Abstract
Amino acid (AA) transporters may act as sensors, as well as carriers, of tissue nutrient supplies. This review considers recent advances in our understanding of the AA-sensing functions of AA transporters in both epithelial and nonepithelial cells. These transporters mediate AA exchanges between extracellular and intracellular fluid compartments, delivering substrates to intracellular AA sensors. AA transporters on endosomal (eg, lysosomal) membranes may themselves function as intracellular AA sensors. AA transporters at the cell surface, particularly those for large neutral AAs such as leucine, interact functionally with intracellular nutrient-signaling pathways that regulate metabolism: for example, the mammalian target of rapamycin complex 1 (mTORC1) pathway, which promotes cell growth, and the general control non-derepressible (GCN) pathway, which is activated by AA starvation. Under some circumstances, upregulation of AA transporter expression [notably a leucine transporter, solute carrier 7A5 (SLC7A5)] is required to initiate AA-dependent activation of the mTORC1 pathway. Certain AA transporters may have dual receptor-transporter functions, operating as “transceptors” to sense extracellular (or intracellular) AA availability upstream of intracellular signaling pathways. New opportunities for nutritional therapy may include targeting of AA transporters (or mechanisms that upregulate their expression) to promote protein-anabolic signals for retention or recovery of lean tissue mass.
INTRODUCTION
The cells of the human body are able to respond to changes in availability of dietary and plasma nutrients by functional adaptations (eg, increasing or decreasing growth, proliferation, and energy expenditure) appropriate to the prevailing conditions. To do this, the systems controlling these responses are capable of direct or indirect monitoring of intracellular and extracellular nutrient concentrations through use of nutrient “sensors.” Numerous enzymes, receptors, and transporter proteins may have nutrient-sensor functions. This review focuses only on amino acid (AA)5 sensing by AA transporters.
The proteolipid surface membrane of cells is a selective barrier to nutrients and, because AAs do not readily diffuse across lipid membranes, membrane-spanning transporter proteins (see Figure 1) are required to help move AAs in and out of a cell and between membrane-bound intracellular compartments [eg, cytosol and lysosome (see references 1 and 2 for review)]. AA transport may be coupled to movements of ions including Na+, H+, K+, and/or Cl−, as well as movement of other AAs by antiport. The modern classification of AA transporters in mammalian cells based on similarity between transporter gene sequences has superseded a classical “systems”-based classification of AA transport mechanisms (1, 3, 4), although both remain in common use. There are 6 major families of AA transporters in the solute carrier (SLC) gene superfamily (SLC1, SLC6, SLC7, SLC36, SLC38, and SLC43 families) and an orphan SLC16 monocarboxylate transporter, which transports aromatic AAs (see, eg, http://www.bioparadigms.org for details of SLC genes). The protein products of these transporter genes are characterized by having multiple (typically 10–12) transmembrane domains (TMDs) organized around a central pore region. Members of the SLC3 gene family are also classed as AA transporters but are atypical in that they form single TMD glycoproteins that act as regulatory subunits for a subfamily of SLC7 transporters. There is also a group of 7-TMD AA transporter proteins from the LCT (lysosomal cystine transporter) gene family expressed at the lysosomal membrane (5, 6). Several AA transporters are linked to inheritable human metabolic disorders (eg, SLC7A9 and cystinuria) (7, 8).
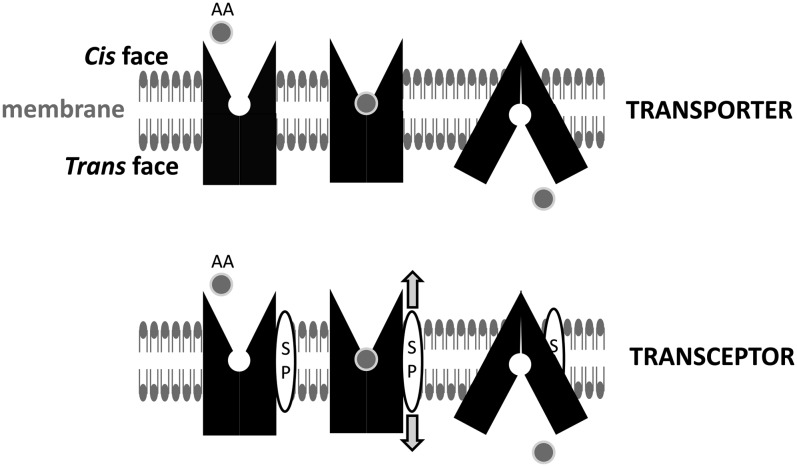
FIGURE 1.
Transporters and transceptors. The AA transport process proceeds through a sequence of steps as follows: 1) binding of AAs to a specific exposed site on the transporter at the (cis) membrane surface; 2) a change in conformation of the transporter, which results in the AA-bound site becoming exposed to the opposite (trans) membrane surface through the central pore; and 3) release of AAs and reorientation of the transporter to the initial cis-facing conformation. AA transport may be coupled to movements of ions including Na+, H+, K+, or Cl− (see Figure 2). Certain AA transporters may also act as transceptors (ie, binding or translocation of AAs is coupled to activation of an intracellular signaling cascade), enabling them to “sense” the size of the cis pool of AAs (10, 19). The signal may be generated directly by the transceptor: for example, by covalent modification (eg, phosphorylation) or proteolytic cleavage of an intracellular-signaling precursor or indirectly through an intermediate signal–generating molecule (represented here as a transmembrane SP). The SP produces a signal (arrows) in response to a conformational change in the transceptor due to AA binding/translocation. AA, amino acid; SP, signaling peptide.
The AA binding sites of mammalian AA transporters generally recognize a range of structurally similar AAs as cargo for transport: for example, large neutral AAs (LNAAs), small neutral AAs (SNAAs), CAAs (cationic AAs), or AAAs (anionic AAs). AA transporter expression is tissue specific, and many cell types express several AA transporters with overlapping specificities, with the result that transport of a specific AA frequently involves the integrated activity of several AA uniporters and antiporters operating in parallel (see references 9 and 10 for review). AA transporter activity and substrate competition are important factors in the determination and regulation of AA fluxes across cell membranes (9).
The major AA sensing-signaling pathways in mammalian cells are the mammalian target of rapamycin complex 1 (mTORC1) and general control non-derepressible (GCN) pathways (see Figure 2). The AA-sensing mechanisms of the mTORC1 pathway, which is activated when certain AAs (eg, leucine) are abundant, appear to involve monitoring AA concentrations in both cytosol and subcellular organelles such as lysosomes (11, 12). The GCN pathway primarily senses intracellular AA availability at the level of AA “charging” on transfer RNA (tRNA) bound to the GCN2 protein kinase and is activated when one or more AAs are scarce (13, 14). AA transporters have important roles upstream and downstream of both mTORC1 and GCN pathways and may help in monitoring both intracellular and extracellular AA abundances (eg, references 15–18; see references 10, 12, and 19) for review). AA transporters may act directly as the initiating sensor for a signaling pathway—for example, activation of mTORC1 signaling by the SLC38A2 transporter (18)—or may serve as a conduit for delivery of AAs to intracellular sensing pathways, notably the leucine transporter SLC7A5 for mTORC1 activation (17, 20). AA transporters may also generate indirect nutrient-related signals related to effects of cotransported solutes on intracellular pH and volume (see reference 10 for review).
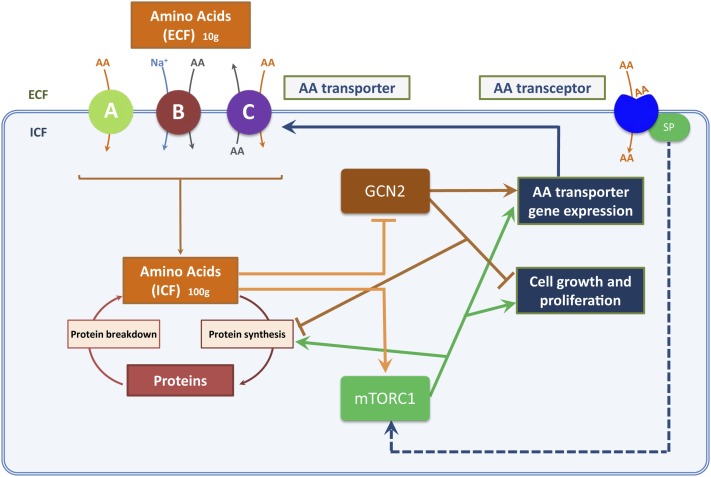
FIGURE 2.
AA pools and nutrient sensing. Homeostasis of ECF and ICF pools of AAs depends on the balance between AA fluxes through transport and metabolic pathways. AA transporters function by specific mechanisms, which include uniport (facilitative transport; denoted as “A”), symport (cotransport; denoted as “B”), and antiport (exchange; denoted as “C”). Net delivery of AAs to the ICF pool by AA transporters in an adult consuming a balanced diet (equivalent to dietary AA intake at steady state) is ∼60–100 g/d (3, 66). The 2 major AA-sensitive signaling proteins in mammalian cells are GCN2 and mTOR (as part of mTORC1), which respond to changes in ICF AA concentrations as shown and regulate protein turnover (and hence cell growth). AA transceptors at the cell surface may also sense size and composition of the ECF pool of AAs. AA transceptors on intracellular membranes (not shown) may perform equivalent roles in sensing ICF pools of AAs. AA, amino acid; ECF, extracellular fluid; GCN2, general control non-derepressible 2; ICF, intracellular fluid; mTOR, mammalian target of rapamycin; mTORC1, mammalian target of rapamycin complex 1; SP, signaling peptide (this may generate or transmit the transceptor signal; see Figure 1 legend for further description).
AA TRANSPORTERS AND AA SENSING BY THE mTORC1 PATHWAY
The mTORC1 pathway is a key anabolic intracellular signaling mechanism responsive to stimuli such as nutrients, growth factors, energy stress, hypoxia, and mechanical strain (12, 21–23). In the nutritional context, mTORC1 has a key role in coordinating the combination of nutrient and endocrine (largely growth factor) signals generated by a protein meal into an anabolic drive to stimulate protein turnover and, under appropriate circumstances, tissue growth (12, 24). The mTORC1 protein complex is composed of mTOR itself and accessory proteins including RAPTOR (regulatory-associated protein of mTOR), mLST8/GβL (mammalian LST8/G-protein β-subunit–like protein), and the inhibitory subunits PRAS40 (proline rich Akt substrate 40 kDa) and DEPTOR (DEP domain-containing mTOR-interacting protein) (eg, see references 12 and 25 for review). Two of the best-characterized downstream targets of mTORC1 are S6K1 (p70-S6 kinase 1) and eukaryotic initiation factor 4E binding protein 1 (4E-BP1) (24, 26). Phosphorylation of S6K1 by mTORC1 stimulates it to interact with and phosphorylate the S6 ribosomal protein components, increasing the rate of cellular protein synthesis. In addition, 4E-BP1 in its nonphosphorylated form is tightly associated with eukaryotic initiation factor (eIF) 4E, a protein involved in the initial coupling of 40S ribosomes to the 5′ end of mRNA strands. This association inhibits eIF4E, but mTORC1 phosphorylation of 4E-BP1 releases it from eIF4E, allowing the latter to promote recruitment of mRNA to ribosomes and hence stimulate protein synthesis (eg, references 11 and 24 for review).
The mechanisms activating mTORC1 in response to AAs and growth factors both involve small GTPases (Rag GTPases and Rheb GTPase, respectively) (27). Rag (Ras-related GTPase) proteins exist as heterodimers of Rag A/B combined with Rag C/D: Rag A/B-GTP and Rag C/D-GDP constitute the “active” forms and the reversed nucleotide orientation the “inactive” forms (27, 28). The Rag heterodimer docks with the Ragulator protein complex (composed of LAMTOR 1–5), which is bound to the lysosome membrane (29). The active forms of Rag GTPase directly interact with “inactive” cytosolic mTORC1 by binding with RAPTOR and cause the localization of mTORC1 to the surface of lysosomes (see Figure 3). The p62 protein is an additional signaling adaptor linking the Rag and mTORC1 protein complexes (30). Recruitment of mTORC1 to the lysosome allows it to be fully activated by binding Rheb-GTP, which provides additional signaling input from growth factor stimuli (24) and makes AA sufficiency a prerequisite for efficient insulin signaling through mTORC1 (27).
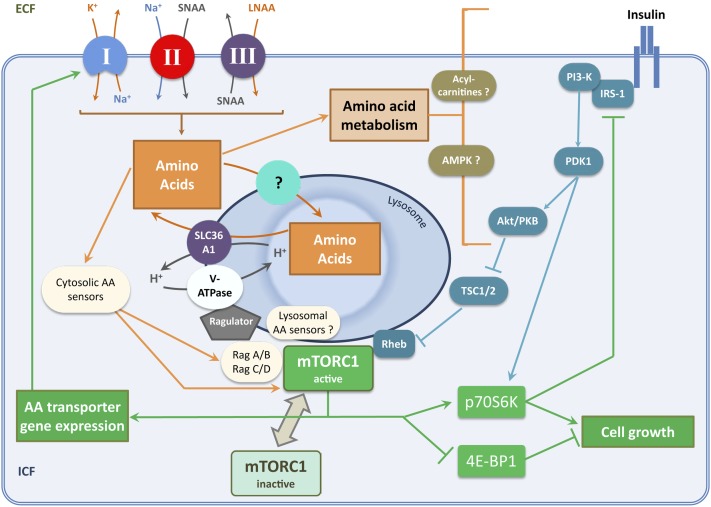
FIGURE 3.
Neutral AA transporters and activation of the mTORC1 signaling pathway. This diagram shows the relation between neutral AA transport, intracellular AA concentration, and the mTORC1 growth signaling pathway in nonepithelial mammalian cells (see text for further details). A sequential relation between primary, secondary, and tertiary active transport systems (denoted as “I,” “II,” and “III,” respectively) contributes substantially to transport of LNAAs across cell membranes. Energy input is provided through ATP hydrolysis by the Na+/K+ pump (primary active transport). Note the operation of symport (cotransport) and antiport (exchange) mechanisms for AAs in series downstream of the Na+/ K+ pump. In epithelial cells, broad-scope neutral AA transporters provide both SNAAs and LNAAs coupled to ion fluxes by secondary active transport, lessening the requirement for step III. AA (principally LNAA) concentration and/or flux within intracellular compartments promotes recruitment of mTORC1 to lysosomes where it is activated by interactions with Rag and Rheb GTPases. Such activation of mTORC1, downstream of nutrient (AA) and growth factor (insulin) signals, stimulates protein synthesis and ribosome biogenesis by effector mechanisms as indicated. Both cytosolic and lysosomal AA sensors have been reported (see sections in text entitled “Plasma membrane AA transporters and cytosolic AA sensing upstream of mTORC1” and “AA transporters and lysosomal AA sensing upstream of mTORC1”). Remarkably little is known about the transporter or transporters mediating neutral AA uptake into lysosomes, although SLC38A7 has recently emerged as a candidate for this role (48). Intracellular AA metabolism may also modulate growth factor signaling upstream of mTORC1 (62, 63). AA, amino acid; Akt/PKB, protein kinase B; AMPK, adenosine monophosphate activated protein kinase; ECF, extracellular fluid; ICF, intracellular fluid; IRS-1, insulin receptor substrate 1; LNAA, large neutral amino acid; mTORC1, mammalian target of rapamycin complex 1; PDK1, 3-phosphoinositide dependent protein kinase 1; PI3-K, phosphatidylinositide 3-kinase; Rag, Ras-related GTPase; Rheb, Ras homolog enriched in brain; SLC36A1, solute carrier 36A1; SNAA, small neutral amino acid; TSC1/2, tuberous sclerosis complex 1/2; V-ATPase, vacuolar H+-ATPase; 4E-BP1, eukaryotic initiation factor 4E binding protein 1.
Plasma membrane AA transporters and cytosolic AA sensing upstream of mTORC1
An increase in intracellular concentration (and/or metabolic flux) of AAs promotes the Rag-dependent translocation of the mTORC1 complex from the cytosol to the surface of lysosomes (see Figure 3). LNAAs (a group including essential AAs such as leucine, isoleucine, valine, phenylalanine, and tryptophan) are key stimulants of mTORC1 activation alongside glutamine and arginine. Leucine is particularly potent and is frequently used as an exemplar to describe AA-dependent activation of mTORC1 (25). Several putative cytosolic AA sensors linked to mTORC1 activation have been reported (12, 31; see Table 1 for a summary), a number of which are capable of directly binding AAs such as leucine [notably leucyl-tRNA synthetase (LRS), GDH (glutamate dehydrogenase), and UBR1-2 (unbranched chain AA receptors 1 and 2)]. When leucine binds to LRS it acts as a GTPase-activating protein (GAP) for RagD heterodimers and helps anchor the Rag proteins to the lysosome membrane. This switches RagD from a GTP to a GDP conformation in the presence of AAs, which promotes the recruitment of mTORC1 to the lysosome membrane (31). Aminoacyl-tRNA synthetases other than LRS do not cause any changes in mTORC1, although LRS may activate mTORC1 for AAs other than leucine: for example, isoleucine can bind to the leucine-binding site of LRS due to misacylation (31). There is some evidence for 2 distinct stimulatory signals from either glutamine or LNAAs for full activation of mTORC1 (32, 33), and indeed glutamine deprivation may reduce mTORC1 activity without decreasing intracellular leucine concentration (32). Glutaminolysis catalyzed by GDH may provide a glutamine-specific, leucine-stimulated signal for mTORC1 activation (33; see Table 1).
TABLE 1.
Cellular proteins associated with cytosolic AA sensing upstream of mTORC11
| AA sensor | Mechanism | Reference |
| LRS | A component of the multi-tRNA synthetase complex involved in tRNA charging. GTPase activating protein domain on LRS hydrolyzes GTP to GDP on RagD in the presence of leucine. Links RagB/RagD heterodimer with Ragulator. | (31) |
| IMPK | Implicated as an AA sensor for mTORC1 signaling by acting as a cofactor between mTOR and RAPTOR in the presence of AAs. | (67) |
| MAP4K3 | Ste20 family kinase regulated by AAs, acting upstream of mTORC1. | (39, 68) |
| VPS34 | Positive regulator of mTORC1 signaling, present on the surface of lysosomes. Functions upstream of mTORC1 as an AA sensor, perhaps linked to AA-induced changes in intracellular calcium ions. | (69, 70) |
| GDH | Leucine activates GDH stimulating glutaminolysis and generation of α-ketoglutarate, which, perhaps through action of prolyl hydroxylases, promotes GTP loading of RagB and mTORC1 recruitment to lysosomes. | (33) |
| RalA | EAA sensor for mTORC1 signaling, involves GTP binding to RalA. May localize to lysosome. | (25, 71) |
| UBR1-2 | Ubiquitin ligases, which are negative regulators of mTORC1 and interact with “N-degrons” (AA residues at amino-terminus of target proteins). Leucine binding to UBR1-2 inhibits their effect and promotes TORC1 signaling. | (72) |
Note that some putative cytosolic AA sensors may also localize to lysosomes. AA, amino acid; EAA, essential amino acid; GDH, glutamate dehydrogenase; IMPK, inositol polyphosphate multikinase; LRS, leucyl–transfer RNA synthetase; MAP4K3, mitogen-activating protein kinase kinase kinase kinase-3; mTORC1, mammalian target of rapamycin complex 1; Rag, Ras-related GTPase; RalA, Ras-like protein A; RAPTOR, regulatory-associated protein of mTOR; Ste20, Sterile 20; tRNA, transfer RNA; UBR1-2, unbranched chain amino acid receptors 1 and 2; VPS34, vacuolar sorting protein-34.
The generation of an anabolic signal by dietary protein requires that AAs are delivered to the intracellular sensor and effector molecules associated with the mTORC1 pathway, and AA transporters at the plasma membrane are an essential component of the delivery pathway (see Figure 3). AA transporters at the plasma membrane of epithelial cells differ from those in nonepithelial tissues, although they are from the same or related SLC gene families. In epithelial cells, broad-scope Na+-coupled AA symporters such as SLC6A19 (system BO, aka BOAT) (34) and SLC6A14 (system BO,+, aka BO,+AT) (35) are able to transport both LNAAs and SNAAs directly into cells upstream of mTORC1 activation. In contrast, LNAAs are taken up into nonepithelial cells by exchange or facilitative mechanisms, whereas SNAAs tend to be transported by concentrative Na+-coupled transport. In consequence, certain SNAAs (especially glutamine and alanine) become highly concentrated in tissues such as skeletal muscle, acting as labile nitrogen stores (9, 36), whereas LNAAs do not accumulate to any great extent and indeed tend to equilibrate between intracellular and extracellular fluids. A sequential relation between primary, secondary, and tertiary active transport systems (see Figure 3) has developed that contributes substantially to transport of LNAAs across cell membranes; this includes symport (cotransport) and antiport (exchange) mechanisms operating in series downstream of the Na+ pump. A key feature of this relation is the ability of a small subset of AAs with intermediate size (notably glutamine) to be transported by both the secondary and tertiary active transporters. The most prevalent and well studied of these AA transporters are SLC38A2 (system A, aka SNAT2), SLC1A5 (system ASC, aka ASCT2), and SLC7A5 (system L, aka LAT1) (17, 20, 37). SLC38A2 and SLC1A5 are Na+-coupled secondary active transporters mediating Na+-SNAA symport, and SLC7A5 is an LNAA antiporter mediating tertiary active transport when downstream of the former. Genetic or functional inactivation of these AA transporters inhibits rapid growth and proliferation of mammalian cells in culture (eg, references 17, 18, and 20), generally linked to reduced mTORC1 signaling. Conversely, induction of functional SLC7A5 gene expression is an initiating factor for activation of the mTORC1 pathway by the proliferation factor HIF2α (hypoxia-inducible factor 2-α) (38) and during T lymphocyte activation (20). The level of SLC7A5 expression in fibroblasts correlates directly with the effectiveness of leucine-induced mTORC1 activation in these cells (39). In some circumstances, intracellular glutamine accumulation by SLC38A2 and/or SLC1A5 may become a limiting factor for mTORC1 activation due to SLC7A5 requiring intracellular glutamine as an obligate antiporter to transport leucine into the cell (17, 37).
Other amino acids, for example, arginine via the SLC7A1-4 (system y+, aka CAT1-4) transporters, may also influence mTORC1 pathway activation (40). Extracellular AAs may also activate mTORC1 through certain AA transporters at the cell surface (eg, SLC38A2) acting as dual-function “AA transceptors” (see Figure 1 and section entitled “AA transporters and AA sensing by the GCN pathway”) (18).
AA transporters and lysosomal AA sensing upstream of mTORC1
The AA-dependent recruitment of the multiprotein mTORC1 complex to lysosomal membrane compartments (Figure 3) appears to be associated with AA accumulation into lysosomes (16), indicating that AA transporters on endosomal membranes play a role in AA sensing. Lysosomal degradation of proteins allows the cell to recycle some of the AAs, and the process of autophagy is linked to mTORC1 signaling (12, 17). The precise relations between AA pools in the cytosol and lysosomal lumen and the lysosomal AA transporters mediating AA exchange between these subcellular compartments are poorly understood. Under AA sufficiency, a lysosomal-anchored “nutrisome” protein complex has been proposed as a sensor of intralysosomal AA concentrations upstream of mTORC1 activation (15, 16). Two putative AA-sensing transporter proteins, the vacuolar H+-ATPase (v-ATPase) and the SLC36A1 H+-coupled AA transporter (aka PAT1), have been reported to function as part of the “nutrisome” (12, 15, 16) and to physically interact with the Rag-Ragulator complex. The v-ATPase helps generate the acidic interior of the lysosomes compared with the slightly alkaline cytosol (pH 5 to pH 7.4) by pumping protons into the lysosome from the cytosol, hydrolyzing ATP to ADP in the process. SLC36A1 has both endosomal and plasma membrane localization (41, 42) and mediates H+-dependent AA efflux from the lysosomal lumen into the cytosol. SLC36A1 appears to be required for AA-induced activation of mTORC1 (43), but it exerts a negative influence on lysosomal mTORC1 signaling when overexpressed in mammalian cell lines (16). The influx/efflux and/or accumulation of AAs into the lysosomal lumen is somehow detected by the nutrisome [a so-called inside-out method of AA sensing (16)], causing activation of the GEF (guanine exchange factor) function of the Ragulator complex, which, in turn, promotes the GTP charging of RagA/B necessary for mTORC1 engagement. SLC36A1 has been suggested to act as an AA transceptor on the lysosomal membrane (43), but although certain LNAAs may act as inhibitors of SLC36 transporter functional activity (44), the AA substrate selectivity of SLC36 transporters does not match the range of AAs activating mTORC1 (41, 44). The AA transporter proteins mediating AA influx to lysosomes, alongside proteins underlying many other lysosomal membrane functions, remain unidentified. AA transport systems having functional resemblance to the T and L carriers for LNAAs in plasma membranes have been characterized in the membranes of intact lysosomes (45–47). These systems (named t, l, and h) are saturable, displaying Km values of 5–30 μmol phenylalanine, leucine, or tryptophan/L. A recent semiquantitative proteomic analysis of rat liver lysosome-enriched membranes (48) identified several AA transporters previously assigned to other subcellular compartments that may reside (at least secondarily) on lysosomes. These include 2 LCT family members characterized by a duplicated motif termed the PQ loop (6): PQLC4 (cystinosin) is the lysosomal cystine exporter defective in cystinosis (8) and PQLC2 is a lysosomal exporter of CAAs (5). Of the relatively few neutral AA transporters identified in this study, SLC38A7 is particularly interesting in that 1) it appears to transport (or at least be inhibited by) a fairly broad range of neutral and cationic AAs including leucine (49) and 2) several of the SLC38 family transporters effectively operate as Na+-dependent H+-AA antiporters (see reference 42 for a recent review), a mechanism that might favor AA accumulation into lysosomes via SLC38A7.
AA TRANSPORTERS AND AA SENSING BY THE GCN PATHWAY
GCN2 is an eIF2α kinase that senses the absence of individual cytosolic AAs by direct binding to uncharged cognate tRNAs (13, 14). Phosphorylation of eIF2α impairs initiation of mRNA translation at the methionine start codon and reduces global protein synthesis. This response to AA starvation also facilitates selective translation of specific mRNAs [eg, the transcription factor ATF4 (activating transcription factor 4)] associated with upregulation of genes with functions in AA biosynthesis, retention, and scavenging. An important effector arm of the GCN pathway in many cell types is upregulation of the SLC38A2 AA transporter as an AA scavenger mechanism during periods of AA deficiency. This process, known as “adaptive regulation,” involves transcriptional upregulation of transporter gene expression (ATF4 binds to the AA-response element in the SLC38A2 gene, activating transcription) (50), maintenance of SLC38A2 mRNA translation through an internal ribosome entry site (51), and increased stability (reduced degradation) of SLC38A2 transporter proteins (eg, 52). The latter feature is regulated by a mechanism intrinsic to the SLC38A2 protein, whereby AA transport activity (or binding of AA substrates to the transporter protein) seemingly acts to promote internalization and degradation of the protein itself when AAs are abundant (52), a feature characteristic of AA “transceptors” in other eukaryotic organisms (19). In fact, both SLC38A2 and SLC36A1 have been proposed to operate as multifunctional AA transceptors (15, 52), whereby AA substrate binding to the transporter protein induces an intracellular nutrient signal independent of AA transport. SLC38A2 may also be involved in the central response to essential AA deficiency within the anterior piriform cortex of the brain, although not as the primary AA sensor (53). The delivery of AAs to the cytosol from lysosomal proteolysis may also affect the GCN pathway: for example, deletion of the PQLC2 gene homolog laat-1 in Caenorhabditis elegans worms limits cytosolic lysine and arginine, causing embryonic lethality when the GCN2 pathway is impaired (54).
AA TRANSPORTERS AND AA SENSING IN A TISSUE-SPECIFIC CONTEXT
Lymphocytes
AA supply may become an increasingly limiting factor for tissue protein synthesis during periods of rapid cell growth or proliferation (eg, for lymphocytes during an immune response), when intracellular availability of both LNAAs (eg, leucine for protein synthesis) and SNAAs (eg, glutamine or glycine for cell metabolism) may become highly dependent on the AA transport capacity at the cell surface. Sustained growth may therefore require substantial upregulation of AA transporter expression, as is seen in the process by which T lymphocytes grow and proliferate after activation through the T cell receptor (TCR). T lymphocyte activation markedly increases protein synthesis associated with a high fold-induction of SLC7A5 mRNA and a resultant induction of high-affinity (low μmol/L Km) LNAA uptake to increase AA supply in the most effective manner (20). Other high-affinity AA transporters (notably SLC7A1 and SLC1A5) are also upregulated to a lesser extent. Sustained uptake of leucine (and other LNAAs) through SLC7A5 in activated T lymphocytes is required for mTORC1 activation and upregulation of the c-Myc mitogen and energy-supplying metabolic pathways (20). The SLC7A5 transporter is recognized as a potential immunosuppressive (55) and antitumor (56) target; indeed, high-affinity (nmol/L) inhibitors of SLC7A5 are potent immunosuppressants (55). Induction of SLC7A5 expression through the TCR in T lymphocytes is blocked by cyclosporin A, an immunosuppressive drug that inhibits calcineurin-mediated signaling, but not by rapamycin, an immunosuppressant that inhibits mTORC1 (20). A combined failure of TCR-activated growth and proliferation in SLC7A5-null T lymphocytes (20) appears to be more severe than the growth failure seen with mTORC1-inhibited (rapamycin-treated) T lymphocytes. These observations indicate that SLC7A5 may have an additional AA-sensing role above that related to mTORC1 activation that is associated with control of cell division and proliferation.
Skeletal muscle
The increase in LNAA availability after protein or AA ingestion is associated with upregulation in expression of AA transporters (eg, SLC38A2, SLC7A5) in human skeletal muscle 2–3 h postmeal (57). SLC7A5, in particular, is a high-affinity LNAA transporter; hence, upregulation of this transporter in muscle during the anabolic phase of the dietary cycle may represent an adaptive response to enhance the mTORC1 growth signal from nutrients and insulin. Indeed, muscle-specific (MCK-Cre) SLC7A5 knockout mice fed a high-protein diet display mild insulin resistance with reduced mTORC1 pathway activation in skeletal muscles (N Poncet, FE Mitchell, Y-B Shi, and PM Taylor, unpublished observations, 2013). Skeletal muscle, in common with several other tissues, expresses both high-affinity and low-affinity system L transporters for LNAAs (SLC7A5 and SLC7A8, respectively) (4). Such dual-transporter systems are suggested to help prolong preparation for cellular starvation and facilitate recovery from starvation, acting to “fine tune” sensing of nutrient depletion through integration of inputs relating to internal and external nutrient availability (58).
Epithelial cells
The broad-scope Na+-coupled neutral AA transporter SLC6A19 provides an important AA supply for epithelial mTORC1 signaling and SLC6A19 knockout in mice produces a phenotype of apparent epithelial cell starvation (34). During mammalian embryonic development, AA activation of mTORC1 is an important aspect of blastocyst activation (59). The broad-scope Na+/Cl−-coupled AA transporter SLC6A14, which accepts both neutral and cationic AAs as substrates, is upregulated at the blastocyst stage; and the enhanced uptake of AAs (especially leucine) that it affords is an important factor for blastocyst activation and trophoblast outgrowth (35). Placental growth is modulated by mTORC1, which regulates the activity of key AA transporters (eg, SLC7A5) by posttranslational modifications or by affecting transporter translocation to the placental surface (60). The activity of placental AA transporters is decreased in intrauterine growth restriction in conjunction with a reduction in placental mTORC1 activity (61).
UNRESOLVED ISSUES
Whereas the importance of AA sensing and signaling to growth and metabolic functioning of mammalian cells and tissues is clear, the extent to which the mTORC1 and GCN pathways influence whole-body homeostasis in healthy, well-nourished human adults on a day-to-day basis remains poorly understood. In vitro cell-based studies are providing remarkable insights into the molecular basis of AA sensing, but they generally use culture media containing LNAAs at concentrations 2–5 times those found in extracellular fluids in vivo and study mTORC1 activation by AA starvation followed by refeeding with leucine or an AA mixture. In such circumstances, the “leucine-repletion capacity” of transporters such as SLC7A5 may be critical for AA sensing (39). Our understanding of the situation in vivo, where lower AA concentrations that fluctuate with diurnal feeding behavior (but will rarely decrease to concentrations constituting AA starvation) are the prevailing condition, requires further investigation.
At the subcellular level, the relative importance of cytosolic and lysosomal AA sensing is still under debate. In terms of AA sensing at the molecular level, the lack of a clear direct relation between intracellular LNAA concentrations and mTORC1 activity in some studies (eg, references 18 and 32) raises the possibility that LNAA flux through a transporter or metabolic pathway (rather than LNAA concentration per se) may be a “sensed” variable. Other gaps in the picture of AA-dependent mTORC1 activation include the following: 1) the mechanism by which AAs such as leucine accumulate within lysosomes in response to increased extracellular AA supply and 2) the mechanistic link between AA accumulation into lysosomes and nutrisome activation upstream of RagA/B GTPases.
NUTRITIONAL IMPLICATIONS
The requirement for sufficiency of LNAAs such as leucine to achieve full activation of mTORC1 signaling downstream of insulin links AA transporter function and insulin action in vivo. This is well illustrated by the observation that SLC6A19-null mice show reduced insulin responsiveness and impaired body-weight control (34). Dietary leucine is now being considered as an adjunct treatment of insulin resistance related to obesity (eg, references 62 and 63). New opportunities for nutritional therapy may lie in targeting nutrient-sensing AA transporters (or mechanisms that upregulate their activity) so as to promote protein-anabolic signals designed to retain lean tissue mass in aging or to regain it during rehabilitation from disease or injury. Postprandial upregulation of expression of AA transporters such as SLC7A5 in skeletal muscle may provide LNAAs as an energy source as well as an anabolic signal and substrate, given that it has recently been shown that administering leucine or carbohydrate supplements after a meal extends the duration of enhanced protein synthesis in skeletal muscle by helping maintain cellular energy status (64). Such maintenance of ATP supply also reduces the risk of activating “stress” sensors such as AMPK (adenosine monophosphate activated protein kinase) and REDD1/2 (regulated in development and DNA damage responses 1/2), both of which activate TSC1/2 (tuberous sclerosis complex 1/2) and hence repress mTORC1 signaling (65). An improved understanding of the time course of such events may help further improve dietary recommendations for the optimal utilization of protein (64, 66).
Acknowledgments
I thank Nadège Poncet for valuable assistance in preparation of the figures.
PMT received an honorarium and expenses from ASN in relation to participation at the ASN Presidential Symposium 2013. The author declared no other conflicts of interest.
Footnotes
Abbreviations used: AA, amino acid; eIF, eukaryotic initiation factor; GCN, general control non-derepressible; LNAA, large neutral amino acid; LRS, leucyl–transfer RNA synthetase; mTORC1, mammalian target of rapamycin complex 1; Rag, Ras-related GTPase; SLC, solute carrier gene superfamily; SNAA, small neutral amino acid; TCR, T cell receptor; TMD, transmembrane domain; tRNA, transfer RNA; 4E-BP1, eukaryotic initiation factor 4E binding protein 1.
REFERENCES
- 1.Bröer S. Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 2008;88:249–86. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs BC, Bode BP. Amino acid transporters ASCT2 and LAT1 in cancer: partners in crime? Semin Cancer Biol 2005;15:254–66. [DOI] [PubMed] [Google Scholar]
- 3.Poncet N, Taylor PM. The role of amino acid transporters in nutrition. Curr Opin Clin Nutr Metab Care 2013;16:57–65. [DOI] [PubMed] [Google Scholar]
- 4.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch Eur J Physiol 2004;447(5):532–42. [DOI] [PubMed] [Google Scholar]
- 5.Jézégou A, Llinares E, Anne C. Kieffer-Jaquinod S, O'Regan S, Aupetit J, Chabli A, Sagné C, Debacker C, Chadefaux-Vekemans B, et al. Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystinosis therapy. Proc Natl Acad Sci USA 2012;109:E3434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhai Y, Heijne WHM, Smith DW, Saier MH., Jr Homologues of archaeal rhodopsins in plants, animals and fungi: structural and functional predications for a putative fungal chaperone protein. Biochim Biophys Acta Biomembr 2001;1511:206–23. [DOI] [PubMed] [Google Scholar]
- 7.Bröer S, Palacin M. The role of amino acid transporters in inherited and acquired diseases. Biochem J 2011;436:193–211. [DOI] [PubMed] [Google Scholar]
- 8.Kalatzis V, Cherqui S, Antignac C, Gasnier B. Cystinosin, the protein defective in cystinosis, is a H+-driven lysosomal cystine transporter. EMBO J 2001;20:5940–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev 1990;70:43–77. [DOI] [PubMed] [Google Scholar]
- 10.Hundal HS, Taylor PM. Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab 2009;296:E603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab 2009;296:E592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SG, Buel G, Blenis J. Nutrient regulation of the mTOR complex 1 signaling pathway. Mol Cell 2013;35:463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 2009;136:731–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gallinetti J, Harputlugil E, Mitchell JR. Amino acid sensing in dietary-restriction-mediated longevity: roles of signal-transducing kinases GCN2 and TOR. Biochem J 2013;449:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ögmundsdóttir MH, Heublein S, Kazi S, Reynolds B, Visvalingam SM, Shaw MK, Goberdhan DCI. Proton-assisted amino acid transporter PAT1 complexes with Rag GTPases and activates TORC1 on late endosomal and lysosomal membranes. PLoS ONE 2012;7:e36616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 2011;334:678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicklin P, Bergman P, Zhang B. Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell 2009;136:521–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinilla J, Aledo JC, Cwiklinski E, Hyde R, Taylor PM, Hundal HS. SNAT2 transceptor signalling via mTOR: a role in cell growth and proliferation? Front Biosci (Elite Ed) 2011;3:1289–99. [DOI] [PubMed] [Google Scholar]
- 19.Kriel J, Haesendonckx S, Rubio-Texeira M, Van Zeebroeck G, Thevelein JM. From transporter to transceptor: signaling from transporters provokes re-evaluation of complex trafficking and regulatory controls. Bioessays 2011;33:870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sinclair LV, Rolf J, Emslie E, Shi Y-B, Taylor PM, Cantrell DA. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat Immunol 2013;14:500–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasiakos SM. Exercise and amino acid anabolic cell signaling and the regulation of skeletal muscle mass. Nutrients 2012;4(7):740–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Inoki K, Kim J, Guan K-L. AMPK and mTOR in cellular energy homeostasis and drug targets. Annu Rev Pharmacol Toxicol 2012;52:381–400. [DOI] [PubMed] [Google Scholar]
- 23.Efeyan A, Zoncu R, Sabatini DM. Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med 2012;18:524–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimball SR, Jefferson LS. Control of translation initiation through integration of signals generated by hormones, nutrients, and exercise. J Biol Chem 2010;285:29027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dodd KM, Tee AR. Leucine and mTORC1: a complex relationship. Am J Physiol Endocrinol Metab 2012;302:E1329–42. [DOI] [PubMed] [Google Scholar]
- 26.Iadevaia V, Huo Y, Zhang Z, Foster LJ, Proud CG. Roles of the mammalian target of rapamycin, mTOR, in controlling ribosome biogenesis and protein synthesis. Biochem Soc Trans 2012;40:168–72. [DOI] [PubMed] [Google Scholar]
- 27.Durán RV, Hall MN. Regulation of TOR by small GTPases. EMBO Rep 2012;13:121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 2010;141:290–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bar-Peled L, Schweitzer Lawrence D, Zoncu R, Sabatini David M. Ragulator is a GEF for the Rag GTPases that signal amino acid levels to mTORC1. Cell 2012;150:1196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duran A, Amanchy R, Linares JF, Joshi J, Abu-Baker S, Porollo A, Hansen M, Moscat J, Diaz-Meco MT. p62 Is a key regulator of nutrient sensing in the mTORC1 pathway. Mol Cell 2011;44:134–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S. Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 2012;149:410–24. [DOI] [PubMed] [Google Scholar]
- 32.Chiu M, Tardito S, Barilli A, Bianchi M, Dall'Asta V, Bussolati O. Glutamine stimulates mTORC1 independent of the cell content of essential amino acids. Amino Acids 2012;43:2561–7. [DOI] [PubMed] [Google Scholar]
- 33.Durán RV, Oppliger W, Robitaille AM, Heiserich L, Skendaj R, Gottlieb E, Hall MN. Glutaminolysis activates Rag-mTORC1 signaling. Mol Cell 2012;47:349–58. [DOI] [PubMed] [Google Scholar]
- 34.Bröer A, Juelich T, Vanslambrouck JM, Tietze N, Solomon PS, Holst J, Bailey CG, Rasko JE, Broer S. Impaired nutrient signaling and body weight control in a Na+ neutral amino acid cotransporter (Slc6a19)-deficient mouse. J Biol Chem 2011;286:26638–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Winkle LJ, Tesch JK, Shah A, Campione AL. System B0,+ amino acid transport regulates the penetration stage of blastocyst implantation with possible long-term developmental consequences through adulthood. Hum Reprod Update 2006;12:145–57. [DOI] [PubMed] [Google Scholar]
- 36.Brosnan JT. Interorgan amino acid transport and its regulation. J Nutr 2003;133(suppl 1):2068S–72S. [DOI] [PubMed] [Google Scholar]
- 37.Baird FE, Bett KJ, MacLean C, Tee AR, Hundal HS, Taylor PM. Tertiary active transport of amino acids reconstituted by coexpression of system A and L transporters in Xenopus oocytes. Am J Physiol Endocrinol Metab 2009;297:E822–9. [DOI] [PubMed] [Google Scholar]
- 38.Elorza A, Soro-Arnáiz I, Meléndez-Rodríguez F. Rodríguez-Vaello V, Marsboom G, de Cárcer G, Acosta-Iborra B, Albacete-Albacete L, Ordóñez A, Serrano-Oviedo L, Giménez-Bachs JM, et al. HIF2α acts as an mTORC1 activator through the amino acid carrier SLC7A5. Mol Cell 2012;48:681–91. [DOI] [PubMed] [Google Scholar]
- 39.Schriever SC, Deutsch MJ, Adamski J, Roscher AA, Ensenauer R. Cellular signaling of amino acids towards mTORC1 activation in impaired human leucine catabolism. J Nutr Biochem 2013;24:824–31. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, Kang BN, Tian J, Liu Y, Luo HR, Hester L, Snyder SH. The cationic amino acid transporters CAT1 and CAT3 mediate NMDA receptor activation-dependent changes in elaboration of neuronal processes via the mammalian target of rapamycin mTOR pathway. J Neurosci 2007;27:449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sagné C, Agulhon C, Ravassard P, Darmon M, Hamon M, El Mestikawy S, Gasnier B, Giros B. Identification and characterization of a lysosomal transporter for small neutral amino acids. Proc Natl Acad Sci USA 2001;98:7206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schiöth HB, Roshanbin S, Hägglund MGA, Fredriksson R. Evolutionary origin of amino acid transporter families SLC32, SLC36 and SLC38 and physiological, pathological and therapeutic aspects. Mol Aspects Med 2013;34:571–85. [DOI] [PubMed] [Google Scholar]
- 43.Heublein S, Kazi S, Ogmundsdottir MH, Attwood EV, Kala S, Boyd CA, Wilson C, Goberdhan DC. Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene 2010;29:4068–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edwards N, Anderson CMH, Gatfield KM, Jevons MP, Ganapathy V, Thwaites DT. Amino acid derivatives are substrates or non-transported inhibitors of the amino acid transporter PAT2 (slc36a2). Biochim Biophys Acta 2011;1808:260–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stewart BH, Collarini EJ, Pisoni RL, Christensen HN. Separate and shared lysosomal transport of branched and aromatic dipolar amino acids. Biochim Biophys Acta 1989;987:145–53. [DOI] [PubMed] [Google Scholar]
- 46.Pisoni RL, Thoene JG. The transport systems of mammalian lysosomes. Biochim Biophys Acta 1991;1071:351–73. [DOI] [PubMed] [Google Scholar]
- 47.Andersson HC, Kohn LD, Bernardini I, Blom HJ, Tietze F, Gahl WA. Characterization of lysosomal monoiodotyrosine transport in rat thyroid cells: evidence for transport by system h. J Biol Chem 1990;265:10950–4. [PubMed] [Google Scholar]
- 48.Chapel A, Kieffer-Jaquinod S, Sagné C, Verdon Q, Ivaldi C, Mellal M, Thirion J, Jadot M, Bruley C, Garin J, et al. An extended proteome map of the lysosomal membrane reveals novel potential transporters. Mol Cell Proteomics 2013;12:1572–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hägglund MGA, Sreedharan S, Nilsson VCO, Shaik JHA, Almkvist IM, Bäcklin S, Wrange Ö, Fredriksson R. Identification of SLC38A7 (SNAT7) protein as a glutamine transporter expressed in neurons. J Biol Chem 2011;286:20500–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Palii SS, Thiaville MM, Pan Y-X, Zhong C, Kilberg MS. Characterization of the amino acid response element within the human sodium-coupled neutral amino acid transporter 2 (SNAT2) system A transporter gene. Biochem J 2006;395:517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gaccioli F, Huang CC, Wang C, Bevilacqua E, Franchi-Gazzola R, Gazzola GC, Bussolati O, Snider MD, Hatzoglou M. Amino acid starvation induces the SNAT2 neutral amino acid transporter by a mechanism that involves eukaryotic initiation factor 2alpha phosphorylation and CAP-independent translation. J Biol Chem 2006;281:17929–40. [DOI] [PubMed] [Google Scholar]
- 52.Hyde R, Cwiklinski EL, MacAulay K, Taylor PM, Hundal HS. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transceptor, by amino acid availability. J Biol Chem 2007;282:19788–98. [DOI] [PubMed] [Google Scholar]
- 53.Gietzen DW, Aja SM. The brain's response to an essential amino acid-deficient diet and the circuitous route to a better meal. Mol Neurobiol 2012;46:332–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu B, Du H, Rutkowski R, Gartner A, Wang X. LAAT-1 is the lysosomal lysine/arginine transporter that maintains amino acid homeostasis. Science 2012;337:351–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Usui T, Nagumo Y, Watanabe A, Kubota T, Komatsu K, Kobayashi J, Osada H. Brasilicardin A, a natural immunosuppressant, targets amino acid transport system L. Chem Biol 2006;13:1153–60. [DOI] [PubMed] [Google Scholar]
- 56.Oda K, Hosoda N, Endo H. Saito K, Tsujihara K, Yamamura M, Sakata T, Anzai N, Wempe MF, Kanai Y, et al. L-type amino acid transporter 1 inhibitors inhibit tumor cell growth. Cancer Sci 2010;101:173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 2010;298:E1011–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levy S, Kafri M, Carmi M, Barkai N. The competitive advantage of a dual-transporter system. Science 2011;334:1408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.González IM, Martin PM, Burdsal C, Sloan JL, Mager S, Harris T, Sutherland AE. Leucine and arginine regulate trophoblast motility through mTOR-dependent and independent pathways in the preimplantation mouse embryo. Dev Biol 2012;361:286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell Physiol 2009;296:C142–50. [DOI] [PubMed] [Google Scholar]
- 61.Roos S, Jansson N, Palmberg I, Säljö K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J Physiol 2007;582:449–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adeva MM, Calvino J, Souto G, Donapetry C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Amino Acids 2012;43:171–81. [DOI] [PubMed] [Google Scholar]
- 63.Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K, Gall W, Kahn CR. Dietary leucine—an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS ONE 2011;6:e21187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wilson GJ, Layman DK, Moulton CJ, Norton LE, Anthony TG, Proud CG, Rupassara SI, Garlick PJ. Leucine or carbohydrate supplementation reduces AMPK and eEF2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am J Physiol Endocrinol Metab 2011;301:E1236–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelleher AR, Kimball SR, Dennis MD, Schilder RJ, Jefferson LS. The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. Am J Physiol Endocrinol Metab 2013;304:E229–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Layman DK. Dietary guidelines should reflect new understandings about adult protein needs. Nutr Metab 2009;6:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim S, Kim SF, Maag D. Maxwell M, Resnick AC, Juluri KR, Chakraborty A, Koldobskiy MA, Cha, SH, Barrow R, et al. Amino acid signaling to mTOR mediated by inositol polyphosphate multikinase. Cell Metab 2011;13:215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan L, Mieulet V, Burgess D. Findlay GM, Sully K, Procter J, Goris J, Janssens V, Morrice NA, Lamb RF. PP2AT61ε is an inhibitor of MAP4K3 in nutrient signaling to mTOR. Mol Cell 2010;37:633–42. [DOI] [PubMed] [Google Scholar]
- 69.Byfield MP, Murray JT, Backer JM. hVps34 is a nutrient-regulated lipid kinase required for activation of p70 S6 kinase. J Biol Chem 2005;280:33076–82. [DOI] [PubMed] [Google Scholar]
- 70.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab 2008;7:456–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maehama T, Tanaka M, Nishina H, Murakami M, Kanaho Y, Hanada K, Ral A. functions as an indispensable signal mediator for the nutrient-sensing system. J Biol Chem 2008;283:35053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kume K, Iizumi Y, Shimada M, Ito Y, Kishi T, Yamaguchi Y, Handa H. Role of N-end rule ubiquitin ligases UBR1 and UBR2 in regulating the leucine-mTOR signaling pathway. Genes Cells 2010;15:339–49. [DOI] [PubMed] [Google Scholar]