Abstract
Objectives
In this secondary analysis of the AIM-HIGH trial, the objectives were to examine the relationship between niacin treatment, lipoproteins, and cardiovascular (CV) outcomes.
Background
During 3-year follow-up in 3,414 patients with established CV disease and low HDL-C, combined niacin + LDL-lowering therapy did not reduce CV events versus LDL-lowering therapy alone.
Methods
Subjects taking simvastatin + ezetimibe were randomized to extended-release (ER) niacin 1500–2000 mg or minimal immediate-release niacin (<150 mg) as placebo at bedtime. LDL-C in both groups was maintained from 40 to 80 mg/dL. Hazard ratios (HR) were estimated by Cox proportional hazards for relationships between lipoproteins and the composite endpoint of CV death, myocardial infarction, acute coronary syndrome, ischemic stroke, or symptom-driven revascularization.
Results
CV outcomes were not associated with ER niacin in any baseline lipoprotein tertile. In a subset of patients in both the highest triglyceride (>198 mg/dl) and lowest HDL-C (<33 mg/dl) tertiles, ER niacin showed a trend toward benefit (HR=0.74, p=0.073). In-trial LDL-C, nonHDL-C, and TC/HDL-C ratio were positively associated with CV events in the control group, but these relationships were absent in the ER niacin group.
Conclusions
Baseline lipoprotein tertiles did not predict differential benefit or harm with ER niacin added to LDL-lowering therapy, but a small dyslipidemic subgroup may benefit. ER niacin attenuated expected relationships of lipoprotein risk factors with CV events, raising the possibility that nonlipoprotein actions of niacin could impact risk.
Clinical trial info
AIM-HIGH; NCT00120289
Keywords: niacin, cardiovascular events, clinical trial, lipoproteins, GPR109A
Introduction
In the Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes (AIM-HIGH) trial, ER niacin added to intensive LDL-C-lowering therapy did not reduce atherothrombotic events compared to intensive LDL-C-lowering therapy alone (1). Recently the Heart Protection Study 2, Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) likewise showed no benefit for combination ER niacin/laropiprant therapy (2,3). However, niacin added to ongoing statin therapy has been associated with atherosclerotic lesion regression (4,5). In an early randomized trial, a niacin monotherapy group experienced fewer events than placebo subjects (6). Combination drug regimens including niacin were associated with event reductions in 3 smaller trials (7–9).
Pharmacologic effects of niacin can be separated into lipoprotein effects thought to be mediated by actions in the liver (10,11) and nonlipoprotein effects mediated by the G-protein coupled receptor 109A (GPR109A) on adipocytes, macrophages, and dermal dendritic cells or by a direct action on endothelial cells (10,12–15). These varying effects of niacin lend importance to the present analysis of the interaction between plasma lipoproteins, niacin treatment, and atherothrombotic events in AIM-HIGH.
Methods
Study design
As described previously, AIM-HIGH participants had established stable atherosclerotic disease with HDL-C <40 mg/dl for men, <50 mg/dl for women, high triglyceride (150 to 400 mg/dl, and LDL-C <180 mg/dl (adjusted for LDL-lowering treatment) (1). All subjects initially received simvastatin 40 mg daily, plus ER niacin at doses increasing weekly from 500 mg to 2000 mg per day. Subjects tolerating at least 1500 mg ER niacin daily were randomized 1:1 to ER niacin or matching placebo tablets. To disguise treatment assignment, placebo tablets included 50 mg immediate-release niacin in each 500 or 1000 mg tablet. In both treatment groups, simvastatin doses were adjusted, and/or ezetimibe 10 mg daily added, to maintain LDL-C within 40 to 80 mg/dl.
Statistical analysis
Lipoprotein values were measured by protocol at baseline, 1, 3, and 6 months and each year after randomization. Baseline lipoprotein tertiles were constructed across all randomized subjects. Baseline was defined as the last measurement prior to randomization.
Relationships between lipoproteins and cardiovascular events were examined using primary study endpoint, which was the first occurrence of death from coronary heart disease, nonfatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome, or symptom-driven coronary or cerebral revascularization. Time to event analyses examined the period from randomization to a primary endpoint event, withdrawal of consent, loss to follow-up, administrative censoring, or the end of the double-blind period. Each lipoprotein was standardized by the overall baseline standard deviation.
Hazard ratios examining the relationship between standardized baseline lipoprotein tertiles and events were calculated from Cox Proportional Hazards models, adjusted for gender and diabetes. Heterogeneity between baseline lipoprotein tertiles and events across randomization assignment was assessed by including lipoprotein-by-treatment interaction terms. A subgroup analysis of subjects simultaneously in the highest tertile of baseline triglycerides and the lowest tertile of baseline HDL-C was specified a priori.
The relationship between in-trial standardized lipoprotein values and events was assessed by averaging values from scheduled visits after randomization and before the first confirmed primary event, study termination, or the date of last contact. For each lipoprotein separately, within-subject averages were included in Cox proportional hazards models, adjusted for gender and diabetes. Sensitivity analyses were performed as described with Online Supplementary Tables 2 and 3. Heterogeneity of joint effects of HDL-C, LDL-C and logTG across treatments was assessed using the likelihood ratio test to compare the reduced model, including terms for randomized treatment assignment, HDL-C, LDL-C, logTG, gender and diabetes, to the full model, including these terms plus the three lipoprotein-by-treatment interactions (16,17).
Two-sided P-values < 0.05 were considered significant, without adjustment for multiple testing. SAS Version 9.2 was used for all analyses.
Results
Study population and lipoprotein changes
All randomized subjects were evaluated (n=3,414). Baseline lipoproteins were assessed on statin therapy in 3196 patients (93.6%) and without statin in 218 (6.4%). These groups were combined for the present analysis. Lipoprotein changes by baseline lipoprotein tertiles are shown in Online Supplementary Table 1.
Effect of treatment on cardiovascular events by baseline lipid/lipoprotein tertiles
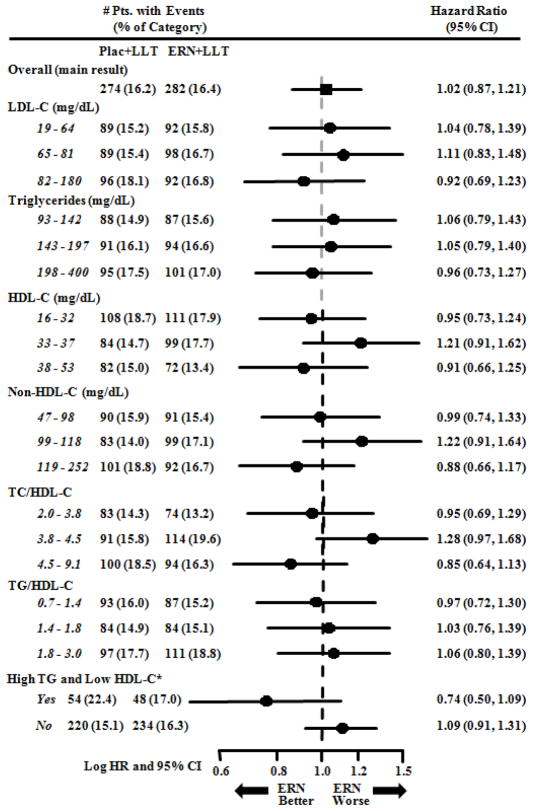
Figure 1 shows that treatment assignment did not significantly affect the primary endpoint of first major CV event in any baseline tertile of lipoprotein or lipoprotein ratio. For the 522 subjects (15.3%) who simultaneously had baseline triglyceride in the highest tertile (≥198 mg/dl) and HDL-C in the lowest tertile (<33 mg/dl), a non-significant trend toward reduction of CV risk was evident in the ER niacin group (HR = 0.74, p = 0.073). In a smaller group (439 subjects, 12.9%) that met somewhat stricter criteria of triglyceride, ≥200 mg/dl and HDL-C < 32 mg/dl, the trend toward reduced events in the niacin group was stronger (HR = 0.64, p = 0.032).
Figure 1. Effect of treatment on cardiovascular events by baseline lipoprotein/lipid tertiles.
Hazard ratios (HR) for the effect of treatment assignment were calculated using Cox Proportional Hazards models, including terms for gender and diabetes. *High TG and Low HDL-C designates subjects with highest tertile triglyceride and lowest tertile HDL-C. Pts = Patients, Pbo = Placebo, ERN = Extended release niacin, CI = confidence interval, TG = triglyceride, TC = total cholesterol. Placebo tablets included 100–150 mg immediate-release niacin.
Relation of cardiovascular events to lipoprotein variables
Baseline and in-trial HDL-C levels were not significantly associated with CV events in either treatment group (Table 1). In-trial LDL-C, non-HDL-C, and TC/HDL-C significantly predicted events only in the control group (p<0.001 to p=0.003).
Table 1.
Relationship of cardiovascular events to baseline and in-trial lipoprotein variables.
| Variable | SD | LLT + Placebo*
|
LLT + ERN
|
Interaction P-value† | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio | (95% CI) | P | Hazard Ratio | (95% CI) | P | |||
| Baseline | ||||||||
| LDL-C‡ | 23 | 1.05 | 0.94, 1.18 | 0.37 | 1.02 | 0.92, 1.14 | 0.66 | 0.60 |
| Log TG‡ | 0.34 | 1.06 | 0.93, 1.19 | 0.38 | 0.98 | 0.87, 1.10 | 0.70 | 0.44 |
| HDL-C‡ | 5.6 | 0.91 | 0.80, 1.05 | 0.19 | 0.96 | 0.85, 1.08 | 0.47 | 0.78 |
| NonHDL-C‡ | 27 | 1.09 | 0.97, 1.22 | 0.16 | 1.01 | 0.90, 1.13 | 0.89 | 0.31 |
| TC/HDL-C ratio | 0.97 | 1.13 | 1.01, 1.27 | 0.035 | 1.02 | 0.92, 1.14 | 0.68 | 0.22 |
| TG/HDL-C ratio | 0.41 | 1.08 | 0.95, 1.23 | 0.22 | 1.00 | 0.89, 1.12 | 0.94 | 0.44 |
| In-trial | ||||||||
| LDL-C‡ | 23 | 1.39 | 1.16, 1.67 | <0.001 | 1.01 | 0.83, 1.22 | 0.96 | 0.01 |
| Log TG‡ | 0.34 | 1.06 | 0.95, 1.19 | 0.28 | 0.97 | 0.87, 1.08 | 0.56 | 0.31 |
| HDL-C‡ | 5.6 | 0.95 | 0.84, 1.07 | 0.37 | 0.99 | 0.91, 1.08 | 0.79 | 0.85 |
| NonHDL-C‡ | 27 | 1.31 | 1.13, 1.52 | <0.001 | 0.98 | 0.83, 1.15 | 0.78 | 0.008 |
| TC/HDL-C ratio | 0.97 | 1.20 | 1.06, 1.35 | 0.003 | 1.04 | 0.89, 1.20 | 0.64 | 0.19 |
| TG/HDL-C ratio | 0.41 | 1.08 | 0.96, 1.21 | 0.20 | 0.99 | 0.89, 1.09 | 0.77 | 0.38 |
HRs for lipoprotein effects on CV events were closer to 1.00 in ER niacin subjects compared to control subjects for every baseline and in-trial variable (Table 1). In particular, in-trial LDL-C and non-HDL-C were associated with CV events in the control group (HRs=1.39 and 1.31, respectively), but not in the ER niacin group (HRs=1.01 and 0.98, respectively), and tests for heterogeneity were significant. Sensitivity analyses either confirmed or did not contradict this conclusion (Online Supplementary Tables 2 and 3). Multivariable analysis was performed to determine whether the overall predictive impact of in-trial lipoprotein variables (including LDL-C, HDL-C, and logTG) differed according to treatment assignment. This analysis showed that the treatment groups differed significantly (p=0.025), suggesting that the use of ER niacin in patients treated with intensive LDL-C lowering therapy reduces the overall impact of lipoproteins on CV events.
Discussion
The primary result of AIM-HIGH was the lack of an effect on CV events despite a 15% higher HDL-C level in the group receiving ER niacin compared to the control group receiving intensive LDL-lowering therapy alone. The present analysis extends the concept of lack of relationship with HDL-C, since HDL-C levels showed no correlation with events.
We found no baseline group defined by lipoprotein tertiles in which combined therapy was significantly better than control LDL-lowering therapy alone. However, a non-significant trend toward better outcomes with ER niacin combined therapy appeared in a small group who had baseline triglyceride in the highest tertile with simultaneous HDL-C in the lowest tertile. This trend toward benefit in a dyslipidemic subgroup has been noted in randomized trials of fibrates, which like niacin lower triglyceride and raise HDL-C (18).
In the AIM-HIGH control group, in-trial LDL-C, non-HDL-C, and total/HDL-C cholesterol ratio predicted atherothrombotic events significantly. In contrast, none of these atherogenic lipoprotein variables predicted atherothrombotic events in the group receiving ER niacin. We also examined the joint impact of in-trial LDL-C, HDL-C, and triglycerides on events, finding a significant difference between the control group and the ER niacin combined therapy group.
The loss of the relation of in-trial atherogenic lipoproteins to CV events in ER niacin-treated patients suggests that niacin may affect the relationship between lipoproteins and events. This altered relationship implies either 1) niacin-induced compositional changes in lipoproteins that make them neutral with regard to atherothrombosis, or 2) an influence of niacin on CV events independent of lipoproteins, or 3) both. We consider the first option to be unlikely as a sole explanation and favor the idea that nonlipoprotein effects of niacin may influence atherothrombotic events, obscuring the effects of lipoproteins and leading to HRs close to unity in the ER niacin combined therapy group.
Recent results from HPS2-THRIVE highlighted the potential for clinical harm from niacin related to a variety of off-target (nonlipoprotein) adverse events (2,3). The present analysis fits with the hypothesis of clinically important nonlipoprotein effects and extends their potential impact to the primary outcome variable of CV events.
A well-recognized nonlipoprotein action of niacin is GPR109A-dependent inhibition of adipocyte triglyceride lipolysis. Plasma nonesterified fatty acid levels rapidly fall more than 60%, then rebound and overshoot after 1–2 hours (10,12). This metabolic perturbation repeated every night could promote CV events via impaired myocardial fuel supply, subsequent excess in fatty acid anion concentrations, and/or a counter-regulatory hormone response including catecholamines (19,20). Myocardial energetics are known to shift from fatty acid to glucose oxidation following niacin administration to fasting humans (12).
Niacin was administered at mealtimes prior to the introduction of ER niacin in the late 1990s. Mealtime dosing may avert the metabolic perturbation just described, since food absorption supports myocardial fuel supply, and epinephrine is specifically suppressed (21). AIM-HIGH was designed largely on the basis of prior niacin trials with mealtime dosing (6–9). However, in both AIM-HIGH and HPS2-THRIVE niacin administration shifted to bedtime, potentially magnifying the consequences of adipocyte lipolysis inhibition. This nonlipoprotein action of niacin needs further study in the fasting and post-prandial states.
Cellular effects of niacin include suppression of inflammatory responses in endothelial cells and macrophages, and increased cholesterol efflux in macrophages (13–15). Niacin inhibited atherogenesis in wild-type, but not GPR109A-negative mice, and the effect was transferable with GPR109A-competent bone marrow cells (13). In contrast to adipocyte lipolysis inhibition, these cellular effects appear generally beneficial. The net effect of multiple nonlipoprotein actions of niacin, together with the relatively small 15% HDL-C increase in AIM-HIGH, could bring about a balance of harm and benefit leading to no overall change in CV events. This hypothesis brings together diverse clinical and basic results on niacin and is testable at multiple levels.
The present study has limitations as a secondary analysis, and results should be considered hypothesis-raising rather than conclusive. The identification of apparent benefit in a small dyslipidemic subgroup is subject to error due to multiplicity. Further insights may be gained by considering apolipoproteins, lipoprotein(a), and HDL and LDL particle concentrations, which are being analyzed and presented separately (22).
In summary, this analysis reinforces a diminished role for niacin-induced HDL-C increase in prevention of atherothrombotic events. Baseline lipoprotein tertiles did not predict differential benefit or harm with ER niacin added to aggressive LDL-lowering therapy, but a small subgroup of subjects with baseline dyslipidemia showed possible benefit. Atherogenic lipoproteins correlated positively with CV events in the control group, but not in the ER niacin-treated group. This observation raises the possibility that nonlipoprotein effects of niacin might have affected CV events in AIM-HIGH.
Supplementary Material
Acknowledgments
Support: Supported by the National Heart, Lung, and Blood Institute (U01-HL-081616 and U01-HL-081649) and by an unrestricted grant from Abbott Laboratories, Chicago, IL. Abbott Laboratories donated the extended-release niacin, the matching placebo, and the ezetimibe; Merck donated the simvastatin. Neither of these companies had any role in the oversight or design of the study, or in the analysis or interpretation of the data.
Abbreviations
- AIM-HIGH trial
Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes trial
- ER niacin
extended-release niacin
- CV
cardiovascular
- LDL-C
low density lipoprotein cholesterol
- HDL-C
high density lipoprotein cholesterol
- HR
hazard ratio
- GPR109A
G-protein coupled receptor 109A
- CABG
coronary artery bypass graft surgery
- PCI
percutaneous coronary intervention
Footnotes
Disclosures: Some authors have received honoraria and consulting fees from Abbott and Merck. None of the authors declare relationships that would bias the results and analysis presented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aim-High Investigators. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. New Engl J Med. 2011;365:2255–67. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 2.Haynes R, Jang L, Hopewell JC, et al. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J. 2103;34:1279–91. doi: 10.1093/eurheartj/eht055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armitage J. HPS2-THRIVE: Randomized placebo-controlled trial of ER niacin and laropiprant in 25,673 patients with pre-existing cardiovascular disease. American College of Cardiology Annual Scientific Sessions; San Francisco: 2013. [Google Scholar]
- 4.Lee JM, Robson MD, Yu LM, et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J Am Coll Cardiol. 2009;54:1787–94. doi: 10.1016/j.jacc.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 5.Taylor AJ, Villines TC, Stanek EJ, et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. New Engl J Med. 2009;361:2113–2122. doi: 10.1056/NEJMoa0907569. [DOI] [PubMed] [Google Scholar]
- 6.Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease. J Am Med Assoc. 1975;231:360–381. [PubMed] [Google Scholar]
- 7.Carlson LA, Rosenhamer G. Reduction of mortality in the Stockholm Ischaemic Heart Disease Secondary Prevention Study by combined treatment with clofibrate and nicotinic acid. Acta Med Scand. 1988;223:405–418. doi: 10.1111/j.0954-6820.1988.tb15891.x. [DOI] [PubMed] [Google Scholar]
- 8.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. New Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 9.Whitney EJ, Krasuski RA, Personius BE, et al. A randomized trial of a strategy for increasing high-density lipoprotein cholesterol levels: effects on progression of coronary heart disease and clinical events. Ann Intern Med. 2005;142:95–104. doi: 10.7326/0003-4819-142-2-200501180-00008. [DOI] [PubMed] [Google Scholar]
- 10.Lauring B, Taggart AK, Tata JR, et al. Niacin lipid efficacy is independent of both the niacin receptor GPR109A and free fatty acid suppression. Sci Transl Med. 2012;4:148ra115. doi: 10.1126/scitranslmed.3003877. [DOI] [PubMed] [Google Scholar]
- 11.Zhang LH, Kamanna VS, Ganji SH, Xiong XM, Kashyap ML. Niacin increases HDL biogenesis by enhancing DR4-dependent transcription of ABCA1 and lipidation of apolipoprotein A-I in HepG2 cells. J Lipid Res. 2012;53:941–50. doi: 10.1194/jlr.M020917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson LA. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J Intern Med. 2005;258:94–114. doi: 10.1111/j.1365-2796.2005.01528.x. [DOI] [PubMed] [Google Scholar]
- 13.Lukasova M, Malaval C, Gille A, Kero J, Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J Clin Invest. 2011;121:1163–73. doi: 10.1172/JCI41651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Digby JE, Martinez F, Jefferson A, et al. Anti-inflammatory effects of nicotinic acid in human monocytes are mediated by GPR109A dependent mechanisms. Arterioscler Thromb Vasc Biol. 2012;32:669–76. doi: 10.1161/ATVBAHA.111.241836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ganji SH, Qin S, Zhang L, Kamanna VS, Kashyap ML. Niacin inhibits vascular oxidative stress, redox-sensitive genes, and monocyte adhesion to human aortic endothelial cells. Atherosclerosis. 2009;202:68–75. doi: 10.1016/j.atherosclerosis.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 16.Neyman J, Pearson E. On the problem of the most efficient tests of statistical hypotheses. Philos Trans Roy Soc London Series A. 1933;231:289–337. [Google Scholar]
- 17.Kassalow LM. Likelihood ratio tests for regression coefficients from the cox proportional hazards model using the phreg procedure. SAS Conference Proceedings: SAS Users Group International; Cary, NC: SAS; 1993. [Google Scholar]
- 18.Accord Study Group. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–74. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vega GL, Cater NB, Meguro S, Grundy SM. Influence of extended-release nicotinic acid on nonesterified fatty acid flux in the metabolic syndrome with atherogenic dyslipidemia. Am J Cardiol. 2005;95:1309–1313. doi: 10.1016/j.amjcard.2005.01.073. [DOI] [PubMed] [Google Scholar]
- 20.Carlson LA, Levi L, Oro L. Plasma lipids and urinary excretion of catecholamines in man during experimentally induced emotional stress, and their modification by nicotinic acid. J Clin Invest. 1968;47:1795–805. doi: 10.1172/JCI105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penev P, Spiegel K, Marcinkowski T, Van Cauter E. Impact of carbohydrate-rich meals on plasma epinephrine levels: dysregulation with aging. J Clin Endocrinol Metab. 2005;90:6198–206. doi: 10.1210/jc.2005-0415. [DOI] [PubMed] [Google Scholar]
- 22.Albers JJ, Slee A, O’Brien KD, et al. Relationship of apolipoproteins A-1 and B and lipoprotein(a) to cardiovascular outcomes in the AIM-HIGH trial. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.06.051. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.