Graphical abstract
Highlights
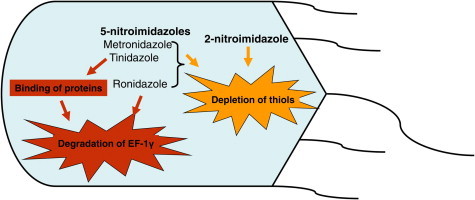
► Metronidazole and tinidazole bind Giardia proteins, including thioredoxin reductase. ► 5-Nitroimidazoles cause degradation of translation elongation factor-1γ (EF-1 γ). ► Nitroimidazoles deplete intracellular thiol levels. ► Nitroimidazoles affect Giardia differently than other parasites.
Keywords: Nitroimidazoles, Protein binding, Translation elongation factor-1γ, Thiol depletion
Abstract
Giardia lamblia (syn. duodenalis, intestinalis) is a globally occurring micro-aerophilic human parasite that causes gastrointestinal disease. Standard treatment of G. lamblia infections is based on the 5-nitroimidazole drugs metronidazole and tinidazole. In two other micro-aerophilic parasites, Entamoeba histolytica and Trichomonas vaginalis, 5-nitroimidazole drugs bind to proteins involved in the thioredoxin-mediated redox network and disrupt the redox equilibrium by inhibiting thioredoxin reductase and depleting intracellular thiol pools. The major aim of this study was to assess whether nitroimidazoles exert a similar toxic effect on G. lamblia physiology.
The 5-nitroimidazoles metronidazole and tinidazole were found to bind to the same subset of proteins including thioredoxin reductase. However, in contrast to E. histolytica and T. vaginalis, none of the other proteins bound are candidates for being involved in the thioredoxin-mediated redox network. Translation elongation factor EF-1γ, an essential factor in protein synthesis, was widely degraded upon treatment with 5-nitroimidazoles. 2-Nitroimidazole (azomycin) and the 5-nitroimidazole ronidazole did not bind to any G. lamblia proteins, which is in contrast to previous findings in E. histolytica and T. vaginalis. All nitroimidazoles tested reduced intracellular thiol pools in G. lamblia, but metronidazole, also in contrast to the situation in the other two parasites, had the slightest effect. Taken together, our results suggest that nitroimidazole drugs affect G. lamblia in a fundamentally different way than E. histolytica and T. vaginalis.
1. Introduction
Giardia lamblia (syn. duodenalis, intestinalis) is considered to be the most common protozoan intestinal parasite worldwide (Buret, 2008). It causes giardiasis, a disease with symptoms such as diarrhea, nausea and failure to thrive in infected children. Treatment of giardiasis is mainly based on metronidazole (Tejman-Yarden and Eckman, 2011), a 5-nitroimidazole drug which is exclusively toxic to microaerophilic and anaerobic microorganisms (Samuelson, 1999). Alternatively, another 5-nitroimidazole drug, tinidazole, is often prescribed (Fung and Doan, 2005).
The selective toxicity of 5-nitroimidazoles to microaerophiles and anaerobes has been ascribed to the existence of factors in these organisms that can transfer electrons to the nitro group and, therefore, activate the prodrug metronidazole to its active state (Müller, 1983). Further, since the single electron transfer product, the nitroradical anion, is easily re-oxidized by oxygen to the parent compound (Pervez-Reyes et al., 1980) effective doses of activated drug are only achieved in organisms with very low intracellular oxygen concentrations. It is still not fully understood whether the nitroradical anion state or a further reduced state, e.g. the nitrosoimidazole state, is the actual agent (Moreno and Docampo, 1985). There is certain evidence that the latter could be the case (West et al., 1982; Wislocki et al., 1984). In G. lamblia, several factors have been suggested to reduce 5-nitroimidazoles, including ferredoxin, which in turn receives electrons from pyruvate:ferredoxin oxidoreductase (Townson et al., 1996), nitroreductase 1 (Nillius et al., 2011), and thioredoxin reductase (Leitsch et al., 2011).
Comparably little is known about the mode of action of 5-nitroimidazoles in G. lamblia. In general, these drugs are considered to damage DNA (Edwards, 1993) but it has as yet not been conclusively demonstrated that this damage is caused by direct attack of 5-nitroimiazoles on DNA rather than by secondary events (LaRusso et al., 1978). Indeed, it was recently suggested that metronidazole induces programmed cell death in G. lamblia which also includes fragmentation of DNA (Ghosh et al., 2009; Bagchi et al., 2012). Further, ultrastructural studies revealed damage to the dorsal surface and the lateral flange upon treatment with metronidazole (Campanati and Monteiro-Real, 2002; Müller et al., 2006). Also damage to the ventral disk was observed (Oxberry et al., 1994) but this effect was not confirmed in other studies (Campanati and Monteiro-Real, 2002; Müller et al., 2006).
In our previous work on metronidazole action in Entamoeba histolytica (Leitsch et al., 2007) and Trichomonas vaginalis (Leitsch et al., 2009) we performed proteomic analyses with nitroimidazole-treated parasites and showed that metronidazole forms covalent adducts with a defined subset of proteins, including thioredoxin reductase and proteins that are known or likely to depend on thioredoxin-mediated reduction. Thioredoxin reductase activity was strongly diminished after nitroimidazole treatment, resulting in impaired removal of hydrogen peroxide by peroxidases (Leitsch et al., 2009). Furthermore, nitroimidazoles were shown to deplete intracellular thiol pools (Leitsch et al., 2007, 2009). Taken together, our results suggested that nitroimidazoles disrupt the redox system in these parasites.
In order to shed more light on metronidazole action in G. lamblia and to find further evidence for our claim that nitroimidazoles strike at the cellular redox system in microaerophilic parasites, we performed an analogous study in G. lamblia using a variety of nitroimidazole drugs, including metronidazole, tinidazole, ronidazole and 2-nitroimidazole (azomycin).
2. Materials and methods
2.1. Nitroimidazoles
Metronidazole, ronidazole, tinidazole, and 2-nitroimidazole (azomycin) were purchased from Sigma–Aldrich.
2.2. Cell culture
G. lamblia BRIS/83/HEPU/106 (referred to as 106) was grown in completely filled and tightly closed 25 ml polysterene culture flasks (Falcon, Becton–Dickinson) in modified TYI-S-33 medium containing bovine bile (Keister, 1983).
2.3. Analysis by two-dimensional gel electrophoresis (2DE)
G. lamblia cultures (2–5 × 107 cells) were harvested at RT by centrifugation at 750g (5 min). Cell pellets were subsequently washed in two centrifugation steps (750g at RT, 5 min) in 1× PBS. Cells were resuspended in 2 ml of 10% trichloroacetic acid, 20% ultrapure water and 70% acetone, and incubated at −20 °C for at least 1 h. Afterwards, the samples were centrifuged at 20,000g at 4 °C and washed twice by in 90% acetone followed by centrifugation at 20,000g (at 4 °C for 20 min). Samples were resolubilised in an appropriate amount of sample buffer (7 M urea, 2 M thiourea, 1% dithithreitol, 1% ampholytes pH 3–10) and insoluble material was removed by centrifugation at 20,000g (at 20 °C for 20 min). Two-dimensional gel electrophoresis (2DE) was performed as described (Leitsch et al., 2005). For isoelectric focussing in 17 cm IPG strips, 500 μg of protein were loaded. The second dimension was run over 20 × 20 cm gels in a Protean II xi cell (BioRad). Gels were Coomassie-stained and images were evaluated using Melanie™ 4 software (Genebio).
2.4. Identification of proteins by mass spectrometric analysis
Protein identification was performed as described recently (Leitsch et al., 2011). Excised protein spots were digested with 0.02 mg/ml trypsin (modified, sequencing grade, Roche). Subsequently, acidified peptides were spotted on a Bruker AnchorChip™ target with matrix ((alpha)-cyano-4-hydroxy-cinnamic acid) 0.5 mg/ml in 90% acetonitrile, 0.1% trifluoroacetic acid (TFA). Samples were washed with 0.5% TFA and re-crystallized in 6:3:1 ethanol:acetone:10 mM ammonium phosphate in 0.1% TFA. Mass spectrometry (MS) and tandem mass spectrometry (MS/MS) data were obtained using a Bruker Daltonics Ultraflex III, MALDI TOF/TOF mass spectrometer. Mascot software (2.2.02) was used to search against the NCBInr database with mass tolerances of 0.4 Da for MS and 0.8 Da for MS/MS.
2.5. Measurement of non-protein thiol pools
Cultures were incubated with the concentrations of nitroimidazoles indicated for 2 h or left untreated. After harvest, pellets were washed once in 1× PBS. Pellets were resuspended in 20 mM EDTA and cells were lysed by addition of 0.1% Triton X-100. Insoluble material was removed by centrifugation at 20,000g for 10 min. Protein concentrations in the supernatants were determined by Bradford assay (Bio-Rad). Subsequently, proteins were removed by precipitation in 5% TCA at room temperature, followed by centrifugation at 20,000g for 5 min. To the supernatants the double volume each of 0.4 M Tris/HCl pH 8.9 and 1.7 μl 100 mM Ellman’s reagent (DTNB) were added per ml reaction mixture. Reduction of DTNB was measured at OD412 in a Perkin Elmer Lambda 25 UV–Vis spectrometer (Δε412 = 13.6 mM−1 cm−1).
3. Results and discussion
3.1. Binding of metronidazole and tinidazole to a small subset of proteins including thioredoxin reductase
In analogy to our previous work on metronidazole action in E. histolytica (Leitsch et al., 2007) and T. vaginalis (Leitsch et al., 2009), two-dimensional gel electrophoresis (2DE) was performed in order to identify G. lamblia proteins that are bound by metronidazole and other nitroimidazoles (Fig. 1). G. lamblia strain 106 cultures were treated for 2 h with two different concentrations of metronidazole (10 μM, 50 μM), followed by harvest and preparation of lysates for 2DE. In total, nine protein spots were found to be shifted to a more basic pI (Fig. 2A) as typically observed with nitroimidazole-protein adducts (Leitsch et al., 2007, 2009). Shifting of proteins in terms of pI depended on the concentration of metronidazole applied, the shifted proportions of all proteins bound being smaller at 10 μM as compared to 50 μM metronidazole (Fig. 2B). With two protein spots, 6 and 7 (Table 1), clearly-defined adducts could only be observed if 10 μM metronidazole were applied (Fig. 2B), whereas treatment with 50 μM metronidazole obviously induced degradation of the protein. In order to rule out that the observed shifts were caused by apoptotic or necrotic processes, nitroimidazole binding G. lamblia cultures were exposed to other nitroimidazoles (Fig. 1) which had been shown to have a similar mode of action as metronidazole in E. histolytica (Leitsch et al., 2007) and T. vaginalis (Leitsch et al., 2009). As observed before in E. histolytica and T. vaginalis, tinidazole bound the same proteins as metronidazole (Fig. 2B) but caused smaller shifts in pI due to its different charge as compared to metronidazole (Leitsch et al., 2007). Surprisingly, another 5-nitroimidazole, ronidazole (Fig. 1), only induced the degradation of EF-1γ but did not lead to any discernible protein shifts (not shown) although it is more toxic to G. lamblia than any other nitroimidazole used in this study (Boreham et al., 1985). It is important to note that in E. histolytica ronidazole readily forms adducts with proteins (unpublished data), ruling out that the 2DE procedure itself resolves ronidazole adducts with proteins. Equally surprising, treatment with 2-nitroimidazole (azomycin) did not lead to any protein shifts (not shown) although it had been found to bind to proteins in E. histolytica (Leitsch et al., 2007) and in T. vaginalis (unpublished data). Nevertheless, 2-nitroimidazole was strongly toxic to G. lamblia and led to total immotility and detachment of most of the cells from the bottom of the culture flasks when being applied at 50 μM for 2 h. Also control samples treated with the nitrofuran drug furazolidone (50 μM for 2 h) and the benzimidazole drug albendazole (20 μM for 3 h) did not display any discernible adduct formation (data not shown), indicating that the protein shifts observed with metronidazole and tinidazole do not reflect any apoptotic or necrotic changes but, rather, direct binding of activated metabolites of these drugs to proteins.
Fig. 1.
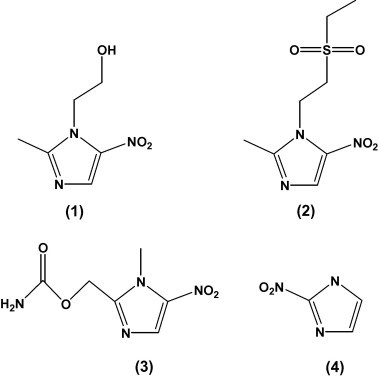
The nitroimidazoles used in this study: (1) Metronidazole; (2) tinidazole; (3) ronidazole; (4) 2-nitroimidazole (azomycin).
Fig. 2.
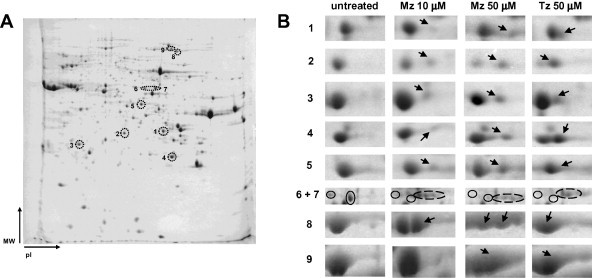
(A) a 2D-gel (20 × 20 cm, pH 5–8) showing all proteins bound by metronidazole and tinidazole. (B) close-up of the affected proteins in untreated cells, and in cells either treated with 10 μM metronidazole (Mz), 50 μM metronidazole, or 50 μM tinidazole (Tz) for 2 h. Covalent adducts of metronidazole and tinidazole are visible as newly appearing protein spots containing the affected protein shifted to a more basic pI (to the right). Shifted portions of proteins are indicated by arrows. In the case of proteins 6 and 7, the original spots are indicated by continuous circles and shifted portions by broken circles. Some of the protein shifts with tinidazole (proteins 5, 8, and 9) are so narrow that no new distinct spots containing tinidazole-bound protein are discernible. Instead, the original spots are extended to a more basic pI. The numbers refer to the following proteins: (1) thioredoxin reductase; (2) alpha-11 giardin; (3) β-giardin; (4) Giardia trophozoite antigen 2; (5) branched-chain amino acid transferase; (6) and (7) elongation factor-1γ; (8) pyruvate phosphate dikinase; (9) alcohol dehydrogenase. For details see Table 1.
Table 1.
The eight proteins bound by metronidazole and tinidazole.
| Spot | Protein | MW (Da) | Theoret. pI | Accession No. | Mowse scoreb |
|---|---|---|---|---|---|
| 1 | Thioredoxin reductasea | 33,869 | 6.16 | XP_001707168 | 166 |
| 2 | Alpha-11 giardin | 35,011 | 5.74 | XP_001705101 | 522 |
| 3 | β-Giardin | 30,877 | 5.25 | XP_001705425 | 634 |
| 4 | Giardia trophozoite antigen 2 | 26,569 | 6.95 | XP_001709922 | 271 |
| 5 | Branched-chain amino acid transferase | 39,065 | 6.14 | XP_001706262 | 207 |
| 6 + 7 | Elongation factor-1γ | 45,217 | 6.05 | XP_001707321 | 320 |
| 8 | Pyruvate phosphate dikinase | 97,659 | 6.31 | XP_001705572 | 416 |
| 9 | Alcohol dehydrogenase | 97,134 | 6.31 | XP_001710238 | 522 |
Already published in Leitsch et al. (2011).
Mascot protein mowse scores.
Protein spots were isolated for mass spectrometric identification by peptide mass fingerprinting. The nine spots contained eight different proteins (Table 1), including thioredoxin reductase. However, with the exception of thioredoxin reductase none of the proteins identified have been reported to be involved in the thioredoxin-mediated redox system as is the case in E. histolytica (Leitsch et al., 2007) and T. vaginalis (Leitsch et al., 2009). Amongst the proteins bound were two structural proteins (giardins) and a Giardia trophozoite antigen (GTA-2) with unknown function. Further, an alcohol dehydrogenase with a coenzyme A acylating aldehyde dehydrogenase (ACDH) domain, pyruvate phosphate dikinase (PPDK), a branched-chain amino acid transferase, and elongation factor-1γ (EF-1γ), which localized to two distinct protein spots, were identified. To the best of our knowledge, none of these proteins have ever been suggested to interact with thioredoxin, neither in G. lamblia, nor in any other organism. It is interesting to note, however, that co-purification experiments suggest that PPDK is associated with pyruvate:ferredoxin oxidoreductase (PFOR) (Anita Burgess, unpublished results), an enzyme known to reduce nitroimidazoles via ferredoxin (Townson et al., 1996; Leitsch et al., 2011). In addition, GTA-2 and the herein identified alcohol dehydrogenase localize in native polyacrylamide gels together with PFOR (Anita Burgess, unpublished results). These observations imply that PPDK, GTA-2, and alcohol dehydrogenase might be targets of nitroimidazoles because they are closely associated with PFOR, a source of reactive nitroimidazole metabolites (Townson et al., 1996; Leitsch et al., 2011).
Unfortunately, our attempts to identify a thioredoxin which is reduced by G. lamblia thioredoxin reductase have so far been unsuccessful (Leitsch et al., 2011). Thus, we could not measure in the present study whether nitroimidazole treatment has a detrimental effect on thioredoxin reductase activity, as previously done in E. histolytica and T. vaginalis (Leitsch et al., 2007, 2009). A highly probable drug target of metronidazole and other 5-nitroimidazoles, however, is EF-1γ, having an essential function in protein synthesis. Whether nitroimidazole binding to the other identified proteins contributes to the toxicity of metronidazole and tinidazole, remains to be determined by application of appropriate enzyme assays. Our previous results with T. vaginalis (Leitsch et al., 2009) and E. histolytica (Leitsch et al., 2007) suggest that nitroimidazole binding only inhibits some enzyme activities, e.g. thioredoxin reductase, whereas other enzymes, e.g. thioredoxin peroxidase, enolase and malate dehydrogenase in T. vaginalis, are not affected (Leitsch et al., 2009).
3.2. Nitroimidazoles decrease the intracellular thiol pools of G. lamblia
Since nitroimidazoles compromise the redox equilibrium in E. histolytica (Leitsch et al., 2007) and T. vaginalis (Leitsch et al., 2009) by strongly decreasing non-protein thiol levels, we wanted to know whether nitroimidazole treatment leads to a similar effect in G. lamblia. To this end, cultures of G. lamblia 106 were treated with either 50 μM of metronidazole, tinidazole, ronidazole, or 2-nitroimidazole for 2 h. Subsequently, reduced thiol pools were determined with Ellman’s reagent (Leitsch et al., 2007, 2009) and compared to untreated controls (Fig. 3). The non-protein thiol content of untreated G. lamblia 106 was 8.8 ± 3.7 nmol (mg protein)−1.
Fig. 3.
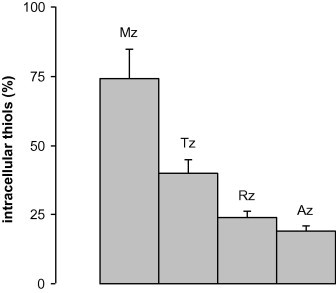
Non-protein thiol content in nitroimidazole-treated cells as compared to untreated controls (100%). Mz, metronidazole; Tz, tinidazole; Rz, ronidazole; Az, 2-nitroimidazole (azomycin). All nitroimidazoles were applied at a concentration of 50 μM for 2 h. All values were determined in at least three independent experiments each. Error bars represent SEM.
All nitroimidazoles tested decreased non-protein thiol levels. However, in stark contrast to E. histolytica (Leitsch et al., 2007) and T. vaginalis (Leitsch et al., 2009), treatment with 2-nitroimidazole had the strongest effect, whereas metronidazole reduced thiol levels by only 25% (Fig. 3).
4. Conclusion
Taken together, our observations strongly suggest that nitroimidazoles are differently metabolized in G. lamblia as compared to the other two parasites. Of the nitroimidazoles tested, only metronidazole and tinidazole formed adducts with proteins (Fig. 2). Treatment with ronidazole only induced the degradation of EF-1γ, and 2-nitroimidazole had no discernible effect on the 2D-profile of treated G. lamblia at all. Further, nitroimidazoles affected intracellular thiol pools in G. lamblia (Fig. 3) fundamentally different as observed in E. histolytica and T. vaginalis (Leitsch et al., 2007, 2009). 2-Nitroimidazole depleted thiol levels in G. lamblia most efficiently (Fig. 3), followed by ronidazole and tinidazole, whereas metronidazole had only a modest effect. This is the exact opposite of the situation in E. histolytica (Leitsch et al., 2007) and T. vaginalis (Leitsch et al., 2009) where metronidazole is more effective than tinidazole and 2-nitroimidazole.
It should be taken into consideration that G. lamblia dwells in the small intestine and, therefore, is adapted to rather high concentrations of oxygen but low concentrations of reactive oxygen species due to the presence of bile which contains high concentrations of glutathione (Mastronicola et al., 2011). As compared to E. histolytica and T. vaginalis, this could be reflected by a different set of factors that influence metabolization of nitroimidazole drugs. Further work will be necessary to identify these factors.
Acknowledgements
This study was supported by grants P22546 (David Leitsch) and P22037 (Sarah Schlosser) of the Austrian Science Fund (FWF). We are very grateful to Jacqui and Peter Upcroft who inspired this study.
References
- Bagchi S., Oniku A.E., Topping K., Mamhoud Z.N., Paget T.A. Programmed cell death in Giardia. Parasitology. 2012;12:1–10. doi: 10.1017/S003118201200011X. [DOI] [PubMed] [Google Scholar]
- Boreham P.F., Phillips R.E., Shepherd R.W. A comparison of the in-vitro activity of some 5-nitroimidazoles and other compounds against Giardia intestinalis. J. Antimicrob. Chemother. 1985;16:589–595. doi: 10.1093/jac/16.5.589. [DOI] [PubMed] [Google Scholar]
- Buret A.G. Pathophysiology of enteric infections with Giardia duodenalis. Parasite. 2008;15:261–265. doi: 10.1051/parasite/2008153261. [DOI] [PubMed] [Google Scholar]
- Campanati L., Monteiro-Real L.H. The effects of the antiprotozoal drugs metronidazole and furazolidone on trophozoites of Giardia lamblia (P1 strain) Parasitol. Res. 2002;88:80–85. doi: 10.1007/s004360100502. [DOI] [PubMed] [Google Scholar]
- Edwards D.I. Nitroimidazole drugs – action and resistance mechanisms. I. Mechanisms of action. J. Antimicrob. Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- Fung H.B., Doan T.L. Tinidazole: a nitroimidazole antiprotozoal agent. Clin. Ther. 2005;27:1859–1884. doi: 10.1016/j.clinthera.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Ghosh E., Ghosh A., Ghosh A.N., Nozaki T., Ganguly S. Oxidative stress-induced cell cycle blockage and a protease-independent programmed cell death in microaerophilic Giardia lamblia. Drug Des. Dev. Ther. 2009;3:103–110. doi: 10.2147/dddt.s5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keister D.B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans. R. Soc. Trop. Med. Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- LaRusso N.F., Tomasz M., Kaplan D., Müller M. Absence of strand breaks in deoxyribonucleic acid treated with metronidazole. Antimicrob. Agents Chemother. 1978;13:19–24. doi: 10.1128/aac.13.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitsch D., Radauer C., Paschinger K., Wilson I.B.H., Breiteneder H., Scheiner O., Duchêne M. Entamoeba histolytica: analysis of the trophozoite proteome by two-dimensional polyacrylamide gel electrophoresis. Exp. Parasitol. 2005;110:191–195. doi: 10.1016/j.exppara.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Leitsch D., Kolarich D., Wilson I.B.H., Altmann F., Duchêne M. Nitroimidazole action in Entamoeba histolytica: a central role for thioredoxin reductase. PLoS Biol. 2007;5:e211. doi: 10.1371/journal.pbio.0050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitsch D., Kolarich D., Binder M., Stadlmann J., Altmann F., Duchêne Trichomonas vaginalis: metronidazole and other nitroimidazole drugs are reduced by the flavin enzyme thioredoxin reductase and disrupt the cellular redox system. Implications for nitroimidazole toxicity and resistance. Mol. Microbiol. 2009;72:518–536. doi: 10.1111/j.1365-2958.2009.06675.x. [DOI] [PubMed] [Google Scholar]
- Leitsch D., Burgess A.G., Dunn L.A., Krauer K.G., Tan K., Duchêne M., Upcroft P., Eckmann L., Upcroft J.A. Pyruvate:ferredoxin oxidoreductase and thioredoxin reductase are involved in 5-nitroimidazole activation while flavin metabolism is linked to 5-nitroimidazole resistance in Giardia lamblia. J. Antimicrob. Chemother. 2011;66:1756–1766. doi: 10.1093/jac/dkr192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronicola D., Giuffrè A., Testa F., Mura A., Forte E., Bordi E., Pucillo L.P., Fiori P.L., Sarti P. Giardia intestinalis escapes oxidative stress by colonizing the small intestine: a molecular hypothesis. IUBMB Life. 2011;63:21–25. doi: 10.1002/iub.409. [DOI] [PubMed] [Google Scholar]
- Moreno S.N.J., Docampo R. Mechanism of toxicity of nitro compounds used in chemotherapy of trichomoniasis. Environ. Health Perspect. 1985;64:199–208. doi: 10.1289/ehp.8564199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. Mode of action of metronidazole on anaerobic bacteria and protozoa. Surgery. 1983;93:165–171. [PubMed] [Google Scholar]
- Müller J., Rühle G., Müller N., Rossignol J.F., Hemphill A. In vitro effects of thiazolides on Giardia lamblia WB clone C6 cultured axenically and in coculture with Caco2 cells. Antimicrob. Agents Chemother. 2006;50:162–170. doi: 10.1128/AAC.50.1.162-170.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nillius N., Müller J., Müller N. Nitroreductase (GlNR1) increases susceptibility of Giardia lamblia and Escherichia coli to nitro drugs. J. Antimicrob. Chemother. 2011;66:1029–1035. doi: 10.1093/jac/dkr029. [DOI] [PubMed] [Google Scholar]
- Oxberry M.E., Thompson R.C., Reynoldson J.A. Evaluation of the effects of albendazole and metronidazole on the ultrastructure of Giardia duodenalis, Trichomonas vaginalis and Spironucleus muris using transmission electron microscopy. Int. J. Parasitol. 1994;24:695–703. doi: 10.1016/0020-7519(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Pervez-Reyes E., Kalyanaraman B., Mason R.P. The reductive metabolism of metronidazole and ronidazole by aerobic liver microsomes. Mol. Pharmacol. 1980;17:239–244. [PubMed] [Google Scholar]
- Samuelson J. Why metronidazole is active against both bacteria and parasites. Antimicrob. Agents Chemother. 1999;43:1533–1541. doi: 10.1128/aac.43.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejman-Yarden N., Eckman L. New approaches to the treatment of giardiasis. Curr. Opin. Infect. Dis. 2011;24:451–456. doi: 10.1097/QCO.0b013e32834ad401. [DOI] [PubMed] [Google Scholar]
- Townson S.M., Upcroft J.A., Upcroft P. Characterisation and purification of pyruvate:ferredoxin oxidoreductase from Giardia duodenalis. Mol. Biochem. Parasitol. 1996;79:183–193. doi: 10.1016/0166-6851(96)02661-8. [DOI] [PubMed] [Google Scholar]
- West S.B., Wislocki P.G., Fiorentini K.M., Alvaro R., Wolf F.J., Lu A.Y.H. Drug residue formation from ronidazole, a 5-nitroimidazole. I. Characterization of in vitro protein alkylation. Chem. Biol. Interact. 1982;41:265–279. doi: 10.1016/0009-2797(82)90105-3. [DOI] [PubMed] [Google Scholar]
- Wislocki P.G., Bagan E.S., Vandenheuvel W.J.A., Walker R.A., Alvaro R.F., Arison B.H., Lu Y.H., Wolf F.J. Drug residue formation from ronidazole, a 5-nitroimidazole. V. Cysteine adducts formed upon reduction of ronidazole by dithionite or rat liver enzymes in the presence of cysteine. Chem. Biol. Interact. 1984;49:13–25. doi: 10.1016/0009-2797(84)90049-8. [DOI] [PubMed] [Google Scholar]


