Abstract
Cognitive styles can be characterized as individual differences in the way people perceive, think, solve problems, learn, and relate to others. Field dependence/independence (FDI) is an important and widely studied dimension of cognitive styles. Although functional imaging studies have investigated the brain activation of FDI cognitive styles, the combined structural and functional correlates with individual differences in a large sample have never been investigated. In the present study, we investigated the neural correlates of individual differences in FDI cognitive styles by analyzing the correlations between Embedded Figures Test (EFT) score and structural neuroimaging data [regional gray matter volume (rGMV) was assessed using voxel-based morphometry (VBM)] / functional neuroimaging data [resting-brain functions were measured by amplitude of low-frequency fluctuation (ALFF)] throughout the whole brain. Results showed that the increased rGMV in the left inferior parietal lobule (IPL) was associated with the EFT score, which might be the structural basis of effective local processing. Additionally, a significant positive correlation between ALFF and EFT score was found in the fronto-parietal network, including the left inferior parietal lobule (IPL) and the medial prefrontal cortex (mPFC). We speculated that the left IPL might be associated with superior feature identification, and mPFC might be related to cognitive inhibition of global processing bias. These results suggested that the underlying neuroanatomical and functional bases were linked to the individual differences in FDI cognitive styles and emphasized the important contribution of superior local processing ability and cognitive inhibition to field-independent style.
Introduction
Cognitive styles refer to individual differences in the way people perceive, think, learn, solve problems, and relate to others [1-3]. Many cognitive style dimensions have been studied in the literature, however, field dependence/independence (FDI) is the most widely studied dimension measured by Embedded Figures Test (EFT) [4,5]. The EFT requires subjects to locate the simple shape that embedded in a complex figure. Based on the EFT score, Witkin et al. [1] identified the field-dependent (FD) and field-independent (FI) visual perceptual styles. FD individuals exhibit more dependency on the surrounding field and cannot easily perceive the embedded part. One the other extreme, FI individuals tend to be less influenced by the information from the visual fields and can perform better in the test [1,5]. The EFT score forms a continuous distribution, and reflects a varying degrees towards one kind of perception tendency mode or the other [1,2]. A person’s tendency to perceive was found to be significantly related to their cognitive functioning, personality characteristic, and their social behavior [5]. At the present time, FDI cognitive styles have been used as an excellent predictor of an individual’s success in a particular situation, particularly in terms of academic achievement, individual and organizational behaviors in numerous applied fields [6,7]. FDI cognitive styles are widely studied in cognitive and educational fields, but the neural underpinnings of FDI cognitive styles have been little investigated.
To date, a limited number of studies employed neuropsychological measures to investigate FDI cognitive styles that mainly focused on visuospatial processing bias and clinical individual differences [8–15]. Early EEG studies showed that FI subjects exhibited smaller between-hemisphere coherence and more hemispheric specialization [8,9]. The successful performance in the EFT reflects individual variations in detecting local features under the circumstance that global perception still dominates [10]. Manjaly et al. [11]used a straightforward shape recognition task as control condition, and found significant activations in the left inferior and superior parietal cortex and left inferior frontal gyrus specific in the EFT. Furthermore, Lee et al. [12]adopted a match task as control condition and suggested that posterior cortical in the left hemisphere was related to the perception of local elements and the medial frontal involved in the suppression of global perceptual processing bias in EFT. Recently, Walter and Dassonville [14] found that FI individuals typically recruit a strongly bilateral frontoparietal network when performing the EFT. Many clinical studies found that children with high-functioning autism exhibited superior performance on the EFT [16-19]. Autism is possibly characterized by a cognitive style biased towards local rather than global information processing [20,21]. Although FI doesn’t equate with autism, neuroimaging studies on autism may help us to understand more specific regional functions. Damarla et al. [15] observed that more activation occurred in left dorsolateral prefrontal and inferior parietal lobe in normal control subjects and more activation occurred in visuospatial areas in autism group. They suggested normal subjects had more functional integration of higher-order executive regions with visuospatial regions, while autism relied more on visuospatial regions to preserve or enhance performance on the EFT. Upon these studies, we supposed that individual differences in FDI cognitive styles might be related to the differential use of medial frontal region for suppression of the irrelevant background information or global processing bias and posterior visual-spatial regions (inferior/superior parietal regions) for local visual processing.
Despite previous task-related neuroimaging studies on FDI cognitive styles described above, these studies depended heavily on inconsistent methodologies and limited by a small sample size, which lead difficult to reconcile the inconsistencies among them [22]. Moreover, previous studies have stated that people are quite stable in their preferred perception mode [23,24]. On the other hand, the superiority on the EFT is the cognitive profile characteristic of autism individual, which has strong heritability [25,26]. Baron-Cohen and Hammer [27] reported that the parents of children with autism or Asperger’s syndrome were also faster on the EFT relative to the matched control parents. Considering its stability, examining structure correlates of FDI cognitive styles would eliminate task-related differences and become especially useful for investigating the anatomical correlates of individual differences.
On a similar note, resting state shows strong activation of several brain areas without an external task [28]. Spontaneous cortical activity can serve as a predictor of individual differences in several cognitive domains, such as perception, problem solving, and memory [29–31]. The spontaneous fluctuations in the blood oxygen level dependent (BLOD) signal of fMRI are not random noise but physiologically meaningful and low-frequency fluctuations (LFFs; 0.01 Hz to 0.08 Hz) reveal spontaneous neuronal activity [32]. Moreover, regional amplitude of low frequency fluctuation (ALFF) was an index for measuring regional spontaneous neuronal activity in the resting-state fMRI [33–35].
Functional imaging studies have shown that the increases in gray matter (GM) are associated with increased or decreased brain activity [36–38]. The structure and function may function differently and change independently [39]. For these reasons, the combination of structural imaging of regional gray matter volumes and ALFF of resting state would provide complementary information and advance our understanding of the FDI cognitive styles. However, the neural substrate of individual differences in FDI cognitive styles that employed the combined methods has never been investigated yet.
In this study, fMRI was performed to examine both structural and resting-state functional brain alterations in FDI cognitive styles measured by EFT. Structural differences related to FDI was first conducted to be examined by standard voxel-based morphometry (VBM) [40]. And the ALFFs [34,41]of resting-state fMRI were used to reflect regional properties of the brain’s intrinsic neural activity. Furthermore, based on previous neuroimaging studies on FDI cognitive styles, we predicted that FDI cognitive styles would be associated with visuospatial processing and high-order suppression, subserved mainly by significant structural and functional alterations in posterior visual-spatial regions and medial frontal region respectively. The combination of structural and resting-state functional data may improve our understanding of the neural correlates of individual differences in FDI cognitive styles.
Methods
Ethics statement
The study was approved by Southwest University Brain Imaging Center Institutional Review Board. In accordance with the Declaration of Helsinki (1991), written informed consents were obtained from all participants. In addition, all of the participants were remunerated for their participation.
Participants
A total of 286 right-handed, healthy volunteers (140 females and 146 males; mean age = 20.01 years, SD = 1.33, aged 18-26 years) participated in the study as part of our ongoing project to examine the association among brain imaging, creativity and mental health. For VBM analyses, all 286 subjects were included in the study. For ALFF analyses, 25 subjects were excluded due to excessive head motions, resulting in 261 subjects (132 females and 129 males; mean age = 20.03 years, SD=1.36, aged 18-26 years). Data derived from the subjects in this study are to be used in other studies irrelevant to the theme of this study. Our project gathered psychological behavioral data and imaging data for every subject. Behavioral measures consist of questionnaires for creativity, personality, intelligence, and mental health and experimental tasks for working memory, attention, reponse inhibition, emotion Stroop task. MRI scans included resting state imaging, T1-weighted image and diffusion tensor imaging. All participants were university students from the local community of Southwest University. They were recruited using adverts on bulletin board at BBS of Southwest University (http://qcjy.swu.edu.cn/bbs/) or by introducing this study and our laboratory’s previous experiments by person in charge in every college to our subjects. No participant had a history of neurological or psychiatric illness.
Embedded figures test
EFT is a timed paper-and-pencil performance test adapted from the individual-administered Embedded Figures Test [42]. The present study employed a Chinese version of the EFT revised by the College of Psychology in Beijing Normal University [43]. The revised EFT adopts the majority of the original EFT items, with a few complex figures slightly modified. It comprises three sections: the first/practice section (9 figures); section B (10 figures); and section C (10 figures). The task is to locate and trace the simple figures in the context of the complex figures, as quickly as possible within three 5-min sections (the practice section, section B, section C). The total number of correct answers on the second and third sections (ranged between 0 and 20) were considered as the EFT score.
These modifications to the original EFT were based on much polit testing in four groups (adults, senior-high-school students, junior-high-school students, primary-school students). Validity had been tested by Pearson’s correlation coefficients between EFT scales and the rod-and-frame test (RFT) [r = 0.49, p < 0.05]. The test reliability was calculated by Pearson’s correlation coefficients between section B and section C of the revised EFT [r = 0.90, p < 0.05]. The difficulty distribution of the revised EFT is 0.97-0.21; the discrimination distribution of it is 0.17-0.94.
Assessment of general intelligence
The Raven’s Progressive Matrices test, which is often regarded as a good marker of the general factor of fluid intelligence [44]. In this study, the Chinese version of the combined Raven’s Progressive Matrices test (CRT) was used for fluid intelligence [45-47]. The CRT is composed of the Colored Progressive Matrices (A, B, and AB sets) and the last three parts of the Standard Progressive Matrices (C, D, and E sets). Each set comprises five items increasingly difficulty. The number of the correct answers given in 40 min was used as the CRT score. The CRT scale has high internal consistency (Cronbach’s α = 0.93) and a good validity (r = 0.56) with another popular general intelligence scale, namely, Wechsler Intelligence Scale [45–47].
MRI Data Acquisition
All of the MR images were acquired on a 3.0 T Siemens Trio MRI scanner (Siemens Medical, Erlangen, Germany) at the Brain Imaging Research Central in Southwest University. For each subject two sets of MR images were acquired in this study. First, subjects completed a resting-state functional scan, during which time they were instructed to close eyes, not to move, think particularly or fall asleep. Each subject reported not having fallen asleep using a simple questionnaire after scanning. BOLD images were obtained using Echo Planar Imaging (EPI) sequence with following parameters: slices = 28; repetition time (TR)/echo time (TE) = 2000/40 ms; flip angle = 90°; FOV = 256 mm × 256 mm; voxel size = 4 ms × 4 ms × 4 ms; thickness/slice gap = 4/1 mm; and matrix = 64 × 64. For each subject, total 242 volumes were collected. Second, a high-resolution T1-weighted anatomical images were acquired using a magnetization-prepared rapid gradient echo (MPRAGE) sequence (TR = 1900ms; TE = 2.52 ms; inversion time = 900 ms; flip angle = 9 degrees; resolution matrix = 256 × 256; slices = 176; thickness = 1.0 mm; voxle size = 1 mm × 1 mm × 1 mm).
VBM analysis
The MR images were processed using the SPM8 (Wellcome Department of Cognitive Neurology, London, UK; www.fil.ion.ucl.ac.uk/spm/) implemented in Matlab 7.8 (MathWorks Inc., Natick, MA, USA). Each MR image was first displayed in SPM8 to screen for artifacts or gross anatomical abnormalities. For better registration, the reorientation of the images was manually set to the anterior commissure. Segmentation of the images into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF) using the new segmentation in SPM8. Subsequently, we performed Diffeomorphic Anatomical Registration through Exponentiated Lie (DARTEL) algebra in SPM8 for registration, normalization, and modulation [48]. To ensure that regional differences in the absolute amount of GM were conserved, the image intensity of each voxel was modulated by the Jacobian determinants. Then, registered images were transformed to standard Montreal Neurological Institute (MNI) space. Finally, the normalized modulated images (gray matter images) were smoothed with a 10-mm full-width at half maximum (FWHM) Gaussian kernel to increase signal to noise ratio.
Statistical analyses of GMV data were performed using SPM8. In the whole-brain analyses, we used a multiple linear regression to identify regions where regional GMV was associated with individual differences in EFT score. In the multiple linear regression analyses, the score of EFT was used as the variable of interest. To control for possible confounds variables, age, sex, the CRT score and global volumes of GM were entered as covariates into the regression model. To reduce the risk of false negatives and achieve maximal sensitivity, we applied explicit masking with an population-specific automatic optimal threshold to restrict the search volume within gray matter achieved using the Masking toolbox in SPM8 (http://www0.cs.ucl.ac.uk/staff/g.ridgway/masking/). This automatic mask-creation strategy is based on maximizing the correlation between the original and thresholded images and attempts to find an optimal threshold to binarize an average image [49].. At the whole-brain level, a multiple comparison correction was performed using the voxel-level False Discovery Rate (FDR) approach, at a threshold of p <0.05 [50].
ALFF analysis
Functional image preprocessing was performed using Data Processing Assistant for Resting-state fMRI (DPARSF,http://www.restfmri.net/forum/DPARSF;[51]) software. The first ten volumes of the functional images were discarded, because of the instability of the initial MR signals and subjects’ adaptation to the circumstances. The remaining images were preprocessed following these steps: slice timing correction, head motion correction, spatial normalization to the Montreal Neurological Institute (MNI) template and then resampling voxel size of 3 mm× 3 mm×3 mm followed by spatial smoothing with a 8-mm full width at half maximum (FWHM) Gaussian kernel. 25 subjects with an estimated maximum displacement in any direction greater than 2.0 mm or a head rotation greater than 2.0° were discarded to minimize movement artifacts in this study. The time series were transformed to the frequency domain using a fast Fourier transform (FFT). Then the power spectrum obtained was square-rooted and averaged across 0.01–0.08 Hz at each voxel. This averaged square root comprised the ALFF. Finally, the ALFF value of each voxel was standardized by dividing the global mean ALFF value within a brain-mask, and other tissues outside the brain were removed [34,52].
A multiple linear regression was performed to identify regions where regional ALFF was associated with EFT score at the whole-brain level. Age, sex and the CRT score were entered as covariates of no interest into the regression model. The score of EFT was used as the variable of interest. A more lenient correction was adopted for multiple comparisons. Clusters were considered significant at the combined voxel-extent threshold of an uncorrected voxel level of p < 0.001 and cluster extent > 49 voxels, which corresponded to a corrected p < 0.005. The AlphaSim correction (cluster radius connection: rmm = 5; number of Monte Carlo simulations = 1000) was conducted using the AlphaSim program in the REST software (http://www.restfmri.net), which applied Monte Carlo simulation [53] to caculate the probability of false positive detection by considering both the individual voxel probability thresholding and cluster size [51].
Results
Sample Descriptive
Table 1 lists the characteristics of demographics of the total sample. A total of 286 healthy subjects (140 females and 146 males) were included in the VBM analysis, and 261 subjects (132 females and 129 males) were included in the ALFF analysis. The EFT score for all subjects ranged from 6 to 20. There were no significant differences between sexes on the EFT score of either the VBM or ALFF analysis (see Table 1).
Table 1. Demographic and behavioral data.
| Items | Total subjects |
|---|---|
| VBM analysis | |
| Number of subjects | 286 |
| Females / males | 140 / 146 |
| Age (years) | 20.01 ± 1.33 (18-26) |
| CRT score | 65.90 ± 3.45 (49-72) |
| EFT score | 13.66 ± 3.03 (6-20) |
| ALFF analysis | |
| Number of subjects | 261 |
| Females / males | 132 / 129 |
| Age (years) | 20.03 ± 1.36 (18-26) |
| CRT score | 65.89 ± 3.42 (49-72) |
| EFT score | 13.63 ± 3.07 (6-20) |
A total of 286 subjects were included in the VBM analysis. From that sample, 261 subjects were included in the ALFF analysis.
Abbreviations: VBM, voxe-based morphometry; ALFF, amplitude of low-frequency fluctuation; EFT, embedded figures test.
Correlation between rGMV/ALFF and EFT Score
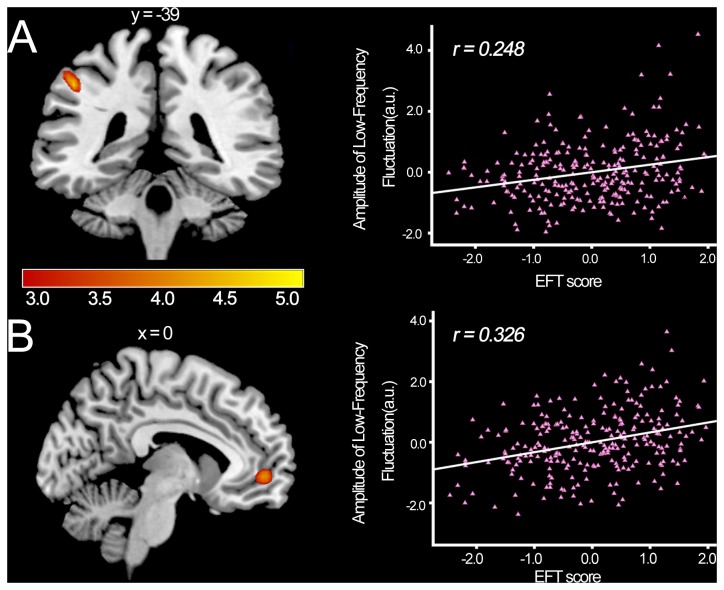
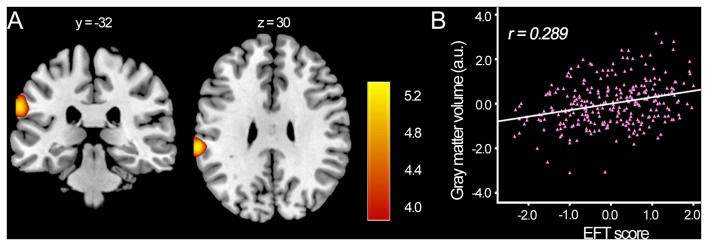
After controlling for age, gender, CRT socre and global GM volumes, EFT score was positively correlated with the gray matter volume in a cluster that mainly included areas in the inferior parietal lobule (IPL) [left: r = 0.289, cluster size = 661, t = 5.34, p (corr) = 0.001; see Figure 1, Table 2]. There was no brain area whose GMV was negatively correlated with EFT score. With a more lenient threshold, EFT score was only positively correlated with ALFF values in the IPL [left: r = 0.248, cluster size = 97, t = 4.74, p (corr) = 0.005; see Figure 2, Table 2] and mPFC [ r = 0.326, cluster size = 63, t = 4.15, p (corr) = 0.005; see Figure 2, Table 2]. Although the increased GMV in left IPL was found almost near the increased ALFF in the same region, there was no overlapping between the GMV and ALFF results, and the GMV in the regions did not correlate with the ALFF.
Figure 1. Regions of correlation between rGMV and EFT score.
(p < 0.05, corrected for FDR).
(A) The left IPL in which variability in rGMV exhibited significant positive correlation with EFT score (n=286) is superimposed on a standard T1-weighted template brain in MNI stereotactic space. (B) A scatterplot between left IPL volume and EFT score adjusted for age, gender, and total gray matter volume is shown for illustration purpose only.
Table 2. Brain regions with significant positive correlations between rGMV/ALFF values and EFT score.
| analysis | Brian regions | BA | Peak coordinates |
Cluster size (voxels) | t-value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| VBM | Left IPL | 40 | -65 | -32 | 30 | 661 | 5.34 |
| ALFF | Left IPL | 40 | -48 | -39 | 45 | 97 | 4.74 |
| mPFC | 10 | 0 | 48 | -6 | 63 | 4.15 | |
No regions showed significant negative correlations between rGMV/ALFF and EFT score.
Abbreviations: rGMV, regional gray matter volume; ALFF, amplitude of low-frequency fluctuation; EFT, embedded figures test; BA, Brodmann areas; IPL, inferior parietal lobule; mPFC, medial prefrontal cortex.
Figure 2. ALFF was positively correlated with individual EFT score.
In the left panel, results are shown with p < 0.0005 uncorrected for visualization purposes. (A) Coronal view. Regions of significant correlation are shown in the left IPL; (B) Sagittal view. Regions of significant correlation are shown in the mPFC. The right panel shows corresponding scatterplots of the relationship between the EFT score and ALFF values of the significant cluster in the region of the left panel. The scatterplots adjusted for age, gender, CRT score are shown for illustration purpose only.
Discussions
To the best of our knowledge, this is the first study combined VBM analysis of structural neuroimaging data and ALFF analysis of resting-state fMRI data to investigate the association between FDI cognitive styles as measured by EFT and rGMV/ALFF in subcortical regions. Our findings showed that increased rGMV in the left IPL was associated with EFT score. Consistent with our expectations, a significant positive correlation between ALFF and EFT score was found in the fronto-parietal network, including left IPL and mPFC. The IPL obtained by VBM and ALFF analyses did not overlap. Furthermore, no negative correlations in neither rGMV nor ALFF were observed. Our results suggested that underlying neuroanatomical and functional bases were linked to the individual differences in FDI cognitive styles.
Firstly, greater rGMV in the left IPL may contribute to superior local visual processing for more efficient performers (FI individuals). This result corresponded to the activation pattern and findings of other functional visual-spatial imaging studies [11,54-60]. Therefore we considered that left IPL might be the core brain region involved in detail-focused visuospatial task (specific in EFT). EFT assesses visuospatial tendency indicative of spatial orienting or global-local processing [60,61]. Fink et al. [62] found that inferior parietal regions were involved in locating an object in space or making judgments about related object properties. Strong local processing requirements in EFT was well-established left-hemispheric dominance in Manjaly’s study [13] and more activation was observed in left posterior parietal cortex, including the intraparietal sulcus (IPS) and left ventral premotor cortex (posterior inferior parietal lobe). Furthermore, previous studies have demonstrated that individuals with autism exhibit superior ability in terms of spatial abilities and attention-to-detail [27,63]. They found that children with autism or Asperger’s syndrome were more accurate and faster than normal on the EFT [17,19] and these superior characteristics of autism has strong heritability [25,27,64]. Damarla et al. [15] reported autism group more relied on visuospatial areas (bilateral superior extending to inferior parietal and right occipital) areas for the intact or superior performance in EFT. These studies suggested that performance on the EFT reflected the efficiency of local visual processing and inferior parietal lobe played a key role in such cognitive visual-spatial function. Some researches shown decreases of gray matter reductions located in superior temporal sulsus, fronto-striatal and parietal network in autism [65,66]. But cerebral gray matter volume (especially in left-lateralized) increases were found for the individuals with autism [67-69], which is in accordance with the results in this study. It is important to note that our sample consisted of a typical population, which was regarded as neurotypical controls in autism researches. FI individuals with more efficient performance in EFT are more able to perceive an element independently from its context. According to our study, this stable personality characteristic may have its structural basis in the left IPL. Thus, increased rGMV in the left IPL in FI individuals might be associated with an excellent function of local visual processing.
Secondly, higher ALFF in the brain areas belonging to the fronto-parietal network, including the left IPL and mPFC might primarily reflect the dis-embedding process during EFT. We found no apparent overlap between structural and functional findings. Haier et al. [38] indicated that structural change in one brain region did not necessarily result in functional change in the same location. No study has used anatomical and functional methods in combination in large normal sample to explore the neural basis of FDI cognitive styles.
The fronto-parietal network, which includes elements of the dorsal attention network elements, mainly consists of IPL, IPS, ventromedial prefrontal cortex (vmPFC), precuneus, dorsal frontal, and midcingulate [70-72]. Evidence has suggested that the fronto-parietal network is responsible for attention, visuospatial working memory, and cognitive control [56,73]. Neuroimaging studies have demonstrated that the posterior parietal cortex area contributes as a key locus of storage of representation of visual information and is associated with feature identification in spatial stimuli processing [60,62,74-76]. Furthermore, many previous studies had indicated that the left temporo-parietal regions attend to local aspect of an object’s shape [12,13,15,77-79]. Regarding human visual system, objects within the visual scene are inclined to be identified and perceived as a whole [10,80], and this global perception tendency would dominate during EFT. FI individuals could perceive the local details less influenced by the global form. Therefore, higher ALFF in the left IPL might be related to superior feature identification of local processing for FI individuals. Moreover, we also found the significant positive correlation between ALFF in the mPFC and EFT score, which may also be comparable to previous neuropsychological studies that reported the involvement of the mPFC in cognitive styles. Previous functional imaging studies have indicated that the mPFC might be involved in high-level conflict monitoring, executive control, and decision making [81-86]. Normative perception has preferential global processing bias and better perform in EFT need inhibition of the global perceptual bias to reach more local processing [87,88]. Liu et al. [89] reported that greater activation in the superior frontal and medial frontal brain region in the line-counting task may reflect inhibition of automatic global processing of 3D configurations information, which may interfere with the concurrent local processing. Walter and Dassonville [14] also indicated that superior frontal gyrus might be involved in top-down control of attention required in search for the embedded figure. Furthermore, mPFC has been posited to reflect the suppression of the global perceptual bias during perception of local aspects of hierarchical stimuli [12,87,88]. In addition, The FI individuals could override the global perceptual bias to disembed a local component successfully. Thus, higher ALFF in mPFC for FI individuals was related to more effective at cognitive inhibition of field/global information, which may bring about detail-focused processing.
Visual perceptual styles could affect an individual’s social cognition. A previous study has revealed that there existed significant relations between perceptual styles and social behavior [5]. Russell-Smith et al. [90] suggested that the superior EFT performance in autism may be strongly linked to the social deficits. “Autistic-like” traits, including the display of superior performance on the EFT, were continuously and normally distributed in the general population [91-93]. In our study, inferior parietal lobe (belonging to the tempoparietal junction, TPJ) and mPFC also served as vital regions of social cognition. Recent studies on autism have identified failing to deactivation at resting-state and found that the amount of functional abnormality in mPFC is positively correlated with that of social impairment [94,95]. However, evidence has suggested seeming different cognitive patterns was found between autistic traits in typical and ASD populations [96,97]. In the present study, a higher rest activation of the network was involved in the social cognition in FI individuals with high EFT scores. This result was consist with von dem Hagen et al.’s study [98]. They also found increased rest activation with greater autistic traits in the typical population. However, this study didn’t have sufficient evidence to test the relationship between local processing bias and social cognition directly. Future studies should address this issue to determine whether or not the differences in autism traits at the neuropsysiological level between the autism and normal populations.
In summary, the present study revealed the neural correlates of cognitive styles (FDI) by combining structural and functional MRI analyses in a large sample of healthy young adults. We found that increased rGMV in left IPL might be the structure basis of excellent local processing. Furthermore, functional results indicated that field-independent individuals might recruit a strong fronto-parietal network, relating to superior feature identification and cognitive inhibition. Finally, we attempted to point out that there might be different relationships between visual perceptual styles and discrepancy of individual’s social cognition in typical population and autism. The combination of structural and functional MRI methods possiblyprovided complementary information and advanced our understanding of the FDI cognitive styles. The correlation is arguably low for the research, which is probably due to the large sample size. The large sample analysis improves the validity of the neuroimaging research to some extent. In the future, we will choose clinical subjects with local brain lesion in the related regions in this research to further investigate the neural basis of FDI cognitive styles.
Funding Statement
The research was supported by the National Natural Science Foundation of China (31070900; 30800293; 30970892; 31170983), the Program for New Century Excellent Talents in University (2011) by the Ministry of Education, the Fundamental Research Funds for the Central Universities (SWU1209101), China Postdoctoral Science Foundation funded project (2012M510098), the Research Funds for Southwest University (SWU09103), the Key Discipline Fund of National 211 Project (NSKD 11007), the Fundamental Research Funds for the Central Universities (swu1209101), the Program for New Century Excellent Talents in University (2011) by the Ministry of Education, China Postdoctoral Science Foundation funded project (2012M510098), and the postgraduate Innovation Foundation of Science and Technology of Southwest University (kb2011002). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Witkin HA, Moore CA, Goodenough DR, Cox PW (1977) Field-dependent and field-independent cognitive styles and their educational implications. Rev Educ Res 47: 1-64. doi: 10.3102/00346543047001001. [DOI] [PubMed] [Google Scholar]
- 2. Witkin HA (1950) Individual differences in ease of perception of embedded figures. J Pers 19: 1-15. doi: 10.1111/j.1467-6494.1950.tb01084.x. PubMed: 14795367. [DOI] [PubMed] [Google Scholar]
- 3. Witkin HA, Lewis H, Hertzman M, Machover K, Meissner P et al. (1972) Personality through perception: An experimental and clinical study. Westport, CT: Greenwood Press. [Google Scholar]
- 4. Ford N (1995) Levels and types of mediation in instructional systems: an individual differences approach. Int J Humcomput St 43: 241-259. [Google Scholar]
- 5. Kozhevnikov M (2007) Cognitive styles in the context of modern psychology: toward an integrated framework of cognitive style. Psychol Bull 133: 464-481. doi: 10.1037/0033-2909.133.3.464. PubMed: 17469987. [DOI] [PubMed] [Google Scholar]
- 6. Zhang L, Sternberg RJ, Sternberg R, Zhang LF (2001) Thinking styles across cultures: Their relationships with student learning. Perspectives on Thinking, learning and cognitive styles. London: Lawrence Erlbaum Associates. [Google Scholar]
- 7. Hayes J, Allinson CW (1994) Cognitive style and its relevance for management practice. Br J Manage 5: 53-71. doi: 10.1111/j.1467-8551.1994.tb00130.x. [DOI] [Google Scholar]
- 8. O'Connor KP, Shaw JC (1978) Field dependence, laterality and the EEG. Biol Psychol 6: 93-109. doi: 10.1016/0301-0511(78)90049-2. PubMed: 647092. [DOI] [PubMed] [Google Scholar]
- 9. Oltman PK, Semple C, Goldstein L (1979) Cognitive style and interhemispheric differentiation in the EEG. Neuropsychologia 17: 699-702. doi: 10.1016/0028-3932(79)90046-0. PubMed: 522985. [DOI] [PubMed] [Google Scholar]
- 10. Milne E, Szczerbinski M (2009) Global and local perceptual style, field-independence, and central coherence: An attempt at concept validation. Adv Cogn Psychol 5: 1-26. doi: 10.2478/v10053-008-0062-8. PubMed: 20523847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manjaly ZM, Marshall JC, Stephan KE, Gurd JM, Zilles K et al. (2003) In search of the hidden: an fMRI study with implications for the study of patients with autism and with acquired brain injury. Neuroimage 19: 674-683. doi: 10.1016/S1053-8119(03)00095-8. PubMed: 12880798. [DOI] [PubMed] [Google Scholar]
- 12. Lee PS, Foss-Feig J, Henderson JG, Kenworthy LE, Gilotty L et al. (2007) Atypical neural substrates of Embedded Figures Task performance in children with Autism Spectrum Disorder. NeuroImage 38: 184-193. doi: 10.1016/j.neuroimage.2007.07.013. PubMed: 17707658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Manjaly ZM, Bruning N, Neufang S, Stephan KE, Brieber S et al. (2007) Neurophysiological correlates of relatively enhanced local visual search in autistic adolescents. NeuroImage 35: 283-291. doi: 10.1016/j.neuroimage.2006.11.036. PubMed: 17240169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walter E, Dassonville P (2011) Activation in a frontoparietal cortical network underlies individual differences in the performance of an embedded figures task. PLOS ONE 6: e20742. doi: 10.1371/journal.pone.0020742. PubMed: 21799729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Damarla SR, Keller TA, Kana RK, Cherkassky VL, Williams DL et al. (2010) Cortical underconnectivity coupled with preserved visuospatial cognition in autism: Evidence from an fMRI study of an embedded figures task. Autism Res 3: 273-279. doi: 10.1002/aur.153. PubMed: 20740492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ring HA, Baron-Cohen S, Wheelwright S, Williams SC, Brammer M et al. (1999) Cerebral correlates of preserved cognitive skills in autism: a functional MRI study of embedded figures task performance. Brain 122 ( 7): 1305-1315. doi: 10.1093/brain/122.7.1305. PubMed: 10388796. [DOI] [PubMed] [Google Scholar]
- 17. Shah A, Frith U (1983) An islet of ability in autistic children: a research note. J Child Psychol Psychiatry 24: 613-620. doi: 10.1111/j.1469-7610.1983.tb00137.x. PubMed: 6630333. [DOI] [PubMed] [Google Scholar]
- 18. Happé F, Frith U (2006) The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord 36: 5-25. doi: 10.1007/s10803-005-0039-0. PubMed: 16450045. [DOI] [PubMed] [Google Scholar]
- 19. Jolliffe T, Baron-Cohen S (1997) Are people with autism and Asperger syndrome faster than normal on the Embedded Figures Test? J Child Psychol Psychiatry 38: 527-534. doi: 10.1111/j.1469-7610.1997.tb01539.x. PubMed: 9255696. [DOI] [PubMed] [Google Scholar]
- 20. Happé F (1999) Autism: cognitive deficit or cognitive style? Trends Cogn Sci 3: 216-222. doi: 10.1016/S1364-6613(99)01318-2. PubMed: 10354574. [DOI] [PubMed] [Google Scholar]
- 21. Happé F, Frith U (2006) The weak coherence account: Detail-focused cognitive style in autism spectrum disorders. J Autism Dev Disord 36: 5-25. doi: 10.1007/s10803-005-0039-0. PubMed: 16450045. [DOI] [PubMed] [Google Scholar]
- 22. Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J et al. (2013) Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14: 1-12. PubMed: 23232605. [DOI] [PubMed] [Google Scholar]
- 23. Faterson HF, Witkin HA (1970) Longitudinal study of development of the body concept. Dev Psychol 2: 429-438. doi: 10.1037/h0029167. [DOI] [Google Scholar]
- 24. Witkin HA, Goodenough DR, Karp SA (1967) Stability of cognitive style from childhood to young adulthood. J Pers Soc Psychol 7: 291-300. doi: 10.1037/h0025070. PubMed: 6065857. [DOI] [PubMed] [Google Scholar]
- 25. Szatmari P, Paterson AD, Zwaigenbaum L, Roberts W, Brian J et al. (2007) Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat Genet 39: 319-328. doi: 10.1038/ng1985. PubMed: 17322880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Losh M, Piven J (2007) Social-cognition and the broad autism phenotype: identifying genetically meaningful phenotypes. J Child Psychol Psychiatry 48: 105-112. doi: 10.1111/j.1469-7610.2006.01594.x. PubMed: 17244276. [DOI] [PubMed] [Google Scholar]
- 27. Baron-Cohen S, Hammer J (1997) Parents of children with Asperger syndrome: What is the cognitive phenotype? J Cogn Neurosci 9: 548-554. doi: 10.1162/jocn.1997.9.4.548. PubMed: 23968217. [DOI] [PubMed] [Google Scholar]
- 28. Raichle ME (2010) Two views of brain function. Trends Cogn Sci 14: 180-190. doi: 10.1016/j.tics.2010.01.008. PubMed: 20206576. [DOI] [PubMed] [Google Scholar]
- 29. Pessoa L, Gutierrez E, Bandettini P, Ungerleider L (2002) Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron 35: 975-987. doi: 10.1016/S0896-6273(02)00817-6. PubMed: 12372290. [DOI] [PubMed] [Google Scholar]
- 30. Baldassarre A, Lewis CM, Committeri G, Snyder AZ, Romani GL et al. (2012) Individual variability in functional connectivity predicts performance of a perceptual task. Proc Natl Acad Sci U S A 109: 3516-3521. doi: 10.1073/pnas.1113148109. PubMed: 22315406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang YY, Rothbart MK, Posner MI (2012) Neural correlates of establishing, maintaining, and switching brain states. Trends Cogn Sci 16: 330-337. doi: 10.1016/j.tics.2012.05.001. PubMed: 22613871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8: 700-711. doi: 10.1038/nrn2201. PubMed: 17704812. [DOI] [PubMed] [Google Scholar]
- 33. Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA et al. (2001) Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. AJNR Am J Neuroradiol 22: 1326-1333. PubMed: 11498421. [PMC free article] [PubMed] [Google Scholar]
- 34. Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ et al. (2007) Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev 29: 83-91. doi: 10.1016/j.braindev.2006.07.002. PubMed: 16919409. [DOI] [PubMed] [Google Scholar]
- 35. Huang XQ, Lui S, Deng W, Chan RC, Wu QZ et al. (2010) Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. Neuroimage 49: 2901-2906. doi: 10.1016/j.neuroimage.2009.11.072. PubMed: 19963069. [DOI] [PubMed] [Google Scholar]
- 36. Goodyear BG, Douglas EA (2009) Decreasing task-related brain activity over repeated functional MRI scans and sessions with no change in performance: implications for serial investigations. Exp Brain Res 192: 231-239. doi: 10.1007/s00221-008-1574-7. PubMed: 18818908. [DOI] [PubMed] [Google Scholar]
- 37. Landau SM, Schumacher EH, Garavan H, Druzgal TJ, D'Esposito M (2004) A functional MRI study of the influence of practice on component processes of working memory. NeuroImage 22: 211-221. doi: 10.1016/j.neuroimage.2004.01.003. PubMed: 15110011. [DOI] [PubMed] [Google Scholar]
- 38. Haier RJ, Karama S, Leyba L, Jung RE (2009) MRI assessment of cortical thickness and functional activity changes in adolescent girls following three months of practice on a visual-spatial task. BMC Res Notes 2: 174. doi: 10.1186/1756-0500-2-174. PubMed: 19723307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Han Y, Lui S, Kuang W, Lang Q, Zou L et al. (2012) Anatomical and functional deficits in patients with amnestic mild cognitive impairment. PLOS ONE 7: e28664. doi: 10.1371/journal.pone.0028664. PubMed: 22319555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ashburner J, Friston KJ (2000) Voxel-based morphometry--the methods. NeuroImage 11: 805-821. doi: 10.1016/S1053-8119(00)91734-8. PubMed: 10860804. [DOI] [PubMed] [Google Scholar]
- 41. Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY et al. (2008) An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods 172: 137-141. doi: 10.1016/j.jneumeth.2008.04.012. PubMed: 18501969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Witkin HA (1971) A manual for the embedded figures tests. Consulting Psychologists Press. [Google Scholar]
- 43. Xie J, Zhang H (1988) Cognitive style: experimental study on a personality dimension. Beijing Normal University Press. [Google Scholar]
- 44. Raven JC (1938) Progressive matrices: A perceptual test of intelligence, Sets A, B, C, D, and E.London:, Lewis Publishing House. [Google Scholar]
- 45. Li D, Hu KD, Chen GP, Jin Y, Li M (1988) The testing results report on the combined Raven's test in Shanghai. Psychol Sci, 4: 27-31. [Google Scholar]
- 46. Wang D, Qian M (1989) The revised report of the combined Raven's test in countryside of china. Reports of the psychological science, 5, 23-27.
- 47. Wang D, Di M, Qian M (2007) A report on the third revision of combined raven's test (CRT- C3) for children in China. Chin J Clin Psychol,15: 559-568. [Google Scholar]
- 48. Ashburner J (2007) A fast diffeomorphic image registration algorithm. NeuroImage 38: 95-113. doi: 10.1016/j.neuroimage.2007.07.007. PubMed: 17761438. [DOI] [PubMed] [Google Scholar]
- 49. Ridgway GR, Omar R, Ourselin Sb, Hill DL, Warren JD, et al (2009) Issues with threshold masking in voxel-based morphometry of atrophied brains. NeuroImage 44: 99-111. doi: 10.1016/j.neuroimage.2008.08.045. PubMed: 18848632. [DOI] [PubMed] [Google Scholar]
- 50. Genovese CR, Lazar NA, Nichols T (2002) Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15: 870-878. doi: 10.1006/nimg.2001.1037. PubMed: 11906227. [DOI] [PubMed] [Google Scholar]
- 51. Chao-Gan Y, Yu-Feng Z (2010) DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci 4: 13 PubMed: 20577591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG et al. (2010) The oscillating brain: complex and reliable. Neuroimage 49: 1432-1445. doi: 10.1016/j.neuroimage.2009.09.037. PubMed: 19782143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ledberg A, Akerman S, Roland PE (1998) Estimation of the probabilities of 3D clusters in functional brain images. Neuroimage 8: 113-128. doi: 10.1006/nimg.1998.0336. PubMed: 9740755. [DOI] [PubMed] [Google Scholar]
- 54. O'Boyle MW, Cunnington R, Silk TJ, Vaughan D, Jackson G et al. (2005) Mathematically gifted male adolescents activate a unique brain network during mental rotation. Cogn Brain Res 25: 583-587. doi: 10.1016/j.cogbrainres.2005.08.004. PubMed: 16150579. [DOI] [PubMed] [Google Scholar]
- 55. Wanzel KR, Anastakis DJ, McAndrews MP, Grober ED, Sidhu RS et al. (2007) Visual–spatial ability and fMRI cortical activation in surgery residents. Am J Surg 193: 507-510. doi: 10.1016/j.amjsurg.2006.11.011. PubMed: 17368300. [DOI] [PubMed] [Google Scholar]
- 56. Desco M, Navas-Sanchez FJ, Sanchez-González J, Reig S, Robles O et al. (2011) Mathematically gifted adolescents use more extensive and more bilateral areas of the fronto-parietal network than controls during executive functioning and fluid reasoning tasks. NeuroImage 57: 281-292. doi: 10.1016/j.neuroimage.2011.03.063. PubMed: 21463696. [DOI] [PubMed] [Google Scholar]
- 57. Weiss MM, Wolbers T, Peller M, Witt K, Marshall L et al. (2009) Rotated alphanumeric characters do not automatically activate frontoparietal areas subserving mental rotation. NeuroImage 44: 1063-1073. doi: 10.1016/j.neuroimage.2008.09.042. PubMed: 18977449. [DOI] [PubMed] [Google Scholar]
- 58. Harris IM, Egan GF, Sonkkila C, Tochon-Danguy HJ, Paxinos G et al. (2000) Selective right parietal lobe activation during mental rotation A parametric PET study. Brain 123: 65-73. doi: 10.1093/brain/123.1.65. PubMed: 10611121. [DOI] [PubMed] [Google Scholar]
- 59. Kovács I, Kozma P, Fehér Á, Benedek G (1999) Late maturation of visual spatial integration in humans. Proc Natl Acad Sci USA 96: 12204-12209. doi: 10.1073/pnas.96.21.12204. PubMed: 10518600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weissman DH, Woldorff MG (2005) Hemispheric asymmetries for different components of global/local attention occur in distinct temporo-parietal loci. Cereb Cortex 15: 870-876. doi: 10.1093/cercor/bhh187. PubMed: 15459080. [DOI] [PubMed] [Google Scholar]
- 61. Bölte S, Holtmann M, Poustka F, Scheurich A, Schmidt L (2007) Gestalt perception and local-global processing in high-functioning autism. J Autism Dev Disord 37: 1493-1504. doi: 10.1007/s10803-006-0231-x. PubMed: 17029017. [DOI] [PubMed] [Google Scholar]
- 62. Fink GR, Dolan RJ, Halligan PW, Marshall JC, Frith CD (1997) Space-based and object-based visual attention: shared and specific neural domains. Brain 120 ( 11): 2013-2028. doi: 10.1093/brain/120.11.2013. PubMed: 9397018. [DOI] [PubMed] [Google Scholar]
- 63. Frith U (1989) Autism: Explaining the enigma. Wiley Online Library. [Google Scholar]
- 64. Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E et al. (1995) Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med 25: 63-77. doi: 10.1017/S0033291700028099. PubMed: 7792363. [DOI] [PubMed] [Google Scholar]
- 65. Boddaert N, Chabane N, Gervais H, Good CD, Bourgeois M et al. (2004) Superior temporal sulcus anatomical abnormalities in childhood autism: a voxel-based morphometry MRI study. NeuroImage 23: 364-369. doi: 10.1016/j.neuroimage.2004.06.016. PubMed: 15325384. [DOI] [PubMed] [Google Scholar]
- 66. McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY et al. (2005) Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain 128: 268-276. PubMed: 15548557. [DOI] [PubMed] [Google Scholar]
- 67. Hazlett HC, Poe MD, Gerig G, Smith RG, Piven J (2006) Cortical gray and white brain tissue volume in adolescents and adults with autism. Biol Psychiatry 59: 1-6. doi: 10.1016/j.biopsych.2005.06.015. PubMed: 16139816. [DOI] [PubMed] [Google Scholar]
- 68. Lotspeich LJ, Kwon H, Schumann CM, Fryer SL, Goodlin-Jones BL et al. (2004) Investigation of neuroanatomical differences between autism and Asperger syndrome. Arch Gen Psychiatry 61: 291–298. doi: 10.1001/archpsyc.61.3.291. PubMed: 14993117. [DOI] [PubMed] [Google Scholar]
- 69. Nickl-Jockschat T, Habel U, Maria Michel T, Manning J, Laird AR et al. (2012) Brain structure anomalies in autism spectrum disorder—a meta-analysis of VBM studies using anatomic likelihood estimation. Hum Brain Mapp 33: 1470-1489. doi: 10.1002/hbm.21299. PubMed: 21692142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liston C, McEwen BS, Casey BJ (2009) Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA 106: 912-917. doi: 10.1073/pnas.0807041106. PubMed: 19139412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fair DA, Dosenbach NU, Church JA, Cohen AL, Brahmbhatt S et al. (2007) Development of distinct control networks through segregation and integration. Proc Natl Acad Sci USA 104: 13507-13512. doi: 10.1073/pnas.0705843104. PubMed: 17679691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK et al. (2007) Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA 104: 11073-11078. doi: 10.1073/pnas.0704320104. PubMed: 17576922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Buckholtz JW, Meyer-Lindenberg A (2012) Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron 74: 990-1004. doi: 10.1016/j.neuron.2012.06.002. PubMed: 22726830. [DOI] [PubMed] [Google Scholar]
- 74. Linden DE, Bittner RA, Muckli L, Waltz JA, Kriegeskorte N et al. (2003) Cortical capacity constraints for visual working memory: dissociation of fMRI load effects in a fronto-parietal network. Neuroimage 20: 1518-1530. doi: 10.1016/j.neuroimage.2003.07.021. PubMed: 14642464. [DOI] [PubMed] [Google Scholar]
- 75. Todd JJ, Marois R (2004) Capacity limit of visual short-term memory in human posterior parietal cortex. Nature 428: 751-754. doi: 10.1038/nature02466. PubMed: 15085133. [DOI] [PubMed] [Google Scholar]
- 76. Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL (2000) Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat Neurosci 3: 292-297. doi: 10.1038/73009. PubMed: 10700263. [DOI] [PubMed] [Google Scholar]
- 77. Martens U, Hübner R (2013) Functional hemispheric asymmetries of global/local processing mirrored by the steady-state visual evoked potential. Brain Cogn 81: 161-166. doi: 10.1016/j.bandc.2012.11.005. PubMed: 23246827. [DOI] [PubMed] [Google Scholar]
- 78. Fink GR, Marshall JC, Halligan PW, Dolan RJ (1999) Hemispheric asymmetries in global/local processing are modulated by perceptual salience. Neuropsychologia 37: 31-40. PubMed: 9920469. [DOI] [PubMed] [Google Scholar]
- 79. Martinez A, Moses P, Frank L, Buxton R, Wong E et al. (1997) Hemispheric asymmetries in global and local processing: evidence from fMRI. Neuroreport 8: 1685-1689. doi: 10.1097/00001756-199705060-00025. PubMed: 9189915. [DOI] [PubMed] [Google Scholar]
- 80. Kimchi R (1992) Primacy of wholistic processing and global/local paradigm: a critical review. Psychol Bull 112: 24-38. doi: 10.1037/0033-2909.112.1.24. PubMed: 1529037. [DOI] [PubMed] [Google Scholar]
- 81. Botvinick MM, Cohen JD, Carter CS (2004) Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci 8: 539-546. doi: 10.1016/j.tics.2004.10.003. PubMed: 15556023. [DOI] [PubMed] [Google Scholar]
- 82. Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004) The role of the medial frontal cortex in cognitive control. Science 306: 443-447. doi: 10.1126/science.1100301. PubMed: 15486290. [DOI] [PubMed] [Google Scholar]
- 83. Narayanan NS, Laubach M (2006) Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron 52: 921-931. doi: 10.1016/j.neuron.2006.10.021. PubMed: 17145511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Horst NK, Laubach M (2012) Working with memory: evidence for a role for the medial prefrontal cortex in performance monitoring during spatial delayed alternation. J Neurophysiol 108: 3276-3288. doi: 10.1152/jn.01192.2011. PubMed: 23019007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Brass M, Wenke D, Spengler S, Waszak F (2009) Neural correlates of overcoming interference from instructed and implemented stimulus-response associations. J Neurosci 29: 1766-1772. doi: 10.1523/JNEUROSCI.5259-08.2009. PubMed: 19211883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cohen MX, Ridderinkhof KR, Haupt S, Elger CE, Fell J (2008) Medial frontal cortex and response conflict: evidence from human intracranial EEG and medial frontal cortex lesion. Brain Res 1238: 127-142. doi: 10.1016/j.brainres.2008.07.114. PubMed: 18760262. [DOI] [PubMed] [Google Scholar]
- 87. Lux S, Marshall JC, Ritzl A, Weiss PH, Pietrzyk U et al. (2004) A functional magnetic resonance imaging study of local/global processing with stimulus presentation in the peripheral visual hemifields. Neuroscience 124: 113-120. doi: 10.1016/j.neuroscience.2003.10.044. PubMed: 14960344. [DOI] [PubMed] [Google Scholar]
- 88. Weissman DH, Giesbrecht B, Song AW, Mangun GR, Woldorff MG (2003) Conflict monitoring in the human anterior cingulate cortex during selective attention to global and local object features. Neuroimage 19: 1361-1368. doi: 10.1016/S1053-8119(03)00167-8. PubMed: 12948694. [DOI] [PubMed] [Google Scholar]
- 89. Liu Y, Cherkassky VL, Minshew NJ, Just MA (2011) Autonomy of lower-level perception from global processing in autism: evidence from brain activation and functional connectivity. Neuropsychologia 49: 2105-2111. doi: 10.1016/j.neuropsychologia.2011.04.005. PubMed: 21513720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Russell-Smith SN, Maybery MT, Bayliss DM, Sng AA (2012) Support for a link between the local processing bias and social deficits in autism: an investigation of embedded figures test performance in non-clinical individuals. J Autism Dev Disord 42: 2420-2430. doi: 10.1007/s10803-012-1506-z. PubMed: 22434280. [DOI] [PubMed] [Google Scholar]
- 91. Almeida RA, Dickinson JE, Maybery MT, Badcock JC, Badcock DR (2010) A new step towards understanding Embedded Figures Test performance in the autism spectrum: The radial frequency search task. Neuropsychologia 48: 374-381. doi: 10.1016/j.neuropsychologia.2009.09.024. PubMed: 19786040. [DOI] [PubMed] [Google Scholar]
- 92. Best CS, Moffat VJ, Power MJ, Owens DG, Johnstone EC (2008) The boundaries of the cognitive phenotype of autism: Theory of mind, central coherence and ambiguous figure perception in young people with autistic traits. J Autism Dev Disord 38: 840-847. doi: 10.1007/s10803-007-0451-8. PubMed: 18004653. [DOI] [PubMed] [Google Scholar]
- 93. Constantino JN, Todd RD (2005) Intergenerational transmission of subthreshold autistic traits in the general population. Biol Psychiatry 57: 655-660. doi: 10.1016/j.biopsych.2004.12.014. PubMed: 15780853. [DOI] [PubMed] [Google Scholar]
- 94. Kennedy DP, Redcay E, Courchesne E (2006) Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci USA 103: 8275-8280. doi: 10.1073/pnas.0600674103. PubMed: 16702548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kennedy DP, Courchesne E (2008) The intrinsic functional organization of the brain is altered in autism. Neuroimage 39: 1877-1885. doi: 10.1016/j.neuroimage.2007.10.052. PubMed: 18083565. [DOI] [PubMed] [Google Scholar]
- 96. Kunihira Y, Senju A, Dairoku H, Wakabayashi A, Hasegawa T (2006) ‘Autistic traits in non-autistic Japanese populations: Relationships with personality traits and cognitive ability. JAutism Dev Disord 36: 553-566. [DOI] [PubMed] [Google Scholar]
- 97. Lombardo MV, Barnes JL, Wheelwright SJ, Baron-Cohen S (2007) Self-referential cognition and empathy in autism. PLOS ONE 2: e883. doi: 10.1371/journal.pone.0000883. PubMed: 17849012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. von dem Hagen EA, Nummenmaa L, Yu R, Engell AD, Ewbank MP et al. (2011) Autism spectrum traits in the typical population predict structure and function in the posterior superior temporal sulcus. Cereb Cortex 21: 493-500. doi: 10.1093/cercor/bhq062. PubMed: 20439317. [DOI] [PMC free article] [PubMed] [Google Scholar]