Graphical abstract
Highlights
► We discuss the role of the natural environment during Leishmania antimony résistance selection. ► We discuss the importance of these determinants in a zoonotic or anthroponotic context. ► We put these in perspectives with recent findings on heavy metal pollution.
Keywords: Leishmania, Drug resistance, Life cycle, Environment
Abstract
Leishmania drug resistance and particularly antimony resistance still continues to emerge in different part of the world. Because visceral and cutaneous leishmaniasis are transmitted in foci with zoonotic or anthroponotic life-cycles, the link between chemotherapeutic resistance and the selection for drug resistance, through drug consumption, cannot be as obvious for all forms of leishmaniasis. The underlying factors that trigger the selection of antimony resistant parasites are poorly studied in regard to environmental aspects. Recently, a correlation between the emergence of antimony unresponsiveness in India and water arsenic contamination has been raised. The presence of some yet unidentified environmental factors driving the selection of antimony resistant Leishmania populations in a zoonotic context of leishmaniasis is also currently questioned. The identification of key molecules involved in the selection of antimony resistance and their importance in the selective process have to be re-evaluated in light of the environment were all the hosts of Leishmania (mammalian and arthropod) evolved. These new insights will help to (i) address the risk of therapeutic failure associated with the emergence of drug-resistance and (ii) propose new therapeutic protocols to aim at reducing the risk of resistance in endemic areas.
1. Introduction
Because antimony (Sb) compounds are the primary antileishmanial drugs in most of developing countries, the emergence of drug resistant strains in different parts of the world poses a public health problem. To date, very little is known about the underlying factors favoring the selection of Sb resistant Leishmania. It is assumed that the therapeutic stress applied during leishmaniasis treatment is the primary selective pressure that drives the selection of drug-resistant Leishmania. This point has been extensively documented in various infectious models, but could be the tip of the iceberg in Leishmania Sb resistance (Blower et al., 1996; Witte, 1998). A recent publication by Aït-Oudhia et al. (in press) concludes that in an area where Sb pressure can be considered as low or absent, in a zoonotic context of leishmaniasis, strains of Leishmania infantum with very low antimony susceptibility can be isolated from dogs at a relatively high frequency. Primary resistance of L. infantum (syn. Leishmania chagasi) isolates among HIV negative patients has also been recently documented (Inocêncio da Luz et al., 2011). Furtheremore, in South America, in a context of zoonotic cutaneous leishmaniasis, Leishmania panamensis and Leishmania braziliensis strains highly resistant to antimony were isolated prior to patient treatment (Rojas et al., 2006). All these recurrent observations suggest that some associated molecules rather than Sb parenteral administration might be involved in the selection of such antimony resistant strains (Aït-Oudhia et al., 2011a, in press). Therefore, an integrated approach that includes identification of key molecules of environmental or host derived origins, as well as their sources and the parasitic stage they act on, may shed light on the genesis of Leishmania drug resistance in endemic foci. These will help to identify potential “hot spot” of antimony resistance.
2. Leishmania life cycle and selective pressure
Visceral and cutaneous leishmaniasis are transmitted in foci with zoonotic or anthroponotic life-cycles, implying that all the hosts that Leishmania colonizes, as well as the environment that surround these hosts can be highly divergent. Therefore, a link between drug consumption and selection of drug resistant parasites may not be as obvious for leishmaniasis as for other microorganisms (see Fig. 1).
Fig. 1.
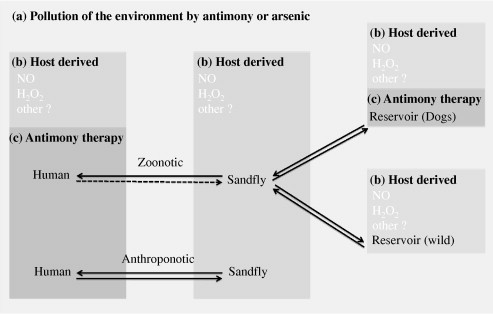
Origin of potential selective agents in the context of anthroponotic and zoonotic life cycles of Leishmania. During its life cycle of Leishmania alternates (arrows) between various host and have two main morphological forms: intracellular amastigotes in the mammalian hosts and motile promastigotes in the sandfly vector. Selective agents that operate to select Sb resistance are present at each parasitic stage. They can originate from the environment (a), the chemotherapeutic pressure applied during antimony therapy (b) and/or from host derived microbicidal factors (c). All of them can select Leishmania parasites with low antimony susceptibility. In zoonotic foci of leishmaniasis caused by L. infantum, the agent responsible for the visceral form of the disease, mammalian hosts can be wild or domestic (cats or dogs). Humans are not considered as reservoir except in Leishmania HIV co-infection where transmission from human to sandfly is documented (dashed arrows). In these cases, only dogs are treated with antimonials. In anthroponotic forms of the disease, humans represent the principal reservoir host for Leishmania amastigotes. In the sandfly, Leishmania promastigotes accomplish their intravectorial development in the lumen of the sandfly gut and heavy metals of environmental origin can reach promastigotes parasites during sandfly feeding. Antimony or other metal ions like arsenic can reach Leishmania amastigotes in mammals when they drink water in polluted environment. In addition, host derived microbicidal molecules also involved in the mode of actions of metal ions like hydrogen peroxide (H2O2) or nitric oxide (NO), can contribute to the selection of antimony resistant parasites. The production of these molecules can be induced in the sandfly, at the level of the intestinal gut epithelium, and in the host cells of Leishmania in their mammalian reservoir host.
In the anthroponotic forms of leishmaniasis, like Leishmania donovani and most foci of Leishmania tropica, parasites are transmitted from patients to patients by the bite of a sandfly. In zoonotic forms of leishmaniasis, that include different cutaneous or mucocutaneous leishmaniasis, parasites are transmitted to patients by sandflies from a reservoir host, represented by domestic or wild mammals. In this context, the main chemotherapeutic pressure that Leishmania will encouter will be during the treatment of individuals, with the exception of zoonotic leishmaniasis caused by L. infantum. In this visceral form of the disease, the therapeutic pressure is applied on a population of parasites that is not transmitted, because humans are generally not considered as a reservoir for L. infantum, with the exeption for HIV/Leishmania co-infection (Molina et al., 1999). Dogs are the main domestic and peridomestic reservoir host of L. infantum. In the occidental part of the Mediterranean (France, Italy, Spain, Portugal…), dogs are treated with the same drugs used to treat human leishmaniasis, while this is not supposed to occur. Infected dogs never achieve parasitological cure, even when their health improves. This, in conjunction with the high percentage of animals that will relapse and repeatedly be treated with the same compound, makes antimony resistant parasites selection and transmission possible (Campino and Maia, 2012). Contradictory results have been obtained in the very few studies made to evaluate the susceptibility to antimonials of L. infantum strains isolated from treated and untreated dogs. While Carrio and Portus (2002) did not find any differences between isolates coming from treated and untreated dogs, others reported a decreased in vitro sensitivity to Sb during and after several treatment courses (Gramiccia et al., 1992). In fact, the risk of emergence and spread of resistant parasites in the canine and human populations should not be neglected, especially when dogs and humans are treated with the same drugs.
Overall, in anthroponotic foci and is some peculiar cases of zoonotic visceral leishmaniasis, the antimony administered during treatment represent a major selective agent for drug-resistant parasites (Fig. 1).
3. Interplay between environmental and host derived selective agents
In many infectious diseases and more particularly in bacterial borne diseases, the emergence of resistance to antibiotics in previously susceptible pathogens is challenging to medicine. There is now a growing body of evidence demonstrating that environmental microbes are highly drug resistant and that the antibiotic resistance genes have the potential to be transferred to pathogenic bacteria via horizontal gene transfer. These genes then evolved under the strong antibiotic selective pressure that occurs during the treatment of infections (Reviewed in Martinez, 2009; Wright, 2010). In bacteria, the elements involved in the resistance to heavy metals are present in bacterial strains like Ralstonia metallidurans (Mergeay et al., 2003), which are well adapted for surviving in naturally heavy metals-rich habitats (e.g. volcanic soils). The strong selective pressure due to anthropogenic pollution has made that chromosomally-encoded determinants for heavy metal resistance are now present in gene transfer units, so they can efficiently spread among bacterial populations (Silver and Phung, 1996, 2005; Nies, 2003). Nonetheless exposure to environmental chemicals is certainly not a contemporary phenomenon as bioactive chemical diversity is certainly ancient and vast.
Leishmania parasite does not evolve in naturally heavy metal rich environment. However, in heavily polluted areas, such as mining sites, swamps and urban environments, sandflies feed on plants that could be contaminated by local atmospheric dust, and wild or feral animal reservoirs that can be exposed to heavy metal such as Sb and Arsenic (As), by drinking polluted water. Concentrations up to 3 mg/l of Sb have been reported in some natural sources (Krachler et al., 2005; Shotyk et al., 2005a,b). The presence of As or Sb in contaminated environments has been hypothesized to be a selective agent involved in antimony resistance selection (Aït-Oudhia et al., 2011a). Likewise, the information given by Perry et al. (2011) is suggestive of the role play by As groundwater contamination on the emergence of decreased antimonial efficacy in India. In this country, mass arsenic poisoning was identified in the 1980s and preceded the emergence of decreased antimony efficacy. If verified, this hypothesis will be the first factual evidence of the involvement of factors present in the environment during the selection of antimony resistant Leishmania. In fact arsenic and antimonial are metalloids that share many common structural and chemical properties and it has long been recognized that selection of Leishmania for arsenic resistance led to antimony cross-resistance (Rosen, 1995). To understand if arsenic pollution has triggered antimony resistance will be far from easy, because no strains representative of the pre-arsenic contamination period, before the 1980s, are present in cryobanks and because no specific markers for arsenic resistance are available. Therefore it will be difficult to determine whether arsenic pressure through water contamination or antimony pressure applied during chemotherapy underlie the selection of antimony resistance (Fig. 1).
Besides the presence of antimony in the ecosystem where Leishmania take place, several host derived microbicidal molecules such as nitric oxide (NO) or reactive oxygen species (ROS) might also contribute to shape antimony susceptibility in natural Leishmania populations. These molecules play a role in the in vivo leishmanicidal activity of antimony formulations (Carter et al., 2005; Mookerjee-Basu et al., 2006; Mandal et al., 2007; Sarkar et al., 2011) and mediate the cytotoxic effect of metal ions, like As (Valko et al., 2005). Leishmania resistance to NO involves trypanothione metabolism, which is crucial for antimony resistance (Bocedi et al., 2010). Experimental evidence supports the notion that NO may be a crucial factor that can modify the antimony response of Leishmania populations. Axenic amastigotes of Leishmania that were selected in vitro for SbIII-resistance are cross-resistant to As (unpublished results) and expressed a cross-resistance to NO (Holzmuller et al., 2005). Although up to now no studies have addressed the effect that NO resistance has on antimony cross-resistance, it was shown/observed that L. braziliensis promastigotes isolated from antimony-unresponsive patients were generally less susceptible to NO (Souza et al., 2010).
Altogether, the environment that Leishmania encounters all over its life cycle might play a more important role for drug resistance selection in zoonotic context of leishmaniasis than in anthroponotic ones, even if they can also in part contribute to drug resistance emergence in both situations.
4. Concluding remarks
The therapeutic arsenal used for the treatment of leishmaniasis is limited. Besides antimony containing drugs, like Pentostam® or Glucantime®, amphotericin B, pentamidine and miltefosine are also used. No cross-resistance between antimony and other antileishmanial molecules was detected in in vitro selected antimony resistant mutant (Sereno et al., 2001) or in naturally resistant L. tropica and L. major (Hadighi et al., 2007). A cross resistance between antimony and amphotericin B or miltefosine was detected in naturally resistant L. donovani parasites and has been experimentally linked to the overexpression of the histone H2A (Kumar et al., 2009; Singh et al., 2010). Altogether these observations reinforce our opinion on the crucial importance of understanding the selective factors triggering Leishmania drug resistance in natura, in order to limit its spread.
Recently, a superior fitness of clinical resistant strains has been documented for antimony resistant field isolates of L. donovani (Vanaerschot et al., 2010). These observations contrast with the in vitro selected parasites where fitness and competitive costs are associated with drug resistance (Agnew et al., 2001; Sereno et al., 2001; Aït-Oudhia et al., 2011b).
The next years will be important to define the diversity and the relative importance of environmental factors that play a role in the selection of antimony resistant Leishmania in endemic areas. These finding will help to better understand the risk of therapeutic failure associated with the emergence of drug-resistance and streamline the development of novel therapeutic protocols aiming at decreasing the risk of selection of resistant strains.
Acknowledgements
DS, is supported by IRD and AOK by the ENSV. C. Maia (SFRH/BPD/44082/2008) holds a fellowship from Fundação para a Ciência e a Tecnologia, Ministério da Ciência, Tecnologia e Ensino Superior, Portugal. This study was partially funded by EU grant FP7-261504 EDENext and is catalogued by the EDENext Steering Committee as EDENext038 (http://www.edenext.eu). The contents of this publication are the sole responsibility of the authors and don’t necessarily reflect the views of the European Commission. This project was partially funded by the DVS de l’IRD (Département Valorisation Sud, AAP Leishmed 2008).
References
- Agnew P., Holzmuller P., Michalakis Y., Sereno D., Lemesre J.L., Renaud F. In vitro growth of Leishmania amazonensis promastigotes resistant to pentamidine is dependent on interactions among strains. Antimicrob. Agents Chemother. 2001;45:1928–1929. doi: 10.1128/AAC.45.6.1928-1929.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aït-Oudhia K., Gazanion E., Vergnes B., Oury B., Sereno D. Leishmania antimony resistance. What we know what we can learn from the field. Parasitol. Res. 2011;109:1225–1232. doi: 10.1007/s00436-011-2555-5. [DOI] [PubMed] [Google Scholar]
- Ait-Oudhia K., Gazanion E., Oury B., Vergnes B., Sereno D. The fitness of antimony-resistant Leishmania parasites: lessons from the field. Trends Parasitol. 2011;27:141–142. doi: 10.1016/j.pt.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Ait-Oudhia K., Gazanion E., Sereno D., Oury B., Pratlong F., Dedet J.P., Lachaud L. In vitro susceptibility to antimonials and amphotericin B of Leishmania infantum strains isolated from dogs in a region lacking drug selection pressure. Vet. Parasitol. 2012;187:386–393. doi: 10.1016/j.vetpar.2012.01.034. [DOI] [PubMed] [Google Scholar]
- Blower S.M., Small P.M., Hopewell P.C. Control strategies for tuberculosis epidemics: new models for old problems. Science. 1996;273:497–500. doi: 10.1126/science.273.5274.497. [DOI] [PubMed] [Google Scholar]
- Bocedi A., Dawood K.F., Fabrini R., Federici G., Gradoni L., Pedersen J.Z., Ricci G. Trypanothione efficiently intercepts nitric oxide as a harmless iron complex in trypanosomatid parasites. FASEB. J. 2010;24:1035–1042. doi: 10.1096/fj.09-146407. [DOI] [PubMed] [Google Scholar]
- Campino L., Maia C. The role of reservoirs: canine leishmaniasis. In: Ponte-Sucre A., Padron-Nieves M., Diaz E., editors. Drug Resistance in Leishmania Parasites – Consequences, Molecular Mechanism, and Possible Treatments. Springer Verlag; Viena, Austria: 2012. [Google Scholar]
- Carrio J., Portus M. In vitro susceptibility to pentavalent antimony in Leishmania infantum strains is not modified during in vitro or in vivo passages but is modified after host treatment with meglumine antimoniate. BMC Pharmacol. 2002;2:E11. doi: 10.1186/1471-2210-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter K.C., Hutchison S., Boitelle A., Murray H.W., Sundar S., Mullen A.B. Sodium stibogluconate resistance in Leishmania donovani correlates with greater tolerance to macrophage antileishmanial responses and trivalent antimony therapy. Parasitology. 2005;131:747–757. doi: 10.1017/S0031182005008486. [DOI] [PubMed] [Google Scholar]
- Gramiccia M., Gradoni L., Orsini S. Decreased sensitivity to meglumine antimoniate (Glucantime) of Leishmania infantum isolated from dogs after several courses of drug treatment. Ann. Trop. Med. Parasitol. 1992;86:613–620. doi: 10.1080/00034983.1992.11812717. [DOI] [PubMed] [Google Scholar]
- Hadighi R., Boucher P., Khamesipour A., Meamar A.R., Roy G., Ouellette M., Mohebali M. Glucantime-resistant Leishmania tropica isolated from Iranian patients with cutaneous leishmaniasis are sensitive to alternative antileishmania drugs. Parasitol. Res. 2007;101:1319–1322. doi: 10.1007/s00436-007-0638-0. [DOI] [PubMed] [Google Scholar]
- Holzmuller P., Sereno D., Lemesre J.L. Lower nitric oxide susceptibility of trivalent antimony-resistant amastigotes of Leishmania infantum. Antimicrob. Agents Chemother. 2005;49:4406–4409. doi: 10.1128/AAC.49.10.4406-4409.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inocêncio da Luz R., Romero G.A., Dorval M.E., Cruz I., Canavate C., Dujardin J.C., VanAssche T., Cos P., Maes L. Drug susceptibility of Leishmania infantum (syn. Leishmania chagasi) isolates from Brazilian HIV-positive and HIV-negative patients. J. Antimicrob. Chemother. 2011;66:677–679. doi: 10.1093/jac/dkq508. [DOI] [PubMed] [Google Scholar]
- Krachler M., Zheng J., Koerner R., Zdanowicz C., Fisher D., Shotyk W. Increasing atmospheric antimony contamination in the northern hemisphere: snow and ice evidence from Devon Island, Arctic Canada. J. Environ. Monit. 2005;7:169–1176. doi: 10.1039/b509373b. [DOI] [PubMed] [Google Scholar]
- Kumar D., Kulshrestha A., Singh R., Salotra P. In vitro susceptibility of field isolates of Leishmania donovani to miltefosine and amphotericin B: correlation with sodium antimony gluconate susceptibility and implications for treatment in areas of endemicity. Antimicrob. Agents Chemother. 2009;53:835–838. doi: 10.1128/AAC.01233-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal G., Wyllie S., Singh N., Sundar S., Fairlamb A.H., Chatterjee M. Increased levels of thiols protect antimony unresponsive Leishmania donovani field isolates against reactive oxygen species generated by trivalent antimony. Parasitology. 2007;134:1679–1687. doi: 10.1017/S0031182007003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J.J. The role of natural environments in the evolution of resistance traits in pahtogenic bacteria. Proc. Biol. Sci. 2009;276:2521–2530. doi: 10.1098/rspb.2009.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergeay P.S., Monchy S., Vallaeys T., Auquier V., Benotmane A., Bertin P., Taghavi S., Dunn J., Van der Lelie D., Wattiez R. Ralstonia metallidurans, a bacterium specifically adapted to toxic metals: Towards a catalogue of metal responsive genes. FEMS Microbiol. Rev. 2003;27:385–410. doi: 10.1016/S0168-6445(03)00045-7. [DOI] [PubMed] [Google Scholar]
- Molina R., Lohse J.M., Pulido F., Laguna F., Lopez-Velez R., Alvar J. Infection of sand flies by humans coinfected with Leishmania infantum and human immunodeficiency virus. Am. J. Trop. Med. Hyg. 1999;60:51–53. doi: 10.4269/ajtmh.1999.60.51. [DOI] [PubMed] [Google Scholar]
- Mookerjee-Basu J., Mookerjee A., Sen P., Bhaumik S., Sen P., Banerjee S., Naskar K., Choudhuri S.K., Saha B., Raha S., Roy S. Sodium antimony gluconate induces generation of reactive oxygen species and nitric oxide via phosphoinositide 3-kinase and mitogen-activated protein kinase activation in Leishmania donovani-infected macrophages Antimicrob. Agents Chemother. 2006;50:1788–1797. doi: 10.1128/AAC.50.5.1788-1797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nies D.H. Efflux-mediated heavy metal resistance in prokaryote. FEMS Microbiol. Rev. 2003;27:313–339. doi: 10.1016/S0168-6445(03)00048-2. [DOI] [PubMed] [Google Scholar]
- Perry M.R., Wyllie S., Prajapati V.K., Feldmann J., Sundar S., Boelaert M., Fairlamb A.H. Visceral leishmaniasis and arsenic: An ancient poison contributing to antimonial treatment failure in the Indian subcontinent? Plos. Negl. Trop. Dis. 2011;5:e1227. doi: 10.1371/journal.pntd.0001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R., Valderrama L., Valderrama M., Varona M.X., Ouellette M., Saravia N.G. Resistance to antimony and treatment failure in human Leishmania (Viannia) infection. J. Infect. Dis. 2006;193:1375–1383. doi: 10.1086/503371. [DOI] [PubMed] [Google Scholar]
- Rosen B.P. Resistance mechanisms to arsenicals and antimonials. J Basic Clin Physio Pharmacol. 1995;6:251–263. doi: 10.1515/jbcpp.1995.6.3-4.251. [DOI] [PubMed] [Google Scholar]
- Sarkar A., Saha P., Mandal G., Mukhopadhyay D., Roy S., Singh S.K., Das S., Goswami R.P., Saha B., Kumar D., Das P., Chatterjee M. Monitoring of intracellular nitric oxide in leishmaniasis: its applicability in patients with visceral leishmaniasis. Cytometry. 2011;79:35–45. doi: 10.1002/cyto.a.21001. [DOI] [PubMed] [Google Scholar]
- Silver S., Phung L.T. Bacterial heavy metal resistance. new surprises. Annu. Rev. Microbiol. 1996;50:753–789. doi: 10.1146/annurev.micro.50.1.753. [DOI] [PubMed] [Google Scholar]
- Silver S., Phung L.T. A bacterial view of the periodic table: genes and proteins for toxic inorganicious. J. Ind. Microbiol. Biotechnol. 2005;32:587–605. doi: 10.1007/s10295-005-0019-6. [DOI] [PubMed] [Google Scholar]
- Sereno D., Guilvard E., Maquaire S., Cavaleyra M., Holzmuller P., Ouaissi A., Lemesre J.L. Experimental studies on the evolution of antimony-resistant phenotype during the in vitro life cycle of Leishmania infantum: implications for the spread of chemoresistance in endemic areas. Acta Trop. 2001;80:195–205. doi: 10.1016/s0001-706x(01)00154-1. [DOI] [PubMed] [Google Scholar]
- Shotyk W., Krachler M., Chen B. Antimony: global environmental contaminant. J. Environ. Monit. 2005;7:1135–1136. doi: 10.1039/b515468p. [DOI] [PubMed] [Google Scholar]
- Shotyk W., Krachler M., Chen B., Zheng J. Natural abundance of Sb and Sc in pristine groundwaters, Springwater Township, Ontario, Canada, and implications for tracing contamination from landfill leachates. J. Environ. Monit. 2005;7:1238–1244. doi: 10.1039/b509352j. [DOI] [PubMed] [Google Scholar]
- Singh R., Kumar D., Duncan R.C., Nakhasi H.L., Salotra P. Overexpression of histone H2A modulates drug susceptibility in Leishmania parasites. Int. J. Antimicrob. Agents. 2010;36:50–57. doi: 10.1016/j.ijantimicag.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Souza A.S., Giudice A., Pereira J.M., Guimaraes L.M., De-Jesus A.R., De-Moura T.R., Wilson M.E., Carvalho E.M., Almeida R.P. Resistance of Leishmania (Viannia) braziliensis to nitric oxide: correlation with antimony therapy and TNF-alpha production. BMC Infect. Dis. 2010;10:209. doi: 10.1186/1471-2334-10-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valko M., Morris H., Cronin M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- Vanaerschot M., Maes I., Ouakad M., Adaui V., Maes L., De Doncker S., Rijal S., Chappuis F., Dujardin J.C., Decuypere S. Linking in vitro and in vivo survival of clinical Leishmania donovani strains. PLoS ONE. 2010;5(8):e12211. doi: 10.1371/journal.pone.0012211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte W. Medical consequences of antibiotic use in agriculture. Science. 1998;279:996–997. doi: 10.1126/science.279.5353.996. [DOI] [PubMed] [Google Scholar]
- Wright G.D. Antibiotic resistance in the environment a link to the clinic. Curr. Opin. Microbiol. 2010;13:589–594. doi: 10.1016/j.mib.2010.08.005. [DOI] [PubMed] [Google Scholar]


