; Williamson et al., 2009). A key issue is that these channels are generated through the accumulation of multiple, and often inter-changeable, proteins (channel subunits), providing for channel complexity and heterogeneity (Martin et al., 1998). This channel heterogeneity makes it difficult to evaluate which conformations of channel are functional (and important) within parasites. Even if this is determined, reconfiguring the relevant stoichiometries in heterologous expression systems suitable for high throughout screens remains a challenge, as described in the case of nicotinic acetylcholine receptors (nAChRs) (Boulin et al., 2008; Boulin et al., 2011). Further, such heterogeneity in the protein complements of ion channels seeds concern that conformations with variable drug sensitivities will readily facilitate Darwinian-based progression towards resistance. Finally, for those making decisions on funding new drug discovery programmes, the selection of ion channels as targets raises the concern that resistance against drugs acting at similar targets is already undermining control such that the search for drugs guaranteed to have a novel mode-of-action would seem preferable. The latter approach should diminish the risk that parasites resistant to established anthelmintics would be resistant to new drug leads. This thinking does assume that the established drug resistance is due to mutation in the target protein rather than a more generic mechanism, for example, enhanced detoxification processes. Regardless, all lead compounds are tested against known anthelmintic resistant isolates at an early stage in development.
Whilst the targets of current anthelmintics centre upon classical neurotransmitter signalling, neuropeptide signalling is a similarly major, but neglected component of all helminth nervous systems. Numerous features of neuropeptide signalling can be used to provide justification for its selection as a drug target resource. Obviously, peptides themselves are not useful as chemotherapeutic targets, although their transcripts could have merit as targets for transgenic plant-based RNAi-approaches to nematode control (Huang et al., 2006; Yadav et al., 2006; Kimber et al., 2007). Narrowing focus to subsets of putative chemotherapeutic targets within neuromuscular signalling, the G-protein coupled receptors (GPCRs) step forward as proteins that are highly ‘druggable’ with estimates that up to 50% of human medicines are directed at these proteins (Flower, 1999; Wise et al., 2002; Jacoby et al., 2006; Overington et al., 2006; Lagerström and Schiöth, 2008). GPCRs have well established utility as drug targets, being suitable for heterologous expression and high throughput screening. Further, helminth GPCRs are documented as being amenable to heterologous expression (Bouchard et al., 2003; Kubiak et al., 2003, 2008; Rogers et al., 2003; Geary and Kubiak, 2005; Greenwood et al., 2005; Mertens et al., 2004, 2005, 2006; Carre-Pierrat et al., 2006; Omar et al., 2007; Kimber et al., 2009; Taman and Ribeiro, 2009, 2011), meaning that parasite GPCRs would meet the first two ‘druggability’ criteria. The ‘druggability’ of other candidate proteins would have to be considered on a case-by-case basis.
With a provisional selection of GPCRs as candidate drug targets, and following our scheme for selection, we would next consider their expression/occurrence within key parasite species and life stages. Unfortunately, detailed information on neuropeptide GPCR expression in helminth parasites is lacking, thus the selection of individual receptors would have to be justified based on initial evidence derived for homologues from model species such as Caenorhabditis elegans. In the absence of appropriate expression data for parasites, data on the occurrence and activity of neuropeptides (receptor ligands) does provide some evidence of receptor expression. An appealing feature of this widespread expression is target accessibility to drug. Whilst widespread/high expression might require higher effective concentrations in order to affect a sufficient portion of the receptor population to have a deleterious effect, requirements for drug penetrance (and potentially effective concentration) could be lower for targets that are expressed adjacent to the surface of the parasite. Further, there is ample evidence from physiology studies and some RNAi-based studies that neuropeptides modulate parasite motor function and that these receptors are expressed on nerve and muscle tissues across key parasite life stages (see Day et al., 1994; Maule et al., 1995, 2002; Moffett et al., 2003; Kimber et al., 2007; Marks and Maule, 2010; Mousley et al., 2010). Determining expression in the target life stage(s) of parasites is a pre-requisite for target selection and progression such that it needs to be established at an early stage.
With respect to resistance, spectrum and toxicity, targets associated with parasite neuropeptide signalling appear to fulfill the selection criteria. Firstly, no available anthelmintics are known to target neuropeptide signalling such that drugs acting on targets drawn from this protein subset have an a priori claim to a novel mode-of-action. Secondly, several BLAST surveys on neuropeptide complements have revealed marked conservation of peptide ligands across nematode parasite species and clades (see McVeigh et al., 2005a, 2008). The ability of individual peptide ligands to act across species and clade boundaries gives hope for the identification of broad spectrum drugs that act on the same receptor targets in multiple parasite species. Feeding confidence further is the fact that some helminth neuropeptide GPCRs are notably promiscuous with respect to activating ligand structure (see Greenwood et al., 2005; McVeigh et al., 2006). Indeed, some neuropeptides have been shown to display inter-phyla activities in arthropods, nematodes and platyhelminths (Marks et al., 1997; Mousley et al., 2004, 2005a,b), highlighting the possibility that drugs acting at associated receptors could have endectocide potential. Thirdly, there is much evidence that the neuropeptide complements of both nematode and platyhelminth parasites are unique and quite distinct to those seen in vertebrate/host species (see McVeigh et al., 2005a,b, 2008, 2009). Again, such differences serve to reduce concerns that toxicity would be an issue for target selective drugs.
Finally, data on functional importance are needed to underpin the initial selection of targets for progression to focussed, target validation experiments. With respect to our example selection of neuropeptide GPCRs, there are much data showing the impact of neuropeptides (in particular those from the FMRFamide-like peptide [FLP] family) on parasitic helminth tissues with evidence of physiological effects through peptide application to muscle strips/muscle fibres/nerves (Day et al., 1994; Cowden and Stretton, 1995; Maule et al., 1995; Pang et al., 1995; Marks et al., 1996; Fellowes et al., 1998; Davis and Stretton, 2001), and behavioural modulation through peptide injection (Reinitz et al., 2000) and RNAi (Kimber et al., 2007; Dalzell et al., 2010). The marked potency and longevity of neuropeptide actions described in these studies adds to the appeal of their receptors as targets in that less drug may be needed to trigger activity and impact worm behaviour. These data broadly support the potential of neuropeptide GPCRs as drug targets.
So within the context of our criteria for drug target candidate selection, targets from within neuropeptide signalling present a prima facie case for selection. This case appears to be particularly strong for neuropeptide-activated GPCRs. However, we still await direct validation that individual neuropeptide GPCRs represent good anthelmintic targets, especially important in light of potential concerns about their utility for this purpose. Amongst concerns raised about their validity as targets is the fact that in human medicine, most drugs acting at GPCRs serve to alter the physiological state of cells or tissues, resulting in modulatory impacts rather than dramatic alterations that could equate to spastic or flaccid paralysis/death, desirable endpoints for anthelmintics (one obvious exception is atropine, a muscarinic AChR antagonist, which can be lethal to mammals). Further, many of the neuropeptide families are diverse with numerous structurally similar peptides (this is especially evident in nematodes), leading to the charge of inherent functional redundancy. Although this is supported by evidence that some neuropeptide mutant C. elegans present with normal or wild-type phenotypes (Li et al., 1999), increasing the diversity of phenotype scoring methods and monitoring the consequences of both knockout and overexpression, are exposing ever-more functionalities and reducing this concern (Li and Kim, 2008).
Knowing that over-expression (rather than knock-out or knock-down) is required to reveal phenotypic aberration for a particular GPCR would demand that screens were tailored to identify agonists rather than antagonists (see Geary, 2010). Conveniently, in such circumstances a primary weakness of RNAi-based screens can become its strength – RNAi lowers the level of target protein, equating to an antagonistic impact on phenotype. Therefore, in a target validation paradigm, RNAi will select for candidate drug targets which disrupt worm biology upon knockdown, making them suitable for antagonistic drug screens.
Whilst there are data supporting the selection of candidate anthelmintic targets from neuropeptide signalling systems, these are relatively fragmented and need more data from basic research if we are to better resolve target selection issues. In an effort to facilitate progress in this area, the remainder of this review discusses the merits for what we consider the most obvious anthelmintic target candidates associated with neuropeptide signalling.
4. Putative anthelmintic targets within the helminth neuropeptidergic system
Neuropeptidergic signalling pathways can be considered in terms of three primary divisions, which are discussed separately below: (1) neuropeptide biosynthesis and synaptic release, (2) receptors and signal transduction, (3) signal termination.
4.1. Neuropeptide biosynthesis and synaptic release
Both flatworms and nematodes display an ever-growing diversity of peptidergic messengers, encoded by several distinct gene families, with different naming systems employed between phyla. Nematodes are currently understood to possess the larger complement of peptides (the free-living model nematode C. elegans possesses ⩾250 neuropeptides, with parasitic nematodes likely displaying similar diversity; McVeigh et al., 2005a, 2006, 2008, 2009; Abad et al., 2008; Kikuchi et al., 2011). Nematode neuropeptide genes span three gene families: flp (FMRF-amide-like peptides), ins (insulin peptide-like), and nlp (neuropeptide-like protein) (see Marks and Maule, 2010; Li and Kim, 2010, for recent reviews). The diversity of flatworm neuropeptides has only recently been fully appreciated, with bioinformatic and proteomic descriptions of more than 100 individual peptides from 10 species (McVeigh et al., 2009; Collins et al., 2010). While increasing knowledge of these amidated bioactive peptides has illustrated the widespread nature and potential functional diversity of helminth neuropeptide signalling, the peptides are not viable drugs because of their poor in vivo instability and poor penetrance of lipid bilayers (Ho et al., 1990; Sheehy et al., 2000). Attention has instead been directed towards screening for non-peptide ligands for helminth neuropeptide receptors, although no such lead compounds have yet been described. Fortunately, neuropeptide-encoding genes are translated and processed by a conserved biosynthetic pathway with homologous proteins recognisable in both helminth phyla. If structurally or pharmacologically distinct from host homologues, these proteins associated with neuropeptide maturation and signal termination may represent valuable anthelmintic targets. However, our ability to target these components is only as good as our knowledge of basic parasite biology, and a recurring theme illustrated below is that while C. elegans and some other models have provided much useful knowledge, our current understanding of the specific effectors of neuropeptide signalling in parasitic helminths is extremely limited. Graphical depictions of neuropeptide processing and signalling pathways have been presented elsewhere (see McVeigh et al., 2005b, 2006; Husson and Schoofs, 2007a,b; Li and Kim, 2008; Geary, 2010), and are not reproduced here.
4.1.1. Transcription and translation of helminth neuropeptide genes
Neuropeptide genes are initially transcribed under the control of cell-specific promoters within neuronal nuclei, yielding complex gene-specific expression patterns which presumably indicate differential functionality. For example, localisation studies in both nematodes and flatworms reveal complex, distinct and sometimes overlapping neuropeptide expression patterns (Yew et al., 2005; Li and Kim, 2008, 2010; Collins et al., 2010; Jarecki et al., 2010; Nanda and Stretton, 2010). Unsurprisingly, neuropeptide genes are additionally subject to developmentally- or environmentally-regulated expression by micro-RNA (miRNA)-based post-transcriptional regulation (several flp-miRNAs have been described in the plant parasitic nematode Bursaphelenchus xylophilus (Huang et al., 2010)). Such mechanisms raise the (currently) remote possibility of anthelmintic therapy based on inhibition of miRNAs – an approach proposed as a possible future anti-cancer therapy (reviewed by Nana-Sinkam and Croce (2010, 2011)), although the applicability of these approaches to helminths remains untested.
Following nuclear export and splicing, neuropeptide mRNAs are translated directly into a precursor protein (also referred to as a prepropeptide), which may incorporate single or multiple copies of mature neuropeptides. The precursor is, during translation, translocated into the endoplasmic reticulum (ER) lumen and thus into the classical regulated secretory pathway, which involves transit through the trans-Golgi network, packaging and concentration into large dense-cored secretory vesicles (LDCVs), axonal transport, and eventual release at the synapse (Emanuelsson et al., 2007; Zhang et al., 2010). Recognition of the prepropeptide’s nascent N-terminal secretory signal peptide is thought to occur upon its translational emergence from the ribosome, by a signal recognition particle (SRP), which binds the signal peptide and transports the entire ribonucleoprotein complex to an ER channel known as a translocon, through which the growing prepropeptide is inserted into the ER lumen. Reverse genetics experiments on a C. elegans SRP homologue (Table 1) reveal a range of aberrant motility and developmental phenotypes consistent with SRP’s role at the apex of the classical secretory pathway. Although this protein represents an appealing target, it is difficult to envisage a mechanism which could therapeutically and specifically inhibit this molecule’s function, short of targeting the protein–protein interaction (a wider strategy proposed by Taylor et al. (2011)) between SRP and prepropeptide. This process of classical secretion/exocytosis is thought to exist in all of the eukarya (Blobel and Dobberstein, 1975; Koch et al., 2003). Within the ER lumen, the signal peptide is first cleaved from the precursor by a membrane-bound signal peptidase complex. Molecular disruption of a C. elegans signal peptidase gene (phi-20; Table 1) demonstrates a range of aberrant phenotypes including paralysis. Similarly, when a subunit of the plant parasitic nematode Meloidogyne incognita signal peptidase (MiSPC3) was targeted by RNAi, a ∼50% reduction in the number of M. incognita recovered from plant roots was reported (Charlton et al., 2010).
Table 1.
Neuropeptide pathway genes that following knockout or RNA interference induce deleterious phenotype in Caenorhabditis elegans. Note that some of these genes do not function exclusively within neuropeptide signalling pathways, and are involved more generally in cellular secretory processes.
| Protein | C. elegans | D. melanogaster | Helminths |
|---|---|---|---|
| Neuropeptide biosynthesis | |||
| Signal recognition particle | F08D12.1, larval arrest/late larval arrest, locomotion variant, embryonic lethal. larval lethal1,2,3,4,5,6 | Srp72, no phenotype available | Schmidtea mediterranea: blastema abnormal; body lesions; lysis; photoreceptors abnormal7 |
| Signal peptidase complex | phi-20, Paralysed, larval lethal, embryonic lethal, maternal sterile2,4,5,8,9,10,11,12 | Spase22-23, no phenotype data available | Meloidogyne incognita: reduced infectivity of plant roots13 |
| Prohormone convertase PC2 | egl-3, Egg retention, egg-laying defective, coiler14 | Amon, developmental defects, larval arrest, defective larval motility15 | S. mediterranea: aberrant motility (paralysis, abnormal flipping, abnormal responses to touch, vibration and light)7 |
| PC2 chaparone, 7B2 | Sbt-1; no deleterious phenotypes reported | 7B2; no phenotype data available. | S. mediterranea: blastema abnormal; behaviour abnormal (sluggish, abnormal flipping, abnormal vibration response, light response abnormal); flattened posture7 |
| Carboxypeptidase E | egl-21, Egg retention, egg-laying defective, coiler14 | No direct orthologue16 | |
| Peptidylglycine-α-amidating monooxygenase | T19B4.1, sterile, lethal [WB] | ||
| Large dense core vesicle kinesin, | unc-104/klp-1, Sterile, locomotion variant, lethal5 [WB] | Unc-104, aberrant larval motility17 | |
| Calcium-activated protein for secretion (CAPS) | unc-31/egl-22, Egg-laying defective, paralysed, flaccid, locomotion variant, egg-retention, sluggish14,18 | CAPS; embryonic/larval lethal19 | |
| Syntaxin | syn-1/syx-3, Sterile, lethal [WB]; Syn-4/syx-4, embryonic lethal, maternal sterile, reduced brood size9,20; unc-64, locomotion variant21 | Syx1A, temperature-dependent paralysis, multiple developmental defects, larval paralysis and lethality22 | |
| Tomosyn | tom-1, Locomotion variant, larval lethal, embryonic lethal2,10,23 | Tomosyn, no deleterious phenotypes reported | |
| Neuropeptide signal transduction | |||
| Insulin receptors | daf-2, Sterile, locomotion variant, larval lethal, reduced brood size, lethal24 | ||
| G-protein α subunits | gsa-1, egl-30, goa-1, Egg laying defective, locomotion variant, sterile progeny, maternal sterile, larval lethal, reduced life-span, sluggish2,6,20,23; gpa-12, egg laying variant, embryonic lethal, locomotion variant25 | G-salpha60A, larval lethal26 | |
| G-protein β and γ subunits | gpb-1, Locomotion variant, larval lethal, egg laying variant, embryonic lethal, maternal sterile2,5; gpb-2, coiler27; gpc-2, sterile, embryonic lethal, sick, lethal28 | Gbeta13F, developmental defects29 | |
| Regulators of G-protein signalling (RGS) proteins | eat-16, Hyperactive egg laying, sterile, body bend variant, hyperactive, early eggs laid, lethal30; rgs-9, embryonic lethal31; rgs-10, embryonic lethal, maternal sterile32 | CG42450, no phenotype available | S. mediterranea: blastema abnormal; photoreceptors abnormal; light response abnormal; variably abnormal [7] |
| Adenylate cyclase | acy-2, locomotion variant2; acy-4, sterile, male mating defective, lethal [WB] | AC13E, AC76E, AC3, CG43373, Ac78C no phenotypic data available; Rut, no deleterious phenotypes reported | |
| Protein kinase A | kin-1, egg-laying variant, larval lethal9 | Pka-C1, embryonic lethality, slow development33; CG32944, no phenotype data available | |
| Phospholipase C-β | egl-8, Sterile, male sterile, egg-laying defective, egg-laying variant, posterior body wall contraction defective28,34 | norpA, no deleterious phenotypes reported | |
| IP3 channel | itr-1, Sterile, maternal sterile, lethal2,5 [WB] | Itp-r83A, no deleterious phenotypes reported | |
| Synaptic peptidases | |||
| Neprilysin | nep-1, Maternal sterile31; C49D10.10, embryonic lethal2 | Nep2, no phenotype data available. CG15485, no phenotype data available | |
Deleterious refers to lethality, reduced lifespan, or aberrant motility or reproduction. Phenotype data from WormBase (http://www.wormbase.org; WS225 16/06/2011), FlyBase (http://www.flybase.org; FB2011_07, 21/07/2011) or as referenced: 1Gaiser et al. (2009), 2Simmer et al. (2003), 3Hamamichi et al. (2008), 4Sonnichsen et al. (2005), 5Kamath et al. (2003), 6Sieburth et al. (2005), 7Reddien et al. (2005), 8Gonczy et al. (2000), 9Rual et al. (2004), 10Balklava et al. (2007), 11Nollen et al. (2004), 12Frand et al. (2005), 13Charlton et al. (2010), 14Trent et al. (1983), 15Rayburn et al. (2003), 16Settle et al. (1995), 17Barkus et al. (2008), 18Speese et al. (2007), 19Renden et al. (2001), 20Ceron et al. (2007), 21Brenner, 1974, 22Littleton et al. (1998), 23Fraser et al. (2000), 24Malone and Thomas, 2004, 25Yau et al. (2003), 26Wolfgang et al. (2001), 27Avery, 1993, 28Lehner et al. (2006), 29Schaefer et al. (2001), 30Porter and Koelle, 2010, 31Maeda et al. (2001), 32Fernandez et al. (2005), 33Perrimon et al. (1996), 34Espelt et al. (2005).
4.1.2. Prepropeptide processing
Once bereft of signal sequence, the peptide precursor is next cleaved by prohormone/proprotein convertase (PC) enzymes (also known as neuroendocrine convertases) situated within LDCVs. These enzymes belong to the subtilisin/kexin-like superfamily of serine proteases which are responsible for post-translational processing of a range of secretory proteins in both prokarya and eukarya. Prohormone convertase PC2 and PC1/3 families are involved in processing within the regulated secretory pathway of invertebrate neuronal and endocrine tissues (Seidah, 2011), and indeed available evidence suggests that PC2 orthologues play a conserved role in helminth neuropeptide processing (Reddien et al., 2005; Husson et al., 2006). Functionally, PC2 excises peptides from their prepropeptide by C-terminal cleavage of basic amino acid motifs which flank mature peptides in prepropeptides. Basic motifs most commonly consist of pairs of the basic amino acids lysine and/or arginine (although some variation on this pattern has been noted (Veenstra, 2000; Duckert et al., 2004; Fricker, 2005; McVeigh et al., 2005a; Rehfeld et al., 2008). PC2 orthologues have been characterised in C. elegans (egl-3, named for its identification from a screen for egg-laying mutants), and Schmidtea mediterranea. In both of these species, reverse genetics analyses (Table 1) have validated disruption of PC2 as having deleterious effects on helminth biology, including effects on motility, behaviour and reproduction (Trent et al., 1983; Kass et al., 2001; Reddien et al., 2005). While such effects are not lethal in these free-living species, therapeutic effects of a similar magnitude in a parasite, resident in a more challenging environment, may result in reduced viability or lethality for the parasite. In C. elegans this disruption almost completely ablates the normal profile of FLP neuropeptides seen in N2 (wild-type) worms (Husson et al., 2006), perhaps explaining the global effects described above. Disruption of PC2’s chaperone protein ‘7B2’ (a protein required for the normal transport, activation and regulation of PC2 (Van Horssen et al., 1995; Muller et al., 1997; Lee and Lindberg, 2008)) has similarly severe effects on the C. elegans FLP profile (Husson et al., 2007). Specific small-molecule inhibitors have been reported as active against murine PC2, demonstrating >90% inhibition with Ki values in the sub-μM range (Kowalska et al., 2009), indicating the possibility of developing similar lead compounds with therapeutic activity against helminth PC2. Although no pharmacological or structural studies have been performed on helminth PC2 enzymes, it is perhaps worth noting that nematode PC2/EGL-3 orthologues exhibit a unique sequence insert within the catalytic domain, which may indicate the possibility of nematode-specific pharmacology (Kovaleva et al., 2002), potentially permissive of pharmacological differentiation between host and helminth enzymes. A Schistosoma mansoni PC2 orthologue was suggested as a drug target candidate in a chemogenomics screen based on proteins displaying deleterious mutant phenotypes in both C. elegans and Drosophila melanogaster (Caffrey et al., 2009), although this was not supported by RNAi studies from the same laboratory (Stefanić et al., 2010), which described the absence of aberrant phenotypes associated with RNAi of 11 schistosome targets.
Since PC2 cleaves C-terminally to dibasic markers, its cleavage results in excision of a glycine-extended peptide intermediate which retains a C-terminal dibasic motif. Before further processing can occur, this extension must be removed by carboxypeptidase E (CPE) activity, as demonstrated by proteomics analysis of C. elegans mutants deficient in the CPE orthologue, egl-21, which displayed a peptide profile consisting almost exclusively of unprocessed, C-terminally extended peptides (Husson et al., 2007), allied to a range of aberrant phenotypes (Table 1). Although small molecule inhibitors have been described for other metallocarboxypeptidases (Fernández et al., 2010), none have yet been discovered with specific activity against CPE.
4.1.3. Neuropeptide amidation
Most helminth neuropeptides are C-terminally amidated, where the sequential actions of PC2 and CPE liberate a C-terminal glycine-extended prepeptide which is then subject to amidation (enzymatic production of a C-terminal NH2 moiety), a process that represents the final step in neuropeptide maturation. Amidation is a two-step process in which two enzymes act sequentially: (1) peptidyl α-hydroxylating monooxygenase (PHM), a copper-, oxygen- and ascorbate-dependent enzyme, first catalyses hydroxylation of the pre-peptide C-terminal glycine, to yield an alpha-hydroxyglycine-extended peptide (Eipper et al., 1992; Kulathila et al., 1999), (2) peptidyl-alpha-hydroxyglycine alpha-amidating lyase (PAL), a zinc dependant enzyme, catalyses the secondary dealkylation of the alcohol amide intermediate to form the alpha-amidated neuropeptide and a glyoxylate by-product (Eipper et al., 1992; Kulathila et al., 1999).
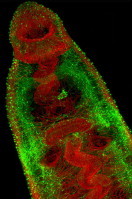
In vertebrates, PHM and PAL exist as a bifunctional protein designated peptidylglycine alpha-amidating monooxygenase (PAM; for review see Prigge et al., 2000). Several invertebrate species, including the flatworms Dugesia japonica (Asada et al., 2005), and S. mansoni (Mair et al., 2004; Atkinson et al., 2010), encode monofunctional PHM and PAL enzymes on distinct genes, while nematodes including C. elegans, encode multiple copies of bi- and monofunctional enzymes. The biological significance of such genetic complexity remains unclear. Amidating enzymes similarly have widespread expression within helminth nervous systems (Mair et al., 2004; Atkinson et al., 2010). Using an anti-PHM antiserum we have been able to visualise the entire amidated neuropeptide system of adult schistosomes (Fig., 1), revealing previously unrecognised levels of neuronal complexity and supporting hypotheses on the importance of amidated neuropeptides to schistosome biology. This importance is directly illustrated by the reduced activity of glycine-extended free-acids (<10%) in comparison to their alpha-amidated counterparts (Eipper et al., 1992; Bowman et al., 1996; Bolkenius and Ganzhorn, 1998; Kulathila et al., 1999; Prigge et al., 2000; Bowman et al., 2002; McVeigh et al., 2011). Further, C-terminal amidation was shown to be critical to the activity of a myoexcitatory peptide on dispersed schistosome muscle fibres (Day et al., 1997), supporting the importance of the C-terminal amide signature for myoactivity in schistosomes. Importantly, no alternative mechanism for the in vivo amidation of neuropeptides has been documented.
Fig. 1.
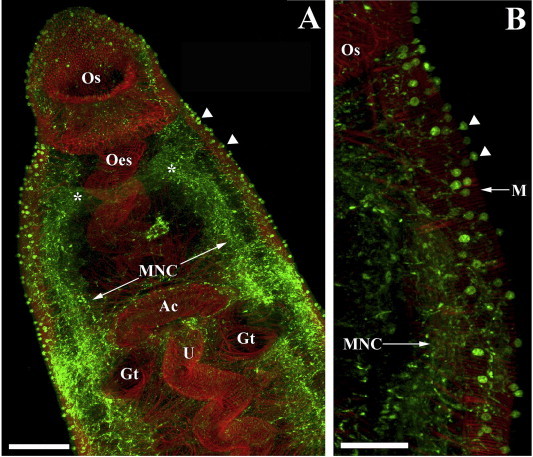
The amidated neuropeptide system of an adult female Schistosoma mansoni. Peptidyl α-hydroxylating monooxygenase (PHM)-immunoreactivity (IR) appears green (fluorescein isothiocyanate [FITC]-labelled secondary antibody) whilst filamentous actin in muscle fibres appears red (tetramethyl rhodamine isothiocyanate [TRITC]-labelled phalloidin). (A) Extensive PHM-IR in the paired cerebral ganglia (∗), main nerve cords (MNC) and nerve processes innervating the oral sucker (Os), oesophagus (Oes), acetabulum (Ac), uterus (U) and gut (Gt) in the anterior region of a female schistosome. The outer surface of the fore body region is covered in immuno positive sensory endings (arrow heads). (B) PHM-IR just posterior to the oral sucker (Os) in one of the main nerve cords (MNC) and associated nerve net that provides branches to the numerous sensory endings (arrow heads) of the anterior-lateral forebody. Note that the tegument is not visible; body wall muscle (M). Scale bars: A = 30 μm; B = 12 μm.
Commercially available inhibitors of PHM/PAL/PAM act by inhibition of conserved active site mechanisms, and would therefore likely be toxic to both parasite and host enzymes. Thus, the identification of differences between host and helminth enzymes is key to unearthing their potential as novel parasite drug targets. Functional data from characterised helminth enzymes such as schistosome PHM and PAL (Mair et al., 2004; Atkinson et al., 2010) indicate that while structural similarities remain evident within conserved active site motifs, marked catalytic and in some cases subtle structural differences between vertebrate and parasite enzymes may provide for successful chemotherapeutic discrimination.
On the face of it, peptide amidation, a process mandatory for the biological activity of most neuropeptides, is an appealing component of the neuropeptide signalling system for anti-parasitic intervention. As such, much of the appeal of targeting either enzyme is the likelihood that their dysfunction would compromise all forms of amidated neuropeptide signalling and, thereby, disrupt all the associated parasite processes and behaviours. One would hypothesise that this would be catastrophic to worm biology and survival. Clearly, using reverse genetic tools to probe this hypothesis is an important step in the drug target validation process. Whilst this has been attempted in S. mansoni, knockdown for Sm-phm-1 and Sm-pal-1 was inconsistent preventing valid phenotypic assessments (Atkinson et al., 2010). To date most of the phenotypic evidence from RNAi based attempts to disrupt the helminth neuropeptide amidation process comes from large scale RNAi experiments in C. elegans, which have reported sterile, lethal and aldicarb resistant phenotypes (see Table 1); Drosophila phm loss-of-function mutants display lethal phenotypes (Jiang et al., 2000). Clearly, the validation of amidating enzymes as targets would be strongly supported by RNAi-based evidence of profound phenotypic aberration in parasites. In the absence of these data the potential of PHM and PAL as anthelmintic targets remains to be established.
4.1.4. Docking and release of synaptic large dense-cored vesicles
Upon arrival in the synaptic terminal, neuropeptide-containing LDCVs are ready for release into the synapse. Available evidence from C. elegans suggests that in nematodes, these processes employ similar molecular mechanisms to those of higher organisms (indeed, all of the proteins mentioned below have clear orthologues in all of the major model organisms, including humans) although there is currently insufficient evidence to enable rational identification of prospects for chemical intercession within this system. Current understanding of the C. elegans LDCV release mechanism is limited, but implicates an UNC-104 kinesin in LDCV antero-grade axonal transport (Zahn et al., 2004) (UNC-116 may also be involved; Li and Kim, 2008). Only three proteins have been identified as specifically required for aspects of LDCV docking, priming and exocytosis: UNC-31/CAPS, a Ca2+-sensing LDCV-membrane docking protein (Speese et al., 2007; Hammarlund et al., 2008; Lin et al., 2010); syntaxin, a component of the SNARE (soluble N-ethyl-maleimide-sensitive fusion protein attachment receptor) complex involved in LDCV-membrane fusion (Hammarlund et al., 2008); and tomosyn, an inhibitor of LDCV exocytosis (Gracheva et al., 2007a,b). Other membrane proteins implicated in exocytosis of synaptic vesicles (carrying classical neurotransmitters; Richmond, 2007) may also have roles in LDCV exocytosis.
4.2. Neuropeptide receptors and signal transduction mechanisms
4.2.1. Insulin-like receptor tyrosine kinases
While the vast majority of neuropeptides in helminths, and throughout nature, are thought to exert their effects via GPCRs, insulin-like peptides signal through conserved receptor tyrosine kinase (RTK)-based mechanisms, which represent proven drug targets for an existing arsenal of anti-phosphorylation drugs used in cancer chemotherapy (Levitzki and Gazit, 1995). Although only a single homologue of mammalian insulin receptors has been described in C. elegans (Kimura et al., 1997), this species appears to possess an additional suite of structurally-divergent insulin-like receptors (Dlakic, 2002). Currently, no insulin receptors have been characterised from parasitic nematodes, although seven insulin-like peptide-encoding gene orthologues were recently identified in the genome of the plant parasitic nematode, B. xylophilus (Kikuchi et al., 2011). In contrast, insulin receptors have been identified from parasitic platyhelminths with some success – an insulin-like RTK (EmIR) has been identified in the cestode Echinococcus multilocularis (Konrad et al., 2003). Unfortunately, similar insulin-binding kinetics of the cestode and human insulin receptors could raise concerns about the potential toxicity of therapeutic agents active at the cestode receptor. Two insulin receptors have been described in S. mansoni (SmIR-1, -2) and Schistosoma japonicum (SjIR-1, -2) (Vicogne and Dissous, 2003; Khayath et al., 2007; Ahier et al., 2008; You et al., 2010, 2011), both of which possess sequence inserts within tyrosine kinase domains that are absent from the human insulin receptor. You et al. (2010) demonstrated that these inserts do not affect the functionality of the TK fold, but are predicted to be highly antigenic and are proposed as potential structural targets for the design of anti-SmIR vaccines or inhibitors.
4.2.2. Neuropeptide-like G protein-coupled receptors
The vast majority of neuropeptide effects are mediated by GPCRs, which, upon ligand binding, recruit a heterotrimeric GTP-binding protein (aka G-protein) through which intracellular signal transduction is mediated. Neuropeptide-activated GPCRs have drawn much attention from industrial and academic drug discovery research groups thanks largely to the proven druggability of this receptor family, and perhaps by the novel anthelmintic emodepside, one target of which appears to be nematode latrophillin-like GPCRs (Saeger et al., 2001; Welz et al., 2005; Krüger et al., 2009; Mühlfeld et al., 2009). From an industrial point of view the appeal of GPCR targets is further enhanced by an array of existing heterologous expression and assay technologies (Greenwood et al., 2005; Minic et al., 2005; Woods et al., 2010). GPCRs are implicated as mediators of neuropeptide signalling events in both helminth phyla, and escape major concerns associated with anthelmintic field-resistance. The value of neuropeptide GPCR drug targets has been recognised by the Bill and Melinda Gates Foundation which is currently funding a project led by Prof Timothy G. Geary (McGill University) in collaboration with two African universities, aiming to identify FLP GPCR ligands from natural product extracts (http://www.gatesfoundation.org/Grants-2008/Pages/McGill-University-OPP52088.aspx).
Despite the undeniable potential of helminth GPCRs as targets for novel anthelmintics, our understanding of their basic biology is limited and fragmentary, such that we can point to specific functions of only a handful of helminth peptide GPCRs, all from C. elegans – at the time of writing, not one peptide GPCR has been functionally characterised from a parasitic worm. Functional data from model helminths have centred on receptor deorphanisation (ligand-receptor matching) and/or reverse genetics analyses, as described below.
4.2.2.1. Peptide GPCRs in Nematoda
Estimates of the extent of the C. elegans peptide-GPCR complement vary widely between published studies (Bargmann, 1998; Keating et al., 2003; Fredriksson and Schiöth, 2005), with the most recent analysis suggesting that C. elegans possesses as many as 91 peptide receptor genes encoding 125 distinct GPCRs (Janssen et al., 2010); similar figures are not yet available for any parasitic nematode. C. elegans therefore represents the source of much of our current understanding of helminth GPCR function, having supported studies into both receptor deorphanisation and functional characterisation. Functional genomics studies in C. elegans have exposed some peptide GPCRs that associate with deleterious phenotypes such as lethality, reduced lifespan, compromised motility or aberrant reproductive function; a subset of these have been deorphanised (Table 2). Assuming functional conservation, parasite orthologues of these receptors would have obvious appeal as candidate chemotherapeutic targets. The C. elegans database (WormBase) identifies 21 putative peptide GPCRs that yield deleterious phenotypes following knockout or RNAi analysis (Table 2), effectively simulating antagonistic pharmacology at these receptors. Perhaps most promising, are five GPCRs for which reduced function leads to lethality and/or sterility, including one of the FLP-18 receptors (Y58G8A.4/FLP18R2). Six GPCRs yield phenotypes associated primarily with altered motility, including the FLP-15 receptor NPR-3 (C10C6.2), which yields reduced egg output, alongside aberrant locomotion and paralysis in some animals (see Keating et al., 2003). Ablation of nine more receptors triggers reduced/compromised reproductive capacity. These lethal, motility and reproductive phenotypes are consistent with deleterious anthelmintic treatment outcomes, and go some way towards validating the utility of antagonistic chemotherapy at neuropeptide GPCRs. The potential for agonist activity at nematode neuropeptide receptors has been demonstrated by a large body of literature describing the effects of FLPs on Ascaris suum neuromuscular bioassays, in which FLPs have been shown to have potent effects on somatic, pharyngeal and ovijector muscle preparations (Maule et al., 1995; Holden-Dye et al., 1997; Fellowes et al., 1998; Marks et al., 1999; Reinitz et al., 2000; Davis and Stretton, 2001; Purcell et al., 2002a,b; Moffett et al., 2003; Mousley et al., 2004; Papaioannou et al., 2005). Promising as these potential targets are, their further validation is hindered by our ignorance of parasite orthologues. Identification and characterisation of such orthologues should be a priority for GPCR-directed anthelmintic discovery programmes. Although there are many other C. elegans peptide GPCRs that correlate with more subtle functions and so lack obvious appeal as anthelmintic targets, their homologues may prove valuable as targets in parasites. More general discussions of the biology of C. elegans peptide GPCRs are available elsewhere (Li and Kim, 2008, 2010; Mousley et al., 2010).
Table 2.
Neuropeptide-like G protein-coupled receptors (GPCRs) that following knockout or RNA interference induce deleterious phenotype in Caenorhabditis elegans.
| GPCR | Most potent ligand | Aberrant phenotypes reported |
|---|---|---|
| Y58G8A.4 (FLP18R2) | FLP-181 | Sterile, lethal |
| C54A12.2 | Embryonic lethal2 | |
| AC7.1/TAG-49 | Embryonic lethal, sick, maternal sterile3,4 | |
| T23B3.4 (CKR-1) | Embryonic lethal, reduced life span, reduced brood size5,6 | |
| C30F12.6 (NMUR-4) | Larval arrest, sterile, embryonic lethal7 | |
| C10C6.2 (NPR-3/FLP-15R) | FLP-158 | Some paralysis, locomotion variant, reduced brood size, sluggish9 |
| F59D12.1 | Paralysed, locomotion variant, sluggish, flat locomotion path, frequency body bend reduced9 | |
| Y54E2A.1 | Locomotion variant, maternal sterile5,6 | |
| T05A1.1 (NPR-2) | Locomotion variant, locomotion reduced, kinker9 | |
| T02E9.1 | Locomotion variant, sluggish, body bend reduced9 | |
| C04E6.9 (SRD-16) | Locomotion variant10 | |
| C16D6.2 (FLP18R1) | Reduced fertility, fewer egg-laying events during active9 | |
| Y59H11AL.1 (VRFaR2) | FLP-711 | Reduced brood size7 |
| T22D1.12 (NPR-12) | Reduced brood size5 | |
| F41E7.3 | Fewer egg-laying events during active9 | |
| C25G6.5 (NPR-11/FLP-21R) | FLP-2112 | Fewer egg laying events during active |
| F21C10.12 | Egg laying defective13 | |
| F54D7.3 (GnRHR) | NLP-4714 | Delayed egg laying14 |
| Y39A3B.5 (CKR-2) | NLP-1215 | Reduced life span6 |
| T27D1.3 (NPR-15) | Organism development variant16 | |
| C26F1.6 (VRFaR1) | FLP-717 | Hyperactive egg laying9 |
| F35G8.1 | Hyperactive egg laying9 |
Deleterious refers to lethality, reduced lifespan, or aberrant motility or reproduction. Where known, most potent ligand is indicated. Note that most of these GPCRs have orthologues in D. melanogaster, but (with the exception of F54D7.3) all are listed on FlyBase as ‘no phenotypic data available’ (http://www.flybase.org; FB2011_07, 21/07/2011). Phenotype data from WormBase (http://www.wormbase.org; WS225 16/06/2011), or as referenced: 1Kubiak et al. (2008), 2Sonnichsen et al. (2005), 3Kamath et al. (2003), 4Simmer et al. (2003), 5Rual et al. (2004), 6Park et al. (2010), 7Ceron et al. (2007), 8Kubiak et al. (2003), 9Keating et al. (2003), 10Zugasti et al. (2005), 11Mertens et al. (2006), 12Lowery et al. (2003), 13Gort et al. (2008), 14Lindemans et al. (2009), 15Janssen et al. (2008), 16Byrne et al. (2007), 17Mertens et al. (2004).
4.2.2.2. Peptide GPCRs in Platyhelminthes
In flatworms, knowledge of peptide GPCRs has recently been accelerated by analysis of the trematode S. mansoni (Berriman et al., 2009) and planarian S. mediterranea (Robb et al., 2008) genomes, from which respectively at least 35 and 81 putative peptide receptors (Rhodopsin-like family GPCRs) have been described (Fitzpatrick et al., 2009; Zamanian et al., in press), as well as a divergent, flatworm-specific clade of 19 S. mansoni and 40 S. mediterranea GPCRs, known as PROF1 (Platyhelminth-specific, Rhodopsin-like, Orphan Family (1) receptors (Zamanian et al., in press). Although these display no homology to any other GPCRs that could inform ligand class/structure, some of them could conceivably be neuropeptide-activated. Given their unique existence in phylum Platyhelminthes, PROF1 GPCRs represent candidate drug targets with potentially unique pharmacology, which could promote exquisite drug selectivity between host and parasite GPCRs. An additional FLP-activated GPCR (Gt-NPR-1) has been described in the planarian flatworm, Girardia tigrina (Omar et al., 2007). Gt-NPR-1, heterologously expressed in Chinese hamster ovary cells, was activated by FLPs emanating from both flatworms and nematodes and as such, currently represents the sole deorphanised neuropeptide receptor from any flatworm. Notably, the existence of clear Gt-NPR-1 orthologues in both S. mansoni and S. mediterranea (Zamanian et al., in press), provides tacit support for employing planarian model systems for the study of parasitic flatworm receptors. This is an important observation, given that presently no flatworm peptide GPCR has been subject to direct functional characterisation, making it impossible to directly validate any individual flatworm receptor as an anthelmintic target – planarians represent useful tools in the push towards functional characterisation of conserved GPCRs in phylum Platyhelminthes.
4.2.3. Peptide-gated ion channels
GPCRs represent the prevalent mechanism of neuropeptide signal reception and transduction, eliciting the long-term, metabotropic, modulatory effects that are characteristic of neuropeptides. However, neuropeptide-gated ion channels have also been reported as mediators of fast synaptic transmission in molluscan (Cottrell et al., 1990; Lingueglia et al., 1995; Jeziorsky et al., 2000; Furukawa et al., 2006) and cnidarian (Golubovic et al., 2007) nervous systems. Intriguingly, similar channels may also exist in parasitic nematodes. One nematode FLP (PF4 - Panagrellus redivivus FLP #4; KPNFIRFamide; Maule et al., 1995), displays a unique activity profile, having been shown in patch-clamp experiments to directly gate a population of low-conductance Cl− channels on A. suum somatic muscle, leading to a fast hyperpolarisation of Ascaris body wall muscle preparations in vitro, with consequent muscular flaccid paralysis (Maule et al., 1995; Holden-Dye et al., 1997; Purcell et al., 2002a,b). This implies the existence in parasitic nematodes of a muscle-expressed peptide-gated Cl− channel (here referred to as a ‘PF4 receptor’). Such receptor channels are not known to exist in mammalian hosts, and therefore represent a target of potentially unique, and consequently selective, pharmacology, against which agonists or antagonists could elicit deleterious effects on parasite neuromuscular function. Other helminth ligand-gated ion channels are of proven druggability since they already represent targets for the majority of current front-line anthelmintics – levamisole, morantel, pyrantel, paraherquamides and the AADs act by modulating the function of nAChRs (Atchison et al., 1992; Zinser et al., 2002; Kaminsky et al., 2008) while the macrocyclic lactones act through GABA – and glutamate-gated Cl− channels (Arena et al., 1992; Wolstenholme and Rogers, 2005). It is clear that helminth ligand-gated ion channels have demonstrable drug-discovery potential and that the PF4 receptor, as a unique example of that group, warrants further research directed towards its identification and characterisation.
4.2.4. GPCR signal transduction mechanisms
GPCRs signal through a conserved mechanism involving recruitment of a heterotrimeric G-protein complex, an event that is triggered by ligand binding to the GPCR, through which signals are transduced in order to initiate an intracellular response. Current understanding of the intimate details of this signalling process have been reviewed elsewhere (McCudden et al., 2005; Bastiani and Mendel, 2006), and are considered only briefly here. G-protein heterotrimers, consisting of α, β and γ subunits exist at rest on the cytosolic face of the membrane, with the alpha subunit bound to GDP. Interaction of a congruent ligand with a GPCR triggers recruitment of this complex, in concert with activation of the G-protein as indicated by the Gα’s exchange of GDP for GTP. The conventional view is that upon activation, the Gα subunit dissociates from the Gβγ complex, allowing Gα and Gβγ to exert separate and distinct downstream effects. Gα’s intrinsic GTPase activity tends to catabolise GTP into GDP (a process that is accelerated both by interaction with effectors and specific regulatory proteins), returning Gα to its resting state following effector interaction, at which time it tends to re-associate with Gβγ. Gα-GTP and Gβγ can both interact with several effector proteins, although diversity within Gα subtype (classified according to the effector enzymes with which each class interacts) is the main determining factor in G-protein functional diversity.
The C. elegans complement of heterotrimeric G-proteins is well characterised, encompassing 21 Gα, 2 Gβ and 2 Gγ subunits. The Gα family includes single orthologues of each of the recognised mammalian Gα subunits: Gs (gsa-1), Gi/o (goa-1), Gq (egl-30) and G12 (gpa-12). C. elegans additionally possesses a divergent clade of 17 genes lacking clear homology to any other Gα class, but most similar to Gi/o-like genes (Bastiani and Mendel, 2006). Reverse genetics of the core C. elegans Gα orthologues expose a variety of aberrant phenotypes befitting of their core role in cell signalling (Table 1), while the divergent Gα genes described above do not yet appear to have been subject to detailed functional interrogation. Both of the C. elegans Gβ and one of the two Gγ subunit genes display aberrant mutant phenotypes. This suggests that functional redundancy is largely absent within Gβ and Gγ genes, and that these proteins may then represent an appealing metabolic ‘choke-point’ against which therapeutics may be targeted (Smrcka et al., 2008; Dessal et al., 2008, 2011). At present there are no descriptions of G-protein sequences from parasitic nematodes. Knowledge of flatworm G-proteins is similarly sparse, with cloned sequences available only for S. mansoni Gαs, Gαi and Gαo subunits (Mansour and Mansour, 1989; Iltzsch et al., 1992). Parasite G-proteins can be targeted therapeutically by suramin, an inhibitor of Gα-GPCR coupling used in both trypanosomiasis and onchocerciasis chemotherapy, and also in human cancer therapy (although suramin is a notoriously promiscuous drug) (Voogd et al., 1993; Höller et al., 1999). Further drug targeting of Gα function seems possible given descriptions of Gα modulators, which have been proposed as drugs for use in inherited human disorders related to Gα malfunction (Chahdi et al., 1998; Ja et al., 2006). Aside from the divergent clade of C. elegans Gα genes described above, no evidence is currently available on the existence of pharmacological or structural differences between mammalian and helminth G-protein function, consistent with parasite-specific drug function. Indeed, cursory BLAST searches indicate high levels of amino-acid sequence identity and similarity in comparisons between human and schistosome Gα subclasses (unpublished data), such that achieving selective interference with parasitic helminth G-protein function might be a challenge. There is growing interest in a family of Gα-regulatory proteins (regulators of G-protein signalling; RGS proteins) as drug targets in human medicine (McCudden et al., 2005; Kimple et al., 2007; Sjögren et al., 2010). RGS proteins are GTPase-accelerating factors that act as desensitisers of G-protein signalling, and as such represent an attractive additional target set within GPCR-related signalling pathways. Data on RGS protein structure and function is completely lacking in parasitic helminths, although C. elegans RGS homologues have been well characterised (Table 1) (Chase and Koelle, 2004; Hess et al., 2004; Hiley et al., 2006; Ferkey et al., 2007; Porter et al., 2010; Esposito et al., 2010).
Physiological investigation of the signal transduction mechanisms employed by helminth neuropeptides have illuminated several potential drug targets functioning downstream of peptide GPCRs. The key postsynaptic intracellular mechanisms implicated in helminth neuropeptide actions are the adenylate cyclase and phosphatidylinositol pathways. In the adenylate cyclase pathway, Gαs initially stimulates adenylate cyclase to catalyse production of cyclic adenosine monophosphate (cAMP) from adenosine triphosphate (ATP). cAMP activates protein kinase A (PKA), which can elicit cellular effects by phosphorylation of enzymes, contractile proteins or ion channels. The first step in the phosphatidylinositol pathway is activation of β-class phospholipase C (PLC) by Gαq,, processing phosphatidylinositol (4, 5) bisphosphate (PIP2) into the second messengers inositol (1, 4, 5) triphosphate (IP3) and diacylglycerol (DAG). IP3 liberates intracellularly sequestered Ca2+ from the sarcoplasmic reticulum (SR) by opening of IP3-gated Ca2+ channels, while DAG simulates protein kinase C (PKC) to phosphorylate cellular effectors. It is these second messengers that trigger the ultimate cellular effects reported of helminth neuropeptides. Studies in flatworms illustrate a dichotomy between signalling pathways employed by two of the primary classes of flatworm neuropeptides: flatworm neuropeptide Fs (NPFs) share a conserved neuropeptide Y (NPY)-like mechanism, signalling through depression of cellular cAMP levels (presumably by inhibition of adenylate cyclase activity) (Humphries et al., 2004), while flatworm FLPs have been shown to signal mostly through phosphatidylinositol-based pathways involving PLC and PKC (Graham et al., 2000; Novozhilova et al., 2010). More specifically, a recent study showed that the flatworm FLP YIRFamide elicits its contractile effect on S. mansoni dispersed muscle fibres by indirect gating of sarcolemmal voltage-operated Ca2+ channels, via a PKC-dependent pathway, which is likely GPCR-mediated (Novozhilova et al., 2010). Similar peptidergic signalling mechanisms in schistosomes may also involve cytosolic liberation of sequestered intracellular Ca2+ from the SR (Day et al., 2000). Conversely, nematode FLPs appear largely to employ adenylate cyclase-mediated signalling pathways, where the diverse FLP effects seen in A. suum or Ascaridia galli bioassays are mediated by either upregulation or downregulation of cellular cAMP levels (Trim et al., 1998; Reinitz et al., 2000; Thompson et al., 2003). However, PKC-1 has also been implicated in the regulation of C. elegans peptidergic secretion (Sieburth et al., 2007). An alternative, nitric oxide (NO)-based signalling mechanism is employed by PF1 (SDPNFLRFamide), which relaxed A. suum somatic muscle via nitric oxide synthase (NOS) activity within the hypodermis, a Ca2+-dependent enzyme that catalyses production of the inhibitory messenger, NO (Bowman et al., 1995; Bascal et al., 2001).
Several of the genes encoding components of these second messenger pathways display mutant phenotypes related to viability or reproductive function in both C. elegans and D. melanogaster (Table 1). Current evidence suggests that adenylate cyclases may be druggable, following description of an isoform-selective lead candidate inhibitor of mammalian adenylate cyclase AC1, which is bioavailable in animal models following both oral and intraperitoneal application (Wang et al., 2011). The isoform selectivity demonstrated by this compound suggests that selective targeting of parasite adenylate cyclase isoforms may be possible. Although components of helminth second-messenger signal transduction pathways appear to represent putative drug targets, knowledge of the parasite homologues of these effectors is conspicuously lacking.
4.3. Neuropeptide signal termination
Following activation of its cognate receptor(s), a neuropeptide must be removed from the receptor’s binding site and cleared from the synapse if further neurotransmission is to occur, in a process designated signal termination. Neuropeptide signal termination is performed by a battery of peptidases that may destroy peptides either within the synaptic cleft, or following peptide/receptor internalisation. The importance of the general process of signal termination is well illustrated by the effects of carbamate-based pesticides on AChEs, the signal terminating enzymes that destroy synaptic ACh and lead to paralysis and death in some pest species (Baron, 1994).
Much neuropeptide breakdown in nematodes is seemingly performed by neprilysin-like zinc metalloendopeptidases (Isaac et al., 2000). C. elegans neprilysin knockouts display deleterious phenotypes (Table 1; Spanier et al., 2005), although the specific synaptic peptidolytic function of these mutants has not yet been examined. Endopeptidase, aminopeptidase and deamidase activities have been reported in A. suum muscle homogenates, where enzymes capable of metabolising FLPs have been detected (Sajid and Isaac, 1994; Sajid et al., 1996, 1997), and similar aminopeptidase activity capable of digesting vertebrate peptide hormone substrates has been reported in C. elegans and P. redivivus extracts (Masler, 2002). Comparative studies are limited to the report of differential peptidolytic potencies between extracts of P. redivivus and M. incognita such that implications for selective chemotherapy or broad-spectrum utility of novel drugs are unknown (Masler, 2010). The specific enzymes responsible for these activities have not yet been purified or cloned, although evidence supports the involvement of a deamidase resembling mammalian serine peptidase (Sajid and Isaac, 1994), a neprilysin-like endopeptidase which does not appear to be membrane-bound (Isaac et al., 2000), and an aminopeptidase with similar pharmacology to mammalian aminopeptidase N (Sajid et al., 1997). Dipeptidyl carboxypeptidase activity, common in other peptidergic signalling systems, has not been described in nematodes (Isaac et al., 2000). The employment of standard inhibitor profiling suggests that nematode peptidases show both similarities and differences in their pharmacology to that of orthologous vertebrate enzymes (Sajid and Isaac, 1995; Sajid et al., 1996, 1997). Further studies will be required to identify helminth-specific inhibitors that might represent novel drug lead candidates.
5. Conclusions
Functional analyses of various components of helminth neuropeptidergic signalling systems indicate their potential utility as targets for anthelmintic drugs. Despite this potential, the overwhelming theme throughout this article is how little we still know about the specific molecular players within parasitic helminths, and how this currently inhibits our ability to test hypotheses relating to drug target validation. While C. elegans is a useful model for many aspects of nematode biology, and has illuminated some structural and functional features of nematode neuropeptide pathways, the species’ utility for elucidating neuropeptide function is limited by poor knowledge of its relationship to parasitic forms, and by clear differences in the complements of some proteins between C. elegans and parasites. Nevertheless, nematode biology has had a head start on flatworm studies, where genome and transcriptome projects have only recently appeared, and where we have yet to reap the understanding of basic organismal and molecular biology enjoyed by the C. elegans and D. melanogaster research communities. The potential for exploitation of drug targets within helminth neuropeptide signalling has yet to be realised. With continued funding for research into basic helminth neuropeptide biology, increased knowledge can perhaps be translated into novel, broad spectrum anthelmintics.
Acknowledgements
Nikki J. Marks, Angela Mousley and Aaron G. Maule acknowledge support from The Department of Agricultural and Rural Development for Northern Ireland, The Department of Education and Learning for Northern Ireland and the Biotechnology and Biological Sciences Research Council.
References
- Abad P., Gouzy J., Aury J.M., Castagnone-Sereno P., Danchin E.G., Deleury E., Perfus-Barbeoch L., Anthouard V., Artiguenave F., Blok V.C., Caillaud M.C., Coutinho P.M., Dasilva C., De Luca F., Deau F., Esquibet M., Flutre T., Goldstone J.V., Hamamouch N., Hewezi T., Jaillon O., Jubin C., Leonetti P., Magliano M., Maier T.R., Markov G.V., McVeigh P., Pesole G., Poulain J., Robinson-Rechavi M., Sallet E., Ségurens B., Steinbach D., Tytgat T., Ugarte E., van Ghelder C., Veronico P., Baum T.J., Blaxter M., Bleve-Zacheo T., Davis E.L., Ewbank J.J., Favery B., Grenier E., Henrissat B., Jones J.T., Laudet V., Maule A.G., Quesneville H., Rosso M.N., Schiex T., Smant G., Weissenbach J., Wincker P. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008;26:909–915. doi: 10.1038/nbt.1482. [DOI] [PubMed] [Google Scholar]
- Ahier A., Khayath N., Vicogne J., Dissous C. Insulin receptors and glucose uptake in the human parasite Schistosoma mansoni. Parasite. 2008;15:573–579. doi: 10.1051/parasite/2008154573. [DOI] [PubMed] [Google Scholar]
- Arena J.P., Liu K.K., Paress P.S., Schaeffer J.M., Cully D.F. Expression of a glutamate-activated chloride current in Xenopus oocytes injected with Caenorhabditis elegans RNA: evidence for modulation by avermectin. Brain Res. Mol. Brain Res. 1992;15:339–348. doi: 10.1016/0169-328x(92)90127-w. [DOI] [PubMed] [Google Scholar]
- Asada A., Orii H., Watanabe K., Tsubaki M. Planarian peptidylglycine-hydroxylating monooxygenase, a neuropeptide processing enzyme, colocalizes with cytochrome b561 along the central nervous system. FEBS J. 2005;272:942–955. doi: 10.1111/j.1742-4658.2004.04528.x. [DOI] [PubMed] [Google Scholar]
- Atchison W.D., Geary T.G., Manning B., VandeWaa E.A., Thompson D.P. Comparative neuromuscular blocking actions of levamisole and pyrantel-type anthelmintics on rat and gastrointestinal nematode somatic muscle. Toxicol. Appl. Pharmacol. 1992;112:133–143. doi: 10.1016/0041-008x(92)90289-5. [DOI] [PubMed] [Google Scholar]
- Atkinson L., McVeigh P., Kimber M.J., Marks N.J., Eipper B.A., Mains R.E., Day T.A., Maule A.G. A PAL for Schistosoma mansoni PHM. Mol. Biochem. Parasitol. 2010;173:97–106. doi: 10.1016/j.molbiopara.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L. The genetics of feeding in Caenorhabditis elegans. Genetics. 1993;133:897–917. doi: 10.1093/genetics/133.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balklava Z., Pant S., Fares H., Grant B.D. Genome-wide analysis identifies a general requirement for polarity proteins in endocytic traffic. Nat. Cell Biol. 2007;9:1066–1073. doi: 10.1038/ncb1627. [DOI] [PubMed] [Google Scholar]
- Bargmann C.I. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- Barkus R.V., Klyachko O., Horiuchi D., Dickson B.J., Saxton W.M. Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol. Biol. Cell. 2008;19:274–283. doi: 10.1091/mbc.E07-03-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R.L. A carbamate insecticide: a case study of aldicarb. Environ. Health Perspect. 1994;102:23–27. doi: 10.1289/ehp.94102s1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascal Z.A., Cunningham J.M., Holden-Dye L., O’Shea M., Walker R.J. Characterisation of a putative nitric oxide synthase in the neuromuscular system of the parasitic nematode Ascaris suum. Parasitology. 2001;122:219–231. doi: 10.1017/s003118200100720x. [DOI] [PubMed] [Google Scholar]
- Bastiani, C., Mendel, J., 2006. Heterotrimeric G proteins in C. elegans (October 13, 2006), WormBook, (Ed.), The C. elegans Research Community, WormBook. Doi: 10.1895/wormbook.1.75.1, <http://www.wormbook.org>. [DOI] [PMC free article] [PubMed]
- Berriman M., Haas B.J., LoVerde P.T., Wilson R.A., Dillon G.P., Cerqueira G.C., Mashiyama S.T., Al-Lazikani B., Andrade L.F., Ashton P.D., Aslett M.A., Bartholomeu D.C., Blandin G., Caffrey C.R., Coghlan A., Coulson R., Day T.A., Delcher A., DeMarco R., Djikeng A., Eyre T., Gamble J.A., Ghedin E., Gu Y., Hertz-Fowler C., Hirai H., Hirai Y., Houston R., Ivens A., Johnston D.A., Lacerda D., Macedo C.D., McVeigh P., Ning Z., Oliveira G., Overington J.P., Parkhill J., Pertea M., Pierce R.J., Protasio A.V., Quail M.A., Rajandream M.A., Rogers J., Sajid M., Salzberg S.L., Stanke M., Tivey A.R., White O., Williams D.L., Wortman J., Wu W., Zamanian M., Zerlotini A., Fraser-Liggett C.M., Barrell B.G., El-Sayed N.M. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–360. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J. Cell Biol. 1975;67:835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkenius F.N., Ganzhorn A.J. Peptidylglycine alpha-amidating mono-oxygenase: neuropeptide amidation as a target for drug design. Gen. Pharmacol. 1998;31:655–659. doi: 10.1016/s0306-3623(98)00192-x. [DOI] [PubMed] [Google Scholar]
- Bouchard C., Ribeiro P., Dubé F., Anctil M. A new G protein-coupled receptor from a primitive metazoan shows homology with vertebrate aminergic receptors and displays constitutive activity in mammalian cells. J. Neurochem. 2003;86(5):1149–1161. doi: 10.1046/j.1471-4159.2003.01924.x. [DOI] [PubMed] [Google Scholar]
- Boulin T., Gielen M., Richmond J.E., Williams D.C., Paoletti P., Bessereau J.L. Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor. Proc. Natl. Acad. Sci. USA. 2008;105:18590–18595. doi: 10.1073/pnas.0806933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulin T., Fauvin A., Charvet C., Cortet J., Cabaret J., Bessereau J.L., Neveau C. Functional reconstitution of Haemonchus contortus acetylcholine receptors in Xenopus oocytes provides mechanistic insights into levamisole resistance. Br. J. Pharmacol. 2011;164:1421–1432. doi: 10.1111/j.1476-5381.2011.01420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman J.W., Winterrowd C.A., Friedman A.R., Thompson D.P., Klein R.D., Davis J.P., Maule A.G., Blair K.L., Geary T.G. Nitric oxide mediates the inhibitory effects of SDPNFLRFamide, a nematode FMRFamide-related neuropeptide, in Ascaris suum. J. Neurophysiol. 1995;74:1880–1888. doi: 10.1152/jn.1995.74.5.1880. [DOI] [PubMed] [Google Scholar]
- Bowman J.W., Friedman A.R., Thompson D.P., Ichhpurani A.K., Kellman M.F., Marks N., Maule A.G., Geary T.G. Structure-activity relationships of KNEFIRFamide (AF1), a nematode FMRFamide-related peptide, on Ascaris suum muscle. Peptides. 1996;17:381–387. doi: 10.1016/0196-9781(96)00007-1. [DOI] [PubMed] [Google Scholar]
- Bowman J.W., Friedman A.R., Thompson D.P., Maule A.G., Alexander-Bowman S.J., Geary T.G. Structure-activity relationships of an inhibitory nematode FMRFamide-related peptide, SDPNFLRFamide (PF1), on Ascaris suum muscle. Int. J. Parasitol. 2002;32:1765–1771. doi: 10.1016/s0020-7519(02)00213-8. [DOI] [PubMed] [Google Scholar]
- Boyle J.P., Wu X.J., Shoemaker C.B., Yoshino T.P. Using RNA interference to manipulate endogenous gene expression in Schistosoma mansoni sporocysts. Mol. Biochem. Parasitol. 2003;128(2):205–215. doi: 10.1016/s0166-6851(03)00078-1. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne A.B., Weirauch M.T., Wong V., Koeva M., Dixon S.J., Stuart J.M., Roy P.J. A global analysis of genetic interactions in Caenorhabditis elegans. J. Biol. 2007;6:8. doi: 10.1186/jbiol58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey C.R., Rohwer A., Oellien F., Marhöfer R.J., Braschi S., Oliveira G., McKerrow J.H., Selzer P.M. A comparative chemogenomics strategy to predict essential drug targets in the metazoan pathogen, Schistosoma mansoni. PLoS One. 2009;4:e4413. doi: 10.1371/journal.pone.0004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre-Pierrat M., Baillie D., Johnsen R., Hyde R., Hart A., Granger L., Ségalat L. Characterization of the Caenorhabditis elegans G protein-coupled serotonin receptors. Invertebr. Neurosci. 2006;6(4):189–205. doi: 10.1007/s10158-006-0033-z. [DOI] [PubMed] [Google Scholar]
- Ceron J., Rual J.F., Chandra A., Dupuy D., Vidal M., van den Heuvel S. Large-scale RNAi screens identify novel genes that interact with the C. elegans retinoblastoma pathway as well as splicing-related components with synMuv B activity. BMC Dev. Biol. 2007;7:30. doi: 10.1186/1471-213X-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahdi A., Daeffler L., Gies J.P., Landry Y. Drugs interacting with G protein alpha subunits: selectivity and perspectives. Fundam. Clin. Pharmacol. 1998;12:121–132. doi: 10.1111/j.1472-8206.1998.tb00932.x. [DOI] [PubMed] [Google Scholar]
- Charlton W.L., Harel H.Y., Bakhetia M., Hibbard J.K., Atkinson H.J., McPherson M.J. Additive effects of plant expressed double-stranded RNAs on root-knot nematode development. Int. J. Parasitol. 2010;40(7):855–864. doi: 10.1016/j.ijpara.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Chase D.L., Koelle M.R. Genetic analysis of RGS protein function in Caenorhabditis elegans. Methods Enzymol. 2004;389:305–320. doi: 10.1016/S0076-6879(04)89018-9. [DOI] [PubMed] [Google Scholar]
- Collins J.J., 3rd., Hou X., Romanova E.V., Lambrus B.G., Miller C.M., Saberi A., Sweedler J.V., Newmark P.A. Genome-wide analyses reveal a role for peptide hormones in planarian germline development. PLoS Biol. 2010;8:e1000509. doi: 10.1371/journal.pbio.1000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell G.A., Green K.A., Davies N.W. The neuropeptide Phe–Met–Arg–Phe–NH2 (FMRFamide) can activate a ligand-gated ion channel in Helix neurones. Pflugers Arch. 1990;416:612–614. doi: 10.1007/BF00382698. [DOI] [PubMed] [Google Scholar]
- Cowden C., Stretton A.O. Eight novel FMRFamide-like neuropeptides isolated from the nematode Ascaris suum. Peptides. 1995;16(3):491–500. doi: 10.1016/0196-9781(94)00211-n. [DOI] [PubMed] [Google Scholar]
- Dalzell J.J., McMaster S., Fleming C.C., Maule A.G. Short interfering RNA-mediated gene silencing in Globodera pallida and Meloidogyne incognita infective stage juveniles. Int. J. Parasitol. 2010;40:91–100. doi: 10.1016/j.ijpara.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Dalzell J.J., McVeigh P., Warnock N.D., Mitreva M., Bird D.McK., Abad P., Fleming C.C., Day T.A., Mousley A., Marks N.J., Maule A.G. RNAi effector diversity in nematodes. PLoS Negl. Trop. Dis. 2011;5(6):e1176. doi: 10.1371/journal.pntd.0001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R.E., Stretton A.O. Structure-activity relationships of 18 endogenous neuropeptides on the motor nervous system of the nematode Ascaris suum. Peptides. 2001;22(1):7–23. doi: 10.1016/s0196-9781(00)00351-x. [DOI] [PubMed] [Google Scholar]
- Day T.A., Maule A.G., Shaw C., Halton D.W., Moore S., Bennett J.L., Pax R.A. Platyhelminth FMRFamide-related peptides (FaRPs) contract Schistosoma mansoni (Trematoda: Digenea) muscle fibres in vitro. Parasitology. 1994;109:455–459. doi: 10.1017/s0031182000080707. [DOI] [PubMed] [Google Scholar]
- Day T.A., Maule A.G., Shaw C., Pax R.A. Structure-activity relationships of FMRFamide-related peptides contracting Schistosoma mansoni muscle. Peptides. 1997;18(7):917–921. doi: 10.1016/s0196-9781(97)00073-9. [DOI] [PubMed] [Google Scholar]
- Day T.A., Haithcock J., Kimber M., Maule A.G. Functional ryanodine receptor channels in flatworm muscle fibres. Parasitology. 2000;120(4):417–422. doi: 10.1017/s0031182099005594. [DOI] [PubMed] [Google Scholar]
- Dessal A.L., Prades R., Giralt E., Smrcka A.V. Rational design of a selective covalent modifier of G protein βγ subunits. Mol. Pharmacol. 2008;79:24–33. doi: 10.1124/mol.110.068155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessal A.L., Prades R., Giralt E., Smrcka A.V. Rational design of a selective covalent modifier of G protein βγ subunits. Mol. Pharmacol. 2011;79(1):24–33. doi: 10.1124/mol.110.068155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlakic M. A new family of putative insulin receptor-like proteins in C. elegans. Curr. Biol. 2002;12:R155–R157. doi: 10.1016/s0960-9822(02)00729-7. [DOI] [PubMed] [Google Scholar]
- Duckert P., Brunak S., Blom N. Prediction of proprotein convertase cleavage sites. Protein Eng. Des. Sel. 2004;17(1):107–112. doi: 10.1093/protein/gzh013. [DOI] [PubMed] [Google Scholar]
- Ducray P., Gauvry N., Pautrat F., Goebel T., Fruechtel J., Desaules Y., Weber S.S., Bouvier J., Wagner T., Froelich O., Kaminsky R. Discovery of amino-acetonitrile derivatives, a new class of synthetic anthelmintic compounds. Bioorg. Med. Chem. Lett. 2008;18:2935–2938. doi: 10.1016/j.bmcl.2008.03.071. [DOI] [PubMed] [Google Scholar]
- Eipper B.A., Stoffers D.A., Mains R.E. The biosynthesis of neuropeptides: peptide alpha-amidation. Annu. Rev. Neurosci. 1992;15:57–85. doi: 10.1146/annurev.ne.15.030192.000421. [DOI] [PubMed] [Google Scholar]
- Emanuelsson O., Brunak S., von Heijne G., Nielsen H. Locating proteins in the cell using Target P, Signal P and related tools. Nat. Protoc. 2007;2:953–971. doi: 10.1038/nprot.2007.131. [DOI] [PubMed] [Google Scholar]
- Espelt M.V., Estevez A.Y., Yin X., Strange K. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: role of the inositol-1,4,5-trisphosphate receptor and phospholipases C beta and gamma. J. Gen. Physiol. 2005;126:379–392. doi: 10.1085/jgp.200509355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G., Amoroso M.R., Bergamasco C., Di Schiavi E., Bazzicalupo P. The G protein regulators EGL-10 and EAT-16, the Giα GOA-1 and the G(q)α EGL-30 modulate the response of the C. elegans ASH polymodal nociceptive sensory neurons to repellents. BMC Biol. 2010;8:138–151. doi: 10.1186/1741-7007-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellowes R., Maule A.G., Marks N.J., Geary T.G., Thompson D.P., Shaw C., Halton D.W. Modulation of the motility of the vagina vera of Ascaras suumin vitro by FMRFamide-related peptides. Parasitology. 1998;116:277–287. doi: 10.1017/s0031182097002229. [DOI] [PubMed] [Google Scholar]
- Ferkey D.M., Hyde R., Haspel G., Dionne H.M., Hess H.A., Suzuki H., Schafer W.R., Koelle M.R., Hart A.C. C. elegans G protein regulatorRGS-3 controls sensitivity to sensory stimuli. Neuron. 2007;53:39–52. doi: 10.1016/j.neuron.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A.G., Gunsalus K.C., Huang J., Chuang L.S., Ying N., Liang H.L., Tang C., Schetter A.J., Zegar C., Rual J.F., Hill D.E., Reinke V., Vidal M., Piano F. New genes with roles in the C. elegans embryo revealed using RNAi of ovary-enriched ORFeome clones. Genome Res. 2005;15:250–259. doi: 10.1101/gr.3194805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández D., Pallarès I., Vendell J., Avilés F.X. Progress in metallocarboxypeptidases and their small molecular weight inhibitors. Biochimie. 2010;92:1484–1500. doi: 10.1016/j.biochi.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick J.M., Peak E., Perally S., Chalmers I.W., Barrett J., Yoshino T.P., Ivens A.C., Hoffmann K.F. Anti-schistosomal intervention targets identified by lifecycle transcriptomic analyses. PLoS Negl. Trop. Dis. 2009;3(11):e543. doi: 10.1371/journal.pntd.0000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower D.R. Modelling G protein-coupled receptors for drug design. Biochim. Biophys. Acta. 1999;1422:207–234. doi: 10.1016/s0304-4157(99)00006-4. [DOI] [PubMed] [Google Scholar]
- Frand A.R., Russel S., Ruvkun G. Functional genomic analysis of C. elegans molting. PLoS Biol. 2005;3:e312. doi: 10.1371/journal.pbio.0030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A.G., Kamath R.S., Zipperlen P., Martinez-Campos M., Sohrmann M., Ahringer J.A. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Fredriksson R., Schiöth H.B. The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol. Pharmacol. 2005;67:1414–1425. doi: 10.1124/mol.104.009001. [DOI] [PubMed] [Google Scholar]
- Fricker L.D. Neuropeptide-processing enzymes: applications for drug discovery. AAPS J. 2005;7:E449–E455. doi: 10.1208/aapsj070244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa Y., Miyawaki Y., Abe G. Molecular cloning and functional characterization of the Aplysia FMRFamide-gated Na+ channel. Pflugers Arch. 2006;451:646–656. doi: 10.1007/s00424-005-1498-z. [DOI] [PubMed] [Google Scholar]
- Gaiser A.M., Brandt F., Richter K. The non-canonical Hop protein from Caenorhabditis elegans exerts essential functions and forms binary complexes with either Hsc70 or Hsp90. J. Mol. Biol. 2009;391:621–634. doi: 10.1016/j.jmb.2009.06.051. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Kubiak T.M. Neuropeptide G-protein-coupled receptors, their cognate ligands and behavior in Caenorhabditis elegans. Trends Pharmacol. Sci. 2005;26(2):56–58. doi: 10.1016/j.tips.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Klein R.D., Vanover L., Bowman J.W., Thompson D.P. The nervous systems of helminths as targets for drugs. J. Parasitol. 1992;78:215–230. [PubMed] [Google Scholar]
- Geary T.G., Bowman J.W., Friedman A.R., Maule A.G., Davis J.P., Winterrowd C.A., Klein R.D., Thompson D.P. The pharmacology of FMRFamide-related neuropeptides in nematodes: new opportunities for rational anthelmintic discovery? Int. J. Parasitol. 1995;25:1273–1280. doi: 10.1016/0020-7519(95)00064-9. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Thompson D.P., Klein R.D. Mechanism-based screening: discovery of the next generation of anthelmintic depends upon more basic research. Int. J. Parasitol. 1999;29:105–112. doi: 10.1016/s0020-7519(98)00170-2. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Conder G.A., Bishop B. The changing landscape of antiparasitic drug discovery for veterinary medicine. Trends Parasitol. 2004;20:449–455. doi: 10.1016/j.pt.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Geary T.G., Woo K., McCarthy J.S., MacKenzie C.D., Horton J., Prichard R.K., de Silva N.R., Olliaro P.L., Lazdins-Helds J.K., Engels D.A., Bundy D.A. Unresolved issues in anthelmintic pharmacology for helminthiases of humans. Int. J. Parasitol. 2010;40:1–13. doi: 10.1016/j.ijpara.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Geary T.G. Nonpeptide ligands for peptidergic G protein-coupled receptors. Adv. Exp. Med. Biol. 2010;692:10–26. doi: 10.1007/978-1-4419-6902-6_2. [DOI] [PubMed] [Google Scholar]
- Golubovic A., Kuhn A., Williamson M., Kalbacher H., Holstein T.W., Grimmelikhuizen C.J., Gründer S. A peptide-gated ion channel from the freshwater polyp Hydra. J. Biol. Chem. 2007;282:35098–35103. doi: 10.1074/jbc.M706849200. [DOI] [PubMed] [Google Scholar]
- Gonczy P., Echeverri C., Oegema K., Coulson A.R., Jones S.J.M., Copley R.R., Duperon J., Oegema J., Brehm M., Cassin E., Hannak E., Kirkham M., Pichler S.C., Flohrs K., Goessen A., Leidel S., Alleaume A.M., Martin C., Ozlu N., Bork P., Hymann A.A. Functional genomic analysis of cell division in C. elegans using RNAi of genes on chromosome III. Nature. 2000;408:331–336. doi: 10.1038/35042526. [DOI] [PubMed] [Google Scholar]
- Gort E.H., Van Haaften G., Verlaan I., Groot A.J., Plasterk R.H., Shvarts A., Suijkerbuijk K.P., van Laar T., van der Wall E., Raman V., van Diest P.J., Tijsterman M., Vooijs M. The TWIST1 oncogene is a direct target of hypoxia-inducible factor-2alpha. Oncogene. 2008;27:1501–1510. doi: 10.1038/sj.onc.1210795. [DOI] [PubMed] [Google Scholar]
- Gracheva E.O., Burdina A.O., Touroutine D., Berthelot-Grosjean M., Parekh H., Richmond J.E. Tomosyn negatively regulates CAPS-dependent peptide release at Caenorhabditis elegans synapses. J. Neurosci. 2007;27:10176–10184. doi: 10.1523/JNEUROSCI.2339-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva E.O., Burdina A.O., Touroutine D., Berthelot-Grosjean M., Parekh H., Richmond J.E. Tomosynnegatively regulates both synaptic transmitter and neuropeptide release at the C. elegans neuromuscular junction. J. Physiol. 2007;585:705–709. doi: 10.1113/jphysiol.2007.138321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M.K., Fairweather I., McGeown J.G. Second messengers mediating mechanical responses to the FaRP GYIRFamide in the fluke Fasciola hepatica. Am. J. Physiol. Regul. Comp. Physiol. 2000;279:R2089–R2094. doi: 10.1152/ajpregu.2000.279.6.R2089. [DOI] [PubMed] [Google Scholar]
- Greenwood K., Williams T., Geary T. Nematode neuropeptide receptors and their development as anthelmintic screens. Parasitology. 2005;131:S169–S177. doi: 10.1017/S003118200500819X. [DOI] [PubMed] [Google Scholar]
- Halton D.W., Shaw C., Maule A.G., Johnston C.F., Fairweather I. Peptidergic messengers: a new perspective of the nervous system of parasitic platyhelminths. J. Parasitol. 1992;78(2):179–193. [PubMed] [Google Scholar]
- Halton D.W., Shaw C., Maule A.G., Smart D. Regulatory peptides in helminth parasites. Adv. Parasitol. 1994;34:163–227. doi: 10.1016/s0065-308x(08)60139-6. [DOI] [PubMed] [Google Scholar]
- Halton D.W. Microscopy and the helminth parasite. Micron. 2004;35(5):361–390. doi: 10.1016/j.micron.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Hamamichi S., Rivas R.N., Knight A.L., Cao S., Caldwell K.A., Caldwell G.A. Hypothesis-based RNAi screening identifies neuroprotective genes in a Parkinson’s disease model. Proc. Natl. Acad. Sci. USA. 2008;105:728–733. doi: 10.1073/pnas.0711018105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund M., Watanabe S., Schuske K., Jorgensen E.M. CAPS and syntaxin dock dense core vesicles to the plasma membrane in neurons. J. Cell. Biol. 2008;180:483–491. doi: 10.1083/jcb.200708018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder A., Schmitt-Wrede H.P., Krücken J., Marinovski P., Wunderlich F., Willson J., Amliwala K., Holden-Dye L., Walker R. Cyclooctadepsipeptides – an anthelmintically active class of compounds exhibiting a novel mode of action. Int. J. Antimicrob. Agents. 2003;22:318–331. doi: 10.1016/s0924-8579(03)00219-x. [DOI] [PubMed] [Google Scholar]
- Hess H.A., Röper J.C., Grill S.W., Koelle M.R. RGS-7 completes a receptor-independent heterotrimeric G protein cycle to asymmetrically regulate mitotic spindle positioning in C. elegans. Cell. 2004;119:209–218. doi: 10.1016/j.cell.2004.09.025. [DOI] [PubMed] [Google Scholar]
- Hiley E., McMullan R., Nurrish S.J. The Galpha12-RGSRhoGEF-RhoA signalling pathway regulates neurotransmitter release in C. elegans. EMBO J. 2006;25:5884–5895. doi: 10.1038/sj.emboj.7601458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho N.F., Geary T.G., Raub T.J., Barsuhn C.L., Thompson D.P. Biophysical transport properties of the cuticle of Ascaris suum. Mol. Biochem. Parasitol. 1990;41(2):153–165. doi: 10.1016/0166-6851(90)90178-o. [DOI] [PubMed] [Google Scholar]
- Holden-Dye L., Brownlee D.J., Walker R.J. The effects of the peptide KPNFIRFamide (PF4) on the somatic muscle cells of the parasitic nematode Ascaris suum. Br. J. Pharmacol. 1997;120:379–386. doi: 10.1038/sj.bjp.0700906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höller C., Freissmuth M., Nanoff C. G proteins as drug targets. Cell. Mol. Life Sci. 1999;55:257–270. doi: 10.1007/s000180050288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoste H., Torres-Acosta J.F. Non chemical control of helminths in ruminants: adapting solutions for changing worms in a changing world. Vet. Parasitol. 2011;180:144–154. doi: 10.1016/j.vetpar.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Hotez P.J., Brindley P.J., Bethony J.M., King C.H., Pearce E.J., Jacobsen J. Helminth infections: the great neglected tropical diseases. J. Clin. Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez P.J., Bethony J.M., Diemert D.J., Pearson M., Loukas A. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat. Rev. Microbiol. 2010;8:814–826. doi: 10.1038/nrmicro2438. [DOI] [PubMed] [Google Scholar]
- Huang G., Allen R., Davis E.L., Baum T.J., Hussey R.S. Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc. Natl. Acad. Sci. USA. 2006;103:14301–14306. doi: 10.1073/pnas.0604698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q.X., Cheng X.Y., Mao Z.C., Wang Y.S., Zhao L.L., Yan X., Ferris V.R., Xu R.M., Xie B.Y. MicroRNA discovery and analysis of pinewood nematode Bursaphelenchus xylophilus by deep sequencing. PLoS One. 2010;5:e13271. doi: 10.1371/journal.pone.0013271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries J.E., Kimber M.J., Barton Y.W., Hsu W., Marks N.J., Greer B., Harriott P., Maule A.G., Day T.A. Structure and bioactivity of neuropeptide F from the human parasites Schistosoma mansoni and Schistosoma japonicum. J. Biol. Chem. 2004;279:39880–39885. doi: 10.1074/jbc.M405624200. [DOI] [PubMed] [Google Scholar]
- Hussein A.S., Kichenin K., Selkirk M.E. Suppression of secreted acetylcholinesterase expression in Nippostrongylus brasiliensis by RNA interference. Mol. Biochem. Parasitol. 2002;122(1):91–94. doi: 10.1016/s0166-6851(02)00068-3. [DOI] [PubMed] [Google Scholar]
- Husson S.J., Schoofs L. Altered neuropeptide profile of Caenorhabditis elegans lacking the chaperone protein 7B2 as analysed by mass spectrometry. FEBS Lett. 2007;581:4288–4292. doi: 10.1016/j.febslet.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Husson S.J., Schoofs L. Processing of neuropeptide precursors in Caenorhabditis elegans. Commun. Agric. Appl. Biol. Sci. 2007;72:199–203. [PubMed] [Google Scholar]
- Husson S.J., Clynen E., Baggerman G., Janssen T., Schoofs L. Defective processing of neuropeptide precursors in Caenorhabditis elegans lacking proprotein convertase 2 (KPC-2/EGL-3): mutant analysis by mass spectrometry. J. Neurochem. 2006;98:1999–2012. doi: 10.1111/j.1471-4159.2006.04014.x. [DOI] [PubMed] [Google Scholar]
- Husson S.J., Janssen T., Baggerman G., Bogert B., Kahn-Kirby A.H., Ashrafi K., Schoofs L. Impaired processing of FLP and NLP peptides in carboxypeptidase E (EGL-21)-deficient Caenorhabditis elegans as analysed by mass spectrometry. J. Neurochem. 2007;102:246–260. doi: 10.1111/j.1471-4159.2007.04474.x. [DOI] [PubMed] [Google Scholar]
- Iltzsch M.H., Bieber D., Vijayasarathy S., Webster P., Zurita M., Ding J., Mansour T.E. Cloning and characterization of a cDNA coding for the alpha-subunit of a stimulatory G protein from Schistosoma mansoni. J. Biol. Chem. 1992;267:14504–14508. [PubMed] [Google Scholar]
- Isaac R.E., Siviter R.J., Stancombe P., Coates D., Shirras A.D. Conserved roles for peptidases in the processing of invertebrate neuropeptides. Biochem. Soc. Trans. 2000;28:460–464. [PubMed] [Google Scholar]
- Ja W.W., Wiser O., Austin R.J., Jan L.Y., Roberts R.W. Turning G proteins on and off using peptide ligands. ACS Chem. Biol. 2006;1:570–574. doi: 10.1021/cb600345k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby E., Bouhelal R., Gerspacher M., Seuwen K. The 7 TM G-protein coupled receptor target family. ChemMedChem. 2006;1:761–782. doi: 10.1002/cmdc.200600134. [DOI] [PubMed] [Google Scholar]
- Janssen T., Meelkop E., Lindemans M., Verstraelen K., Husson S.J., Temmerman L., Nachman R.J., Schoofs L. Discovery of a cholecystokinin-gastrin-like signaling system in nematodes. Endocrinology. 2008;149(6):2826–2839. doi: 10.1210/en.2007-1772. [DOI] [PubMed] [Google Scholar]
- Janssen T., Lindemans M., Meelkop E., Temmerman L., Schoofs L. Coevolution of neuropeptidergic signaling systems: from worm to man. Ann. NY Acad. Sci. 2010;1200:1–14. doi: 10.1111/j.1749-6632.2010.05506.x. [DOI] [PubMed] [Google Scholar]
- Jarecki J.L., Andersen K., Konop C.J., Knickelbine J.J., Vestling M.M., Stretton A.O. Mapping neuropeptide expression by mass spectrometry in single dissected identified neurons from the dorsal ganglion of the nematode Ascaris suum. ACS Chem. Neurosci. 2010;1:505–519. doi: 10.1021/cn1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeziorsky M.C., Green K.A., Sommerville J., Cottrell G.A. Cloning and expression of a FMRFamide-gated Na(+) channel from Helisoma trivolvis and comparison with the native neuronal channel. J. Physiol. 2000;526:13–25. doi: 10.1111/j.1469-7793.2000.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N., Kolhekar A.S., Jacobs P.S., Mains R.E., Eipper B.A., Taghert P.H. PHM is required for normal developmental transitions and for biosynthesis of secretory peptides in Drosophila. Dev. Biol. 2000;226:118–136. doi: 10.1006/dbio.2000.9832. [DOI] [PubMed] [Google Scholar]
- Kamath R.S., Fraser A.G., Dong Y., Poulin G., Durbin R., Gotta M., Kanapin A., Le Bot N., Moreno S., Sohrmann M., Welchman D.P., Ziperlen P., Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- Kaminsky R., Ducray P., Jung M., Clover R., Rufener L., Bouvier J., Weber S.S., Wenger A., Wieland-Berghausen S., Goebel T., Gauvry N., Pautrat F., Skripsky T., Froelich O., Komoin-Oka C., Westlund B., Sluder A., Mäser P. A new class of anthelmintics effective against drug-resistant nematodes. Nature. 2008;452:176–180. doi: 10.1038/nature06722. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kass J., Jacob T.C., Kim P., Kaplan J.M. The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J. Neurosci. 2001;21:9265–9272. doi: 10.1523/JNEUROSCI.21-23-09265.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating C.D., Kriek N., Daniels M., Ashcroft N.R., Hopper N.A., Siney E.J., Holden-Dye L., Burke J.F. Whole-genome analysis of 60 G protein-coupled receptors in Caenorhabditis elegans by gene knockout with RNAi. Curr. Biol. 2003;13:1715–1720. doi: 10.1016/j.cub.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Keiser J., Utzinger J. Food-borne trematodiasis: current chemotherapy and advances with artemisinins and synthetic trioxolanes. Trends Parasitol. 2007;23(11):555–562. doi: 10.1016/j.pt.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Keiser J., Duthaler U., Utzinger J. Update on the diagnosis and treatment of food-borne trematode infections. Curr. Opin. Infect. Dis. 2010;23(5):513–520. doi: 10.1097/QCO.0b013e32833de06a. [DOI] [PubMed] [Google Scholar]
- Khayath N., Vicogne J., Ahier A., BenYounes A., Konrad C., Trolet J., Viscogliosi E., Brehm K., Dissous C. Diversification of the insulin receptor family in the helminth parasite Schistosoma mansoni. FEBS. J. 2007;274:659–676. doi: 10.1111/j.1742-4658.2006.05610.x. [DOI] [PubMed] [Google Scholar]
- Kikuchi T., Cotton J.A., Dalzell J.J., Hasegawa K., Kanzaki N., McVeigh P., Takanashi T., Tsai I.J., Assefa S.A., Cock P.J.A., Otto T.D., Hunt M., Reid A.J., Sanchez-Flores A., Tsuchihara K., Yokoi T., Larsson M.C., Miwa J., Maule A.G., Sahashi N., Jones J.T., Berriman M. Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PLoS Pathog. 2011;7(9):e1002219. doi: 10.1371/journal.ppat.1002219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimber M.J., McKinney S., McMaster S.M., Day T.A., Fleming C.C., Maule A.G. flp Gene disruption in a parasitic nematode reveals motor dysfunction and neuronal sensitivity to RNA interference. FASEB J. 2007;21(4):1233–1243. doi: 10.1096/fj.06-7343com. [DOI] [PubMed] [Google Scholar]
- Kimber M.J., Sayegh L., El-Shehabi F., Song C., Zamanian M., Woods D.J., Day T.A., Ribeiro P. Identification of an Ascaris G protein-coupled acetylcholine receptor with atypical muscarinic pharmacology. Int. J. Parasitol. 2009;39(11):1215–1222. doi: 10.1016/j.ijpara.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimple A.J., Willard F.S., Giguère P.M., Johnston C.A., Mocanu V., Siderovski D.P. The RGS protein inhibitor CCG-4986 is a covalent modifier of the RGS4 Galpha-interaction face. Biochim. Biophys. Acta. 2007;1774(9):1213–1220. doi: 10.1016/j.bbapap.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K.D., Tissenbaum H.A., Liu Y., Ruvkun G. Daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. doi: 10.1126/science.277.5328.942. [DOI] [PubMed] [Google Scholar]
- Knowles J., Gromo G. Target selection in drug discovery. Nat. Rev. Drug Discov. 2002;2:63–69. doi: 10.1038/nrd986. [DOI] [PubMed] [Google Scholar]
- Koch H.G., Moser M., Müller M. Signal recognition particle-dependent protein targeting, universal to all kingdoms of life. Rev. Physiol. Biochem. Pharmacol. 2003;146:55–94. doi: 10.1007/s10254-002-0002-9. [DOI] [PubMed] [Google Scholar]
- Konrad C., Kroner A., Spiliotis M., Zavala-Góngora R., Brehm K. Identification and molecular characterisation of a gene encoding a member of the insulin receptor family in Echinococcus multilocularis. Int. J. Parasitol. 2003;33(3):301–312. doi: 10.1016/s0020-7519(02)00265-5. [DOI] [PubMed] [Google Scholar]
- Kovaleva E.S., Yakovlev A.G., Masler E.P., Chitwood D.J. Human proprotein convertase 2 homologue from a plant nematode: cloning, characterization, and comparison with other species. FASEB J. 2002;16:1099–1101. doi: 10.1096/fj.01-0940fje. [DOI] [PubMed] [Google Scholar]
- Kowalska D., Liu J., Appel J.R., Ozawa A., Nefzi A., Mackin R.B., Houghten R.A., Lindberg I. Synthetic small molecule prohormone convertase 2 inhibitors. Mol. Pharmacol. 2009;75:617–625. doi: 10.1124/mol.108.051334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger N., Harder A., von Samson-Himmelstjerna G. The putative cyclooctadepsipeptide receptor depsiphilin of the canine hookworm Ancylostoma caninum. Parasitol. Res. 2009;105(S1):S91–S100. doi: 10.1007/s00436-009-1500-3. [DOI] [PubMed] [Google Scholar]
- Kubiak T.M., Larsen M.J., Zantello M.R., Bowman J.W., Nulf S.C., Lowery D.E. Functional annotation of the putative orphan Caenorhabditis elegans G-protein-coupled receptor C10C6.2 as a FLP15 peptide receptor. J. Biol. Chem. 2003;278:42115–42120. doi: 10.1074/jbc.M304056200. [DOI] [PubMed] [Google Scholar]
- Kubiak T.M., Larsen M.J., Bowman J.W., Geary T.G., Lowery D.E. FMRFamide-like peptides encoded on the flp-18 precursor gene activate two isoforms of the orphan Caenorhabditis elegans G-protein-coupled receptor Y58G8A.4 heterologously expressed in mammalian cells. Biopolymers. 2008;90:339–348. doi: 10.1002/bip.20850. [DOI] [PubMed] [Google Scholar]
- Kulathila R., Merkler K.A., Merkler D.J. Enzymatic formation of C-terminal amides. Nat. Prod. Rep. 1999;16:145–154. doi: 10.1039/a801346b. [DOI] [PubMed] [Google Scholar]
- Lagerström M.C., Schiöth H.B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- Lee S.N., Lindberg I. 7B2 prevents unfolding and aggregation of prohormone convertase 2. Endocrinology. 2008;149:4116–4127. doi: 10.1210/en.2008-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.H., Clothier M.F., Dutton F.E., Nelson S.J., Johnson S.S., Thompson D.P., Geary T.G., Whaley H.D., Haber C.L., Marshall V.P., Kornis G.I., McNally P.L., Ciadella J.I., Martin D.G., Bowman J.W., Baker C.A., Coscarelli E.M., Alexander-Bowman S.J., Davis J.P., Zinser E.W., Wiley V., Lipton M.F., Mauragis M.A. Marcfortine andparaherquamideclass of anthelmintics: discovery of PNU-141962. Curr. Top. Med. Chem. 2002;2:779–793. doi: 10.2174/1568026023393705. [DOI] [PubMed] [Google Scholar]
- Lehner B., Crombie C., Tischler J., Fortunato A., Fraser A.G. Systematic mapping of genetic interactions in Caenorhabditis elegans identifies common modifiers of diverse signaling pathways. Nat. Genet. 2006;38:896–903. doi: 10.1038/ng1844. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- Li, C., Kim, K., 2008. Neuropeptides, September 25, 2008. WormBook (Ed.), The C. elegans Research Community, WormBook. Doi: 10.1895/wormbook.1.142.1, <http://www.wormbook.org>.
- Li C., Kim K. Neuropeptide gene families in Caenorhabditis elegans. Adv. Exp. Med. Biol. 2010;692:98–137. doi: 10.1007/978-1-4419-6902-6_6. [DOI] [PubMed] [Google Scholar]
- Li C., Kim K., Nelson L.S. FMRFamide-related neuropeptide gene family in Caenorhabditis elegans. Brain Res. 1999;848(1–2):26–34. doi: 10.1016/s0006-8993(99)01972-1. [DOI] [PubMed] [Google Scholar]
- Lin X.G., Ming M., Chen M.R., Niu W.P., Zhang Y.D., Liu B., Jiu Y.M., Yu J.W., Xu T., Wu Z.X. UNC-31/CAPS docks and primes dense core vesicles in C. elegans neurons. Biochem. Biophys. Res. Commun. 2010;397(3):526–531. doi: 10.1016/j.bbrc.2010.05.148. [DOI] [PubMed] [Google Scholar]
- Lindemans M., Liu F., Janssen T., Husson S.J., Mertens I., Gäde G., Schoofs L. Adipokinetic hormone signaling through the gonadotropin-releasing hormone receptor modulates egg-laying in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2009;106(5):1642–1647. doi: 10.1073/pnas.0809881106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingueglia E., Champigny G., Lazdunsky M., Barbry P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature. 1995;378:730–733. doi: 10.1038/378730a0. [DOI] [PubMed] [Google Scholar]
- Littleton J.T., Chapman E.R., Kreber R., Garment M.B., Carlson S.D. Temperature-sensitive paralytic mutations demonstrate that synaptic exocytosis requires SNARE complex assembly and disassembly. Neuron. 1998;21:401–413. doi: 10.1016/s0896-6273(00)80549-8. [DOI] [PubMed] [Google Scholar]
- Lowery, D.E., Geary, T.G., Kubiak, T.M., Larsen, M.J., 2003. G protein-coupled receptor-like receptors and modulators thereof. Pharmacia and Upjohn Company, United States. Patent US 6632621 B1.
- Maeda I., Kohara Y., Yamamoto M., Sugimoto A. Large-scale analysis of gene function in Caenorhabditis elegans by high-throughput RNAi. Curr. Biol. 2001;11:171–176. doi: 10.1016/s0960-9822(01)00052-5. [DOI] [PubMed] [Google Scholar]
- Mair G.R., Niciu M.J., Stewart M.T., Brennan G., Omar H., Halton D.W., Mains R., Eipper B.A., Maule A.G., Day T.A. A functionally atypical amidating enzyme from the human parasite Schistosoma mansoni. FASEB J. 2004;18:114–121. doi: 10.1096/fj.03-0429com. [DOI] [PubMed] [Google Scholar]
- Malone E.A., Thomas J.H. A screen for nonconditional dauer-constitutive mutations in Caenorhabditis elegans. Genetics. 1994;136:879–886. doi: 10.1093/genetics/136.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour J.M., Mansour T.E. Identification of GTP-binding proteins in Fasciola hepatica and Schistosoma mansoni by immunoblotting. Mol. Biochem. Parasitol. 1989;36:11–18. doi: 10.1016/0166-6851(89)90195-3. [DOI] [PubMed] [Google Scholar]
- Marks N.J., Maule A.G. Neuropeptides in helminths: occurrence and distribution. Adv. Exp. Med. Biol. 2010;692:49–77. doi: 10.1007/978-1-4419-6902-6_4. [DOI] [PubMed] [Google Scholar]
- Marks N.J., Johnson S., Maule A.G., Halton D.W., Shaw C., Geary T.G., Moore S., Thompson D.P. Physiological effects of platyhelminth RFamide peptides on muscle-strip preparations of Fasciola hepatica (Trematoda: Digenea) Parasitology. 1996;113:393–401. [Google Scholar]
- Marks N.J., Maule A.G., Halton D.W., Geary T.G., Shaw C., Thompson D.P. Pharmacological effects of nematode FMRFamide-related peptides (FaRPs) on muscle contractility of the trematode, Fasciola hepatica. Parasitology. 1997;114:531–539. [PubMed] [Google Scholar]
- Marks N.J., Sangster N.C., Maule A.G., Halton D.W., Thompson D.P., Geary T.G., Shaw C. Structural characterisation and pharmacology of KHEYLRFamide (AF2) and KSAYMRFamide (PF3/AF8) from Haemonchus contortus. Mol. Biochem. Parasitol. 1999;100:185–194. doi: 10.1016/s0166-6851(99)00057-2. [DOI] [PubMed] [Google Scholar]
- Martin R.J., Robertson A.P. Control of nematode parasites with agents acting on neuro-musculature systems: lessons for neuropeptide ligand discovery. Adv. Exp. Med. Biol. 2010;692:138–154. doi: 10.1007/978-1-4419-6902-6_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R.J., Murray I., Robertson A.P., Bjorn H., Sangster N. Anthelmintics and ion-channels: after a puncture, use a patch.Int. J. Parasitol. 1998;28(6):849–862. doi: 10.1016/s0020-7519(98)00048-4. [DOI] [PubMed] [Google Scholar]
- Masler E.P. Aminopeptidases in Caenorhabditis elegans and Panagrellus redivivus: detection using peptide and non-peptide substrates. J. Helminthol. 2002;76:45–52. doi: 10.1079/JOH200193. [DOI] [PubMed] [Google Scholar]
- Masler E.P. In vitro comparison of protease activities in preparations from free-living (Panagrellus redivivus) and plant-parasitic (Meloidogyne incognita) nematodes using FMRFa and FMRFa-like peptides as substrates. J. Helminthol. 2010;84:425–433. doi: 10.1017/S0022149X1000012X. [DOI] [PubMed] [Google Scholar]
- Maule A.G., Shaw C., Halton D.W., Thim L., Johnston C.F., Fairweather I., Buchanan K.D. Neuropeptide F: a novel parasitic flatworm regulatory peptide from Moniezia expansa (Cestoda: Cyclophyllidea) Parasitology. 1991;102:309–316. [Google Scholar]
- Maule A.G., Shaw C., Halton D.W., Thim L. GNFFRFamide: A novel FMRFamide-immunoreactive peptide isolated from the sheep tapeworm, Moniezia expansa. Biochem. Biophys. Res. Commun. 1993;193:1054–1060. doi: 10.1006/bbrc.1993.1732. [DOI] [PubMed] [Google Scholar]
- Maule A.G., Geary T.G., Bowman J.W., Marks N.J., Blair K.L., Halton D.W., Shaw C., Thompson D.P. Inhibitory effects of nematode FMRFamide-related peptides (FaRPs) on muscle strips from Ascaris suum. Invertebr. Neurosci. 1995;1:255–265. doi: 10.1007/BF02211027. [DOI] [PubMed] [Google Scholar]
- Maule A.G., Bowman J.W., Thompson D.P., Marks N.J., Friedman A.R., Geary T.G. FMRFamide-related peptides (FaRPs) in nematodes: occurrence and neuromuscular physiology. Parasitology. 1996;113:S119–S135. doi: 10.1017/s0031182000077933. [DOI] [PubMed] [Google Scholar]
- Maule A.G., Geary T.G., Bowman J.W., Shaw C., Halton D.W., Thompson D.P. The pharmacology of nematode FMRFamide-related peptides. Parasitol. Today. 1996;12(9):351–357. doi: 10.1016/0169-4758(96)10051-x. [DOI] [PubMed] [Google Scholar]
- Maule A.G., Mousley A., Marks N.J., Day T.A., Thompson D.P., Geary T.G., Halton D.W. Neuropeptide signaling systems – potential drug targets for parasite and pest control. Curr. Top. Med. Chem. 2002;2(7):733–758. doi: 10.2174/1568026023393697. [DOI] [PubMed] [Google Scholar]
- McCudden C.R., Hains M.D., Kimple R.J., Siderovski D.P., Willard F.S. G-protein signaling: back to the future. Cell Mol. Life Sci. 2005;62(5):551–577. doi: 10.1007/s00018-004-4462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGonigle L., Mousley A., Marks N.J., Brennan G.P., Dalton J.P., Spithill T.W., Day T.A., Maule A.G. The silencing of cysteine proteases in Fasciola hepatica newly excysted juveniles using RNA interference. Int. J. Parasitol. 2008;38(2):149–155. doi: 10.1016/j.ijpara.2007.10.007. [DOI] [PubMed] [Google Scholar]
- McVeigh P., Kimber M.J., Novozhilova E., Day T.A. Neuropeptide signalling systems in flatworms. Parasitology. 2005;131:S41–S55. doi: 10.1017/S0031182005008851. [DOI] [PubMed] [Google Scholar]
- McVeigh P., Leech S., Mair G.R., Marks N.J., Geary T.G., Maule A.G. Analysis of FMRFamide-like peptide (FLP) diversity in phylum Nematoda. Int. J. Parasitol. 2005;35:1043–1060. doi: 10.1016/j.ijpara.2005.05.010. [DOI] [PubMed] [Google Scholar]
- McVeigh P., Geary T.G., Marks N.J., Maule A.G. The FLP-side of nematodes. Trends Parasitol. 2006;22:385–396. doi: 10.1016/j.pt.2006.06.010. [DOI] [PubMed] [Google Scholar]
- McVeigh P., Alexander-Bowman S., Veal E., Mousley A., Marks N.J., Maule A.G. Neuropeptide-like protein diversity in phylum Nematoda. Int. J. Parasitol. 2008;38:1493–1503. doi: 10.1016/j.ijpara.2008.05.006. [DOI] [PubMed] [Google Scholar]
- McVeigh P., Mair G.R., Atkinson L., Zamanian M., Ladurner P., Novozhilova E., Marks N.J., Day T.A., Maule A.G. Discovery of multiple neuropeptide families in phylum Platyhelminthes. Int. J. Parasitol. 2009;39:1243–1252. doi: 10.1016/j.ijpara.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVeigh P., Mair G.R., Novozhilova E., Day A., Zamanian M., Marks N.J., Kimber M.J., Day T.A., Maule A.G. Schistosome I/Lamides – a new family of bioactive helminth neuropeptides. Int. J. Parasitol. 2011;41:905–913. doi: 10.1016/j.ijpara.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens I., Vandingenen A., Meeusen T., Janssen T., Luyten W., Nachman R.J., De Loof A., Schoofs L. Functional characterisation of the putative orphan neuropeptide G-protein coupled receptor C26F1.6 in Caenorhabditis elegans. FEBS. Lett. 2004;573:55–60. doi: 10.1016/j.febslet.2004.07.058. [DOI] [PubMed] [Google Scholar]
- Mertens I., Meeusen T., Janssen T., Nachman R., Schoofs L. Molecular characterization of two G protein-coupled receptor splice variants as FLP2 receptors in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2005;330(3):967–974. doi: 10.1016/j.bbrc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- Mertens I., Clinckspoor I., Janssen T., Nachman R., Schoofs L. FMRFamide related peptide ligands activate the Caenorhabditis elegans orphan GPCR Y59H11AL 1. Peptides. 2006;27(6):1291–1296. doi: 10.1016/j.peptides.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Minic J., Sautel M., Salesse R., Pajot-Augy E. Yeast system as a screening tool for pharmacological assessment of G protein coupled receptors. Curr. Med. Chem. 2005;12(8):961–969. doi: 10.2174/0929867053507261. [DOI] [PubMed] [Google Scholar]
- Moffett C.L., Beckett A.M., Mousley A., Marks N.J., Thompson D.P., Geary T.G., Halton D.W., Maule A.G. The ovijector of Ascaris suum: multiple response types revealed by C. elegans FMRFamide-related peptides. Int. J. Parasitol. 2003;33:859–876. doi: 10.1016/s0020-7519(03)00109-7. [DOI] [PubMed] [Google Scholar]
- Mousley A., Marks N.J., Halton D.W., Geary T.G., Thompson D.P., Maule A.G. Arthropod FMRFamide-related peptide modulate muscle activity in helminths. Int. J. Parasitol. 2004;34:755–768. doi: 10.1016/j.ijpara.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Mousley A., Maule A.G., Halton D.W., Marks N.J. Inter-phyla studies on neuropeptides: the potential for broad spectrum anthelmintic and/or endectocide discovery. Parasitology. 2005;131:S143–S167. doi: 10.1017/S0031182005008553. [DOI] [PubMed] [Google Scholar]
- Mousley A., Moffett C.L., Duve H., Thorpe A., Halton D.W., Geary T.G., Thompson D.P., Maule A.G., Marks N.J. Expression and bioactivity of allatostatin-like neuropeptides in helminths. Int. J. Parasitol. 2005;35:1557–1567. doi: 10.1016/j.ijpara.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Mousley A., Novozhilova E., Kimber M.J., Day T.A. Neuropeptide physiology in helminths. Adv. Exp. Med. Biol. 2010;692:78–97. doi: 10.1007/978-1-4419-6902-6_5. [DOI] [PubMed] [Google Scholar]
- Mühlfeld S., Schmitt-Wrede H.B., Harder A., Wunderlich F. FMRFamide-like neuropeptides as putative ligands of latrophilin-like HC110-R from Haemonchus contortus. Mol. Biochem. Parasitol. 2009;164:162–164. doi: 10.1016/j.molbiopara.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Muller L., Zhu X., Lindberg I. Mechanism of the facilitation of PC2 maturation by 7B2: involvement in ProPC2 transport and activation but not folding. J. Cell Biol. 1997;139:625–638. doi: 10.1083/jcb.139.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nana-Sinkam S.P., Croce C.M. MicroRNA dysregulation in cancer: opportunities for the development of microRNA-based drugs. IDrugs. 2010;13(12):843–846. [PubMed] [Google Scholar]
- Nana-Sinkam S.P., Croce C.M. MicroRNAs as therapeutic targets in cancer. Transl. Res. 2011;157:216–225. doi: 10.1016/j.trsl.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Nanda J.C., Stretton A.O. In situ hybridization of neuropeptide-encoding transcripts afp-1, afp-3, and afp-4 in neurons of the nematode Ascaris suum. J. Comp. Neurol. 2010;518:896–910. doi: 10.1002/cne.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollen E.A.A., Garcia S.M., Van Haaften G., Kim S., Chavez A., Morimoto R.I., Plasterk R.H.A. Genome-wide RNA interference screen identifies previously undescribed regulators of polyglutamine aggregation. Proc. Natl Acad. Sci. USA. 2004;101:6403–6408. doi: 10.1073/pnas.0307697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novozhilova E., Kimber M.J., Qian H., McVeigh P., Robertson A.P., Zamanian M., Maule A.G., Day T.A. FMRFamide-like peptides enhance voltage-gated calcium currents to elicit muscle contraction in the human parasite Schistosoma mansoni. PLoS Neg. Trop. Dis. 2010;4:e790. doi: 10.1371/journal.pntd.0000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omar H.H., Humphries J.E., Larsen M.J., Kubiak T.M., Geary T.G., Maule A.G., Kimber M.J., Day T.A. Identification of a platyhelminth neuropeptide receptor. Int. J. Parasitol. 2007;37:725–733. doi: 10.1016/j.ijpara.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Overington J.P., Al-Lazikani B., Hopkins A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006;5:993–996. doi: 10.1038/nrd2199. [DOI] [PubMed] [Google Scholar]
- Pang F.Y., Mason J., Holden-Dye L., Franks C.J., Williams R.G., Walker R.J. The effects of the nematode peptide, KHEYLRFamide (AF2), on the somatic musculature of the parasitic nematode Ascaris suum. Parasitology. 1995;110:353–362. doi: 10.1017/s003118200008094x. [DOI] [PubMed] [Google Scholar]
- Papaioannou S., Marsden D., Franks C.J., Walker R.J., Holden-Dye L. Role of a FMRFamide-like family of neuropeptides in the pharyngeal nervous system of Caenorhabditis elegans. J. Neurobiol. 2005;65:304–319. doi: 10.1002/neu.20201. [DOI] [PubMed] [Google Scholar]
- Park S.K., Link C.D., Johnson T.E. Life-span extension by dietary restriction is mediated by NLP-7 signaling and coelomocyte endocytosis in C. elegans. FASEB J. 2010;24:383–392. doi: 10.1096/fj.09-142984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pax R., Bennett J.L., Fetterer R. A benzodiazepine derivative and praziquantel: effects on musculature of Schistosoma mansoni and Schistosoma japonicum. Naunyn Schmiedebergs Arch. Pharmacol. 1978;304(3):309–315. doi: 10.1007/BF00507974. [DOI] [PubMed] [Google Scholar]
- Perrimon N., Lanjuin A., Arnold C., Noll E. Zygotic lethal mutations with maternal effect phenotypes in Drosophila melanogaster. II. Loci on the second and third chromosomes identified by P-element-induced mutations. Genetics. 1996;144:1681–1692. doi: 10.1093/genetics/144.4.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson L., Mousley A., Divine L., Day T.A., Marks N.J., Maule A.G. RNA interference in a cestode reveals specific silencing of selected highly expressed gene transcripts. Int. J. Parasitol. 2010;40:605–615. doi: 10.1016/j.ijpara.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Porter M.Y., Koelle M.R. RSBP-1 is a membrane-targeting subunit required by the Galpha(q)-specific but not the Galpha(o)-specific R7 regulator of G protein signaling in Caenorhabditis elegans. Mol. Biol. Cell. 2010;21:232–243. doi: 10.1091/mbc.E09-07-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter M.Y., Xie K., Pozharski E., Koelle M.R., Martemyanov K.A. A conserved protein interaction interface on the type 5 G protein beta subunit controls proteolytic stability and activity of R7 family regulator of G protein signaling proteins. J. Biol. Chem. 2010;285:41100–41112. doi: 10.1074/jbc.M110.163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge S.T., Mains R.E., Eipper B.A., Amzel L.M. New insights into copper monooxygenases and peptide amidation: structure, mechanism and function. Cell Mol. Life Sci. 2000;57:1236–1259. doi: 10.1007/PL00000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell J., Robertson A.P., Thompson D.P., Martin R.J. The time-course of the response to the FMRFamide-related peptide PF4 in Ascaris suum muscle cells indicates direct gating of a chloride ion-channel. Parasitology. 2002;124:649–656. doi: 10.1017/s0031182002001695. [DOI] [PubMed] [Google Scholar]
- Purcell J., Robertson A.P., Thompson D.P., Martin R.J. PF4, a FMRFamide-related peptide, gates low-conductance Cl(−) channels in Ascaris suum. Eur. J. Pharmacol. 2002;456:11–17. doi: 10.1016/s0014-2999(02)02622-5. [DOI] [PubMed] [Google Scholar]
- Rayburn L.Y.M., Gooding H.C., Choksi S.P., Maloney D., Kidd A.R., Siekhaus D.E., Bender M., Bender M. Amontillado, the Drosophila homolog of the prohormone processing protease PC2, is required during embryogenesis and early larval development. Genetics. 2003;163:227–237. doi: 10.1093/genetics/163.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddien P.W., Bermange A.L., Murfitt K.J., Jennings J.R., Sánchez Alvarado A. Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev. Cell. 2005;8:635–649. doi: 10.1016/j.devcel.2005.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehfeld J.F., Zhu X., Norrbom C., Bundgaard J.R., Johnsen A.H., Nielsen J.E., Vikesaa J., Stein J., Dey A., Steiner D.F., Friis-Hansen L. Prohormone convertases 1/3 and 2 together orchestrate the site-specific cleavages of progastrin to release gastrin-34 and gastrin-17. Biochem. J. 2008;415(1):35–43. doi: 10.1042/BJ20080881. [DOI] [PubMed] [Google Scholar]
- Reinitz C.A., Herfel H.G., Messinger L.A., Stretton A.O. Changes in locomotory behaviour and cAMP produced in Ascaris suum by neuropeptides from Ascarissuum or Caenorhabditis elegans. Mol. Biochem. Parasitol. 2000;111:185–197. doi: 10.1016/s0166-6851(00)00317-0. [DOI] [PubMed] [Google Scholar]
- Renden R., Berwin B., Davis W., Ann K., Chin C.T., Kreber R., Ganetzky B., Martin T.F., Broadie K. Drosophila CAPS is an essential gene that regulates dense-core vesicle release and synaptic vesicle fusion. Neuron. 2001;31:421–437. doi: 10.1016/s0896-6273(01)00382-8. [DOI] [PubMed] [Google Scholar]
- Richmond, J., 2007. Synaptic function (December 7, 2007). In: WormBook (Ed.), The C. elegans Research Community, Wormbook, doi: 10.1895/wormbook.1.69.1, <http://www.wormbook.org>.
- Robb S.M., Ross E., Sánchez-Alvarado A. SmedGD: the Schmidtea mediterranea genome database. Nucleic Acids Res. 2008;36:D599–D606. doi: 10.1093/nar/gkm684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson A.P., Puttachary S., Buxton S.K., Martin R.J. Electrophysiological recording from parasitic nematode muscle. Invertebr. Neurosci. 2008;8(4):167–175. doi: 10.1007/s10158-008-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C., Reale V., Kim K., Chatwin H., Li C., Evans P., de Bono M. Inhibition of Caenorhabditis elegans social feeding by FMRFamide-related peptide activation of NPR-1. Nat. Neurosci. 2003;6(11):1178–1185. doi: 10.1038/nn1140. [DOI] [PubMed] [Google Scholar]
- Rual J.F., Ceron J., Koreth J., Hao T., Nicot A.S., Hirozane-Kishikawa T., Vandenhaute J., Orkin S.H., Hill D.E., van den Heuvel S., Vidal M. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 2004;14:2162–2168. doi: 10.1101/gr.2505604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeger B., Schmitt-Wrede H.P., Dehnhardt M., Benten W.P., Krücken J., Harder A., Von Samson-Himmelstjerna G., Wiegand H., Wunderlich F. Latrophilin-like receptor from the parasitic nematode Haemonchus contortus as target for the anthelmintic depsipeptide PF1022A. FASEB J. 2001;15:1332–1334. doi: 10.1096/fj.00-0664fje. [DOI] [PubMed] [Google Scholar]
- Sajid M., Isaac R.E. Metabolism of AF1 (HNEFIRF–NH2) in the nematode Ascaris suum. Biochem. Soc. Trans. 1994;22:293S. doi: 10.1042/bst022293s. [DOI] [PubMed] [Google Scholar]
- Sajid M., Isaac R.E. Identification and properties of a neuropeptide-degrading endopeptidase (neprilysin) of Ascaris suum muscle. Parasitology. 1995;111:599–608. doi: 10.1017/s0031182000077088. [DOI] [PubMed] [Google Scholar]
- Sajid M., Keating C., Holden-Dye L., Harrow I.D., Isaac R.E. Metabolism of AF1 (KNEFIRF–NH2) in the nematode, Ascaris suum, by aminopeptidase, endopeptidase and deamidase enzymes. Mol. Biochem. Parasitol. 1996;75:159–168. doi: 10.1016/0166-6851(95)02521-9. [DOI] [PubMed] [Google Scholar]
- Sajid M., Isaac R.E., Harrow I.D. Purification and properties of a membrane aminopeptidase from Ascaris suum muscle that degrades neuropeptides AF1 and AF2. Mol. Biochem. Parasitol. 1997;89:225–234. doi: 10.1016/s0166-6851(97)00119-9. [DOI] [PubMed] [Google Scholar]
- Sayed A.A., Simeonov A., Thomas C.J., Inglese J., Austin C.P., Williams D.L. Identification of oxadiazoles as new drug leads for the control of schistosomiasis. Nat. Med. 2008;14(4):407–412. doi: 10.1038/nm1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer M., Petronczki M., Dorner D., Forte M., Knoblich J.A. Heterotrimeric G proteins direct two modes of asymmetric cell division in the Drosophila nervous system. Cell. 2001;107:183–194. doi: 10.1016/s0092-8674(01)00521-9. [DOI] [PubMed] [Google Scholar]
- Seidah N.G. What lies ahead for the proprotein convertases? Ann. NY Acad. Sci. 2011;1220:149–161. doi: 10.1111/j.1749-6632.2010.05883.x. [DOI] [PubMed] [Google Scholar]
- Settle S.H., Jr., Green M.M., Burtis K.C. The silver gene of Drosophilamelanogaster encodes multiple carboxypeptidases similar to mammalian prohormone-processing enzymes. Proc. Natl. Acad. Sci. USA. 1995;92:9470–9474. doi: 10.1073/pnas.92.21.9470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy B.A., Ho N.F., Burton P.S., Day J.S., Geary T.G., Thompson D.P. Transport of model peptides across Ascaris suum cuticle. Mol. Biochem. Parasitol. 2000;105(1):39–49. doi: 10.1016/s0166-6851(99)00161-9. [DOI] [PubMed] [Google Scholar]
- Sieburth D., Ch’ng Q., Dybbs M., Tavazoie M., Kennedy S., Wang D., Dupuy D., Rual J.F., Hill D.E., Vidal M., Ruvkun G., Kaplan J.M. Systematic analysis of genes required for synapse structure and function. Nature. 2005;436:510–517. doi: 10.1038/nature03809. [DOI] [PubMed] [Google Scholar]
- Sieburth D., Madison J.M., Kaplan J.M. PKC-1 regulates secretion of neuropeptides. Nat. Neurosci. 2007;10:49–57. doi: 10.1038/nn1810. [DOI] [PubMed] [Google Scholar]
- Simmer F., Moorman C., van der Linden A.M., Kuijk E., van den Berghe P.V.E., Kamath F.S., Fraser A.G., Ahringer J., Plasterk R.H.A. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS Biol. 2003;1:e12. doi: 10.1371/journal.pbio.0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren B., Blazer L.L., Neubig R.R. Regulators of G protein signaling proteins as targets for drug discovery. Prog. Mol. Biol. Transl. Sci. 2010;91:81–119. doi: 10.1016/S1877-1173(10)91004-1. [DOI] [PubMed] [Google Scholar]
- Skelly P.J., Da’dara A., Harn D.A. Suppression of cathepsin B expression in Schistosoma mansoni by RNA interference. Int. J. Parasitol. 2003;33(4):363–369. doi: 10.1016/s0020-7519(03)00030-4. [DOI] [PubMed] [Google Scholar]
- Smrcka A.V., Lehmann D.M., Dessal A.L. G protein betagamma subunits as targets for small molecule therapeutic development. Comb. Chem. High Throughput Screen. 2008;11:382–395. doi: 10.2174/138620708784534761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen B., Koski L.B., Walsh A., Marschall P., Neumann B., Brehm M., Alleaume A.M., Artelt J., Bettencourt P., Cassin E., Hewitson M., Holz C., Khan M., Lazik S., Martin C., Nitzsche B., Ruer M., Stamford J., Winzi M., Heinkel R., Roder M., Finell J., Hantsch H., Jones S.J., Jones M., Piano F., Gunsalus K.C., Oegema K., Gonczy P., Coulson A., Hyman A.A., Echeverri C.J. Full-genome RNAi profiling of early embryogenesis in Caenorhabditis elegans. Nature. 2005;434:462–469. doi: 10.1038/nature03353. [DOI] [PubMed] [Google Scholar]
- Spanier B., Stürzenbaum S.R., Holden-Dye L.M., Baumeister R. Caenorhabditis elegans neprilysin NEP-1: an effector of locomotion and pharyngeal pumping. Mol. Biol. 2005;352(2):429–437. doi: 10.1016/j.jmb.2005.06.063. [DOI] [PubMed] [Google Scholar]
- Speese S., Petrie M., Schuske K., Ailion M., Ann K., Iwasaki K., Jorgensen E.M., Martin T.F. UNC-31 (CAPS) is required fordense-core vesiclebut not synaptic vesicle exocytosis in Caenorhabditis elegans. J. Neurosci. 2007;27:6150–6162. doi: 10.1523/JNEUROSCI.1466-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanić S.S., Dvořák J., Horn M., Braschi S., Sojka D., Ruelas D.S., Suzuki B., Lim K.C., Hopkins S.D., McKerrow J.H., Caffrey C.R. RNA interference in Schistosoma mansoni schistosomula: selectivity, sensitivity and operation for larger-scale screening. PLoS Negl. Trop. Dis. 2010;4:e850. doi: 10.1371/journal.pntd.0000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I.A., Leathwick D.M. Anthelmintic resistance in nematode parasites of cattle: a global issue? Trends Parasitol. 2011;37:176–181. doi: 10.1016/j.pt.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Taman A., Ribeiro P. Investigation of a dopamine receptor in Schistosoma mansoni: functional studies and immunolocalization. Mol. Biochem. Parasitol. 2009;168(1):24–33. doi: 10.1016/j.molbiopara.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Taman A., Ribeiro P. Glutamate-mediated signaling in Schistosoma mansoni: a novel glutamate receptor is expressed in neurons and the female reproductive tract. Mol. Biochem. Parasitol. 2011;176(1):42–50. doi: 10.1016/j.molbiopara.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Taylor C.M., Fischer K., Abubucker A., Wang Z., Martin J., Jiang D., Magliano M., Rosso M.N., Li B.W., Fischer P.U., Mitreva M. Targeting protein–protein interactions for parasite control. PLoS One. 2011;6:e18381. doi: 10.1371/journal.pone.0018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.P., Klein R.D., Geary T.G. Prospects for rational approaches to anthelmintic discovery. Parasitology. 1996;113:S217–S238. doi: 10.1017/s0031182000077994. [DOI] [PubMed] [Google Scholar]
- Thompson D.P., Davis J.P., Larsen M.J., Coscarelli E.M., Zinser E.W., Bowman J.W., Alexander-Bowman S.J., Marks N.J., Geary T.G. Effects of KHEYLRFamide and KNEFIRFamide on cyclic adenosine monophosphate levels in Ascaris suum somatic muscle. Int. J. Parasitol. 2003;33:199–208. doi: 10.1016/s0020-7519(02)00259-x. [DOI] [PubMed] [Google Scholar]
- Trent C., Tsuing N., Horvitz H.R. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics. 1983;104:619–647. doi: 10.1093/genetics/104.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trim N., Brooman J.E., Holden-Dye L., Walker R.J. The role of cAMP in the actions of the peptide AF3 in the parasitic nematodes Ascaris suum and Ascaridia galli. Mol. Biochem. Parasitol. 1998;93:263–271. doi: 10.1016/s0166-6851(98)00039-5. [DOI] [PubMed] [Google Scholar]
- Urwin P.E., Lilley C.J., Atkinson H.J. Ingestion of double-stranded RNA by pre-parasitic juvenile cyst nematodes leads to RNA interference. Mol. Plant Microbe Interact. 2002;15:747–752. doi: 10.1094/MPMI.2002.15.8.747. [DOI] [PubMed] [Google Scholar]
- Van Horssen A.M., van den Hurk W.H., Bailyes E.M., Hutton J.C., Martens G.J., Lindberg I. Identification of the region within the neuroendocrine polypeptide 7B2 responsible for the inhibition of prohormone convertase PC2. J. Biol. Chem. 1995;270:14292–14296. doi: 10.1074/jbc.270.24.14292. [DOI] [PubMed] [Google Scholar]
- Veenstra J.L. Mono- and dibasic proteolytic cleavage sites in insect neuroendocrine precursors. Arch. Insect Biochem. Physiol. 2000;43:49–63. doi: 10.1002/(SICI)1520-6327(200002)43:2<49::AID-ARCH1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Vicogne J., Dissous C. Schistosoma mansoni receptor tyrosine kinases: towards new therapeutic targets. J. Soc. Biol. 2003;197(4):367–373. [PubMed] [Google Scholar]
- Voogd T.E., Vansterkenburg E.L.M., Wilting J., Janssen L.H.M. Recent research on the biological activity of suramin. Pharmacol. Rev. 1993;45:177–203. [PubMed] [Google Scholar]
- Wang H., Xu H., Wu L.J., Kim S.S., Chen T., Koga K., Descalzi G., Gong B., Vadakkan K.I., Zhang X., Kaang B.K., Zhuo M. Identification of an adenylyl cyclaseinhibitor for treating neuropathic and inflammatory pain. Sci. Transl. Med. 2011;3:65ra3. doi: 10.1126/scitranslmed.3001269. [DOI] [PubMed] [Google Scholar]
- Welz C., Harder A., Schnieder T., Hoglund J., von Samson-Himmelstjerna G. Putative G protein-coupled receptors in parasitic nematodes–potential targets for the new anthelmintic class cyclooctadepsipeptides? Parasitol. Res. 2005;97:S22–S32. doi: 10.1007/s00436-005-1441-4. [DOI] [PubMed] [Google Scholar]
- Williamson S.M., Robertson A.P., Brown L., Williams T., Woods D.J., Martin R.J., Sattelle D.B., Wolstenholme A.J. The nicotinic acetylcholine receptors of the parasitic nematode Ascaris suum: formation of two distinct drug targets by varying the relative expression levels of two subunits. PLoS Pathog. 2009;5(7):e1000517. doi: 10.1371/journal.ppat.1000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise A., Gearing K., Rees S. Target validation of G protein-coupled receptors. Drug Discov. Today. 2002;7:235–246. doi: 10.1016/s1359-6446(01)02131-6. [DOI] [PubMed] [Google Scholar]
- Wolfgang W.J., Hoskote A., Roberts I.J.H., Jackson S., Forte M. Genetic analysis of the Drosophila Gs gene. Genetics. 2001;158:1189–1201. doi: 10.1093/genetics/158.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme A.J., Rogers A.T. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology. 2005;131:S85–S95. doi: 10.1017/S0031182005008218. [DOI] [PubMed] [Google Scholar]
- Woods D., Butler C., Williams T., Greenwood K. Receptor-based discovery strategies for insecticides and parasiticides: a review. Adv. Exp. Med. Biol. 2010;692:1–9. doi: 10.1007/978-1-4419-6902-6_1. [DOI] [PubMed] [Google Scholar]
- Yadav B.C., Veluthambi K., Subramaniam K. Host-generated double stranded RNA induces RNAi in plant-parasitic nematodes and protects the host from infection. Mol. Biochem. Parasitol. 2006;148:219–222. doi: 10.1016/j.molbiopara.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Yau D.M., Yokoyama N., Goshima Y., Siddiqui Z.K., Siddiqui S.S., Kozasa T. Identification and molecular characterization of the G alpha12-Rho guanine nucleotide exchange factor pathway in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA. 2003;100:14748–14753. doi: 10.1073/pnas.2533143100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew J.Y., Kutz K.K., Dikler S., Messinger L., Li L., Stretton A.O. Mass spectrometric map of neuropeptide expression in Ascaris suum. J. Comp. Neurol. 2005;488(4):396–413. doi: 10.1002/cne.20587. [DOI] [PubMed] [Google Scholar]
- You H., Zhang W., Jones M.K., Gobert G.N., Mulvenna J., Rees G., Spanevello M., Blair D., Duke M., Brehm K., McManus D.P. Cloning and characterisation of Schistosoma japonicum insulin receptors. PLoS One. 2010;5:e9868. doi: 10.1371/journal.pone.0009868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H., Gobert G.N., Jones M.K., Zhang W., McManus D.P. Signalling pathways and the host-parasite relationship: putative targets for control interventions against schistosomiasis: signalling pathways and future anti-schistosome therapies. Bioessays. 2011;33:203–214. doi: 10.1002/bies.201000077. [DOI] [PubMed] [Google Scholar]
- Zahn T.R., Angleson J.K., MacMorris M.A., Domke E., Hutton J.F., Scwartz C., Hutton J.C. Dense core vesicle dynamics in Caenorhabditis elegans neurons and the role of kinesin UNC-104. Traffic. 2004;5:544–559. doi: 10.1111/j.1600-0854.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- Zamanian, M., Kimber, M.J., McVeigh, P., Carlson, A., Maule, A.G., Day, T.A., in press. The repertoire of G protein-coupled receptors in the human parasite Schistosoma mansoni and the model organism Schmidtea mediterranea. BMC Genomics. [DOI] [PMC free article] [PubMed]
- Zhang X., Bao L., Ma G.Q. Sorting of neuropeptides and neuropeptide receptors into secretory pathways. Prog. Neurobiol. 2010;90:276–283. doi: 10.1016/j.pneurobio.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Zinser E.W., Wolf M.L., Alexander-Bowman S.J., Thomas E.M., Davis J.P., Groppi V.E., Lee B.H., Thompson D.P., Geary T.G. Anthelmintic paraherquamides are cholinergic antagonists in gastrointestinal nematodes and mammals. J. Vet. Pharmacol. Ther. 2002;25:241–250. doi: 10.1046/j.1365-2885.2002.00423.x. [DOI] [PubMed] [Google Scholar]
- Zugasti O., Rajan J., Kuwabara P.E. The function and expansion of the Patched- and Hedgehog-related homologs in C. elegans. Genome Res. 2005;15:1402–1410. doi: 10.1101/gr.3935405. [DOI] [PMC free article] [PubMed] [Google Scholar]


