Abstract
Background: Haiti has experienced rapid urbanization that has exacerbated poverty and undernutrition in large slum areas. Stunting affects 1 in 5 young children.
Objective: We aimed to test the efficacy of a daily lipid-based nutrient supplement (LNS) for increased linear growth in young children.
Design: Healthy, singleton infants aged 6–11 mo (n = 589) were recruited from an urban slum of Cap Haitien and randomly assigned to receive: 1) a control; 2) a 3-mo LNS; or 3) a 6-mo LNS. The LNS provided 108 kcal and other nutrients including vitamin A, vitamin B-12, iron, and zinc at ≥80% of the recommended amounts. Infants were followed monthly on growth, morbidity, and developmental outcomes over a 6-mo intervention period and at one additional time point 6 mo postintervention to assess sustained effects. The Bonferroni multiple comparisons test was applied, and generalized least-squares (GLS) regressions with mixed effects was used to examine impacts longitudinally.
Results: Baseline characteristics did not differ by trial arm except for a higher mean age in the 6-mo LNS group. GLS modeling showed LNS supplementation for 6 mo significantly increased the length-for-age z score (±SE) by 0.13 ± 0.05 and the weight-for-age z score by 0.12 ± 0.02 compared with in the control group after adjustment for child age (P < 0.001). The effects were sustained 6 mo postintervention. Morbidity and developmental outcomes did not differ by trial arm.
Conclusion: A low-energy, fortified product improved the linear growth of young children in this urban setting. The trial was registered at clinicaltrials.gov as NCT01552512.
INTRODUCTION
Urbanization trends around the world have altered the array of factors that affect the nutrition of young children. The demographic shift coincides with a decreasing prevalence of underweight in young children as households gain better access to energy-dense, although not necessarily higher-quality, foods (1). A high stunting prevalence and micronutrient deficiencies remain important public health problems in urban areas overlaid with the infectious diseases related to the squalor and crowded condition of slum areas. In Haiti, more than one-half (52%) of the population lives in urban areas, and urbanization is growing at a rate of 3.9% annually compared with the worldwide trend of 1.9% (2). Stunting affects 21.9% of children <5 y old, and acute diarrhea that occurs within the past 2 wk is prevalent in 21% of children, with little change over the past 5 y (3, 4).
Food supplementation of vulnerable groups has a long history in Haiti, although there is limited empirical information for the nuances of appropriate food selection, dosing, and timing (5). Ready-to-use therapeutic foods (RUTFs)4 such as Plumpy'Nut (Nutriset), have proven successful in the treatment of severe acute malnutrition (SAM) in many parts of the world, but the use of similar products such as ready-to-use supplemental foods (RUSFs) in larger quantities or lipid-based nutrient supplements (LNSs) in smaller quantities for prevention remains minimal (6–10). LNS products are specifically designed to ensure nutrient adequacy while simultaneously upholding other complementary feeding practices such as breastfeeding and dietary diversity. In Ghana, children supplemented with Nutributter (Nutriset) from 6 to 12 mo of age were shown to have a significantly higher length-for-age z score (LAZ) and weight-for-age z score (WAZ) (mean ± SD: LAZ, −0.20 ±0.54; WAZ, −0.49 ± 0.54) compared with scores for Nutritabs (UNICEF) and Sprinkles (Ped Med Inc) micronutrient-powder groups combined (mean ± SD: LAZ, −0.38 ±0.54; WAZ: −0.65 ± 0.54) (10). Our study aimed to build on this evidence by examining a LNS delivered in a different context of a more-urban setting and over differing time periods of supplementation.
Since the earthquake of January 2010 in Haiti, high-energy RUTF and RUSF products such as Plumpy'Sup (500 kcal/d; Nutriset) have been applied widely in nutrition programming with the potential for displacing breast-milk consumption and other complementary foods in the diet (11). We hypothesized that providing a daily fortified complementary food supplement to children 6–11 mo of age for 3 and 6 mo would improve linear growth over the course of supplementation. Two time periods of LNS supplementation were tested to assess whether the shorter and more-feasible period could produce similar growth effects, with a view toward including an LNS in the package of well-baby services of the Ministry of Public Health and Population (MSPP). The study was also designed to examine secondary morbidity and developmental outcomes and included an additional follow-up visit 6 mo after intervention cessation to test for sustained effects.
SUBJECTS AND METHODS
Study area and participants
This study was conducted in the Fort Saint Michel (FSM) Health Center catchment area of Cap Haitien, which is the second largest city in Haiti after Port-au-Prince. FSM is located in the poorest communal section of the city, Petite Anse, with a population of >80,000 people. The area is a low-lying, densely populated area bordered by a small international airport, a major road, and a canal. During the rainy season, the area is vulnerable to flooding because of the topography and lack of waste and drain-water management infrastructure. Much of the housing is poor, unfinished, and lacking in sanitation, water, and public power. In Cap Haitien, there is one public hospital (ie, Justinien University Hospital) and 2 public clinics. The MSPP integrated package (IP) of well-baby services is primarily provided at clinics or rally posts at temporarily established locations in communities.
Recruitment for this trial was open to all mothers with children <1 y of age from Petite Anse and took place at the FSM clinic, rally posts, and within communities over a period of 7 mo from May to December 2011. Eligibility criteria included the following: infant age between 6–11 mo, the infant was in good health (no fever, congenital health condition, or peanut allergy), a singleton birth, the infant was not severely malnourished [weight-for-length z score (WLZ) less than −3], the household was not receiving other food aid, and residence within the FSM catchment area. Eligible mothers were informed of the study protocol. Written consent forms were read aloud to all mothers in case of illiteracy, and signatures or crosses signifying consent were obtained by the enumerator team.
Sample-size calculations were based on a longitudinal study design with one baseline and 6 follow-up visits. Drawing from the literature that summarized evidence from complementary food supplementation, we estimated that, in this urban population, there would be a 0.20 difference in LAZ between the 6-mo LNS and control groups and between the 3-mo LNS and control groups (5). With the assumption of a 25% attrition rate in this highly mobile slum area, a mean LAZ SD of 1.2, and 0.8 correlation between repeated measures, we calculated 180 subjects/group would be required (α = 0.05 and 1 β = 0.90) to detect a 0.20 difference in the LAZ. Thus, the enrollment goal of 200 subjects/group was set as a conservative estimate for the required sample size. The study was approved by the National Bioethics Committee of the MSPP in Haiti and the Institutional Review Board of the Human Resource Protection Office of Washington University, St Louis, MO.
Study design
The study, which was conducted from May 2011 to December 2012, was a randomized controlled trial with a parallel design (12). Eligible and consenting mother-baby pairs were randomly assigned into the following 3 groups: a control group, 3 mo of daily LNS supplementation (3-mo LNS group), and 6 mo of daily LNS supplementation (6-mo LNS group). All 3 groups received the MSPP standard of care of the IP of well-baby services. Random assignment was carried out through an allocation-concealment mechanism whereby sealed paper forms that masked group assignments were drawn from a container by mothers by using a simple random assignment ratio of 1:1:1 for group assignments. Mother-child pairs were followed monthly during the intervention period (visits 1–6) and one additional time (visit 7) 6 mo postintervention (Figure 1).
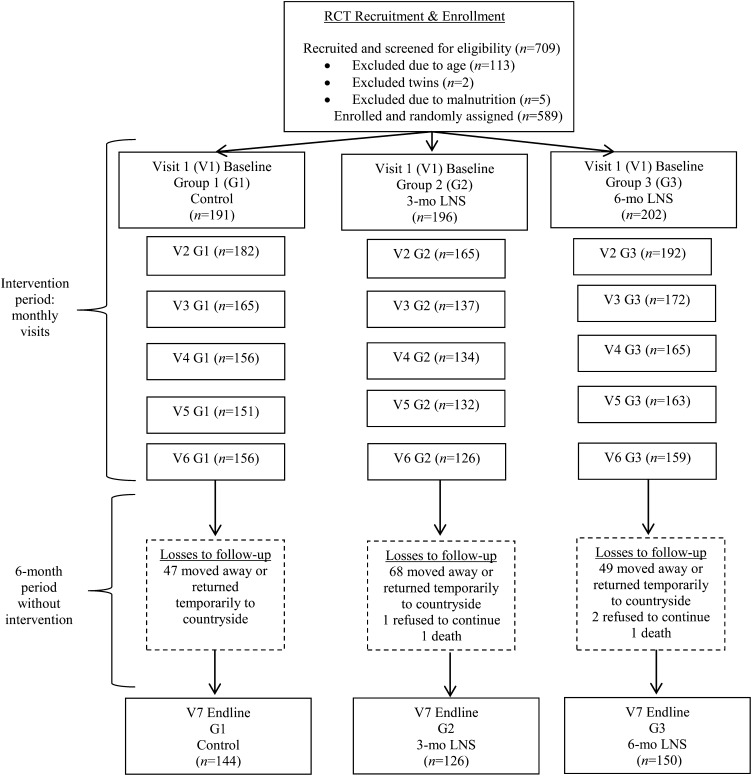
FIGURE 1.
Flow diagram of participant progression through the RCT. During the intervention period from baseline to visit 6, children were followed monthly. At each visit, mothers were surveyed on child diet, morbidities, and developmental outcomes, and child weights and lengths were measured. Mothers in the 3-mo LNS group received a monthly supply of the LNS at each of visits 1–3, and mothers in the 6-mo LNS group received a monthly supply of the LNS during each of visits 1–6. At visit 6, the intervention period ceased, and all mother-child pairs were asked to return 6 mo later for end-line visit 7, when the end-line survey was administered, and final child weight and length measures were taken. LNS, lipid-based nutrient supplement; RCT, randomized controlled trial.
The study team was comprised of one study coordinator and 3 enumerators who participated in a 1-wk training session at the beginning of the trial and another refresher training midway through covering the protocol of anthropometric measures, survey administration, and ethics. The study coordinator supervised all data collection and measures at the FSM clinic site with periodic visits by the principal investigator. Survey data for socioeconomic, demographic, and environmental factors were collected at baseline and again in an adapted form at end line (visit 7) to assess changes in these household- and community-level conditions. Child diet and feeding practices, morbidities, developmental outcomes, and LNS acceptability and use (for 3-mo and 6-mo LNS groups only) were assessed monthly for 6 mo (visits 1–6) and again at end line 6 mo postintervention (visit 7). Qualitative data were also collected at 2 time points during the study and will be reported elsewhere.
Real-time data entry was used at the study site to minimize errors and increase the likelihood of accurate and comprehensive reporting. Two onsite data managers entered data from surveys collected each day. When potential errors or missing data were identified, managers could inquire with enumerators and mothers to either provide appropriate responses or explanatory notes in the data set. Double data entry was conducted on a subset of surveys to check for entry error; > 97% of responses matched those entered for subset analyses.
Interventions
The LNS (Nutributter; Edesia: Global Nutrition Solutions) contains peanuts, sugar, soybean oil, nonfat milk powder, whey, maltodextrin, a vitamin and mineral complex, and the emulsifier lecithin. One sachet of LNS provides 108 kcal/d, which is approximately one-half to one-third of the required energy needed for breastfeeding infants 6–11 mo of age (Table 1).
TABLE 1.
LNS content and Dietary Reference Intakes for infants 7–11 mo old (13)1
| Nutrients | AI/RDA (7–11 mo of age) | LNS | AI/RDA provided by the LNS |
| % | |||
| Protein (g) | 11 | 2.56 | 23 |
| Fat (g) | 30 | 7.08 | 24 |
| Linoleic acid (g) | 4.6 | 1.29 | 28 |
| α-Linoleic acid (g) | 0.5 | 0.29 | 58 |
| Vitamin A (μg) | 500 | 400 | 80 |
| Thiamin (mg) | 0.3 | 0.3 | 100 |
| Riboflavin (mg) | 0.4 | 0.4 | 100 |
| Niacin (vitamin B-3) (mg) | 4 | 4 | 100 |
| Pantothenic acid (vitamin B-5) (mg) | 1.8 | 1.8 | 100 |
| Vitamin B-6 (mg) | 0.3 | 0.3 | 100 |
| Vitamin B-12 (μg) | 0.5 | 0.5 | 100 |
| Folic acid (μg) | 80 | 80 | 100 |
| Vitamin C (mg) | 50 | 30 | 60 |
| Vitamin D (μg) | 10 | 0 | 0 |
| Vitamin E (mg) | 5 | 0 | 0 |
| Vitamin K (μg) | 2.5 | 0 | 0 |
| Calcium (mg) | 260 | 100 | 38 |
| Copper (mg) | 0.22 | 0.2 | 91 |
| Iodine (μg) | 130 | 90 | 69 |
| Iron (mg) | 11 | 9 | 82 |
| Magnesium (mg) | 75 | 16 | 21 |
| Manganese (mg) | 0.6 | 0.08 | 13 |
| Phosphorus (mg) | 275 | 82.2 | 30 |
| Potassium (mg) | 700 | 152 | 22 |
| Selenium (μg) | 20 | 10 | 50 |
| Zinc (mg) | 2.5 | 4 | 160 |
LNS supplementation (20 g/d) provided 108 kcal, protein, fat, and 19 vitamins and minerals. AI, adequate intake; LNS, lipid-based nutrient supplement; RDA, Recommended Dietary Allowance.
During the intervention period, mother-child pairs in LNS trial arms received an exact monthly supply of LNS at their FSM clinic visits (1–6). At baseline, the following simple messages related to the use of the product were communicated to subjects in LNS groups: offer the child one-half of the LNS sachet in the morning and one-half of the LNS sachet in the afternoon; wash hands and the sachet before giving the LNS to the child; store the LNS in a clean and covered container; and the LNS is intended to provide important vitamins and minerals and should not replace other foods. All groups received the following simple messages about appropriate complementary feeding and hygiene practices: continue breastfeeding; offer the child a variety of other complementary feeding foods such as eggs, milk, fish, fruit, and vegetables; and wash hands before feeding. At the end of the study, after the 6-mo postintervention period was completed, mother-child pairs in the control group received a full 6-mo supply of the LNS, and mother-child pairs in the 3-mo LNS group received another 3-mo LNS supply to reach an equitable food distribution across groups. Mothers were informed of this design aspect at baseline to also help motivate them to attend follow-up visits.
Compliance data were collected in module 3 through a series of questions about LNS consumption, protocol adherence, and acceptability. Mothers were asked if their child consumed all of the LNS supply for the month, whether the child finished each daily LNS sachet in its entirety, how many times in a day the LNS was given, and if there was any sharing of the LNS with other household members. A reporting bias may have been present in this data because mothers were encouraged to follow protocols at baseline.
All children in the study received the IP of well-baby services of MSPP, which was one of the partners in the study. The IP is based on the internationally established model Integrated Management of Childhood Illness. At FSM during the study period, the IP routinely included reproductive health services, vaccinations, vitamin A supplementation, minimal nutrition education, growth monitoring, and referrals to Justinien University Hospital for rehabilitation of SAM with RUTF. Both qualitative and quantitative data were collected from mothers to understand the delivery and used of MSPP services, in particular that infant and young-child feeding (IYCF) messages were communicated and understood.
Protocols and variable definitions
An internationally established protocol was applied for the primary outcomes, anthropometric measures of length, height, and weight, which were converted to z scores for length for age, weight for age, weight for height, and BMI (14, 15). Stunting was defined as a an LAZ less than −2, underweight was defined as a WAZ less than −2, and wasting was defined as a WLZ less than −2. Weights were taken by using a Seca Model 874 Electronic Digital Scale (Seca) with a mother/child tare feature (to the nearest 0.1 kg), child lengths (to the nearest 1 mm) were measured by using the Infant/Child ShorrBoard (Shorr Productions LLC), and standing heights (to the nearest 1 mm) for mothers were measured by using a ShorrBoard Portable Height-Length Board (Shorr Productions LLC). Child lengths were measured twice, and if there was a >0.5-mm difference in measures, a third measure was taken.
Children identified during the study with SAM (WLZ < −3) were removed from the study and referred to Justinien University Hospital. A total of 6 children were referred for SAM during the study as follows: one child from the control group, 1 child from the 3-mo LNS group, and 4 children from the 6-mo LNS group. When a child recovered, he or she reentered the LNS study. Another 24 children became moderately malnourished during the study and were referred to the FSM program, although no Plumpy'Sup or other supplements were available during this period. Therefore, these children remained in the study until recovery.
Survey questions were first developed in English and translated into French and Haitian Creole. Each question was screened by the enumerator team, a pilot group of mothers, and representatives from the community for intent, cultural acceptability, and language clarity. Surveys and anthropometric measures were pilot tested at FSM with a subgroup of mothers (n = 20) during which time a series of anthropometric measures were taken to determine interreliability and intrareliability and validity indexes for the enumerator team (16).
Secondary child health and development outcomes assessed by the trial were IYCF practices, dietary consumption patterns, morbidities, and outcomes of language and motor development. IYCF indicators were drawn from internationally established indicators and those standardly applied by Demographic and Health Surveys (14, 17). We examined breastfeeding initiation, exclusive breastfeeding, and continued breastfeeding. The frequency of intake of the most commonly consumed complementary foods, identified through formative research, was collected for the following: cereals (rice, millet, wheat, maize, and other); poultry (chicken, duck, guinea fowl, and other); meat (beef, goat, sheep, pork, and other); fish and shellfish; eggs; milk (cow and goat); infant formula; yogurt or fermented milk; cheese and other dairy products; legumes or pulses (beans, peas, groundnuts, grams, and other); roots and tubers (cassava, potatoes, yams, and cooking bananas), fruit (mangos, bananas, oranges, and other); vegetables (eggplant, tomatoes, cabbage, and carrots); oils, butter, and other fats; and peanut butter. A dietary diversity indicator was created by summing the number of different foods reported consumed in the previous 24 h (15, 18). Finally, a seasonality variable was generated by partitioning the enrollment period into 2 planting and harvesting seasons from May to August and from September to December, respectively.
A range of morbidity outcomes by using a 2-week recall were assessed including diarrhea, respiratory infection, cough, fever, helminthes infection, and eye and skin conditions. At each monthly visit, mothers were asked whether children experienced these morbidities in the previous 14 d. Particular emphasis was placed on diarrhea morbidities in view of the water, hygiene, and sanitation conditions in FSM. Acute diarrhea was defined as ≥3 liquid or semiliquid stools in a day. Prolonged diarrhea was defined as acute diarrhea for 7–14 d, and persistent diarrhea was defined as acute diarrhea for ≥14 d (19). Longitudinal diarrhea prevalence was also calculated by the number of days with acute diarrhea over the number of observation days (20). Other morbidity definitions were respiratory infection (cough plus rapid, difficult breathing in the past 2 wk) and eye or skin conditions (related symptoms of redness, pain, itching, or swelling). Vaccination and micronutrient supplementation coverage was also assessed.
Motor- and simple language-development outcomes were assessed. WHO motor development milestones were applied with the following standard definitions: child crawling on his or her hands and knees, child standing with assistance, child walking with assistance, child stands alone, and child walks alone (14). We collected both mother-reported and enumerator-observed data, when possible, for each milestone.
Statistics
Descriptive statistics were first applied to examine characteristics of the sample and primary and secondary outcomes at each follow-up visit. Univariate tests including chi-square and t tests were used to compare characteristics across trial arms, and ANOVA with the Bonferroni multiple comparisons test examined growth, morbidity, and development outcomes by visit across control, 3-mo LNS, and 6-mo LNS groups (21). Statistics were repeated on subsamples such as mother-infant pairs lost to follow-up or subsamples measured by a single enumerator to understand any potential biases in outcomes. Kernel-density estimation plots were applied to graphically represent the distribution of anthropometric outcomes at each time point, and lowess curves derived from nonparametric, locally weighted regression were generated to illustrate trajectories and patterns of growth across the 3 groups (22).
Longitudinal modeling for the primary anthropometric outcomes of LAZ, WAZ, and WLZ was conducted with generalized least-squares (GLS) regression with random effects and mixed (random and fixed) effects. Both types of models were run, and the mixed-effects model was selected because the following assumptions for the random-effects model were not upheld: individual specific effects were uncorrelated with independent variables (compound symmetry); and the dropout or attrition was random (23). We examined the preventative effect of LNS supplementation on reducing adjusted odds of stunting, underweight, and wasting by using logit models with mixed effects. Similarly, logit models with mixed effects were applied to examine adjusted odds of morbidity and motor-development outcomes. All inference analyses for the efficacy of LNS on child growth used intention-to-treat analyses.
All socioeconomic (number of household members, maternal and paternal employment, income, and wealth and assets), environmental (water, hygiene, and sanitation; housing materials; electricity; and month and season of enrollment), maternal (anthropometric measures, education, parity, and living with a partner or spouse), and child-level (age, sex, IYCF, and diet, anthropometric measures, morbidities, health care received, vaccinations) variables were tested in regression models as independent variables to derive comprehensive models with maximum-likelihood estimation for the influences on growth, morbidities, and development. Only coefficients that achieved significance at P < 0.05 and did not potentially mediate the effect of the LNS on growth were included in the final models. Functional forms of the age variable were assessed, and the ln of age was selected as the best fit for the decelerating growth trajectory in these children. Interaction and spline terms were also generated but not shown to be significant and, therefore, were not included in the model. The Sobel-Goodman mediation test was used in pathway analyses of intervening variables to effect specifically diarrhea and IYCF variables (24).
Independent terms that were highly correlated (r ≥ 0.7) were not included simultaneously in regression models to avoid the problem of colinearity, which was further tested with variance inflation-factor statistics by using a cutoff of 10. Regression diagnostics by using residual plots and checking assumptions were also conducted to assess the goodness of fit for covariates (21). Data analyses were performed with STATA software (version 11.1; StataCorp).
RESULTS
There were 709 mother-infant pairs screened for eligibility, and 120 pairs were excluded because of age, singleton (being a twin), and SAM criteria (Figure 1). We enrolled all eligible mother-infant pairs (n = 589) from May 2011 to December 2011. By the 6-mo visit, there had been a 25% loss to follow-up that increased to 29% by the 12 mo follow-up visit (from May 2012 to December 2012). Mothers lost to follow-up were contacted either by using mobile phones or through home visits to inquire for reasons; 90% of mothers were reached and indicated that they had moved away, returned temporarily to the countryside, or refused to continue because of problems encountered at the clinic. Two child deaths occurred as a result of causes unrelated to the study. Potential biases associated with attrition were assessed. Child, maternal, household, and socioeconomic status characteristics were similar in the mother-child pairs lost to follow-up by group compared with the characteristics by group for mother-child pairs who remained in the study, with the following exceptions: a higher percentage of the control group lost to follow-up had electricity; and in the 3-mo LNS group lost to follow-up, the mean age of mothers was younger, and children showed a significantly greater WAZ (P < 0.05). By visit 5, children who remained in the trial from the 3-mo LNS group were significantly younger than those in the control group (P < 0.05).
Baseline characteristics did not differ by trial arm with the exception of an older mean age in the 6-mo LNS group compared with that of children in 3-mo LNS and control groups (Table 2). The prevalence of stunting was 9.4%, underweight was 5.2%, and wasting was 2.2% with no differences evident by group. Acute diarrhea was prevalent in greater than one-third of children. Education levels completed by mothers were predominantly primary and secondary levels. There was limited variability in the sample for the drinking water source and toilet type. The percentage of households reporting income at or below the internationally established poverty level ($2/d) was 22.5%, and in abject poverty (<$1/d), the percentage was 5.1%. Asset ownership in the form of a house, land, livestock, radio, television, cell phone, appliances, and transportation vehicles was examined and not shown to differ by group.
TABLE 2.
Baseline socioeconomic, health, and demographic characteristics by group1
| Control (n = 191) | 3-mo LNS (n = 196) | 6-mo LNS (n = 202) | |
| Child characteristics | |||
| Age (mo) | 7.2 ± 1.72,a | 7.0 ± 1.5a | 7.8 ± 1.7b |
| F (%) | 58.1 | 53.6 | 53.0 |
| Primiparous (%) | 36.1 | 38.8 | 42.6 |
| Anthropometric measure | |||
| Length-for-age z score | −0.44 ± 1.29 | −0.49 ± 1.13 | −0.39 ± 1.20 |
| Weight-for-age z score | −0.22 ± 1.15 | −0.26 ± 1.15 | −0.21 ± 1.06 |
| Weight-for-length z score | 0.15 ± 1.10 | 0.14 ± 1.12 | 0.10 ± 1.04 |
| Breastfeeding frequency (/d) | 8.2 ± 3.6 | 8.6 ± 3.9 | 8.0 ± 3.7 |
| Poultry consumption frequency (/d) | 0.08 ± 0.36 | 0.03 ± 0.17 | 0.06 ± 0.32 |
| Acute diarrhea (%) | 34.0 | 33.2 | 43.7 |
| Fever (%) | 22.1 | 23.0 | 26.7 |
| Caregiver characteristics | |||
| Maternal age (y) | 28.7 ± 7.2 | 28.2 ± 6.6 | 27.5 ± 6.8 |
| Maternal height (cm) | 158.4 | 157.9 | 157.7 |
| Maternal education completed (%) | |||
| No education | 16.8 | 16.3 | 16.3 |
| Primary school | 36.7 | 35.2 | 35.2 |
| Secondary school | 42.9 | 44.9 | 46.5 |
| Postsecondary | 3.7 | 3.6 | 2.0 |
| Maternal employment (%) | 48.2 | 50.0 | 56.4 |
| Household characteristics | |||
| Electricity (%) | 42.4 | 48.7 | 52.6 |
| Income ($/d) | 3.57 | 3.57 | 3.57 |
| Poverty (income/d <$2) (%) | 22.2 | 21.1 | 24.0 |
| Drinking water source for child (%) | |||
| Public pump | 17.3 | 17.4 | 17.2 |
| Purchased water | 72.3 | 68.4 | 76.1 |
| Other | 10.4 | 14.2 | 6.7 |
| Toilet type (%) | |||
| Automatic flush | 6.8 | 6.1 | 6.6 |
| Latrine | 87.4 | 88.3 | 86.6 |
| No toilet | 4.7 | 3.6 | 4.0 |
| Household members (total n) | 5.5 ± 2.2 | 5.7 ± 2.2 | 5.7 ± 2.9 |
LNS supplementation (20 g/d) provided 108 kcal, protein, fat, and 19 vitamins and minerals. Acute diarrhea (≥3 liquid or semiliquid stools in a 24-h period) and fever (temperature >37°C) prevalence over the past 2 wk. Values in the same row with different superscript letters are significantly different, P < 0.05 (chi-square or t test). LNS, lipid-based nutrient supplement.
Mean ± SD (all such values).
Children were reported to have consumed all of the monthly supply of the LNS during the supplementation period in 98.0% (95% CI: 97.0%, 100.0%) of mothers in the 3-mo LNS group and 97.0% (95% CI: 92.2%, 98.8%) of children in the 6-mo LNS group. By contrast, compliance with twice-daily LNS consumption (protocol of one-half of the LNS sachet in the morning, and one-half of the LNS sachet in the afternoon) was low. In both groups, 6.0% (95% CI: 2.6%, 9.4%) of mothers followed this protocol.
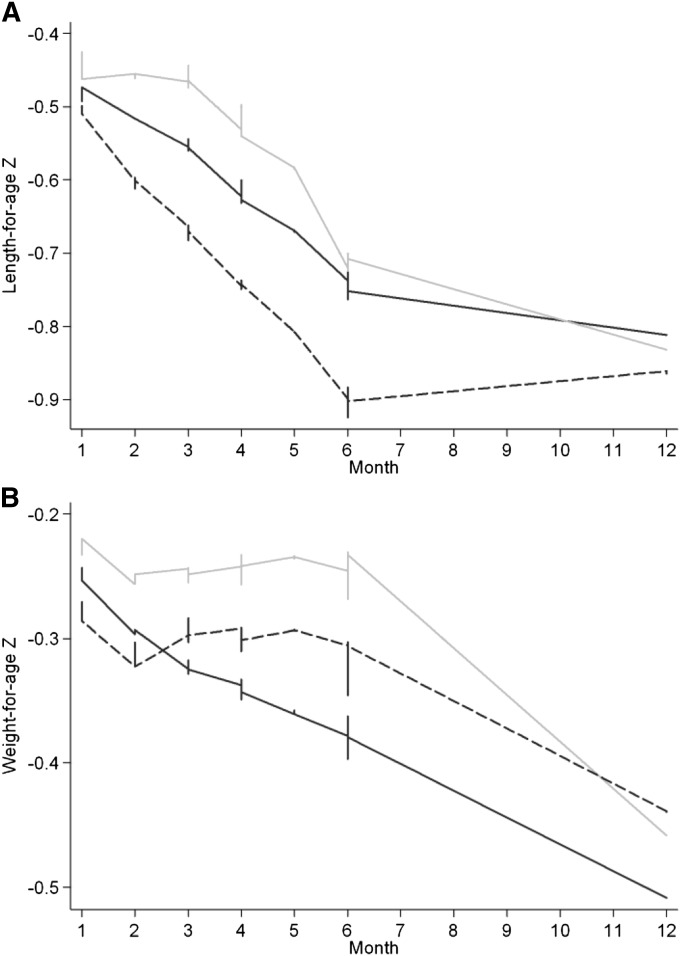
Descriptive data showed that the LAZ in the 6-mo LNS group was maintained during the first one-half of the supplementation period and showed a flat linear pattern compared with the negative slopes of the LAZ for control and 3-mo LNS groups (Figure 2A). By month 4, all groups showed declining LAZ trajectories. At end line, after the postintervention period, the 3 groups converged for the LAZ. The kernel-density estimation of child lengths at visits 6 and 7 (end line) across groups showed a narrowing of distributions for both LNS groups, whereas the control group remained relatively more dispersed and flatter as exemplified in the reduced length SD for the 6-mo LNS group (SD: 1.15) than control (SD 1.29) and 3-mo LNS (SD 1.27) groups. The WAZ dropped initially in all groups and continued to decline in the control group, and during the postintervention period, the WAZ slope was negative for all groups (Figure 2B). These patterns were similarly evident in the cross-sectional comparisons of anthropometric measures at each monthly visit (Table 3).
FIGURE 2.
Lowess curves derived from nonparametric, locally weighted regression illustrate trajectories of growth for the control group (black line), 3-mo LNS group (dashed line), and 6-mo LNS group (gray line) for length-for-age z score (A) and weight-for-age z score (B) for children followed monthly (visits 1–6) and again at end line (visit 7) 6 mo postintervention. The lowess graph applies least-squares smoothing at each visit and, thus, differs from the mean length-for-age and weight-for-age z scores shown in Table 3. In this descriptive data that were not adjusted for age, the 6-mo LNS group showed a length-for-age z score that was maintained at higher levels than in the control and 3-mo LNS groups until the end of the intervention period and converged with the other groups at visit 7 at 12 mo (A). The weight-for-age z score was also shown to be maintained in LNS groups during the supplementation period, whereas in the control group, there was a steadily descending slope until end line, visit 7, at 12 mo (B). LNS, lipid-based nutrient supplement.
TABLE 3.
Child LAZ and WAZ by visit and group1
| Visit 1 (1, n = 191; 2, n = 196; 3, n = 202) | Visit 2 (1, n = 182; 2, n = 165; 3, n = 192) | Visit 3 (1, n = 165; 2, n = 137; 3, n = 172) | Visit 4 (1, n = 156; 2, n = 134; 3, n = 165) | Visit 5 (1, n = 151; 2, n = 132; 3, n = 163) | Visit 6 (1, n = 156; 2, n = 126; 3, n = 159) | Visit 7 (1, n = 144; 2, n = 126; 3, n = 150) | |
| LAZ | |||||||
| Control | −0.45 ± 1.29 | −0.57 ± 1.29 | −0.49 ± 1.16 | −0.61 ± 1.14 | −0.68 ± 1.15 | −0.74 ± 1.19 | −0.81 ± 1.21 |
| 3-mo LNS | −0.49 ± 1.13 | −0.64 ± 1.15 | −0.68 ± 1.06 | −0.70 ± 1.10 | −0.76 ± 1.08 | −0.93 ± 1.00 | −0.86 ± 1.35 |
| 6-mo LNS | −0.39 ± 1.20 | −0.56 ± 1.36 | −0.34 ± 1.272 | −0.46 ± 1.20 | −0.58 ± 1.17 | −0.70 ± 1.16 | −0.83 ± 1.19 |
| WAZ | |||||||
| Control | −0.22 ± 1.15 | −0.32 ± 1.21 | −0.31 ± 1.18 | −0.36 ± 1.17 | −0.37 ± 1.14 | −0.37 ± 1.12 | −0.51 ± 1.21 |
| 3-mo LNS | −0.26 ± 1.15 | −0.37 ± 1.14 | −0.31 ± 1.16 | −0.24 ± 1.21 | −0.27 ± 1.17 | −0.34 ± 1.20 | −0.44 ± 1.21 |
| 6-mo LNS | −0.21 ± 1.06 | −0.29 ± 1.11 | −0.24 ± 1.14 | −0.22 ± 1.08 | −0.21 ± 0.99 | −0.26 ± 1.05 | −0.46 ± 1.03 |
All values are means ± SDs. LNS supplementation (20 g/d) provided 108 kcal, protein, fat, and 19 vitamins and minerals. Child anthropometric measures of length and weight were taken at monthly visits to the clinic during the intervention period (visits 1–6) and again 6 mo postintervention at end line (visit 7). Sample sizes by group number are as follows: 1 denotes the control group; 2 denotes the 3-mo LNS group; and 3 denotes the 6-mo LNS group. LAZ, length-for-age z score; LNS, lipid-based nutrient supplement; WAZ, weight-for-age z score.
LAZ was significantly different by group, P < 0.05 (ANOVA with the Bonferroni multiple comparisons test).
GLS regression modeling showed a significant, positive increase in the LAZ and WAZ for children in the 6-mo LNS group compared with in the control group after adjustment for child age through visit 6 (Table 4). Positive effects on the LAZ and WAZ were reduced by visit 7 (after the 6-mo postintervention period) but remained significant. Compared with the control group, children in the 3-mo LNS group showed reduced growth longitudinally in the LAZ. Results from WLZ modeling are not presented because of a very low prevalence of wasting in the population. Other significant determinants of growth that did not mediate the LNS effect and were shown to improve the model likelihood estimation were poultry consumption frequency per day, maternal height and weight, maternal education level attained, total number of household members, and the season of enrollment. The breastfeeding frequency positively improved growth but was shown to be a significant negative mediating factor by using the Sobel-Goodman mediation test and was removed from the model. Similarly, the incidence of acute diarrhea reduced the LAZ and WAZ, but because incidence of acute diarrhea reduced the LNS effect size on the LAZ by 7% from 0.14% to 0.13%, the variable was not included in the final model. No interaction effects were identified (P > 0.05). At visit 6, prevalences of undernutrition were as follows: stunting, 13.0%; underweight, 5.8%; and wasting, 1.6%. At visit 7 (6 mo postintervention), prevalences were as follows: stunting, 17.9%; underweight, 7.4%; and wasting, 3.1%. No differences by group were evident.
TABLE 4.
Longitudinal regression models for the LNS effect on child-growth outcomes1
| 3-mo LNS compared with control |
6-mo LNS compared with control |
|||||||
| Unadjusted | P | Adjusted | P | Unadjusted | P | Adjusted | P | |
| At visit 6 | ||||||||
| LAZ | −0.10 ± 0.05 | 0.06 | −0.12 ± 0.05 | 0.03 | 0.08 ± 0.05 | 0.16 | 0.13 ± 0.05 | 0.02 |
| WAZ | 0.02 ± 0.05 | 0.65 | 0.01 ± 0.05 | 0.86 | 0.08 ± 0.05 | 0.09 | 0.12 ± 0.05 | 0.02 |
| At visit 7 (6 mo postintervention) | ||||||||
| LAZ | −0.09 ± 0.05 | 0.06 | −0.11 ± 0.05 | 0.03 | 0.06 ± 0.05 | 0.20 | 0.10 ± 0.05 | 0.04 |
| WAZ | 0.03 ± 0.05 | 0.54 | 0.02 ± 0.05 | 0.73 | 0.08 ± 0.04 | 0.09 | 0.11 ± 0.04 | 0.02 |
All values are βs ± SEs. Adjusted values were adjusted for the ln of child age. LNS supplementation (20 g/d) provided 108 kcal, protein, lipids, and 19 vitamins and minerals. Generalized least-squares with mixed-effects longitudinal models were applied to examine LNS supplementation (3-mo and 6-mo LNS) compared with a control on LAZ and WAZ outcomes (P < 0.001; Wald's chi-square test). LAZ, length-for-age z score; LNS, lipid-based nutrient supplement; WAZ, weight-for-age z score.
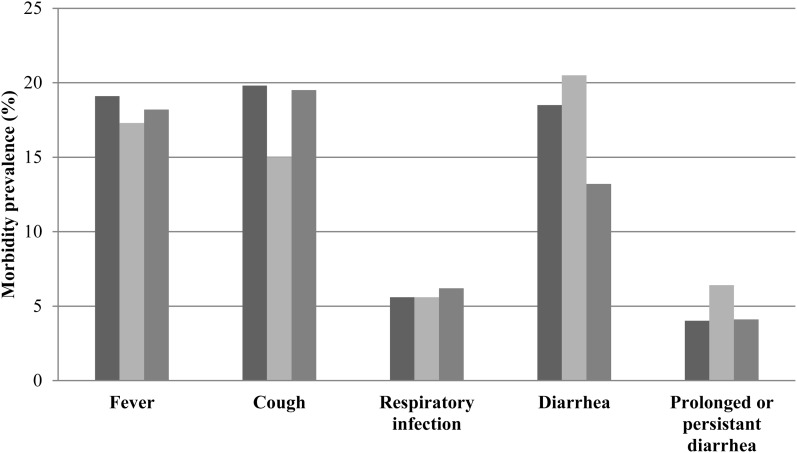
Prevalences of morbidities were not shown to differ by study group in univariate analyses (Figure 3). At visit 6, there was a trend toward reduced acute diarrhea in the 6-mo LNS group and an increased prevalence of prolonged and persistent diarrhea in the 3-mo LNS group (P = 0.06; chi-square test). After adjustment for age in logit regression modeling, no significant effects were shown for morbidity outcomes longitudinally (P > 0.05). In children reported to have experienced acute diarrhea, the mean number of days with acute diarrhea was 4.5 ± 3.3 d, and the longitudinal prevalence was 7.2 ± 15.0%. In the entire sample, the longitudinal prevalence of acute diarrhea was 2.5 ± 8.6%. No significant differences in the longitudinal prevalence of diarrhea were shown across groups.
FIGURE 3.
Child morbidity prevalence at visit 6 for control (dark gray), 3-mo LNS (light gray), and 6-mo LNS (gray) groups. Morbidities were derived from a maternal 2-wk recall for fever, cough, respiratory infection (cough plus rapid, difficult breathing), acute diarrhea (≥3 liquid or semiliquid stools/d), and prolonged diarrhea (acute diarrhea for 7–14 d) or persistent diarrhea (acute diarrhea for >14 d). There were no significant differences in morbidity prevalences in groups, P < 0.05 (chi-square test). LNS, lipid-based nutrient supplement.
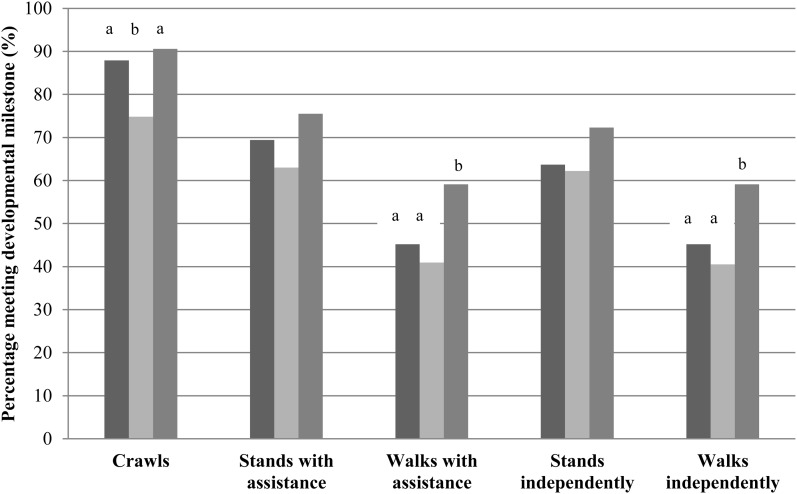
At visit 6, there was a significantly increased percentage of children in the 6-mo LNS group who achieved motor milestones of crawling than in the 3-mo LNS group and walking with assistance and walking independently than in control and 3-mo LNS groups (P < 0.05; chi-square test) (Figure 4). In logit regression modeling, after adjustment for the age of the child and maternal educational level attained, the significance of developmental outcomes was no longer present.
FIGURE 4.
Percentages of children meeting the motor development milestone for control (dark gray), 3-mo LNS (light gray), and 6-mo LNS (gray) groups at visit 6 on the basis of maternal reporting and enumerator observation. The following WHO developmental milestone definitions were applied: crawls (≥3 consecutive movements in a row and stomach does not touch ground); stands with assistance (upright position on both feet holding onto an object and, legs do not touch object for ≥10 s); walks with assistance (upright with back straight, move sideways or forward holding an object, and one leg moves and the other leg supports weight for ≥5 steps); stands independently (upright with back straight on feet and not toes; legs support 100% of weight; no contact with people or objects for 10 s); walks independently (child takes 5 steps independently without the use of people or objects and one leg forward while the other leg supports weight). Bars with different lowercase letters signify statistically different prevalences, P < 0.05 (chi-square test). After adjustment for child age and maternal education level attained in regression modeling, the significance of developmental outcomes was no longer present. LNS, lipid-based nutrient supplement.
DISCUSSION
In this urban slum of Haiti, we showed that a small-quantity LNS introduced early during the complementary feeding period increased growth by 0.13 of LAZ and 0.12 of WAZ in children in the 6-mo LNS group compared with the control group. Both effects, although lessened, were sustained 6 mo postintervention. The 3-mo LNS supplementation group showed a negative effect on the LAZ, and neither LNS arm was shown to affect morbidity and developmental outcomes. Acute diarrhea was highly prevalent in all children in the study and likely limited the LNS effect on the LAZ.
Although underweight and wasting prevalences continues to drop in Haiti, stunting still affects one-fifth of children <5 y of age (3, 4). In our study population, by end line visit 7, the prevalence of underweight was 7.4%, and the prevalence of wasting was 3.1%, but the prevalence of stunting had reached 17.9%. We explicitly aimed to increase linear growth by targeting the problem of poor-quality complementary feeding diets and micronutrient inadequacies (25). Our findings yielded a positive LAZ effect comparable to that in other community-based trials with short periods of supplementation (26). The effect, although reduced, was shown to be sustained 6 mo after the intervention period had ended. We did not show significant results for reduced stunting, which was likely because of the lower prevalence in urban compared with rural areas and insufficient statistical power to detect a change.
Few studies have successfully shown the use of RUTF, RUSF, and LNS products for promoting linear growth. In Chad, there was a small but significant increase in the height-for-age z score by 0.03 per month associated with a 46-g/d dose of RUSF over 4 mo (6). Another study in Malawi showed that, although length gains were not greater with LNS supplementation compared with fortified maize-soy flour (9), there were reduced odds of severe stunting in the LNS group that was sustained after a 2-y nonintervention period (27). The Ghana study described previously most closely matched our design with some differences in design and comparison groups (10). This study showed, through an adjusted, longitudinal comparison, significant differences in the LAZ between Nutributter (−0.20 ± 0.54) and combined Nutritab and Sprinkles groups (−0.38 ± 0.54). Micronutrient powder has been shown to reduce anemia in Haiti (28), but we are not aware of any impacts on linear growth (29).
The negative effect on the LAZ in the 3-mo LNS group merits attention. One potential explanation for this finding may lie with the mother-infant pairs lost to follow-up in this group. Mothers from the 3-mo LNS group who dropped out of the study were significantly younger and had infants with a greater WAZ. At baseline, the LAZ and WAZ did not differ by group, which affirmed the successful random assignment, but immediate losses to follow-up that occurred by visit 2 coincided with immediate decreases in the LAZ and WAZ. For younger mothers with larger babies assigned to the 3-mo LNS group, the perceived advantages of participation may not have outweighed the real costs in terms of their time and lost wages. And for mother-infant pairs who remained in the study, the short period of supplementation may not have been sufficient to overcome micronutrient deficiencies and other nutritional insults through infancy and into young childhood. Finally, we observed that, by visit 5, children who remained in the study from the 3-mo LNS group were significantly younger than those in the control group. Younger children would have shown greater reductions in the LAZ in longitudinal models on the basis of typical growth trajectories in Haiti.
The LNS did not reduce diarrhea morbidities, which are the leading cause of child mortality in Haiti and a rampant problem across the entire population (30, 31). We hypothesized that the LNS would improve the micronutrient nutrition with positive implications for reduced diarrheal prevalence. However, the exceptionally poor water and sanitation conditions in the FSM slum area may be an environmental factor too large to overcome with a nutrition intervention alone. Urban areas tend to have reduced child mortality relative to rural areas in many developing countries, but large slum areas are the exception (32).
Trends toward improved developmental milestones were evident for LNS supplementation. In support of previous studies, there was a significantly higher percentage of children who walked independently in the 6-mo LNS group compared with other groups, although the effect was no longer present with maternal education in GLS regression models. This result may have been due to an aspirational effect in which more educated mothers in intervention groups provided extra stimulation and play motivated by the LNS investment. Nonetheless, the effect on linear growth that was potentially indicative of improved micronutrient nutrition may lead to future improvements in developmental outcomes (33, 34).
Some study limitations were present. Child age at baseline differed across groups, and the 6-mo LNS group began the study at an older age than the other groups. It is unclear why this difference occurred in the randomization process, which was evidently successful across all other characteristics. The older age could have produced a more-conservative LNS effect on the LAZ, which is generally more responsive in younger children (5). The exact age of the child was adjusted for in all regression modeling. Another potential bias may have been losses to follow-up. Relocation was the predominant reason cited for dropping out across all groups, which suggested that the slum area is prone to high population mobility. Characteristics that differed in groups lost to follow-up were either NS for growth outcomes (household electricity) or acknowledged as a potential reason for findings in the 3-mo LNS group (younger mothers and children with a higher baseline WAZ and older age by visit 5). Finally, questions regarding external validity and generalizability often arise with a randomized controlled trial design. We purposefully situated the study at the FSM clinic, where IP services are delivered, with the explicit strategy of future integration of the LNS into MSPP programming, depending on the results of the study. The study was clinic based, which may have precluded some of the operational findings from being translated into more remote rural areas. The effectiveness of the LNS in rural areas as well as other urban areas through community-based rally posts should be assessed to understand logistics of distribution and uptake of services.
In conclusion, this trial showed that an LNS could improve young child linear growth in an urban population and sustain the effects 6 mo postintervention. Findings suggest complementary interventions may be needed together with the LNS to address diarrheal morbidities and promote continued breastfeeding. With the increasing use of RUSF and LNS products, our study suggests a preference for small-quantity products aimed at improving micronutrient nutrition and promoting healthy growth over longer supplementation periods.
Acknowledgments
We acknowledge the other partners in the trial, the Ministry of Public Health and Population (MSPP) in Haiti and Meds and Food for Kids (MFK). We appreciate the valuable contributions made by mothers and children taking part in the study. We also acknowledge K Dewey, E Kuyper, B Vitta (University of California, Davis), N Henretty (Edesia), P Wolff (MFK), C Smith (CARE), L Caulfield (The Johns Hopkins Bloomberg School of Public Health), and J Siekmann (Global Alliance for Improved Nutrition) for technical inputs and D Hedeker (University of Illinois, Chicago) and D Brown (Washington University in St Louis) for their review of statistical methods.
The authors’ responsibilities were as follows—LLI, NMN, JG, and SJLD: designed the research; SJLD, JG, SJ, JF, CL, and M-LA: conducted the research; LLI: analyzed data, wrote the manuscript, and had primary responsibility for the final content of the manuscript; JM and NMN: contributed to the writing of the manuscript; and all authors: read and approved the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: FSM, Fort Saint Michel; GLS, generalized least squares; IP, integrated package; IYCF, infant and young-child feeding; LAZ, length-for-age z score; LNS, lipid-based nutrient supplement; MSPP, Ministry of Public Health and Population; RUSF, ready-to-use supplemental food; RUTF, ready-to-use therapeutic food; SAM, severe acute malnutrition; WAZ, weight-for-age z score; WLZ, weight-for-length z score.
REFERENCES
- 1.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 2.The Central Intelligence Agency (CIA). The world factbook: Central America and the Caribbean, Haiti. Version current 1 April 2013. Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/ha.html cited 1 March 2013).
- 3.Ministere de la Sante Publique et de la Population (MSPP), Institut Haitien de l'Enfance (IHE), ICF International. [Mortality, Morbidity, and Utilization of Services Study, Haiti, 2012. ] Calverton, MD: ICF International, 2013. (in French). [Google Scholar]
- 4.Ministere de la Sante Publique et de la Population (MSPP), Institut Haitien de l'Enfance (IHE), Macro International Inc. Enquête mortalité, morbidité et utilisation des services, Haiti, 2005-2006. Calverton, MD: Macro International Inc., 2007. [Google Scholar]
- 5.Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, Webb P, Lartey A, Black RE; The Lancet Nutrition Interventions Review Group, et al. Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost? Lancet 2013;382:452–77. [DOI] [PubMed] [Google Scholar]
- 6.Huybregts L, Houngbé F, Salpéteur C, Brown R, Roberfroid D, Ait-Aissa M, Kolsteren P. The effect of adding ready-to-use supplementary food to a general food distribution on child nutritional status and morbidity: a cluster-randomized controlled trial. PLoS Med 2012;9:e1001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Defourny I, Minetti A, Harczi G, Doyon S, Shepherd S, Tectonidis M, Bradol JH, Golden M. A large-scale distribution of milk-based fortified spreads: evidence for a new approach in regions with high burden of acute malnutrition. PLoS ONE 2009;4:e5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isanaka S, Nombela N, Djibo A, Poupard M, Van Beckhoven D, Gaboulaud V, Guerin PJ, Grais RF. Effect of preventive supplementation with ready-to-use therapeutic food on the nutritional status, mortality, and morbidity of children aged 6 to 60 months in Niger: a cluster randomized trial. JAMA 2009;301:277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary M, Ashorn P. Complementary feeding with fortified spread and incidence of severe stunting in 6- to 18-month-old rural Malawians. Arch Pediatr Adolesc Med 2008;162:619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adu-Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey K. Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. Am J Clin Nutr 2007;86:412–20. [DOI] [PubMed] [Google Scholar]
- 11.World Food Programme (WFP). Haiti. Available from: http://www.wfp.org/countries/haiti (cited 3 July 2013).
- 12.Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, Oxman AD, Moher D; CONSORT group; Pragmatic Trials in Healthcare (Practihc) group. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337:a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Food and Nutrition Board, Institute for Medicine, National Academy of Sciences. Dietary reference intakes: estimated average requirements. Available from: http://www.iom.edu/Activities/Nutrition/SummaryDRIs/~/media/Files/Activity%20Files/Nutrition/DRIs/5_Summary%20Table%20Tables%201-4.pdf (cited 4 July 2013).
- 14.World Health Organization (WHO). The WHO child growth standards. Version current 1 April 2013. Available from: http://www.who.int/childgrowth/en/ (cited 1 March 2013).
- 15.FANTA. Publications: household dietary diversity score (HDDS) and months of adequate household food provisioning (MAHFP). Version current 1 April 2013. Available from: http://www.fantaproject.org/publications/hdds_mahfp.shtml (cited 18 February 2013).
- 16.Ulijaszek SJ, Kerr DA. Anthropometric measurement error and the assessment of nutritional status. Br J Nutr 1999;82:165–77. [DOI] [PubMed] [Google Scholar]
- 17.Measure DHS Demographic and Health Surveys. DHS overview. Version current 1 April 2013. Available from: http://www.measuredhs.com/What-We-Do/Survey-Types/DHS.cfm (cited 1 February 2013).
- 18.Arimond M, Wiesmann D, Becquey E, Carriquiry A, Daniels MC, Deitchler M, Fanou-Fogny N, Joseph ML, Kennedy G, Martin-Previl Y, et al. Simple food group diversity indicators predict micronutrient adequacy of women's diets in 5 diverse, resource-poor settings. J Nutr 2010;140:2059S–69S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iannotti LL, Zavaleta N, León Z, Huasquiche C, Shankar AH, Caulfield LE. Maternal zinc supplementation reduces diarrheal morbidity in Peruvian infants. J Pediatr 2010;156:960–4. [DOI] [PubMed] [Google Scholar]
- 20.Morris SS, Cousens SN, Kirkwood BR, Arthur P, Ross DA. Is prevalence of diarrhea a better predictor of subsequent mortality and weight gain than diarrhea incidence? Am J Epidemiol 1996;144:582–8. [DOI] [PubMed] [Google Scholar]
- 21.Hamilton LC. Belmont, CA: Brooks/Cole, Division of Thomson Learning Inc, 2004. [Google Scholar]
- 22.StataCorp. Stata: release 11. College Station, TX: StataCorp LP, 2009. [Google Scholar]
- 23.Hedeker D, Gibbons R . 1st ed. Hoboken, NJ: Wiley, John & Sons, Incorporated, 2006. [Google Scholar]
- 24.UCLA, Institute for Digital Research and Communication. Stata FAQ: how to preform Sobel-Goodman mediation tests in Stata. Version current 1 April 2013. Available from: http://www.ats.ucla.edu/stat/stata/faq/sgmediation.htm (cited 27 March 2013).
- 25.USAID. Haiti prospective food security assessment. Washington, DC: FANTA-2 Bridge/FHI 360, 2011. [Google Scholar]
- 26.Sguassero Y, de Onis M, Bonotti AM, Carroli G. Community-based supplementary feeding for promoting the growth of children under five years of age in low and middle income countries. Cochrane Database Syst Rev 2012;6:CD005039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, Ashorn P. Postintervention growth of Malawian children who received 12-mo dietary complementation with a lipid-based nutrient supplement or maize-soy flour. Am J Clin Nutr 2009;89:382–90. [DOI] [PubMed] [Google Scholar]
- 28.Menon P, Ruel MT, Loechl CU, Arimond M, Habicht JP, Pelto G, Michaud L. Micronutrient Sprinkles reduce anemia among 9- to 24-mo-old children when delivered through an integrated health and nutrition program in rural Haiti. J Nutr 2007;137:1023–30. [DOI] [PubMed] [Google Scholar]
- 29.De-Regil LM, Suchdev PS, Vist GE, Walleser S, Pena-Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database Syst Rev 2011;9:CD008959. [DOI] [PubMed] [Google Scholar]
- 30.Harshfield E, Lantagne D, Turbes A, Null C. Evaluating the sustained health impact of household chlorination of drinking water in rural Haiti. Am J Trop Med Hyg 2012;87:786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brasseur P, Agnamey P, Emmanuel E, Pape JW, Vaillant M, Raccurt CP. Cryptosporidium contamination of surface and water supplies in Haiti. Arch Environ Occup Health 2011;66:12–7. [DOI] [PubMed] [Google Scholar]
- 32.Garenne M. Urbanisation and child health in resource poor settings with special reference to under-five mortality in Africa. Arch Dis Child 2010;95:464–8. [DOI] [PubMed] [Google Scholar]
- 33.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B; International Child Development Steering Group. Developmental potential in the first 5 years for children in developing countries. Lancet 2007;369:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, Carter JA; International Child Development Steering Group. Child development: risk factors for adverse outcomes in developing countries. Lancet 2007;369:145–57. [DOI] [PubMed] [Google Scholar]