Abstract
Background: Chemicals with endocrine-disrupting abilities may act as obesogens and interfere with the body's natural weight-control mechanisms, especially if exposure occurs during prenatal life.
Objective: We examined the association between prenatal exposure to polychlorinated biphenyls (PCBs) and p,p′-dichlorodiphenyldichloroethylene (DDE) and subsequent obesity at 5 and 7 y of age.
Design: From 1997 to 2000, 656 pregnant Faroese women were recruited. PCB and DDE were measured in maternal serum and breast milk, and children's weight, height, and waist circumference (WC) were measured at clinical examinations at 5 and 7 y of age. The change in body mass index (BMI) from 5 to 7 y of age was calculated. Analyses were performed by using multiple linear regression models for girls and boys separately, taking into account maternal prepregnancy BMI.
Results: For 7-y-old girls who had overweight mothers, PCB was associated with increased BMI (β = 2.07, P = 0.007), and PCB and DDE were associated with an increased change in BMI from 5 to 7 y of age (PCB: β = 1.23, P = 0.003; DDE: β = 1.11, P = 0.008). No association was observed with BMI in girls with normal-weight mothers. PCB was associated with increased WC in girls with overweight mothers (β = 2.48, P = 0.001) and normal-weight mothers (β = 1.25, P = 0.04); DDE was associated with increased WC only in girls with overweight mothers (β = 2.21, P = 0.002). No associations were observed between PCB or DDE and BMI in 5-y-old girls. For boys, no associations were observed.
Conclusions: Results suggest that prenatal exposure to PCB and DDE may play a role for subsequent obesity development. Girls whose mothers have a high prepregnancy BMI seem most affected.
INTRODUCTION
Emerging evidence suggests that the dramatic increase in the prevalence of obesity over the past 4–5 decades (1) cannot be solely explained by changes in genetic and lifestyle factors. It has been suggested that exposure to chemicals with endocrine-disrupting abilities at vulnerable time periods may be significantly contributing to the obesity epidemic (2, 3). Experimental research has documented that risk of obesity may be influenced by fetal and early postnatal environments that may induce persistent alterations in the metabolism and physiology of offspring (4, 5). Thus, the prenatal period seems to constitute an important window of vulnerability for later obesity. Endocrine-disrupting chemicals (EDCs)5 may interfere with the programming of endocrine-signaling pathways and change metabolic set points established during early vulnerable periods (6, 7). Indeed, in animal studies, exposure to EDCs during critical phases of fetal development has been shown to be linked to later obesity (7, 8). Recent findings have indicated that this association is also present in humans (9). Of chemicals with suspected endocrine-disrupting abilities are polychlorinated biphenyls (PCBs), which were used as lubricants and coolants in electrical appliances (10), and p,p′-dichlorodiphenyldichloroethylene (DDE), which is the main metabolite of the insecticide 1,1,1-trichloro-2,2,bis(p-chlorophenyl)ethane (11). The use of both compounds was prohibited in many countries in the 1970s, but because of high lipophilicity and slow metabolic degradation, the compounds bioaccumulate in the food chain. Humans are mainly exposed to these chemicals through the dietary intake of marine food (12).
Only a few human studies have investigated associations between PCB and DDE exposure and subsequent obesity, and results have been diverging, probably because the adverse effect seems to be associated to both the exposure level and sex (9, 13–15).
The Faroese population consumes high amounts of seafood, and traditional diets include pilot whale blubber with a high concentration of PCB, DDE, and other persistent pollutants. Thus, this population is highly exposed to PCB and DDE but with a wide exposure range because of individual differences in whale-blubber intake. Therefore, the aim of this study was to examine the association between prenatal PCB and DDE exposure and obesity at 5 and 7 y of age in this population.
SUBJECTS AND METHODS
Subjects
The cohort consisted of 640 (8 pairs of twins were excluded) singleton pregnant women in the Faroe Islands who gave birth between November 1997 to March 2000 and had a blood sample drawn at gestational week 34. Obstetric variables, including the date of birth, birth weight, parity, maternal age, and prepregnancy weight and height, were obtained as was information on maternal smoking and alcohol use during pregnancy. In addition, 594 mothers delivered transition milk 4–5 d after parturition. Detailed follow-up examinations were scheduled for the whole cohort when children were (mean ± SD) 4.95 ± 0.06 and 7.5 ± 0.10 y of age. Examinations included a clinical examination, blood sampling, and a maternal interview on the child's current health and past medical history, including the duration of breastfeeding [exclusive and total (in mo)]. The current report was based on cohort members who were examined at 5 and 7.5 y of age (60 and 90 mo of age, respectively) and provided transition milk and a blood sample sufficient for PCB and DDE analyses. For the 60 cohort members who did not participate in the 5-y examination, main reasons were a decision to leave the follow-up study (n = 14), the child did not want to participate (n = 14), a current residence abroad (n = 30), and the death of a child (n = 2) (undiagnosed primary carnitine deficiency: n = 1; SUCLA2 deficiency, which is the gene coding for the b subunit of ADP-forming succinyl-CoA synthetase: n = 1). In 13 children, a chronic disorder, characterized as any kind of somatical or behavioral disorder that might have influenced the child's eating habits or impair growth, had been diagnosed; therefore, growth data of these children were excluded from the analysis. For 6 of the children examined, a venipuncture resulted in an insufficient blood sample, which left a final sample of 561 (88% of the original cohort). For the 73 cohort members who did not participate in the 7-y examination, main reasons were a decision to leave the follow-up study (n = 29), the child did not want to participate (n = 30), a current residence abroad (n = 13), and the death of a child (n = 3). The clinical examination at age 5 y led to additional testing and diagnosis, and after exclusion of 24 children who were suffering from a chronic disorder and 4 children in whom it was impossible to collect enough blood, reliable clinical data were available for 539 children (84% of original cohort). The Ethical Committee of the Faroe Islands approved the study protocol.
Anthropometric measurements
At follow-up examinations at 5 and 7.5 y of age (60 and 90 mo of age, respectively), body weight was measured in kilograms on an electronic scale to the nearest single digit after the decimal point. BMI (in kg/m2) was calculated as weight divided by the square of height. Children were classified as overweight and obese if their BMI exceeded the age- and sex-specific cutoff percentiles defined by the International Obesity Task Force (16). At the age of 5 y (60 mo) cutoffs were BMI of 17.39 and 19.27, respectively, in boys and 17.23 and 19.2, respectively, in girls. At the age of 7 y (90 mo), cutoffs were BMI of 18.12 and 21.06, respectively, in boys and 17.96 and 20.89, respectively, in girls. Waist circumference (WC) was measured to the nearest 0.5 cm with a flexible tape at the end of normal expiration while the subjects were standing. At the age of 7 y, according to age references for WC from a Dutch study, overweight was defined when WC was >62.12 cm in boys and >61.86 cm in girls, and obesity was defined when WC was >69.53 cm in boys and >69.70 cm in girls (17). According to WHO recommendations, mothers were classified as overweight if prepregnancy BMI was ≥25.
Measurements of exposure
PCBs and DDE were assessed from the analysis of maternal serum (430 samples) and milk (542 samples). Serum analyses were conducted by using gas chromatography with electron-capture detection at the University of Southern Denmark (18). Milk analyses were performed by using a similar methodology at the Department of Health, State Agency for Health and Occupational Safety, Schleswig-Holstein, Germany (19). To avoid problems with congeners not assessed and concentrations below the detection limit, a simplified concentration of the sum of PCBs (ΣPCB) was calculated as the sum of congeners PCBs 138, 153, and 180 multiplied by 2 (20). ΣPCB and DDE concentrations were expressed in relation to the total lipid concentration in serum. Concentrations of other persistent organic pollutants, including hexachlorobenzene and 1,1,1-trichloro-2,2,bis(p-chlorophenyl)ethane, were close to or below the limit of detection, and therefore, they were not included in the data analysis.
Statistics
Prenatal PCB and DDE exposure were divided into tertiles, and means (±SDs) or proportions were used to describe characteristics of the population according to tertiles of prenatal PCB exposure and maternal prepregnancy BMI. ANOVA and Pearson's chi-square tests were applied as appropriate to assess statistical significance (P < 0.05) between tertiles.
The correlation between the PCB concentration in maternal serum and milk was assessed by using Pearson's correlation test. Because of the high correlation between concentrations in maternal serum and milk (ΣPCB: r = 0.78; DDE: r = 0.90), missing serum data (ΣPCB: n = 155; DDE: n = 154) were calculated from milk results by using the average ratio (ΣPCB: 0.88; DDE: 0.93) between the two. To assess the robustness of conclusions derived from this method, a multiple-imputation analysis was applied as a sensitivity analysis.
Exposure variables were log transformed to obtain normally distributed residuals with a homogeneous variance. For analyses of changes in BMI from 5 to 7 y of age, the variable change in BMI was generated (BMI at 7 y of age − BMI at 5 y of age). Associations between prenatal PCB and DDE exposure and obesity outcomes (BMI at 5 and 7 y of age; change in BMI and WC at 7 y of age) were analyzed by using multiple linear regression taking into account potential covariates. To examine dose-response relations, PCB and DDE were divided into quartiles and quintiles. The effect of covariate adjustment was explored for those host factors that modified β estimates >10%. Accordingly, covariates retained in the final models were parity and maternal age. BMI at age 7 y was furthermore included as a covariate in the analyses of WC as an outcome as was BMI at age 5 y in the analyses of change of BMI from 5 to 7 y of age. Covariates such as the duration of breastfeeding, socioeconomic status, smoking and alcohol consumption during pregnancy, maternal weight gain during pregnancy, birth weight, and exercise were not adjusted for because they did not modify the β estimate >10%.
Analyses of interactions between PCB and DDE and sex and between PCB and DDE and maternal prepregnancy BMI were modeled by a multiplicative term (P < 0.10 used to define interactions). Significance was assumed at P < 0.05 (2 sided). All analyses were performed with STATA software (version 12; STATA Corp).
RESULTS
A total of 290 boys and 271 girls participated at 5 y of age, and 280 boys and 259 girls participated at 7 y of age (see Supplemental Figure 1 under “Supplemental data” in the online issue). At 5 y of age, 11% of boys and 14% of girls were overweight, and 1.4% of boys and 3% of girls were obese. The corresponding numbers at 7 y of age were 14.3% and 24.3% and 2.1% and 4.2%, respectively. A total of 32.3% and 38.6%, respectively, had a WC corresponding to overweight, and 5.4% and 8.1%, respectively, had a high WC corresponding to obesity. In boys, the mean change in BMI from 5 to 7 y of age was 0.5 ± 1.0, and in girls, the mean change in BMI from 5 to 7 y of age was 0.7 ± 1.2 (see Supplemental Figure 2 under “Supplemental data” in the online issue).
The range of PCB and DDE concentrations derived from milk and serum were as follows: PCBs from milk: 0.08–17.59 μg/g; PCBs from serum: 0.18–15.15 μg/g; DDE from milk: 0.06–11.35 μg/g; DDE from serum: 0.04–11.41 μg/g. In boys, the median (range) was 1.19 μg/g (0.22–15.48 μg/g) for ΣPCB and 0.56 μg/g (0.04–11.41 μg/g) for DDE, and in girls, was 1.19 μg/g (0.07–8.31 μg/g) for ΣPCB and 0.57 μg/g (0.05–5.18 μg/g) for DDE.
The distribution of maternal and child characteristics in relation to tertiles of maternal PCB and DDE concentrations in pregnancy is shown in Table 1. Mothers in the highest tertile of PCB and DDE were more likely to be older at the child's birth and to have a higher parity and BMI in comparison with mothers in the lowest tertile of PCB and DDE. An association between maternal serum PCB and DDE concentrations in pregnancy and child serum concentration at 5 y of age was observed. In Table 2, the distribution of maternal and child characteristics in relation to prepregnancy maternal weight is seen. Overweight mothers were more likely to give birth to children with a higher birth weight, higher BMI, and WC at 7 y of age. Overweight mothers had a higher age at their children's births and a lower weight gain during pregnancy and less often consumed alcohol.
TABLE 1.
Maternal and child characteristics according to tertiles of prenatal PCB and DDE concentrations, Faroese Cohort 1996–2001 (n = 585)1
| PCBs (μg/g lipid) |
DDE (μg/g lipid) |
|||||||
| Characteristics | <0.90 | 0.90–1.65 | >1.65 | P | <0.40 | 0.40–0.77 | >0.77 | P |
| Girls (%) | 47.2 | 48.7 | 47.7 | 0.95 | 47.7 | 48.7 | 47.2 | 0.95 |
| Birth weight (g) | 3719 ± 5132 | 3758 ± 497 | 3708 ± 465 | 0.58 | 3718 ± 497 | 3729 ± 502 | 3738 ± 478 | 0.92 |
| BMI (kg/m2) | ||||||||
| 5 y of age | 16.0 ± 1.4 | 16.0 ± 1.3 | 15.9 ± 1.3 | 0.71 | 16.0 ± 1.4 | 16.0 ± 1.3 | 16.0 ± 1.4 | 0.93 |
| 7 y of age | 16.5 ± 1.8 | 16.6 ± 2.0 | 16.6 ± 1.9 | 0.85 | 16.6 ± 1.8 | 16.6 ± 2.0 | 16.6 ± 1.9 | 0.97 |
| Change in BMI from 5 to 7 y of age (kg/m2) | 0.44 ± 1.0 | 0.68 ± 1.2 | 0.69 ± 1.1 | 0.07 | 0.53 ± 1.0 | 0.62 ± 1.2 | 0.67 ± 1.1 | 0.54 |
| Waist circumference (cm) | 60.8 ± 4.7 | 61.6 ± 5.1 | 61.7 ± 5.0 | 0.19 | 61.1 ± 4.9 | 61.5 ± 5.2 | 61.6 ± 4.8 | 0.53 |
| Maternal age at child's birth (y) | 27.1 ± 4.7 | 29.4 ± 5.0 | 31.3 ± 4.8 | <0.001 | 27.5 ± 5.1 | 29.0 ± 4.7 | 31.2 ± 4.9 | <0.001 |
| Maternal prepregnancy BMI (kg/m2) | 23.9 ± 3.9 | 23.6 ± 3.7 | 24.3 ± 4.5 | 0.18 | 23.2 ± 3.7 | 23.8 ± 3.8 | 24.8 ± 4.5 | 0.001 |
| Maternal weight gain during pregnancy (kg) | 15.3 ± 5.0 | 15.1 ± 5.1 | 14.4 ± 8.1 | 0.35 | 15.4 ± 4.8 | 15.0 ± 5.1 | 14.3 ± 8.3 | 0.19 |
| PCBs and DDE at 5 y of age (μg/g lipid)3 | 0.79 ± 0.5 | 1.37 ± 0.8 | 2.41 ± 1.7 | <0.001 | 0.30 ± 0.2 | 0.57 ± 0.4 | 1.35 ± 1.1 | <0.001 |
| Parity (%)4 | 68.5 | 73.9 | 80.7 | 0.03 | 71.0 | 71.7 | 80.5 | 0.07 |
| SES (high/intermediate/low) (%) | 22/25/53 | 25/26/49 | 26/31/43 | 0.35 | 23/25/52 | 24/26/50 | 27/31/42 | 0.23 |
| Maternal alcohol consumption during pregnancy (%) | 15.4 | 11.8 | 14.4 | 0.57 | 14.9 | 13.8 | 12.8 | 0.84 |
| Maternal smoking during pregnancy (%) | 24.6 | 32.8 | 29.7 | 0.20 | 30.3 | 27.7 | 29.2 | 0.85 |
| No good conditions for playing outdoors (%)5 | 16.5 | 18.2 | 13.2 | 0.40 | 16.7 | 19.0 | 12.2 | 0.18 |
PCB and DDE concentrations were measured in maternal serum and log transformed. The PCB concentration was based on the sum of PCB congeners 138, 153, and 180. P values were determined by using ANOVA and Pearson's chi-square tests of differences across tertiles of PCB and DDE. DDE, p,p′-dichlorodiphenyldichloroethylene; PCB, polychlorinated biphenyl; SES, socioeconomic status.
Mean ± SD (all such values).
Fifty-nine missing values.
Percentage of women with pregnancies before the current one.
Twenty-six missing values.
TABLE 2.
Maternal and child characteristics according to maternal prepregnancy BMI, Faroese Cohort 1996–2001 (n = 585)1
| Characteristics | Normal-weight mothers (BMI <25 kg/m2; n = 390) | Overweight mothers (BMI ≥25 kg/m2; n = 195) | P |
| Girls (%) | 46.7 | 50.3 | 0.41 |
| Birth weight (g) | 3697 ± 4732 | 3791 ± 523 | 0.03 |
| BMI (kg/m2) | |||
| 5 y of age | 15.8 ± 1.2 | 16.3 ± 1.5 | <0.001 |
| 7 y of age | 16.3 ± 1.7 | 17.1 ± 2.1 | <0.001 |
| Waist circumference (cm) | 60.8 ± 4.6 | 62.6 ± 5.5 | <0.001 |
| Maternal age at child's birth (y) | 29.0 ± 5.2 | 29.9 ± 4.9 | 0.05 |
| Maternal prepregnancy BMI (kg/m2) | 21.7 ± 1.8 | 28.4 ± 3.6 | <0.001 |
| Maternal weight gain during pregnancy (kg) | 15.6 ± 4.5 | 13.6 ± 8.7 | <0.001 |
| Prenatal PCBs and DDE (μg/g lipid)3 | |||
| PCBs | 1.56 ± 1.4 | 1.61 ± 1.6 | 0.64 |
| DDE | 0.78 ± 1.0 | 0.94 ± 1.1 | 0.08 |
| Parity (%)4 | 72.9 | 77.7 | 0.22 |
| SES (high/intermediate/low) (%) | 27/27/46 | 19/29/52 | 0.06 |
| Maternal alcohol consumption during pregnancy (%) | 16.4 | 8.7 | 0.01 |
| Maternal smoking during pregnancy (%) | 27.9 | 31.3 | 0.40 |
| No good conditions for playing outdoors (%)5 | 17.2 | 13.4 | 0.24 |
P values were determined by using ANOVA and Pearson's chi-square tests of differences across normal-weight and overweight mothers. DDE, p,p′-dichlorodiphenyldichloroethylene; PCB, polychlorinated biphenyl; SES, socioeconomic status.
Mean ± SD (all such values).
PCB and DDE concentrations were measured in maternal serum and log transformed.
Percentage of women with pregnancies before the current one.
Twenty-six missing values.
We showed significant sex interactions between prenatal PCB and DDE concentrations and BMI at 7 y of age (PCB: P = 0.06; DDE: P = 0.03), the change in BMI (PCB: P = 0.01; DDE: P = 0.007), and WC at 7 y of age (PCB: P = 0.006; DDE: P = 0.06). Maternal prepregnancy BMI was also shown to interact with the association between prenatal PCB and BMI at 7 y of age (P = 0.04) and the change in BMI from 5 to 7 y of age (P = 0.003) and between prenatal DDE and change in BMI at 5–7 y of age (P = 0.006) and WC at 7 y of age in girls (P = 0.07). Because we observed significant interactions, we present associations between PCB and DDE exposure and BMI, the change in BMI, and WC separately for boys and girls and for normal-weight and overweight mothers.
Prenatal PCB and DDE exposure was associated with obesity development at 7 y of age in girls with overweight mothers. After multivariable adjustment, the highest quartile of prenatal PCB exposure was associated with increased BMI (2.3 units of BMI, equivalent to 5.75 kg) (β = 2.07; 95% CI: 0.59, 3.55; P = 0.007) (Table 3). Furthermore, high prenatal PCB and DDE exposure was associated with an increased change in BMI from 5 to 7 y of age (PCB: 1 unit of BMI; DDE: 1.5 units of BMI; equivalent to 2.5 and 3.75 kg, respectively) (PCB: β = 1.23; 95% CI: 0.42, 2.05; P = 0.003. DDE: β = 1.11; 95% CI: 0.30, 1.92; P = 0.008) (Table 3, Figure 1) and increased WC (PCB and DDE: 2.5 cm) (PCB: β = 2.48; 95% CI: 1.10, 3.85; P = 0.001. DDE: β = 2.21; 95% CI: 0.84, 3.56; P = 0.002) (Table 4). Prenatal PCB exposure was associated with an increase in WC at low exposure, although more moderate (second quartile: 1 cm) (β = 1.37; 95% CI: 0.13, 2.61; P = 0.03). The association between prenatal PCB and WC was also apparent in girls with normal-weight mothers, but the association was weaker and only significant in the highest quartile (1.5 cm) (β = 1.25; 95% CI: 0.04, 2.45; P = 0.04). Low prenatal DDE exposure was associated to a decrease in WC in girls with normal-weight mothers (1 cm) (β = −1.46; 95% CI: −2.48, −0.44; P = 0.005). All associations were essentially similar before and after adjustment for maternal BMI in the lean and obese maternal group. A high correlation between BMI at 5 and 7 y of age was observed (r = 0.83) (see Supplemental Tables 1 and 2 under “Supplemental data” in the online issue). No significant associations were shown in girls at 5 y of age or boys at 5 or 7 y of age. Essentially similar findings were given by stratifying in terms of maternal prepregnancy BMI (all P > 0.05).
TABLE 3.
Associations between prenatal PCB and DDE exposure in quartiles and BMI in children 5 and 7 y of age and the change in BMI from 5 to 7 y of age according to maternal prepregnancy BMI, Faroese Cohort 1996–20011
| Girls (regression coefficient) |
Boys (regression coefficient) |
|||||||
| Normal-weight mothers |
Overweight mothers |
Normal-weight mothers |
Overweight mothers |
|||||
| PCBs and DDE in quartiles | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P |
| BMI 5 y of age [n = 561 (girls: n = 271; overweight mothers: n = 89) (boys: n = 290; overweight mothers: n = 98)] | ||||||||
| PCBs | ||||||||
| Second quartile (0.75–1.18 μg/g lipid) | 0.10 (−0.53, 0.74) | 0.95 | 0.19 (−0.81, 1.20) | 0.76 | 0.39 (−0.10, 0.89) | 0.23 | −0.60 (−1.47, 0.27) | 0.18 |
| Third quartile (1.19–1.95 μg/g lipid) | 0.10 (−0.52, 0.72) | 0.79 | 0.13 (−1.06, 1.32) | 0.64 | 0.40 (−0.12, 0.92) | 0.13 | 0.12 (−0.72, 0.96) | 0.70 |
| Fourth quartile (>1.95 μg/g lipid) | −0.12 (−0.82, 0.58) | 0.33 | 0.59 (−0.47, 1.65) | 0.31 | −0.01 (−0.53, 0.51) | 0.74 | 0.01 (−0.84, 0.87) | 0.92 |
| Continuous | −0.10 (−0.43, 0.22) | 0.23 | 0.32 (−0.26, 0.89) | 0.35 | −0.08 (−0.35, 0.20) | 0.53 | −0.06 (−0.46, 0.33) | 0.78 |
| DDE | ||||||||
| Second quartile (0.34–0.56 μg/g lipid) | 0.48 (−0.13, 1.09) | 0.18 | −0.66 (−1.65, 0.34) | 0.16 | 0.40 (−0.09, 0.89) | 0.09 | −0.54 (−1.45, 0.37) | 0.35 |
| Third quartile (0.57–0.92 μg/g lipid) | 0.40 (−0.18, 0.97) | 0.41 | −0.27 (−1.51, 0.98) | 0.70 | 0.16 (−0.33, 0.64) | 0.45 | −0.03 (−0.89, 0.83) | 0.89 |
| Fourth quartile (>0.92 μg/g lipid) | −0.18 (−0.82, 0.45) | 0.29 | 0.15 (−0.90, 1.20) | 0.67 | −0.25 (−0.77, 0.27) | 0.41 | 0.33 (−0.51, 1.16) | 0.42 |
| Continuous | 0.01 (−0.28, 0.30) | 0.67 | 0.10 (−0.34, 0.55) | 0.64 | −0.12 (−0.34, 0.11) | 0.39 | −0.06 (−0.27, 0.39) | 0.76 |
| BMI at 7 y of age [n = 539 (girls: n = 259; overweight mothers: n = 87) (boys: n = 280; overweight mothers: n = 92)] | ||||||||
| PCBs | ||||||||
| Second quartile (0.75–1.18 μg/g lipid) | 0.33 (−0.55, 1.20) | 0.46 | 0.76 (−0.63, 2.15) | 0.28 | 0.51 (−0.15, 1.18) | 0.14 | −0.79 (−1.86, 0.28) | 0.15 |
| Third quartile (1.19–1.95 μg/g lipid) | 0.14 (−0.71, 0.99) | 0.75 | 0.66 (−0.98, 2.29) | 0.43 | 0.11 (−0.58, 0.81) | 0.73 | 0.26 (−0.78, 1.30) | 0.62 |
| Fourth quartile (>1.95 μg/g lipid) | 0.04 (−0.93, 1.01) | 0.93 | 2.07 (0.59, 3.55) | 0.007 | −0.33 (−1.03, 0.36) | 0.42 | 0.04 (−1.02, 1.11) | 0.94 |
| Continuous | −0.04 (−0.49, 0.40) | 0.85 | 1.13 (0.33, 1.93) | 0.006 | −0.28 (−0.64, 0.08) | 0.16 | −0.15 (−0.64, 0.34) | 0.54 |
| DDE | ||||||||
| Second quartile (0.34–0.56 μg/g lipid) | 0.30 (−0.56, 1.15) | 0.49 | −0.06 (−1.49, 1.38) | 0.94 | 0.47 (−0.11, 1.21) | 0.13 | −1.21 (−2.34, 0.09) | 0.17 |
| Third quartile (0.57–0.92 μg/g lipid) | 0.45 (−0.36, 1.26) | 0.28 | −0.09 (−1.91, 1.74) | 0.92 | −0.09 (−0.74, 0.57) | 0.65 | −0.04 (−1.13, 1.05) | 0.95 |
| Fourth quartile (>0.92 μg/g lipid) | −0.18 (−1.08, 0.71) | 0.69 | 1.29 (−0.24, 2.81) | 0.10 | −0.37 (−1.09, 0.34) | 0.21 | −0.24 (−1.31, 0.83) | 0.66 |
| Continuous | 0.05 (−0.35, 0.46) | 0.80 | 0.61 (−0.03, 1.24) | 0.06 | −0.23 (−0.54, 0.07) | 0.09 | −0.12 (−0.55, 0.30) | 0.56 |
| Change in BMI from 5 to 7 y of age [n = 515 (girls: n = 250; overweight mothers: n = 83) (boys: n = 265; overweight mothers: n = 89)] | ||||||||
| PCBs | ||||||||
| Second quartile (0.75–1.18 μg/g lipid) | 0.21 (−0.27, 0.70) | 0.39 | 0.28 (−0.49, 1.04) | 0.47 | 0.34 (−0.09, 0.77) | 0.12 | −0.28 (−0.93, 0.38) | 0.40 |
| Third quartile (1.19–1.95 μg/g lipid) | 0.09 (−0.39, 0.57) | 0.71 | 0.56 (−0.34, 1.46) | 0.22 | 0.01 (−0.46, 0.45) | 0.95 | −0.01 (−0.64, 0.64) | 0.99 |
| Fourth quartile (>1.95 μg/g lipid) | 0.17 (−0.37, 0.71) | 0.53 | 1.23 (0.42, 2.05) | 0.003 | −0.05 (−0.50, 0.39) | 0.98 | −0.06 (−0.70, 0.59) | 0.87 |
| Continuous | 0.06 (−0.19, 0.31) | 0.62 | 0.70 (0.26, 1.14) | 0.002 | −0.08 (−0.32, 0.15) | 0.63 | −0.12 (−0.41, 0.16) | 0.40 |
| DDE | ||||||||
| Second quartile (0.34–0.56 μg/g lipid) | −0.27 (−0.59, 0.06) | 0.11 | 0.51 (−0.26, 1.29) | 0.19 | 0.32 (−0.11, 0.75) | 0.26 | −0.51 (−1.19, 0.18) | 0.15 |
| Third quartile (0.57–0.92 μg/g lipid) | −0.06 (−0.37, 0.25) | 0.70 | 0.14 (0.82, 1.10) | 0.77 | −0.04 (−0.46, 0.38) | 0.65 | 0.10 (−0.54, 0.74) | 0.75 |
| Fourth quartile (>0.92 μg/g lipid) | 0.09 (−0.25, 0.43) | 0.61 | 1.11 (0.30, 1.92) | 0.008 | 0.15 (−0.30, 0.61) | 0.70 | −0.40 (−1.03, 0.23) | 0.21 |
| Continuous | 0.05 (−0.11, 0.20) | 0.57 | 0.46 (0.12, 0.80) | 0.008 | −0.04 (−0.23, 0.16) | 0.63 | −1.13 (−0.38, 0.12) | 0.31 |
PCB and DDE concentrations (in μg/g lipid) were measured in maternal serum and log transformed. The PCB concentration was based on the sum of PCB congeners 138, 153, and 180. Sex interactions between PCB and DDE concentrations and BMI at 7 y of age and the change in BMI from 5 to 7 y of age were significant at P < 0.10. Maternal normal weight was defined as maternal prepregnancy BMI (in kg/m2) <25, and maternal overweight was defined as maternal prepregnancy BMI ≥25. Interactions with maternal prepregnancy BMI between the PCB concentration and BMI at 7 y of age, the change in BMI from 5 to 7 y of age, and between the DDE concentration and change in BMI from 5 to 7 y of age were significant at P < 0.10. Regression coefficients were derived by using a multiple linear regression model adjusted for parity (pregnancies before the current one) and maternal age. Continuous estimated coefficients are the absolute change in BMI expected when the log-transformed PCB variable was multiplied by 10. DDE, p,p′-dichlorodiphenyldichloroethylene; PCB, polychlorinated biphenyl.
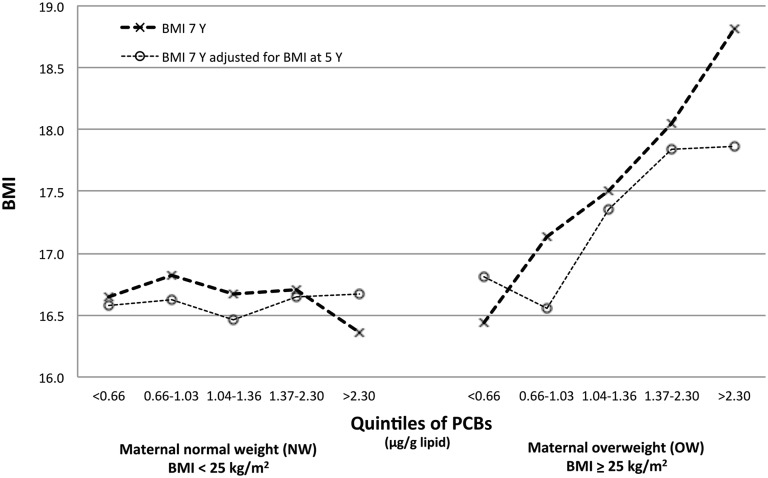
FIGURE 1.
Adjusted associations between prenatal PCB exposure in quintiles and BMI in 7-y-old girls and BMI in 7-y-old girls adjusted for BMI at 5 y of age according to maternal prepregnancy BMI [Faroese cohort 1996–2001 (n = 259)]. Interactions with maternal prepregnancy BMI were significant at P < 0.10. Values were adjusted for parity and maternal age by using multiple linear regression. P-trend for BMI at 7 y of age: NW, P = 0.60; OW, P = 0.003; P-trend for BMI at 7 y of age adjusted for BMI at 5 y of age: NW, P = 0.73; OW, P = 0.001. NW, normal-weight mothers; OW, overweight mothers; PCB, polychlorinated biphenyl.
TABLE 4.
Associations between prenatal PCB and DDE exposure in quartiles and waist circumference in children 7 y of age according to maternal prepregnancy BMI, Faroese Cohort 1996–2001 (n = 539)1
| Waist circumference |
|||||||||
| Girls (regression coefficient; n = 259) |
Boys (regression coefficient; n = 280) |
||||||||
| Normal-weight mothers (n = 172) |
Overweight mothers (n = 87) |
Normal-weight mothers (n = 188) |
Overweight mothers (n = 92) |
||||||
| PCBs and DDE in quartiles | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | Estimate (95% CI) | P | |
| PCBs | |||||||||
| Second quartile (0.75–1.18 μg/g lipid) | 0.80 (−0.29, 1.89) | 0.17 | 1.37 (0.13, 2.61) | 0.03 | 0.41 (−0.46, 1.27) | 0.36 | −0.44 (−2.08, 1.20) | 0.60 | |
| Third quartile (1.19–1.95 μg/g lipid) | 1.00 (−0.07, 2.06) | 0.08 | 2.20 (0.74, 3.65) | 0.004 | −0.05 (−0.95, 0.85) | 0.94 | −1.46 (−3.03, 0.11) | 0.07 | |
| Fourth quartile (>1.95 μg/g lipid) | 1.25 (0.04, 2.45) | 0.04 | 2.48 (1.10, 3.85) | 0.001 | −0.15 (−1.05, 0.75) | 0.97 | 0.12 (−1.49, 1.72) | 0.89 | |
| Continuous | 0.60 (0.05, 1.16) | 0.03 | 1.15 (0.38, 1.92) | 0.004 | −0.12 (−0.58, 0.35) | 0.87 | −0.36 (−1.09, 0.38) | 0.34 | |
| DDE | |||||||||
| Second quartile (0.34–0.56 μg/g lipid) | −1.46 (−2.48, −0.44) | 0.005 | 0.77 (−0.48, 2.03) | 0.22 | 0.30 (−0.56, 1.16) | 0.71 | 0.04 (−1.77, 1,85) | 0.96 | |
| Third quartile (0.57–0.92 μg/g lipid) | 0.81 (−0.16, 1.78) | 0.12 | 2.20 (0.60, 3.80) | 0.008 | 0.29 (−0.56, 1.15) | 0.68 | −0.29 (−1.20, 1.42) | 0.74 | |
| Fourth quartile (>0.92 μg/g lipid) | 0.03 (−1.04, 1.10) | 0.86 | 2.21 (0.84, 3.56) | 0.002 | −0.01 (−0.92, 0.94) | 0.82 | 0.02 (−1.66, 1.69) | 0.98 | |
| Continuous | 0.28 (−0.23, 0.79) | 0.28 | 0.93 (0.36, 1.51) | 0.002 | −0.01 (−0.38, 0.40) | 0.99 | −0.26 (−0.91, 0.38) | 0.94 | |
Waist circumference was measured only at 7 y of age. PCB and DDE concentrations (in μg/g lipid) were measured in maternal serum and log transformed. The PCB concentration was based on the sum of PCB congeners 138, 153, and 180. Sex interactions between PCB and DDE concentrations and waist circumference at 7 y of age were significant at P < 0.10. Maternal normal weight was defined as maternal prepregnancy BMI (in kg/m2) <25, and maternal overweight was defined as maternal prepregnancy BMI ≥25. The interaction with maternal prepregnancy BMI between the DDE concentration and waist circumference was significant at P < 0.10. Regression coefficients were derived by using a multiple linear regression model adjusted for parity (pregnancies before the current one), maternal age, and child BMI at 7 y of age. Continuous estimated coefficients are the absolute change in BMI expected when the log-transformed PCB variable was multiplied by 10. DDE, p,p′-dichlorodiphenyldichloroethylene; PCB, polychlorinated biphenyl.
DISCUSSION
We showed that prenatal PCB exposure was associated with increased BMI and PCB and DDE with increased WC at 7 y of age and a change in BMI from 5 to 7 y of age in girls with overweight mothers. No association was shown for daughters of normal-weight mothers or in boys, which was consistent with a higher susceptibility of the female sex observed in other studies (21, 22).
Only few prospective studies have hitherto investigated associations between prenatal PCB and DDE exposure and subsequent obesity development in humans, and the results have been divergent and related to the exposure level and sex so that a high exposure was associated with weight loss, whereas low exposure was associated with weight gain (9). Our results were consistent with 4 earlier prospective studies in which children were exposed to relatively low PCB exposure (<1.0 ng/mg lipid). Associations between prenatal PCB exposure and overweight in girls at 6 y of age (22), an increase in BMI z scores in children at 3 y of age (15), an increase in weight in 5-y-old girls (13) and girls at puberty (23) were reported. By contrast, 4 prospective studies in which children were exposed to higher PCB concentrations (>4.0 ng/mg lipid) showed associations between prenatal PCB exposure and decreases in weight in girls at 5–24 y of age (24), girls at 4 and 7 y of age (25), girls at 4 y of age (14) and girls at 4, 7, and 17 y of age (26). Our results agree with these findings because girls in our population had PCB exposures that ranged from 0.07 to 8.31 ng/mg lipid, of which only 7 girls had an exposure >4.0 ng/mg lipid. In addition, the BMI of 6 of these girls was less than the mean BMI of the population, which supported the hypothesis of an association between high PCB exposure and weight loss. However, some of the previous studies suffered from a short follow-up, and it is possible that PCB exposure needs a prolonged latency period, and thus, the follow-up time in some of these studies may have been too short to show adverse effects. This possibility was substantiated by our findings of no association between prenatal PCB exposure and BMI at 5 y of age and an association with a change in BMI from 5 to 7 y of age. In addition, previously studies did not take into account maternal prepregnancy weight and, thereby, underestimated the effect of genetic factors that may have explained the observed differences between studies.
Our findings that prenatal DDE exposure similarly was associated with later weight gain in girls with overweight mothers were in accordance with other prospective studies that reported an association with overweight, particularly in girls 6 y of age (22), an increased risk of rapid growth in the first 6 mo of life, an elevated BMI at 14 mo (27), an increased BMI in children 3 y of age (15) and an increased weight and BMI in adult female offspring (28). A differential association between prenatal DDE exposure and later obesity for individuals with and without maternal prepregnancy obesity was also shown in another study; although in contrast to our findings, this association was only apparent in the children of normal-weight mothers. However, the study examined an association between DDE and rapid growth in the first 6 mo of life, and therefore, results may not be directly comparable (27).
We could not offer any explanations for differential associations between prenatal PCB and DDE exposure and BMI and WC observed in relation to maternal prepregnancy BMI. However, certain subgroups might be genetically more sensitive to EDC exposure than other subgroups. The endocrine system may function differently in obese and normal-weight persons (29). For instance, the adipocyte production of hormones such as estrogen and leptin is higher in obese than normal-weight people (30). Furthermore, children of overweight parents are more susceptible to develop obesity, which could explain the discrepancy in the response to prenatal PCB and DDE exposure. Potentially, maternal obesity and PCB or DDE act in synergy to program the offspring to a predisposition for later obesity, but this effect could not be further explored in the current study. Nevertheless, our findings suggested that the association between prenatal PCB and DDE exposure and the subsequent development of obesity may be dependent on maternal weight status, and future studies should take this possibility into account.
It has been suggested that the period of adiposity rebound that occurs between 5 and 7 y of age is a critical period in which to develop obesity (31). Our findings that a high prenatal exposure to PCB and DDE was associated with a greater change in BMI from 5 to 7 y of age in girls with overweight mothers implied that the prenatal exposure of PCB and DDE may be associated with an early adiposity rebound, which has been shown to associate with greater BMI in adolescence and adulthood (32). With consideration that 5–7 y of age is a vulnerable life stage for growth, it could be hypothesized that the chemical compounds may influence some of the hormonal changes that occur from 5 to 7 y of age.
The exposure to PCBs and DDE has been declining since the ban of the chemicals in the 1970s, but they are still a source of exposure because of the slow metabolic degradation. In addition, research has shown that the relation between EDCs and health outcomes may have an inverted U-shaped dose-response curve. As such, lower doses of PCBs and DDE may be more potent than higher doses (9, 33, 34). The fact that a high prenatal PCB and DDE exposure was associated with a higher BMI and WC at 7 y of age and an increase in BMI from 5 to 7 y of age was consistent with the hypothesis that early-life exposure to EDCs may promote obesogenic changes in metabolism (35). Although the mechanisms involved are not yet fully understood, it has been suggested that some EDCs may upregulate adipogenesis or promote fat storage through mechanisms such as the activation of retinoid-X receptor, peroxisome proliferator activated receptor-γ, or glucocorticoid-receptor pathways or the disruption of estrogen and other signaling pathways (36).
Major strengths of this study were the prospective design and the homogenous well-described cohort (37, 38). Moreover, the length of follow-up and the few losses to follow-up enabled us to conclude on subsequent obesity development in the later stages of childhood, with almost complete follow-up. However, there were limitations to the study because a seafood diet is contaminated with other chemical compounds than PCB and DDE (eg, hexachlorobenzene), and most of these compounds are highly correlated. Thus, it is possible that other chemicals than PCB and DDE could have contributed to the observed effects. For example, prenatal exposure to hexachlorobenzene, which is a persistent organic pollutant highly correlated to PCB and DDE, has been reported to cause overweight in children (39). However, the serum concentration of hexachlorobenzene in this study was close to the limit of detection and much lower than that of PCB and DDE, and therefore, we believe it was unlikely that hexachlorobenzene was responsible for the observed effects. Our analyses included many subgroups and, hence, a possibility of a type 1 error. However, most of the results were significant according to a calculated Bonferroni-adjusted significance level (P ≤ 0.006). Also, the use of average ratios between PCB and DDE in milk and serum to calculate missing serum variables has likely induced some errors. Nevertheless, the use of multiple imputation to generate data, which yielded essentially similar results (see Supplemental Tables 3 and 4 under “Supplemental data” in the online issue), did not change the conclusions. Finally, although we adjusted for a number of confounders, residual confounding from unmeasured covariates was still possible. Indeed, unmeasured maternal factors as, eg, the consumption of pilot whale blubber, which is part of a traditional diet and an important source of PCBs, could have differed between normal-weight and overweight mothers. However, because differences in PCB concentrations across socioeconomic status groups or across normal-weight and overweight mothers were not observed, residual confounding from unmeasured factors was less likely. Note that, although a high BMI during childhood is known to be associated with increased risk of obesity in adulthood (40), the current study only followed children for 7 y, and it remains to be seen whether the effects on obesity of PCB and DDE exposure still persist later in life.
In conclusion, we showed that prenatal PCB and DDE exposure was associated with obesity development in 7-y-old girls and changes in BMI from 5 to 7 y of age and WC at age 7 y in girls of mothers with high prepregnancy BMI. These findings have important public health implications because of the high and increasing prevalence of obesity and ubiquity of EDC exposure and also emphasize the need for future prospective studies with long-term follow-up.
Supplementary Material
Acknowledgments
We thank all participants in the Faroese PCB studies and their parents as well as those in the Public Health Department in Tórshavn who helped to organize examinations. We thank C Holst for statistical assistance.
The authors’ responsibilities were as follows—US, PG, and PW: were responsible for cohort formation and data collection; JLT-P, BLH, HRA, and TKJ: were involved in the design of the current study; JLT-P: analyzed data and wrote the first draft of the manuscript; BLH, HRA, and TKJ: supervised the work; and all authors: were involved in data interpretation, writing of the manuscript, and approval of the final version of the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: DDE, p,p′-dichlorodiphenyldichloroethylene; EDC, endocrine-disrupting chemical; PCB, polychlorinated biphenyl; WC, waist circumference.
REFERENCES
- 1.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006;295:1549–55. [DOI] [PubMed] [Google Scholar]
- 2.Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med 2002;8:185–92. [DOI] [PubMed] [Google Scholar]
- 3.Elobeid MA, Allison DB. Putative environmental-endocrine disruptors and obesity: a review. Curr Opin Endocrinol Diabetes Obes 2008;15:403–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJP. Mothers, babies and health in later life. 2nd ed. London, United Kingdom: Churchill Livingstone, 1998. [Google Scholar]
- 5.Lillycrop KA, Burdge GC. Epigenetic changes in early life and future risk of obesity. Int J Obes (Lond) 2011;35:72–83. [DOI] [PubMed] [Google Scholar]
- 6.Grün F, Blumberg B. Endocrine disrupters as obesogens. Mol Cell Endocrinol 2009;304:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newbold RR, Padilla-Banks E, Snyder RJ, Jefferson WN. Perinatal exposure to environmental estrogens and the development of obesity. Mol Nutr Food Res 2007;51:912–7. [DOI] [PubMed] [Google Scholar]
- 8.Grün F, Watanabe H, Zamanian Z, Maeda L, Arima K, Cubacha R, Gardiner DM, Kanno J, Iguchi T, Blumberg B. Endocrine disrupting organotin compounds are potent inducers of adipogenesis in vertebrates. Mol Endocrinol 2006;20:2141–55. [DOI] [PubMed] [Google Scholar]
- 9.Tang-Péronard JL, Andersen HR, Jensen TK, Heitmann BL. Endocrine-disrupting chemicals and obesity development in humans: a review. Obes Rev 2011;12:622–36. [DOI] [PubMed] [Google Scholar]
- 10.Apostoli P, Magoni M, Bergonzi R, Carasi S, Indelicato A, Scarcella C, Donato F. Assessment of reference values for polychlorinated biphenyl concentration in human blood. Chemosphere 2005;61:413–21. [DOI] [PubMed] [Google Scholar]
- 11.Turusov V. Dichlorodiphenyltrichloroethane (DDT): ubiquity, persistence, and risks. Environ Health Perspect 2002;110:125–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelletier C, Imbeault P, Tremblay A. Energy balance and pollution by organochlorines and polychlorinated biphenyls. Obes Rev 2003;4:17–24. [DOI] [PubMed] [Google Scholar]
- 13.Hertz-Picciotto I, Charles MJ, James RA, Keller JA, Willman E, Teplin S. In utero polychlorinated biphenyl exposures in relation to fetal and early childhood growth. Epidemiology 2005;16:648–56. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson JL, Jacobson SW, Humphrey HE. Effects of exposure to PCBs and related compounds on growth and activity in children. Neurotoxicol Teratol 1990;12:319–26. [DOI] [PubMed] [Google Scholar]
- 15.Verhulst SL, Nelen V, Den Hond E, Koppen G, Beunckens C, Vael C, Schoeters G, Desager K. Intrauterine exposure to environmental pollutants and body mass index during the first 3 years of life. Environ Health Perspect 2009;117:122–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole TJ, Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr Obes 2012;7:284–94. [DOI] [PubMed] [Google Scholar]
- 17.Fredriks AM, van Buuren S, Fekkes M, Verloove-Vanhorick SP, Wit JM. Are age references for waist circumference, hip circumference and waist-hip ratio in Dutch children useful in clinical practice? Eur J Pediatr 2005;164:216–22. [DOI] [PubMed] [Google Scholar]
- 18.Heilmann C, Grandjean P, Weihe P, Nielsen F, Budtz-Jørgensen E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS Med 2006;3:e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schade G, Heinzow B. Organochlorine pesticides and polychlorinated biphenyls in human milk of mothers living in northern Germany: current extent of contamination, time trend from 1986 to 1997 and factors that influence the levels of contamination. Sci Total Environ 1998;215:31–9. [DOI] [PubMed] [Google Scholar]
- 20.Grandjean P, Weihe P, Needham LL, Burse VW, Patterson DGJ, Sampson EJ, Jørgensen PJ, Vahter M. Relation of a seefood diet to mercury, selenium, arsenic, and polychlorinated biphenyl and other organochlorine concentrations in human milk. Environ Res 1995;71:29–38. [DOI] [PubMed] [Google Scholar]
- 21.Halldorsson TI, Rytter D, Haug LS, Bech BH, Danielsen I, Becher G, Henriksen TB, Olsen SF. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect 2012;120:668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valvi D, Mendez MA, Martinez D, Grimalt JO, Torrent M, Sunyer J, Vrijheid M. Prenatal concentrations of polychlorinated biphenyls, DDE, and DDT and overweight in children: a prospective birth cohort study. Environ Health Perspect 2012;120:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gladen BC, Ragan NB, Rogan WJ. Pubertal growth and development and prenatal and lactational exposure to polychlorinated biphenyls and dichlorodiphenyl dichloroethene. J Pediatr 2000;136:490–6. [DOI] [PubMed] [Google Scholar]
- 24.Blanck HM, Marcus M, Rubin C, Tolbert PE, Hertzberg VS, Henderson AK, Zhang RH. Growth in girls in utero and postnatally to polybrominated biphenyls and polychlorinated biphenyls. Epidemiology 2002;13:205–10. [DOI] [PubMed] [Google Scholar]
- 25.Rylander L, Strömberg U, Hagmar L. Weight and height at 4 and 7 years of age in children born to mothers with a high intake of fish contaminated with persistent organochlorine pollutants. Chemosphere 2007;67:498–504. [DOI] [PubMed] [Google Scholar]
- 26.Lamb MR, Taylor S, Liu X, Wolff MS, Borrell L, Matte TD, Susser ES, Factor-Litvak P. Prenatal exposure to polychlorinated biphenyls and postnatal growth: a structural analysis. Environ Health Perspect 2006;114:779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mendez MA, Garcia R, Guxens M, Vrijheid M, Kogevinas M, Goni F, Fochs S, Sunyer J. Prenatal organochlorine compound exposure, rapid weight gain and overweight in infancy. Environ Health Perspect 2011;119:272–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karmaus W, Osuch JR, Eneli I, Mudd LM, Zhang J, Mikucki D, Haan P, Davis S. Maternal levels of dichlorodiphenyl-dichloroethylene (DDE) may increase weight and body mass index in adult female offspring. Occup Environ Med 2009;66:143–9. [DOI] [PubMed] [Google Scholar]
- 29.Kuchta KF. Pathophysiologic changes of obesity. Anesthesiol Clin North America 2005;23:421–9. [DOI] [PubMed] [Google Scholar]
- 30.La Merrill M, Birnbaum LS. Childhood obesity and environmental chemicals. Mt Sinai J Med 2011;78:22–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr 1994;59:955–9. [DOI] [PubMed] [Google Scholar]
- 32.Rolland-Cachera MF, Deheeger M, Maillot M, Bellisle F. Early adiposity rebound: causes and consequences for obesity in children and adults. Int J Obes (Lond) 2006;30(suppl 4):S11–7. [DOI] [PubMed] [Google Scholar]
- 33.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 2012;33:378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ Health Perspect 2003;111:994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heindel JJ. Endocrine disruptors and the obesity epidemic. Toxicol Sci 2003;76:247–9. [DOI] [PubMed] [Google Scholar]
- 36.Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012;122:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grandjean P, Bjerve K, Weihe P, Steuerwald U. Duration of pregnancy, birth weight and placenta weight in relation to maternal marine diet. Int J Epidemiol 2001;30:1272–8. [DOI] [PubMed] [Google Scholar]
- 38.Steuerwald U, Weihe P, Jørgensen PJ, Bjerve K, Brock J, Heinzow B, Budtz-Jørgensen E, Grandjean P. Maternal seafod diet, methylmercury exposure, and neonatal neurological function. J Pediatr 2000;136:599–605. [DOI] [PubMed] [Google Scholar]
- 39.Smink A, Ribas-Fito N, Garcia R, Torrent M, Mendez MA, Grimalt JO, Sunyer J. Exposure to hexachlorobenzene during pregnancy increases the risk of overweight in children aged 6 years. Acta Paediatr 2008;97:1465–9. [DOI] [PubMed] [Google Scholar]
- 40.Guo SS, Wu W, Chumlea WC, Roche AF. Predicting overweight and obesity in adulthood from body mass index values in childhood and adolescence. Am J Clin Nutr 2002;76:653–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.