Graphical abstract
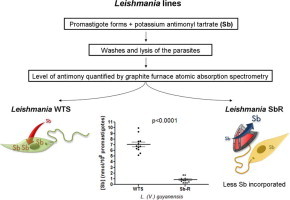
Abbreviations: SbIII, potassium antimonyl tartrate; WTS, Wild-type susceptible; SbR, SbIII-resistant; Lg, L. (V.) guyanensis; Lb, L. (V.) braziliensis; La, L. (L.) amazonensis; Li, L. (L.) infantum; MRPA, multidrug-resistance protein A; Pgp, phosphoglycoprotein; AQP1, aquaglyceroporin-1
Keywords: Leishmania spp., Drug resistance, Potassium antimonyl tartrate, MRPA transporter
Highlights
-
•
Level of expression of Pgp is increased in the SbIII-resistant L. guyanensis and L. amazonensis lines.
-
•
Incorporation of antimony was reduced in the SbIII-resistant L. guyanensis, L. amazonensis and L. braziliensis lines.
-
•
Down-regulation of AQP1 protein was observed in the SbIII-resistant L. guyanensis and L. amazonensis lines.
-
•
Rates of SbIII efflux are higher in the SbIII-resistant lines of L. guyanensis and L. braziliensis.
-
•
Mechanisms of antimony-resistance of the MRPA gene are different among species of Leishmania analyzed.
Abstract
ATP-binding cassette (ABC) transporters have been associated with drug resistance in various diseases. The MRPA gene, a transporter of ABCC subfamily, is involved in the resistance by sequestering metal-thiol conjugates in intracellular vesicles of Leishmania parasite. In this study, we performed the molecular characterization of the MRPA transporter, analysis of P-glycoprotein (Pgp) and aquaglyceroporin-1 (AQP1) expression, and determination of antimony level in antimony-susceptible and -resistant lines of L. (V.) guyanensis, L. (L.) amazonensis, L. (V.) braziliensis and L. (L.) infantum. PFGE analysis revealed an association of chromosomal amplification of MRPA gene with the drug resistance phenotype in all SbIII-resistant Leishmania lines analyzed. Levels of mRNA from MRPA gene determined by real-time quantitative RT-PCR showed an increased expression of two fold in SbIII-resistant lines of Leishmania guyanensis, Leishmania amazonensis and Leishmania braziliensis. Western blot analysis revealed that Pgp is increased in the SbIII-resistant L. guyanensis and L. amazonensis lines. The intracellular level of antimony quantified by graphite furnace atomic absorption spectrometry showed a reduction in the accumulation of this element in SbIII-resistant L. guyanensis, L. amazonensis and L. braziliensis lines when compared to their susceptible counterparts. Interestingly, a down-regulation of AQP1 protein was observed in the SbIII-resistant L. guyanensis and L. amazonensis lines, contributing for decreasing of SbIII entry in these lines. In addition, efflux experiments revealed that the rates of SbIII efflux are higher in the SbIII-resistant lines of L. guyanensis and L. braziliensis, that may explain also the low SbIII concentration within of these parasites. The BSO, an inhibitor of γ-glutamylcysteine synthetase enzyme, reversed the SbIII-resistance phenotype of L. braziliensis and caused an increasing in the Sb intracellular level in the LbSbR line. Our data indicate that the mechanisms of antimony-resistance are different among species of Leishmania analyzed in this study.
1. Introduction
Leishmaniasis is a complex of diseases caused by different species of protozoan parasites belonging to the genus Leishmania. This neglected tropical disease comprises clinical manifestations that range from self-healing cutaneous (CL) and mucocutaneous (MCL) skin lesions to a visceral (VL) form, which is lethal if untreated (Ashutosh et al., 2007). In the New World, L. (Leishmania) infantum (syn. L. (L.) chagasi) (Kuhls et al., 2007) is the causative agent of VL, whereas L. (L.) amazonensis and L. (Viannia) guyanensis cause CL, and L. (V.) braziliensis causes CL and MCL (Marzochi and Marzochi, 1994; Murray et al., 2005; Shaw, 2006). The disease is endemic in 98 countries, especially in northern Africa, Asia, the Mediterranean and Latin America, with more than 350 million people at risk (Ashford et al., 1992; Alvar et al., 2012). There are an estimated 12 million people infected worldwide (World Health Organization, 2012). The estimated incidence of leishmaniasis is approximately 0.2–0.4 million VL cases and 0.7–1.2 million CL cases per year. More than 90% of global VL cases occur in six countries: India, Bangladesh, Sudan, South Sudan, Ethiopia and Brazil. Whereas Afghanistan, Algeria, Colombia, Brazil, Iran, Syria, Ethiopia, North Sudan, Costa Rica and Peru account for 70–75% of global estimated CL incidence (Alvar et al., 2012).
The control of leishmaniasis relies mainly on chemotherapy. The first line choice of treatment against all forms of the disease is based on the administration of pentavalent antimony-containing compounds, such as sodium stibogluconate (SSG) (Pentostam®) and N-methyl-glucamine (Glucantime®). The mechanisms of action of antimony are not fully elucidated. It is known that SbV acts on the amastigote form, inhibiting enzyme activity and the oxidative pathway of fatty acids (Herwaldt, 1999). It is generally agreed that trivalent antimony (SbIII) is the active form of the drug against Leishmania amastigote and promastigote forms (Frézard et al., 2009). Murray and Nathan (1988) demonstrated that macrophage activation has a significant effect on intracellular parasite killing. It has been reported that SSG induces the macrophage to produce leishmanicidal molecules like nitric oxide (NO) and reactive oxygen species (ROS) by activation of signalling pathways, leading to the elimination of intracellular Leishmania donovani amastigotes (Basu et al., 2006). In an animal infection model, the mode of action of SbV is dependent on a number of factors including T cell subsets and cytokines (reviewed by Murray, (2001)). Furthermore, SbV was found to be a potent inhibitor of protein tyrosine phosphatases, leading to an increase in cytokine responses (Pathak and Yi, 2001). Thus, these results suggest that SSG may kill the parasites by both direct and indirect mechanisms. Studies suggest that SbIII causes disturbances in the thiol redox potential of the parasite by inducing the efflux of intracellular thiols and by inhibiting trypanothione reductase, leading the cell to death by oxidative stress (Wyllie et al., 2004; Moreira et al., 2011). It has been shown that antimony kills the parasite by a process involving DNA fragmentation and externalization of phosphatidylserine on the outer surface of membrane (Sereno et al., 2001; Lee et al., 2002; Sudhandiran and Shaha, 2003).
Drug resistance is one of the major clinical problems for the treatment of various diseases ranging from bacteria and parasite infections to cancer. Treatment failure with pentavalent antimonials has been reported recently in several countries. In India, over 60% of patients with VL do not respond to treatment with pentavalent antimony drugs, due to acquired resistance (Sundar et al., 2000). The mechanisms by which species of Leishmania acquire resistance to antimonials have been subject of intensive research for several decades. It has been described that resistance is an interplay between uptake, efflux and sequestration of active molecules (reviewed by Croft et al., 2006). A down-regulation of aquaglyceroporin-1 (AQP1) is correlated with lower SbIII uptake, decreasing the drug concentration within the cell (Marquis et al., 2005). On the other hand, amplification of DNA segments has been observed in several Leishmania species selected for drug resistance (Beverley, 1991; Ouellette and Borst, 1991).
ATP-binding cassette (ABC) transporters have been associated with drug resistance in various diseases. These transporters comprise an ancient superfamily of evolutionarily conserved proteins spanning from bacteria to humans (Dassa and Bouige, 2001). They are integral membrane proteins involved in the energy-dependent transport of a variety of molecules across biological membranes, including amino acids, sugars, peptides, lipids, ions and chemotherapeutic drugs (Higgins, 1992). In mammals, these transporters are associated with chemoresistance mainly through overexpression of the multidrug-resistance (MDR) proteins (Borst et al., 2000). In Leishmania, the first ABC protein identified was MRPA (PgpA) (Ouellette et al., 1990) which is a member of the ABCC subfamily able to confer resistance to antimonials by sequestering metal-thiol conjugates into an intracellular vesicle (Légaré et al., 2001).
Different mechanisms of drug resistance have been identified in Old Word Leishmania species (reviewed by Croft et al., 2006). Although there is considerable evidence showing variability in the response to antimony chemotherapy in New World pathogenic Leishmania species (Moreira et al., 1998; Romero et al., 2001), the mechanisms of drug resistance in these species have not been extensively analyzed. Therefore, understanding the biological diversity and responses to chemotherapy of different New World Leishmania species is necessary to ultimately overcome current limitations in anti-parasitic drug treatment. Thus, in this study we performed the molecular characterization of the MRPA transporter and determination of antimony level in antimony-susceptible and -resistant lines, which were experimentally induced, in four New World Leishmania species: Leishmania guyanensis, Leishmania amazonensis, Leishmania braziliensis and Leishmania infantum. Promastigote forms were characterized for: chromosomal location, analysis of amplification and mRNA levels of the MRPA gene; Pgp and APQ1 protein expression; measurement of intracellular antimony level, efflux rates of SbIII and susceptibility of L. braziliensis lines to BSO, an inhibitor of γ-glutamylcysteine synthetase (GCS) enzyme.
2. Materials and methods
2.1. Leishmania strains
In this study, we used promastigote forms of four different New World Leishmania species: L. guyanensis, L. amazonensis, L. braziliensis and L. infantum (Table 1). These lines were selected in vitro to trivalent antimony (SbIII) by step-wise drug pressure and the resistance index varied from 4 to 20-fold higher than of their wild-type counterparts (Liarte and Murta, 2010). This previous study showed that the SbIII-resistant lines of L. amazonensis, L. braziliensis and L. infantum have cross-resistance to paromomycin. Parasites were grown at 26 °C in M199 medium (Liarte and Murta, 2010).
Table 1.
Leishmania species used in this study⁎.
| Species | Strains | IC50 (μM) |
Resistance index | |
|---|---|---|---|---|
| WTS | SbR | |||
| L. (V.) guyanensis | IUMB/BR/85/M9945 | 0.09 ± 0.04 | 1.64 ± 0.14 | 19 |
| L. (L.) amazonensis | IFLA/BR/67/PH8 | 0.28 ± 0.15 | 1.71 ± 0.11 | 6 |
| L. (V.) braziliensis | MHOM/BR/75/M2904 | 0.15 ± 0.15 | 3.04 ± 0.13 | 20 |
| L. (L.) infantum | MHOM/BR/74/PP75 | 0.33 ± 0.09 | 1.40 ± 0.04 | 4 |
IC50 is the concentration (μM) of SbIII that decreases the rate of cell growth by 50%.The values represent the means ± CI (confidence interval) of three independent experiments in triplicate.
Reference: Liarte and Murta (2010).
2.2. Pulsed field gel electrophoresis (PFGE)
Chromosomal DNA from Leishmania lines (2.0 × 109 cells/mL) was prepared in low-melting temperature agarose plugs as described by Smith et al. (1988). The agarose blocks containing intact Leishmania chromosomes were separated by PFGE in 1% agarose gels in 0.5x TBE (45 mM Tris-HCl, 45 mM boric acid, 1 mM EDTA, pH 8.3) using the Gene Navigator TM System (Amersham Pharmacia, Buckinghamshire, UK). The running buffer (0.5x TBE) was maintained at 9 °C throughout the electrophoresis. Saccharomyces cerevisiae chromosomes were used as size markers (Bio-Rad Inc., Hercules, CA, USA). Electrophoresis conditions were standardized to allow the separation of the greatest number of parasite chromosomes in a single gel, as follows: 90 s for 18 h, 200 s for 18 h, 400 s for 22 h and 600 s for 7 h at 90 V. A range from 365 to 2200 kb was used for wide separation of Leishmania chromosomes. After electrophoresis, gels were stained with ethidium bromide (10 μg/mL) and bands were then transferred onto nylon membrane (Sambrook et al., 1989). The MRPA gene was identified by incubation of the membrane with the 32P-labelled MRPA gene probe. For this probe, was used a 452 bp MRPA fragment (LbrM23_V2.0280) amplified with the primers: forward 5’-TCGTGATTATTCCGTGCGCGTT-3’ and reverse 5’-ACGCTCCACGCTGTTCATGTTT-3’.
2.3. Southern blot
Genomic DNA was isolated from the antimony-susceptible and -resistant Leishmania species according to the protocol described by Sambrook et al. (1989). Approximately 10 μg total DNA of Leishmania lines were digested with the restriction enzyme BamHI (Invitrogen, Carlsbad, CA, USA). The fragments were separated by electrophoresis on a 1% agarose gel and transferred to nylon membrane (Sambrook et al., 1989). The blots were hybridized with [α-32P]dCTP labeled MRPA gene probe as described above. Leishmania DNA polymerase gene (LbrM.16.1600) was used as quantitative control. A fragment of 483 bp from this gene, after amplification using primers: forward 5’-GAAGACAGAGAAGGATGCGA-3’ and reverse 5’-GAGAGCGGGCACCAATCAC-3’, was used as probe. The band intensities were analyzed using the software CP ATLAS 2.0 (http://lazarsoftware.com/download.html).
2.4. Quantitative real-time RT-PCR
Reverse transcription reactions for first strand complementary DNA (cDNA) synthesis were carried out as described below. Each reaction contained 2 μg total RNA, 0.5 μg oligo d(T), 1x first strand buffer, 10 mM DTT, 0.5 mM dNTP, 40 U RNasin and 200 U Superscript II reverse transcriptase in a final volume of 20 μL. All the reagents used were obtained from Invitrogen (Life Technologies, Carlsbad, CA, USA). All reactions were allowed to proceed for 1 h at 42 °C before being stopped by incubation at 70 °C for 20 min. The obtained cDNA was then diluted 15x in water and 5 μL of the reaction product was amplified by real-time PCR using specific primers. Amplification reaction was carried out using the 7000 System SDS Software (PE Applied Biosystems, Foster City, CA, USA). The primers, MRPA forward: 5’-AAGTGGGCAGCGACTCAAA-3’ and MRPA reverse: 5’-CCAGTTCAGCGTCTCCGTT-3’, were the same described by Torres et al. (2010) and Adaui et al. (2011). The DNA polymerase constitutive gene from L. braziliensis (LbrM.16.1600) was used to normalize the amount of sample analyzed. A fragment of 69 bp of the DNA polymerase gene was amplified with the primers forward: 5’-CGAGGGCAAGACATAC-3’ and reverse: 5’-GAGAGCGGGCACCAATCAC-3’. Both pair of primers MRPA and DNA polymerase amplified fragments of 81 and 69 bp respectively, in all four Leishmania species analyzed (data not shown). PCR was carried out in a final volume of 20 μL of reaction mixture containing 10 pmol of forward and reverse primers, 1x SYBR GREEN reaction mix (Applied Biosystems, Life Technologies, Carlsbad, CA, USA) and 5 μL of template DNA. The PCR conditions were as follows: an initial denaturation step at 95 °C for 10 min followed by 40 cycles of denaturation at 95 °C for 15 s and annealing/extension at 60 °C for 1 min. Standard curves were prepared for each run using known quantities of TOPO PCR 2.1 plasmids (Invitrogen, Life Technologies, Carlsbad, CA, USA) containing the MRPA and DNA polymerase genes. Estimates of DNA levels were obtained using the Sequence Detection System data analysis software. Values were normalized to those obtained for DNA polymerase for each sample.
2.5. Western blot analysis
Total proteins and membrane protein fractions from different Leishmania lines were extracted according to the protocols described by Gamarro et al. (1994) and Grogl et al. (1991), respectively. Protein extracts were separated by electrophoresis on a 12% SDS polyacrylamide gel and electrotransferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA). The blots were blocked by incubation with 5% instant non-fat dry milk in PBS supplemented with 0.05% Tween 20 (PBS-T) for 1 h. The blots were then washed twice in PBS-T and incubated for 16 h at 4 °C in the presence of antibodies: monoclonal antibody C219 (1:100) (Abcam, Cambridge, UK) or rabbit polyclonal antibody anti-AQP1 (1:5) (kindly provided by Andrade et al. – in preparation). The blots were washed and incubated with anti-IgG mouse HRP-conjugate (1:2,000) (GE Healthcare) or HRP-conjugated anti-rabbit IgG (1:5,000) (GE Healthcare) for 1 h at room temperature. After, the blots were exposed to Amersham ECL Plus detection agent according to the manufacturer’s instructions and exposed to an X-ray film (Amersham, Buckinghamshire, UK). The results were normalized using the anti-α-tubulin monoclonal antibody (Sigma, St. Louis, USA). The intensity of the bands was analyzed using the software CP ATLAS 2.0.
2.6. Antimony transport assays
2.6.1. Uptake assay
Before performing the uptake and efflux assays, the SbIII-resistant lines are maintained at least two passages in M199 medium in the absence of SbIII, in order to eliminate the residual drug. The antimony uptake assay was based on two protocols previously described (Roberts and Rainey, 1993; Wang et al., 2003). Log phase Leishmania promastigotes were washed twice in a HEPES/Glucose (HG) buffer (20 mM HEPES, 0.15 M NaCl, 10 mM glucose, pH 7.2) and resuspended in this buffer at a density of 1.0 × 108 cells/mL. The volume of 1 mL of this parasite suspension was aliquoted into tubes in quadruplicate: tubes containing only parasites (blank) and tubes with parasites in the presence of 540 μM SbIII (potassium antimonyl tartrate) (Sigma, Saint Louis, MO, USA). Cells were then incubated at 26 °C for 1 h under agitation. Subsequently, the cells were centrifuged at 1816g for 5 min at 4 °C and washed three times with HG buffer. Pellets were then resuspended with 100 μL HG buffer. One aliquot of 10 μL of each tube was used for normalization (parasite quantification) and the remaining volume (90 μL) was submitted to digestion with nitric acid (65%). Antimony concentration was quantified by graphite furnace atomic absorption spectrometry (Perkin Elmer AAnalyst 600). Each uptake assay was performed three times and the signal from blanks was used for background subtraction.
In addition, the antimony-susceptible and -resistant lines of the species L. guyanensis and L. braziliensis were evaluated for the kinetics of incorporation of Sb. The parasites were incubated with 540 μM SbIII and, at different time intervals (0, 0.5, 1, 2 and 3 h), 1 mL aliquots of the parasite suspension were taken and they were submitted to the protocol described above. This assay was also performed to identify the time points at which the WTS and SbR lines exhibit the same intracellular concentration of Sb, to be explored in the efflux protocol.
2.6.2. Efflux assay
Log phase antimony-susceptible and -resistant L. guyanensis and L. braziliensis cells were washed twice with HG buffer and resuspended in this buffer at a density of 1.0 × 108 cells/mL. Aliquots of 1 mL containing only parasites (blank) were taken and the remaining cells were incubated with 540 μM SbIII for each 1 mL (potassium antimonyl tartrate) (Sigma, Saint Louis, MO, USA) at 26 °C under agitation. The WTS lines of L. guyanensis and L. braziliensis were incubated during 2 min and 30 min, respectively. Both SbR lines of these species were incubated for 70 min. After incubation, the cells were centrifuged at 890g for 5 min at 4 °C and washed two times with HG buffer. The pellets were then resuspended with HG buffer at the original density and the cells were incubated at 26 °C under agitation. Aliquots of 1 mL were taken from the parasite suspension at the times 0, 1 and 2 h. Subsequently, these aliquots and the blanks were centrifuged at 890g for 5 min at 4 °C and washed three times with HG buffer. After the last centrifugation, the pellets were resuspended with 100 μL HG buffer. One aliquot of 10 μL of each tube was used for normalization (parasite quantification) and the remaining volume (90 μL) was submitted to digestion with nitric acid (65%). Antimony concentration was quantified by graphite furnace atomic absorption spectrometry (Perkin Elmer AAnalyst 600). Each efflux assay was performed three times in triplicate and the SbIII dosage from blanks was used for background subtraction.
2.6.3. Test of susceptibility to BSO and SbIII uptake in L. braziliensis lines pre-treated with this inhibitor
Approximately 2.0 × 106 cells/mL of L. braziliensis were grown in M199 medium containing various concentrations (2.5–30 mg/mL) of buthionine-sulphoximine (BSO) (Sigma) during 48 h at 26 °C. BSO is an inhibitor of γ-glutamylcysteine synthetase (γ-GCS), a rate-limiting enzyme in thiol biosynthesis (Griffith and Meister, 1979). The drug concentration that decreases cell growth by 50% (IC50) was determined by counting the parasites in the presence and absence of the BSO. After pre-treatment with BSO, the uptake of Sb was investigated in both susceptible and resistant L. braziliensis lines. These parasites were first grown in M199 medium for 48 h in the presence BSO at their respective IC50. Subsequently, the cells were washed twice with HG buffer, resuspended in this buffer at a density of 1.0 × 108 cells/mL and submitted to the uptake assay.
2.7. Statistical analysis
Data were analyzed by Student’s t test performed using the software GraphPad Prism 5.0. A p value of <0.05 was considered statistically significant.
3. Results
3.1. MRPA amplification in SbR Leishmania parasites
The molecular karyotype of antimony-resistant and -susceptible Leishmania lines, obtained through PFGE is presented in Fig. 1A. Overall, the pattern of the physical chromosomal map obtained for Sb-resistant and -susceptible Leishmania parasites is similar except for LgSbR, which, in comparison with its parental WT strain, showed a supplementary band of approximately 1900 kb (Fig. 1A white arrow). The pattern of hybridization of the chromosomes showed that the MRPA gene probe recognized a chromosomal band of approximately 800 kb in all lines of Leishmania analyzed (Fig. 1B) which fits with the size of chromosome 23 (795 kb). In a preliminary analysis, the intensity of this band was increased in the SbIII-resistant line of L. amazonensis. An interesting observation in the antimony-resistant line of L. braziliensis (LbSbR) is that the MRPA gene probe recognized in this sample a DNA smear and two other bands of approximately 200 kb and 1500 kb (Fig. 1B). This result indicates an extrachromosomal amplification of MRPA gene in LbSbR. In agreement with this observation, another band of approximately 700 kb was observed in the resistant sample of L. infantum (Fig. 1B).
Fig. 1.
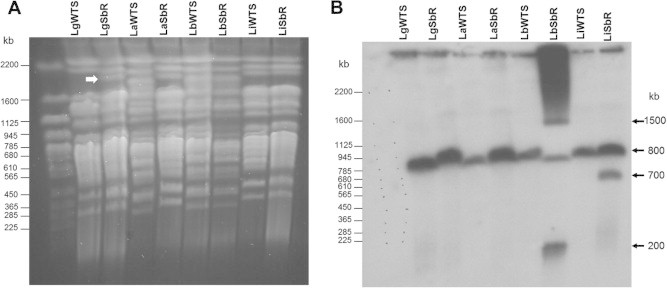
Chromosomal location of the MRPA gene in the antimony-susceptible (WTS) and -resistant (Sb-R) lines of Leishmania. (A) Chromosomal bands from the Leishmania species were separated by PFGE and stained with ethidium bromide. (B) Profile of the chromosomal bands hybridized with a 32P-labelled MRPA-specific probe. Whole chromosomes from Saccharomyces cerevisiae were used as molecular weight markers. The white arrow shows an additional band of approximately 1900 kb in the LgSbR line.
Southern blot assays were carried out using samples of genomic DNA from different Leishmania lines previously digested with the endonuclease BamHI, which has one restriction site within MRPA gene (LbrM23_V2.0280) in a conserved region. Hybridization of nylon membranes containing BamHI-digested DNA against a MRPA-specific probe revealed a major band of 11.0 kb and other faint band of 5.0 kb in all samples analyzed (Fig. 2A). Considering the 11 kb band, after normalization using a DNA polymerase gene probe (Fig. 2B), the densitometry revealed an increased intensity of three- and ten-fold in antimony-resistant L. amazonensis and L. braziliensis lines, respectively, when compared to their susceptible counterparts. This data confirms MRPA gene amplification in these antimony-resistant Leishmania lines. No difference was observed in the other Leishmania lines analyzed.
Fig. 2.
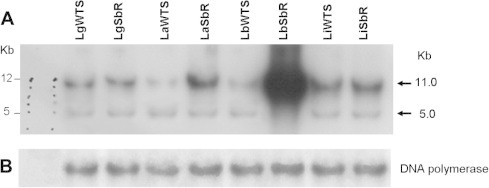
Southern blot analysis of the MRPA gene from antimony-susceptible (WTS) and -resistant (Sb-R) Leishmania lines. Total DNA were digested with the BamHI (A) endonuclease, subject to electrophoresis on a 1% agarose gel. Southern blots were hybridized with a 32P-labelled MRPA-specific probe. As control, the membrane was exposed to a Leishmania DNA polymerase gene probe (B).
3.2. Increased MRPA transcripts in SbR Leishmania
MRPA mRNA levels in the Leishmania lines were evaluated by real-time RT-PCR. Measurements of total RNA were normalized by comparison with those obtained for the housekeeping gene DNA polymerase (LbrM.16.1600). Then, a standard curve was obtained using serial dilutions (107–102 molecules) of plasmids containing the DNA polymerase and MRPA genes cloned into pCR2.1 TOPO vector. We obtained a standard curve with good linearity for both genes (r2 = 0.998 to DNA polymerase and r2 = 0.973 to MRPA). Our data showed a slope of −3.78 and −3.60 for the DNA polymerase and MRPA genes, respectively, indicating high efficiency of PCR (results not shown). The specificity of PCR was analyzed by plots of the temperature-dependent dissociation of the SYBR GREEN dye from the MRPA and DNA polymerase PCR products. The results revealed that fluorescence was only emitted at one temperature, suggesting that only one PCR product was generated in each reaction (results not shown).
The amount of cDNA amplified in the samples of Leishmania spp. was determined by linear regression analysis using the Ct values obtained from the standard curve generated with known amounts of the plasmids of the MRPA gene, normalized with DNA polymerase values. The results showed that mRNA level from MRPA gene is increased two-fold in the antimony-resistant L. guyanensis, L. amazonensis and L. braziliensis lines compared with their respective susceptible pairs. No difference in the MRPA gene expression between antimony-resistant and -susceptible lines of L. infantum was observed (Fig. 3).
Fig. 3.
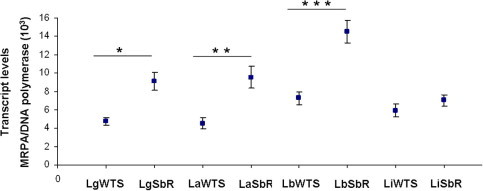
Levels of transcription of the MRPA gene in Leishmania spp. susceptible and resistant lines. Levels of MRPA mRNA as determined quantitatively (relative to the DNA polymerase Leishmania gene) by real-time RT-PCR. Mean values of the transcript levels MRPA/DNA polymerase ± standard deviations from three independent experiments are indicated. The mean values for LgWTS and LgSbR, LaWTS and LaSbR and LbWTS versus LbSbR are significantly different (∗p < 0.002, ∗∗p < 0.003 and ∗∗∗p < 0.001, respectively), whilst the LiWTS versus LiSbR mean values show no difference.
3.3. Expression level of Pgp
We also determined the levels of Pgp in the antimony-susceptible and -resistant Leishmania lines by Western blot analysis using a monoclonal antibody (C219). According to manufacturer’s instructions (Abcam), this antibody recognizes an internal, highly conserved amino acid sequence: VQEALD and VQAALD, corresponding to the C-terminal and N-terminal regions, respectively, found in both MDR1 and MDR3 isoforms of P-glycoprotein of mammals. The results revealed that the C219 antibody recognized a polypeptide of 170 kDa in some Leishmania samples analyzed (Fig. 4B). According to literature data, this polypeptide corresponds to the expected size of Pgp (Cornwell et al., 1987). Interestingly, this antibody detected Pgp only in antimony-resistant L. guyanensis and L. amazonensis lines, but not in the susceptible ones. This result indicates that Pgp is more expressed in these antimony-resistant Leishmania lines, whereas the Pgp levels in the susceptible lines were not high enough for detection. On the other hand, this antibody recognized Pgp in both antimony-susceptible and -resistant L. infantum lines. Densitometry using α-tubulin levels for normalization showed the same level of Pgp expression between both L. infantum lines (Fig. 4C). Additionally, we observed that the C219 antibody also detected another polypeptide of about 48 kDa in all antimony-susceptible and -resistant Leishmania lines analyzed. However, the intensity of this polypeptide was very faint for both L. braziliensis lines (Fig. 4B). The presence of this 48 kDa polypeptide can be due to antibody recognition of a common epitope of another protein of Leishmania or an original fragment of Pgp. It is described in the literature that this same anti-Pgp C219 antibody recognizes smaller polypeptides named as “Pgp-like” components (Grogl et al., 1991; Murta et al., 2001; Anacleto et al., 2003).
Fig. 4.
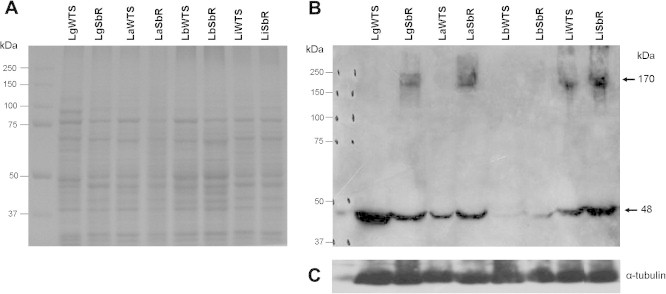
Pgp expression levels in Leishmania species. (A) Electrophoretic profile of proteins from antimony-susceptible (WTS) and -resistant (Sb-R) Leishmania lines. Proteins were separated by SDS-PAGE on 12% gel and stained with Coomassie blue. (B) Western blot analysis using the monoclonal antibody C219. (C) The membrane was normalized with the α-tubulin antibody.
It is important to emphasize that the anti-Pgp antibody did not detect the protein of 170 kDa in any of the two L. braziliensis lines. This result may be due to the absence of overexpression of Pgp in the LbSbR line or changes may have occurred in the amino acid sequence of the epitope region of the protein, preventing its recognition by the C219 antibody. We have also questioned whether the protocol used for total protein extraction was appropriate or not to investigate the Pgp expression that is a transmembrane protein. Thus, a protocol described by Grogl et al. (1991) was also used to obtain enriched fraction of membrane proteins separate from soluble protein fraction. After extraction, the membrane proteins fractions of all Leishmania lines were submitted to western blot analysis using the C219 antibody. However, the results obtained with this protocol (results not shown) were similar from those previously obtained using total protein (Fig. 4B).
3.4. Intracellular accumulation of antimony
In order to compare the level of antimony uptake between antimony-susceptible and -resistant Leishmania lines, the parasites were incubated for 1 h in the presence of 540 μM antimony and, after washing, the intracellular antimony level was quantified by graphite furnace atomic absorption spectrometry. Fig. 5 shows that the uptake of antimony in the resistant lines was significantly reduced in three out of four species analyzed. The data indicate that the antimony levels were approximately seven-, five- and two-fold lower in the antimony-resistant L. guyanensis (Fig. 5A), L. amazonensis (Fig. 5B) and L. braziliensis (Fig. 5C) lines, when compared to their susceptible pairs, respectively. However, the susceptible and resistant lines of L. infantum (Fig. 5D) showed no statistically significant difference in the antimony incorporation. The graphics also show marked differences in the level of antimony incorporation between the different species studied.
Fig. 5.
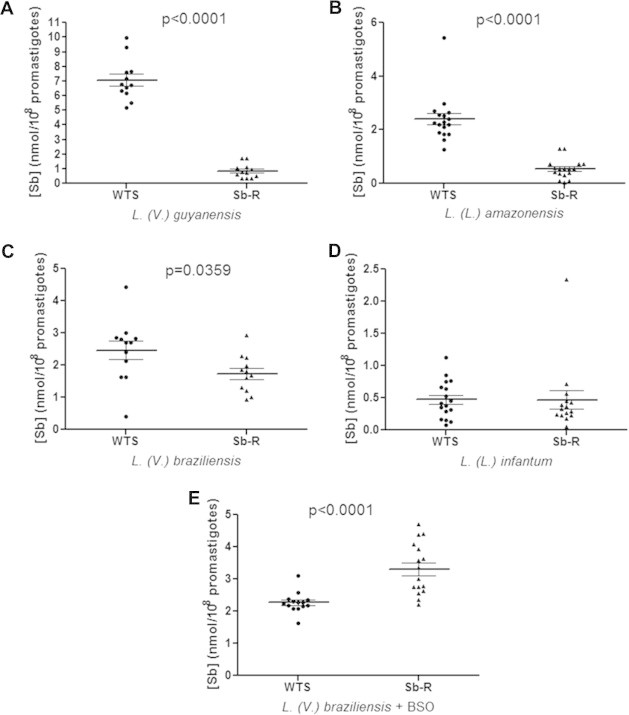
Antimony uptake in promastigote forms of antimony-susceptible (WTS) and -resistant (Sb-R) lines from four different species of Leishmania. Intracellular antimony was quantified by graphite furnace atomic absorption spectrometry after incubation with 540 μM SbIII for 1 h. The values obtained from three independent experiments in quadruplicate, the mean antimony concentration ± SEM and the value p are represented in the graphics for the species: L. (V.) guyanensis (A), L. (L.) amazonensis (B), L. (V.) braziliensis (C) e L. (L.) infantum (D). The effect of BSO in the SbIII uptake by parasites pre-treated with this inhibitor was also investigated in both L. (V.) braziliensis lines (E).
In addition, these results of antimony intracellular accumulation demonstrate that there is an inverse correlation between the intrinsic antimony susceptibility of the Leishmania wild-type lines used in this study and their ability to accumulate antimony. For example, the LgWTS line, which is relatively more Sb sensitive when compared to LiWTS (IC50 0.09 μM versus IC50 0.33 μM, respectively, a 3.7-fold difference), accumulated 14-fold less Sb. The same remark can be made for other lines of Leishmania. The LgWTS line accumulated 2.8-fold less Sb than the LaWTS line (IC50 0.28 μM, a 3-fold difference) and the LbWTS line (IC50 0.15 μM) accumulated 5-fold less Sb than the LiWTS line (a 2.2-fold difference in IC50).
3.5. Susceptibility of L. braziliensis lines to BSO and the effect of this inhibitor in the SbIII uptake
We also investigated the effect of BSO, an inhibitor of γ-glutamylcysteine synthetase (GCS) enzyme, in the intracellular accumulation of antimony by LbWTS and LbSbR lines pre-treated with this inhibitor. Interestingly, the BSO susceptibility assay showed that the LbSbR line is more susceptible to BSO than its susceptible counterpart LbWTS. The IC50 obtained for LbWTS and LbSbR lines were 15 mM and 2.5 mM, respectively. Then, these lines were pre-treated with BSO during 48 h, incubated with SbIII by 1 h and then submitted to antimony uptake measurement (uptake assay). Our results revealed that LbSbR line accumulated more SbIII compared to its susceptible pair (LbWTS) (Fig. 5E). On the other hand, without BSO, the LbSbR line accumulates less SbIII (Fig. 5C). These results suggest that BSO probably decreases the intracellular concentration of thiols, interfering directly on the SbIII-thiol complex formation and leading to the SbIII accumulation. In addition, we determined the SbIII IC50 for the LbWTS and LbSbR lines in the absence and presence of BSO. The LbWTS and LbSbR lines were pre-treated with 15 mM and 2.5 mM BSO, respectively, during 48 h. Subsequently, we incubated these parasites with different SbIII concentrations for 48 h. The IC50 obtained for the LbWTS line in the absence of BSO was 0.025 mg/mL and in the presence of BSO was 0.00625 mg/mL (4-fold lower). On the other hand, for the LbSbR line the IC50 was 2 mg/mL and after pre-treatment with BSO it was 0.0625 mg/mL (32-fold lower), indicating that the BSO reversed the SbIII resistance phenotype.
3.6. Influx and efflux of antimony
Initially, we performed influx kinetics analysis for the lines of L. guyanensis and L. braziliensis. Our results showed that both SbR lines exhibited lower rate of influx of SbIII than their respective susceptible counterparts, the difference being much more pronounced in the case of L. guyanensis species (Figs. 6A and B). In order to compare the efflux of Sb between susceptible and resistant lines, cells were first loaded with about the same level of antimony. Subsequently, the parasites were washed with HG buffer and aliquots were taken at the times 0, 1 and 2 h to analyze the efflux of antimony by graphite furnace atomic absorption spectrometry. The results demonstrate that the rates of SbIII efflux were higher in both antimony-resistant lines of L. guyanensis and L. braziliensis, when compared their susceptible pairs (Fig. 6C and D, respectively). This efflux data explains, at least in part, the lower SbIII concentration found in both SbIII-resistant lines, as presented in the Fig. 5A and C, respectively.
Fig. 6.
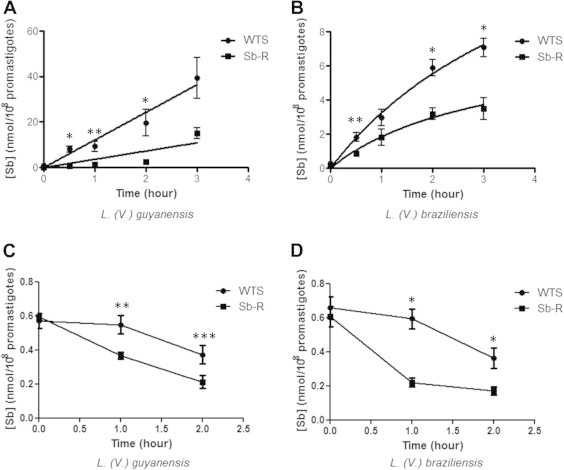
Time course of SbIII uptake and efflux in promastigote forms from antimony-susceptible (WTS) and -resistant (Sb-R) Leishmania lines. The kinetics of uptake and efflux of SbIII were determined by graphite furnace atomic absorption spectrometry in WTS and Sb-R lines of L. (V.) guyanensis (A) and L. (V.) braziliensis (B) during 0–3 h of SbIII incubation. The efflux rates of L. (V.) guyanensis (C) and L. (V.) braziliensis (D) were determined during 0–2 h. Data shown are the means of three independent experiments in triplicate of antimony concentration ± SEM. Statistically different values are highlighted as follows: ∗p < 0.01, ∗∗p < 0.02 and ∗∗∗p < 0.05.
3.7. Decreased expression of AQP1 in SbR Leishmania
In order to investigate whether the lower level of intracellular antimony in the SbIII-resistant lines was due to down-regulation of AQP1 protein, we evaluated the expression level of this protein in the four Leishmania species studied. Western blot analyses with membrane proteins fractions were performed using a rabbit polyclonal antibody anti-AQP1. Our results showed that this antibody recognized a polypeptide of approximately 35 kDa in all antimony-susceptible and -resistant lines of Leishmania analyzed (Fig. 7). After normalization using an α-tubulin antibody, quantification of the band intensity demonstrated that AQP1 expression level is decreased 1.7 and 3-fold in SbIII-resistant lines of L. amazonensis and L. guyanensis, respectively, when, compared to their respective susceptible pairs (Fig. 7). These data are consistent with the lowest SbIII accumulation presented by these two lines among the species studied (Fig. 5). On the other hand, the expression level of AQP1 was similar between the SbIII-susceptible and -resistant lines of L. braziliensis and L. infantum showing band intensities ratio (WT/SbR) values of 0.9 and 0.8, respectively (Fig. 7).
Fig. 7.
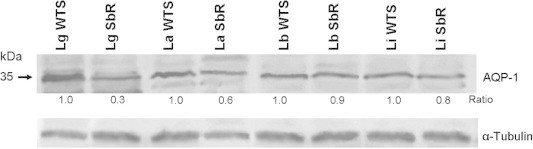
Expression level of AQP1 protein in Leishmania spp. susceptible and resistant lines. Analysis of Western blot with the rabbit polyclonal antibody against the AQP1 protein. The intensity of bands was quantified using ImageJ (http://rsbweb.nih.gov/ij/). Bands were normalized using α-tubulin and the intensity ratio is shown relative to the WTS band (SbR/WTS).
4. Discussion
The phenomenon of resistance to antimonials in Leishmania is complex, multifactorial and involves several pathways, which have similar features with other microorganisms. These pathways include the entry, metabolism, efflux and/or sequestration, as well as cell death through the drug action (Jeddi et al., 2011). MRPA is one of the most studied ABC transporters in Leishmania and its role in the resistance has been well established in vitro (Ouellette et al., 1990). However, the majority of these studies were performed using Old World drug-resistant Leishmania lines.
Our PFGE results showed that the MRPA gene is located in an 800 kb band corresponding to chromosome 23 (795 kb) of Leishmania, corroborating literature data (Leprohon et al., 2009; Monte Neto et al., 2011). Interestingly, the MRPA gene probe also recognized two other bands of different sizes, 200 and 1500 kb, only in the antimony-resistant L. braziliensis line. These supplementary bands indicate that MRPA is rearranged or present as an extrachromosomal amplification in LbSbR (Fig. 1B). Additionally, the smear observed exclusively in this resistant line, provide an evidence of circular DNA amplification as indicated by this particular migration in PFGE possibly corresponding to various topoisomers of the circles (White et al., 1988). According to Beverley (1988), large supercoiled circular DNAs appear to exhibit unusual electrophoretic mobility in PFGE, when compared to the large linear chromosomes. Then, an explanation for the presence of approximately 1500 kb chromosomal band in the antimony-resistant L. braziliensis line may be related to the integration of this band into large linear chromosomal DNA or the formation of large concatenated networks of circular DNA. Moreover, Grondin et al. (1998) demonstrated the formation of extrachromosomal circular DNA amplification derived from precursors linear amplicons in methotrexate-resistant L. tarentolae. This possibility could explain the presence of additional bands concomitant with the smear detected by MRPA gene probe in the same LbSbR line. It is also important to note that the MRPA gene probe recognized a band of approximately 700 kb only in the antimony-resistant L. infantum line. It is described in the literature that the variability in the chromosomal location can be due to the presence of homologous chromosomes of different sizes, variation in the size of the telomeric regions, small deletions or insertions (Henriksson et al., 1993). However, the formation of a MRPA-containing linear extrachromosomal DNA amplification in LiSbR is not discarded. Further studies could confirm the presence of supernumerary chromosomes in these New World Leishmania parasites as suggested by MRPA hybridization as well as by the presence of a new band in LgSbR compared to LgWTS (Fig. 1A, white arrow). Aneuploidy was a phenomenon previously described in drug-resistant Old World Leishmania parasites (Ubeda et al., 2008; Leprohon et al., 2009; Downing et al., 2011) and it supports the evidence of the genome structural plasticity requirement to adapt and survive in the presence of the drug.
Literature data also show the presence of extrachromosomal MRPA amplification in other Leishmania species. Anacleto et al. (2003) reported the presence of extrachromosomal amplicon of 30 kb containing the PgpA gene in L. guyanensis with in vitro-induced resistance to Glucantime. Mukherjee et al. (2007) observed that the MRPA gene was amplified in clinical isolates of sodium antimony gluconate (SAG)-resistant L. donovani lines studied. Recently, Monte Neto et al. (2011) observed that the MRPA gene is present in extrachromosomal linear amplicons in two independent mutants of antimony-resistant L. amazonensis. Sampaio and Traub-Cseko (2003) reported the presence of amplification of the biopterin transporter in a linear and stable chromosome of 245 kb in L. braziliensis (M2903 strain). However, the results obtained by Dias et al. (2007) suggest that in L. braziliensis the generation of amplicons is not a common phenomenon when this specie is subjected to drug pressure. Nevertheless, our results in antimony-resistant L. braziliensis line indicate that extrachromosomal amplification of the MRPA gene may occur, corroborating the data of Sampaio and Traub-Cseko (2003). A possible explanation for the lack of amplification in the samples of L. braziliensis analyzed by Dias et al. (2007) may be due to differences in the antimony-resistance level and the protocol used to obtain the resistant parasites when compared to our study.
Our data showed that MRPA transcripts are twice as high in the antimony-resistant L. guyanensis, L. amazonensis and L. braziliensis lines, compared to their wild-type pairs. This data correlates with the amplification of this gene in antimony-resistant lines, as shown in PFGE and Southern blot assays. However, the MRPA gene amplification of SbIII-resistant L. braziliensis line (10-fold higher) does not reflect in the level of mRNA that was only two-fold higher than its susceptible counterpart. This could also be due to post-transcriptional control that decreases the RNA levels of this gene (Teixeira, 1998). It was shown by targeted DNA microarray that the MRPA transporter is overexpressed in axenic amastigotes of antimony-resistant L. infantum (El Fadili et al., 2005). Recently, Monte Neto et al. (2011) reported an overexpression of this gene in antimony-resistant L. amazonensis, using whole-genome DNA microarrays. Transcriptome data of L. donovani from antimony-resistant clinical isolates demonstrated that the MRPA gene is not increased in these resistant parasites samples (Decuypere et al., 2005). These differences could be explained by the diversity in resistance indexes as well as in the resistance phenotype stability from different species and genetic background (Decuypere et al., 2012).
A multidrug resistance phenotype associated with the overexpression of Pgp was found in tumoral cell lines and in different pathogenic protozoa (Ullman, 1995). In this study, we investigated whether Pgp, which is a product of the MDR1 gene, was also overexpressed in our antimony-resistant Leishmania lines. Our results showed that the anti-Pgp antibody C219 detected a 170 kDa polypeptide corresponding to the expected size for Pgp. Interestingly, this protein is more expressed in the antimony-resistant L. guyanensis and L. amazonensis lines. However, no difference in Pgp expression between antimony-susceptible and -resistant lines of L. infantum was detected, suggesting that Pgp may be not involved in drug resistance in this Leishmania species.
In this study, we used the technique of graphite furnace atomic absorption spectrometry to measure the amount of antimony accumulated in the Leishmania species. Our data show that the incorporation of antimony was reduced in the antimony-resistant L. guyanensis, L. amazonensis and L. braziliensis lines (Fig. 5). Literature data demonstrated that antimony-resistant L. infantum and L. panamensis lines incorporated less antimony, when compared to their susceptible pairs (Brochu et al., 2003). Maharjan et al. (2008) also showed that the SAG-resistant clinical isolates of L. donovani accumulated about three- to seven-fold less antimony compared to clinical isolates susceptible to this drug. All these literature data corroborate our findings, showing that the resistant parasites accumulate less antimony compared with the respective susceptible pairs.
It has been reported that AQP1 is an important carrier for which cells can accumulate SbIII in Leishmania (Borgnia et al., 1999; Gourbal et al., 2004; Marquis et al., 2005). Our results revealed reduced expression of the AQP1 protein in the SbIII-resistant lines of L. guyanensis and L. amazonensis. Thus, this result may explain, at least in part, the reduced uptake of antimony in these resistant lines of the parasite. In fact, previous studies have shown that the reduced accumulation of SbIII in resistant mutants to this compound could be due to decreased activity of AQP1 and that loss of an allele of this gene can cause an increase in the resistance (Gourbal et al., 2004; Marquis et al., 2005; Mukherjee et al., 2013). It is also possible that another transporter may be involved in the low accumulation of antimony in resistant Leishmania lines. On the other hand, no significant difference of antimony incorporation was observed between the antimony-susceptible and -resistant lines of L. infantum. This can be explained due to the low resistance index of this antimony-resistant line. These data of antimony accumulation suggest the presence of different antimony-resistance mechanisms among these Leishmania species analyzed.
We investigated the SbIII efflux rates in lines of L. guyanensis and L. braziliensis. The results revealed that the efflux rates are higher in the antimony-resistant lines of these parasites, contributing to the lower accumulation of SbIII in these Leishmania species studied. Although, the SbIII-resistant L. braziliensis line present low antimony concentration, the level of expression of AQP1 was similar between both SbIII-susceptible and -resistant lines, suggesting that the AQP1 is not involved in the antimony-resistance phenotype. However, the SbIII-resistant L. braziliensis line showed an increased rate of antimony efflux, which could explain in part the lower SbIII accumulation in this line.
Interestingly, susceptibility test to BSO, an inhibitor of γ-glutamylcysteine synthetase (GCS), showed that this inhibitor reversed the SbIII-resistance phenotype of L. braziliensis. The LbSbR line was more susceptible to BSO than its susceptible counterpart LbWTS. More importantly, with BSO pre-treatment, the resistant parasites LbSbR became 32-fold more susceptible to SbIII than those without treatment. Additionally, we analyzed the effect of the BSO in the uptake of antimony in the lines of L. braziliensis pre-treated with this inhibitor. Our results showed that the SbIII-resistant line accumulated more antimony than its wild-type counterpart, suggesting this inhibitor interferes in the polyamine metabolism, changing the intracellular concentration of thiols and antimony. Indeed, in vitro studies have shown that BSO, a specific inhibitor of GCS, an enzyme involved in glutathione and trypanothione biosynthesis, can reverse resistance to trivalent antimony in the parasite L. tarentolae (Grondin et al., 1997).
In conclusion, our data show that the antimony resistance mechanisms are different in the New World Leishmania species analyzed in this study. Functional analysis studies will be performed to investigate the involvement of the MRPA gene in our Leishmania samples.
Acknowledgements
The authors thank the Program for Technological Development in Tools for Health-PDTIS-FIOCRUZ for use of its facilities. We also thank Juvana M. Andrade for kindling provided the polyclonal antibody anti-AQP1. This investigation received financial support from the following agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Universal 475782/2012-7), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG – CBB-PPM 00536/11 and 00196/13) and UNICEF/UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases (TDR) and P3D-Programa de descoberta e desenvolvimento de drogas (PROEP/CNPq/FIOCRUZ 401988/2012-0). S.M.F. Murta and F. Frézard are research fellows supported by CNPq (National Council for the Development of Research of Brazil); D.S. Moreira is supported by CNPq (140435/2013-1) and J.M. Andrade by FIOCRUZ.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Adaui V., Schnorbusch K., Zimic M., Gutiérrez A., Decuypere S., Vanaerschot M., Doncker D.E., Maes S., Llanos-Cuentas I., Chappuis A., Arévalo F., Dujardin J. Comparison of gene expression patterns among Leishmania braziliensis clinical isolates showing a different in vitro susceptibility to pentavalent antimony. Parasitology. 2011;138:183–193. doi: 10.1017/S0031182010001095. [DOI] [PubMed] [Google Scholar]
- Alvar J., Vélez I.D., Bern C., Herrero M., Desjeux P., Cano J., Jannin J., den Boer M., W.H.O. Leishmaniasis Control Team Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacleto C., Abdo M.C., Ferreira A.V., Murta S.M., Romanha A.J., Fernandes A.P., Moreira E.S. Structural and functional analysis of an amplification containing a PGPA gene in a glucantime-resistant Leishmania (Viannia) guyanensis cell line. Parasitol. Res. 2003;90:110–118. doi: 10.1007/s00436-002-0798-x. [DOI] [PubMed] [Google Scholar]
- Ashford R.W., Seaman J., Schorscher J., Pratlong F. Epidemic visceral leishmaniasis in southern Sudan: identity and systematic position of the parasites from patients and vectors. Trans. R. Soc. Trop. Med. Hyg. 1992;86:379–380. doi: 10.1016/0035-9203(92)90229-6. [DOI] [PubMed] [Google Scholar]
- Ashutosh, Sundar S., Goyal N. Molecular mechanisms of antimony resistance in Leishmania. J. Med. Microbiol. 2007;56:143–153. doi: 10.1099/jmm.0.46841-0. [DOI] [PubMed] [Google Scholar]
- Basu J.M., Mookerjee A., Sen P., Bhaumik S., Banerjee S., Naskar K., Choudhuri S.K., Saha B., Raha S., Roy S. Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages. Antimicrob. Agents Chemother. 2006;50:1788–1797. doi: 10.1128/AAC.50.5.1788-1797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S.M. Characterization of the ‘unusual’ mobility of large circular DNAs in pulse-field gradient electrophoresis. Nucleic Acids Res. 1988;16:925–939. doi: 10.1093/nar/16.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S.M. Gene amplification in Leishmania. Annu. Rev. Microbiol. 1991;45:417–444. doi: 10.1146/annurev.mi.45.100191.002221. [DOI] [PubMed] [Google Scholar]
- Borgnia M., Nielsen S., Engel A., Agre P. Cellular and molecular biology of aquaporin water channels. Annu. Rev. Biochem. 1999;68:425–458. doi: 10.1146/annurev.biochem.68.1.425. [DOI] [PubMed] [Google Scholar]
- Borst P., Evers R., Kool M., Winholds J. A family of drug transporters: the multidrug resistance-associated proteins. J. Natl. Cancer Inst. 2000;92:1295–1302. doi: 10.1093/jnci/92.16.1295. [DOI] [PubMed] [Google Scholar]
- Brochu C., Wang J., Roy G., Messier N., Wang X.Y., Saravia N.G., Ouellette M. Antimony uptake systems in the protozoan parasite Leishmania and accumulation differences in antimony-resistant parasites. Antimicrob. Agents Chemother. 2003;47:3073–3079. doi: 10.1128/AAC.47.10.3073-3079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell M.M., Pastan I., Gottesman M.M. Certain calcium channel blockers bind specifically to multidrug-resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J. Biol. Chem. 1987;262:2166–2170. [PubMed] [Google Scholar]
- Croft S.L., Sundar S., Fairlamb A.H. Drug resistance in Leishmaniasis. Clin. Microbiol. Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassa E., Bouige P. The ABC of ABCs: a phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 2001;152:211–229. doi: 10.1016/s0923-2508(01)01194-9. [DOI] [PubMed] [Google Scholar]
- Decuypere S., Rijal S., Yardley V., De Doncker S., Laurent T., Khanal B., Chappuis F., Dujardin J.C. Gene expression analysis of the mechanism of natural Sb(V) resistance in Leishmania donovani isolates from Nepal. Antimicrob. Agents Chemother. 2005;49:4616–4621. doi: 10.1128/AAC.49.11.4616-4621.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decuypere S., Vanaerschot M., Brunker K., Imamura H., Muller S., Khanal B., Rijal S., Dujardin J.C., Coombs G.H. Molecular mechanisms of drug resistance in natural Leishmania populations vary with genetic background. PLoS Negl. Trop. Dis. 2012;6:e1514. doi: 10.1371/journal.pntd.0001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias F.C., Ruiz J.C., Lopes W.C., Squina F.M., Renzi A., Cruz A.K., Tosi L.R. Organization of H locus conserved repeats in Leishmania (Viannia) braziliensis correlates with lack of gene amplification and drug resistance. Parasitol. Res. 2007;101:667–676. doi: 10.1007/s00436-007-0528-5. [DOI] [PubMed] [Google Scholar]
- Downing T., Imamura H., Decuypere S., Clark T.G., Coombs G.H., Cotton J.A., Hilley J.D., de Doncker S., Maes I., Mottram J.C., Quail M.A., Rijal S., Sanders M., Schönian G., Stark O., Sundar S., Vanaerschot M., Hertz-Fowler C., Dujardin J.C., Berriman M. Whole genome sequencing of multiple Leishmania donovani clinical isolates provides insights into population structure and mechanisms of drug resistance. Genome Res. 2011;21:2143–2156. doi: 10.1101/gr.123430.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Fadili K., Messier N., Leprohon P., Roy G., Guimond C., Trudel N., Saravia N.G., Papadopoulou B., Légaré D., Ouellette M. Role of the ABC transporter MRPA (PGPA) in antimony resistance in Leishmania infantum axenic and intracellular amastigotes. Antimicrob. Agents Chemother. 2005;49:1988–1993. doi: 10.1128/AAC.49.5.1988-1993.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frézard F., Demicheli C., Ribeiro R.R. Pentavalent antimonials: new perspectives for old drugs. Molecules. 2009;14:2317–2336. doi: 10.3390/molecules14072317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamarro F., Chiquero M.J., Amador M.V., Légaré D., Ouellette M., Castanys S. P-glycoprotein overexpression in methotrexate-resistant Leishmania tropica. Biochem. Pharmacol. 1994;47:1939–1947. doi: 10.1016/0006-2952(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Gourbal B., Sonuc N., Bhattacharjee H., Légaré D., Sundar S., Ouellette M., Rosen B.P., Mukhopadhyay R. Drug uptake and modulation of drug resistance in Leishmania by an aquaglyceroporin. J. Biol. Chem. 2004;279:31010–31017. doi: 10.1074/jbc.M403959200. [DOI] [PubMed] [Google Scholar]
- Griffith O.W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine) J. Biol. Chem. 1979;254:7558–7560. [PubMed] [Google Scholar]
- Grogl M., Martin R.K., Oduola A.M., Milhous W.K., Kyle D.E. Characteristics of multidrug resistance in Plasmodium and Leishmania: detection of P-glycoprotein-like components. Am. J. Trop. Med. Hyg. 1991;45:98–111. doi: 10.4269/ajtmh.1991.45.98. [DOI] [PubMed] [Google Scholar]
- Grondin K., Haimeur A., Mukhopadhyay R., Rosen B.P., Ouellette M. Co-amplification of the gamma-glutamylcysteine synthetase gene gsh1 and of the ABC transporter gene pgpA in arsenite-resistant Leishmania tarentolae. EMBO J. 1997;16:3057–3065. doi: 10.1093/emboj/16.11.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grondin K., Kundig C., Roy G., Ouellette M. Linear amplicons as precursors of amplified circles in methotrexate-resistant Leishmania tarentolae. Nucleic Acids Res. 1998;26:3372–3378. doi: 10.1093/nar/26.14.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksson J., Pettersson U., Solari A. Trypanosoma cruzi: correlation between karyotype variability and isoenzyme classification. Exp. Parasitol. 1993;77:334–348. doi: 10.1006/expr.1993.1091. [DOI] [PubMed] [Google Scholar]
- Herwaldt B.L. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- Higgins C.F. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Jeddi F., Piarroux R., Mary C. Antimony resistance in Leishmania, focusing on experimental research. J. Trop. Med. 2011:695382. doi: 10.1155/2011/695382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhls K., Keilonat L., Ochsenreither S., Schaar M., Schweynoch C., Presber W., Schönian G. Multilocus microsatellite typing (MLMT) reveals genetically isolated populations between and within the main endemic regions of visceral leishmaniasis. Microbes Infect. 2007;9:334–343. doi: 10.1016/j.micinf.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Lee N., Bertholet S., Debrabant A., Muller J., Duncan R., Nakhasi H.L. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differ. 2002;9:53–64. doi: 10.1038/sj.cdd.4400952. [DOI] [PubMed] [Google Scholar]
- Légaré D., Richard D., Mukhopadhyay R., Stierhof Y.D., Rosen B.P., Haimeur A., Papadopoulou B., Ouellette M. The Leishmania ABC protein PGPA is an intracellular metal-thiol transporter ATPase. J. Biol. Chem. 2001;276:26301–26307. doi: 10.1074/jbc.M102351200. [DOI] [PubMed] [Google Scholar]
- Leprohon P., Légaré D., Raymond F., Madore F., Hardiman G., Corbeil J., Ouellette M. Gene expression modulation is associated with gene amplification, supernumerary chromosomes and chromosome loss in antimony-resistant Leishmania infantum. Nucleic Acids Res. 2009;37:1387–1399. doi: 10.1093/nar/gkn1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liarte D.B., Murta S.M.F. Selection and phenotype characterization of potassium antimony tartrate-resistant populations of four New World Leishmania species. Parasitol. Res. 2010;107:205–212. doi: 10.1007/s00436-010-1852-8. [DOI] [PubMed] [Google Scholar]
- Maharjan M., Singh S., Chatterjee M., Madhubala R. Role of aquaglyceroporin (AQP1) gene and drug uptake in antimony-resistant clinical isolates of Leishmania donovani. Am. J. Trop. Med. Hyg. 2008;79:69–75. [PubMed] [Google Scholar]
- Marquis N., Gourbal B., Rosen B.P., Mukhopadhyay R., Ouellette M. Modulation in aquaglyceroporin AQP1 gene transcript levels in drug-resistant Leishmania. Mol. Microbiol. 2005;57:1690–1699. doi: 10.1111/j.1365-2958.2005.04782.x. [DOI] [PubMed] [Google Scholar]
- Marzochi M.C., Marzochi K.B. Tegumentary and visceral leishmaniases in Brazil: emerging anthropozoonosis and possibilities for their control. Cad Saude Publica. 1994;2:359–375. doi: 10.1590/s0102-311x1994000800014. [DOI] [PubMed] [Google Scholar]
- Monte Neto R.L., Coelho A.C., Raymond F., Légaré D., Corbeil J., Melo M.N., Frézard F., Ouellette M. Gene expression profiling and molecular characterization of antimony resistance in Leishmania amazonensis. PLoS Negl. Trop. Dis. 2011;5:e1167. doi: 10.1371/journal.pntd.0001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira E.S., Anacleto C., Petrillo-Peixoto M.L. Effect of glucantime on field and patient isolates of New World Leishmania: use of growth parameters of promastigotes to assess antimony susceptibility. Parasitol. Res. 1998;84:720–726. doi: 10.1007/s004360050476. [DOI] [PubMed] [Google Scholar]
- Moreira W., Leprohon P., Ouellette M. Tolerance to drug-induced cell death favours the acquisition of multidrug resistance in Leishmania. Cell Death Dis. 2011;2:e201. doi: 10.1038/cddis.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A., Padmanabhan P.K., Singh S., Roy G., Girard I., Chatterjee M., Ouellette M., Madhubala R. Role of ABC transporter MRPA, γ-glutamylcysteine synthetase and ornithine decarboxylase in natural antimony-resistant isolates of Leishmania donovani. J. Antimicrob. Chemother. 2007;59:204–211. doi: 10.1093/jac/dkl494. [DOI] [PubMed] [Google Scholar]
- Mukherjee A., Boisvert S., Monte-Neto R.L., Coelho A.C., Raymond F., Mukhopadhyay R., Corbeil J., Ouellette M. Telomeric gene deletion and intrachromosomal amplification in antimony-resistant Leishmania. Mol. Microbiol. 2013;88:189–202. doi: 10.1111/mmi.12178. [DOI] [PubMed] [Google Scholar]
- Murray H.W. Clinical and experimental advances in treatment of visceral leishmaniasis. Antimicrob. Agents Chemother. 2001;45:2185–2197. doi: 10.1128/AAC.45.8.2185-2197.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H.W., Nathan C.F. In vivo killing of intracellular visceral Leishmania donovani by a macrophage-targeted hydrogen peroxide-generating system. J. Infect. Dis. 1988;158:1372–1375. doi: 10.1093/infdis/158.6.1372. [DOI] [PubMed] [Google Scholar]
- Murray H.W., Berman J.D., Davies C.R., Saravia N.G. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- Murta S.M., dos Santos W.G., Anacleto C., Nirdé P., Moreira E.S., Romanha A.J. Drug resistance in Trypanosoma cruzi is not associated with amplification or overexpression of P-glycoprotein (PGP) genes. Mol. Biochem. Parasitol. 2001;117:223–228. doi: 10.1016/s0166-6851(01)00350-4. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Borst P. Drug resistance and P-glycorpotein in the protozoan parasite Leishmania. Res. Microbiol. 1991;142:737–746. doi: 10.1016/0923-2508(91)90089-s. [DOI] [PubMed] [Google Scholar]
- Ouellette M., Fase-Fowler F., Borst P. The amplified H circle of methotrexate-resistant Leishmania tarentolae contains a novel P-glycoprotein gene. EMBO J. 1990;9:1027–1033. doi: 10.1002/j.1460-2075.1990.tb08206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak M.K., Yi T. Sodium stibogluconate is a potent inhibitor of protein tyrosine phosphatases and augments cytokine respondes in hemopoietic cell lines. J. Immunol. 2001;167:3391–3397. doi: 10.4049/jimmunol.167.6.3391. [DOI] [PubMed] [Google Scholar]
- Roberts W.L., Rainey P.M. Antimony quantification in Leishmania by electrothermal atomic absorption spectroscopy. Anal. Biochem. 1993;211:1–6. doi: 10.1006/abio.1993.1223. [DOI] [PubMed] [Google Scholar]
- Romero G.A.S., Guerra M.V.F., Paes M.G., Macedo V.O. Comparison of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis and L. (V.) guyanensis in Brazil: therapeutic response to meglumine antimoniate. Am. J. Trop. Med. Hyg. 2001;65:456–465. doi: 10.4269/ajtmh.2001.65.456. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Fritsch E.F., Maniatis T. second ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor: 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- Sampaio M.C., Traub-Cseko Y.M. The 245 kb amplified chromosome of Leishmania (V.) braziliensis contains a biopterin transporter gene. Mem. Inst. Oswaldo Cruz. 2003;98:377–378. doi: 10.1590/s0074-02762003000300014. [DOI] [PubMed] [Google Scholar]
- Sereno D., Holzmuller P., Mangot I., Cuny G., Ouaissi A., Lemesre J.L. Antimonial-mediated DNA fragmentation in Leishmania infantum amastigotes. Antimicrob. Agents Chemother. 2001;45:2064–2069. doi: 10.1128/AAC.45.7.2064-2069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J.J. Further thoughts on the use of the name Leishmania (Leishmania) infantum chagasi for the aetiological agent of American visceral leishmaniasis. Mem. Inst. Oswaldo Cruz. 2006;101:577–579. doi: 10.1590/s0074-02762006000500017. [DOI] [PubMed] [Google Scholar]
- Smith, C.L., Klco, S.R., Cantor, C.R., 1988. Pulsed-field electrophoresis and the technology of large DNA molecules. In: Davies, K.E., (Ed.), Genome Analysis, a pratical approach, IRL Press, Oxford, England. pp. 41–72.
- Sudhandiran G., Shaha C. Antimonial-induced increase in intracellular Ca2+ through non-selective cation channels in the host and the parasite is responsible for apoptosis of intracellular Leishmania donovani amastigotes. J. Biol. Chem. 2003;278:25120–25132. doi: 10.1074/jbc.M301975200. [DOI] [PubMed] [Google Scholar]
- Sundar S., More D.K., Singh M.K., Singh V.P., Sharma S., Makharia A., Kumar P.C., Murray H.W. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 2000;31:1104–1106. doi: 10.1086/318121. [DOI] [PubMed] [Google Scholar]
- Teixeira S.M. Control of gene expression in Trypanosomatidae. Braz. J. Med. Biol. Res. 1998;31:1503–1516. doi: 10.1590/s0100-879x1998001200001. [DOI] [PubMed] [Google Scholar]
- Torres D.C., Adaui V., Ribeiro-Alves M., Romero G.A., Arévalo J., Cupolillo E., Dujardin J.C. Targeted gene expression profiling in Leishmania braziliensis and Leishmania guyanensis parasites isolated from Brazilian patients with different antimonial treatment outcomes. Infect. Genet. Evol. 2010;10:727–733. doi: 10.1016/j.meegid.2010.05.006. [DOI] [PubMed] [Google Scholar]
- Ubeda J.M., Légaré D., Raymond F., Ouamer A.A., Boisavert S., Rigault P., Corbeil J., Tremblay M.J., Olivier M., Papadopoulou B., Ouellette M. Modulation of gene expression in drug resistant Leishmania is associated with gene amplification gene deletion and chromosome aneuploidy. Genome Biol. 2008;9:R115. doi: 10.1186/gb-2008-9-7-r115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman B. Multidrug resistance and P-glycoprotein in parasitic Protozoan. J. Bioenerg. Biomembr. 1995;27:77–84. doi: 10.1007/BF02110334. [DOI] [PubMed] [Google Scholar]
- Wang J., Brochu C., Wang X., Wang N., Ouellette M. Determination of trace antimony in cells by inductively coupled plasma mass spectrometry for drug resistance study of protozoan parasite. Chin. J. Anal. Lab. 2003;22(suppl.):107–112. [Google Scholar]
- White T.C., Fase-Fowler G., van Luenen H., Calafat J., Borst P. The H circles of Leishmania tarentolae are unique amplifiable system of oligomeric DNAs associated with drug resistance. J. Biol. Chem. 1988;263:16977–16983. [PubMed] [Google Scholar]
- World Health Organization. Strategic direction for leishmaniasis research: disease burden and epidemiological trends. Available at: <http://www.who.int/tdr/diseases/leish/direction/en/> (Last access 30.06.12).
- Wyllie S., Cunningham M.L., Fairlamb A.H. Dual action of antimonial drugs on thiol redox metabolism in the human pathogen Leishmania donovani. J. Biol. Chem. 2004;279:39925–39932. doi: 10.1074/jbc.M405635200. [DOI] [PubMed] [Google Scholar]


