Graphical abstract

Highlights
► The dicationic arylimidamide DB745 is highly active against Neospora caninum tachyzoites in vitro. ► The drug inhibits host cell invasion and intracellular proliferation. ► DB745 interferes in the structural integrity of the parasite plasma membrane and the nucleolus. ► The two N. caninum isolates Nc-1 and Nc-Liverpool differ in their ability to adapt to DB745 in vitro. ► In vivo treatment of Nc-1 infected mice with DB745 reduces clinical signs and cerebral parasite load.
Keywords: Neospora caninum, Arylimidamides, Tachyzoites, Proliferation, Invasion, Nucleolus, Membrane integrity, In vivo activity
Abstract
Neospora caninum is considered to be the main cause of bovine abortion in Europe and the USA, leading to considerable financial impact. Losses are caused directly by abortions or indirectly through breeding of calves with impaired viability. Due to the lack of effective chemotherapy against bovine neosporosis, there is a need to develop new anti-protozoal compounds, which would either eliminate the parasite or avoid its transmission. In order to identify compounds of interest, the in vitro activities of 41 di-cationic pentamidine derivatives were studied employing a transgenic N. caninum clone expressing beta-galactosidase as a reporter gene. The arylimidamide DB745, previously shown to be highly active against Leishmania donovani in vitro and in vivo, appeared as the most promising compound, with an IC50 of 80 nM in 3-day growth assays and severely affecting both host cell invasion as well as intracellular proliferation. TEM of intracellular tachyzoites identified distinct alterations related to the nucleolus and the nuclear and cellular membrane. Long-term growth assays showed that DB745 acted parasiticidal upon the Nc-Liv isolate, but not against the Nc-1 isolate of N. caninum. In vivo studies in N. caninum (Nc-1 isolate) infected mice showed that daily intraperitoneal application of DB745 for a period of 14 days resulted in a decreased number of clinically affected animals, and lower cerebral parasite burdens in DB745-treated mice compared to non-treated mice. These results illustrate the potential of dicationic arylimidamides for the treatment of N. caninum infections.
1. Introduction
The obligatory intracellular apicomplexan parasite Neospora caninum represents a major cause of stillbirth, abortion, or birth of weak calves all around the planet, with considerable economic implications. Besides abortion, other factors such as reduced milk yield, premature culling and reduced post-weaning weight gain in beef calves have been published, although conflicting results regarding the importance of milk yield and weight gain have been reported (Dubey and Schares, 2011). Due to the large economic impact of neosporosis especially in cattle, considerable efforts have been put into research on accurate diagnosis, prevention and treatment of N. caninum infection (Dubey et al., 2007). Vaccination and chemotherapy have been identified as economically promising options for intervention, provided that suitable targets and effective reagents are developed (Häsler et al., 2006a,b; Monney et al., 2011a).
Infection by N. caninum in herbivores such as cattle occurs in two ways: on one hand through oral uptake of oocysts containing infective sporozoites, which are shed by canine definitive hosts and subsequently disseminate and differentiate into the proliferative tachyzoite stage, or through transplacental infection by rapidly proliferating tachyzoites from dam to the fetus. In an immuno-competent host, the proliferative tachyzoite stage is replaced by the quiescent bradyzoites at the onset of the host immune response, and bradyzoites then form tissue cysts predominantly located in the central nervous system (CNS) and muscle tissue, where they do not cause apparent immunopathology and can persist for many years, up to a lifetime (Hemphill et al., 2006; Dubey et al., 2007). During pregnancy, and associated immunomodulation, recrudescence can occur, followed by bradyzoite-to-tachyzoite interconversion, and subsequent endogenous trans-placental infection represents the main source of neosporosis in cattle.
The chemotherapeutical options for the treatment of N. caninum infections are limited. The main problem lies in the fact that an effective compound should be able to cross the blood–brain barrier and also affect the encysted bradyzoites stage in the chronic phase of infection. In addition, cost-effectiveness and potential residues in meat or milk are important issues. In the past, a wide range of compounds were investigated in vitro, such as lasalocid, monensin, pirithrexim, pyrimethamine, clindamycin, robenidine and trimethoprim (Lindsay et al., 1994), artemisinin (Kim et al., 2002), depudecin (Kwon et al., 2003), toltrazuril, ponazuril (Darius et al., 2004), nitazoxanide and other nitro-and bromo-thiazolides (Esposito et al., 2005, 2007a,b), alcoholic herbal extracts (Youn et al., 2004), di-cationic arylimidamides (Leepin et al., 2008), and miltefosine (Debache and Hemphill, 2012). Only few drugs have been used in vivo. In clinical settings, clindamycin, potentiated sulphonamides and pyrimethamine, drugs that are in use the treatment of toxoplasmosis caused by the closely related Toxoplasma gondii, were successful in eliminating clinical signs in 10 of 27 cases of canine neosporosis (Barber and Trees, 1996). Other compounds have been experimentally evaluated in small animal models, including sulfadiazine and amprolium in mice, which did not eliminate the parasite (Lindsay and Dubey, 1990). Inclusion of toltrazuril into the drinking water eliminated parasites in the central nervous system of C57Bl/6 mice, but cell-mediated immunity was required to achieve its full efficacy (Ammann et al., 2004). Toltrazuril treatment also controlled dia-placental N. caninum transmission in experimentally infected pregnant mice (Gottstein et al., 2005; Strohbusch et al., 2009). In addition, studies on prophylactic toltrazuril administration in newborn calves suggested that this treatment regime could exhibit a certain degree of protective efficacy (Härdi et al., 2006; Kritzner et al., 2002). Intragastric application of miltefosine was shown to be effective in reducing clinical signs of neosporosis in Balb/c mice and resulted in reduced cerebral parasite burden (Debache and Hemphill, 2012).
Pentamidine and its analogues represent a class of broad-spectrum antimicrobial compounds, with activities against a wide range of intracellular and extracellular protozoan parasites (Buckner and Navabi, 2010; Soeiro et al., 2005; Wilson et al., 2008). Since its discovery, pentamidine has been successfully applied to treat African trypanosomiasis, leishmaniasis, and malaria in humans, and the pentamidine-derivative diminazene aceturate is commonly used for trypanosome chemotherapy in livestock (Werbovetz, 2006). More recently, novel analogues, known as arylimidamides, with more favorable pharmacokinetic profiles, improved bioavailability and lower host toxicity were shown to be effective against Leishmania donovani, Trypanosoma cruzi, T. gondii and Besnoitia besnoiti (Batista et al., 2010a,b; Cortes et al., 2011; De Souza et al., 2011; Kropf et al., 2012; Wang et al., 2010). Earlier reports had demonstrated good in vitro efficacy of DB750 and related compounds against N. caninum, with IC50 levels of at submicromolar concentrations ranging between 0.16 and 0.66 μM, indicating the potential of these di-cationic compounds for chemotherapeutical purposes. Debache et al. (2011) showed that treatment of N. caninum-infected mice with the diamidine DB750 led to a significant reduction of clinical signs of disease and cerebral parasite burden, indicating that this compound could cross the blood–brain barrier. Here, we further explored the in vitro and in vivo characteristics of a set of related di-cationic compounds, and identified the arylimidamide DB745 as an interesting candidate for further development to treat N. caninum infections.
2. Materials and methods
Unless otherwise stated, all tissue culture media were purchased from Gibco-BRL (Zurich, Switzerland) and biochemical reagents were from Sigma (St. Louis, MO). The di-cationic compounds used in this study were synthesized at the Department of Chemistry and Center for Biotechnology and Drug Design, Georgia State University, USA, or at the Department of Chemistry and Physics, Augusta State University, USA. They were kept as dry powder or as stock solutions of 1 mg/ml in dimethyl sulfoxide and were stored at −20 °C.
2.1. Cell culture and parasite purification
Vero cells were maintained in RPMI 1640 medium supplemented with 5% foetal calf serum (FCS), 2 mM l-glutamine, 50 U of penicillin/ml, and 50 μg of streptomycin/ml at 37 °C with 5% CO2 in tissue culture flasks and were trypsinized 3 times a week. Human foreskin fibroblasts (HFF) were maintained in Dulbecco’s modified Eagles’s medium (DMEM) with 10% FCS, 50 U of penicillin/ml, and 50 μg of streptomycin/ml at 37 °C with 5% CO2 in tissue culture flasks. Cultures were trypsinized once a week. Transgenic N. caninum Nc-1 (Nc-1 isolate) expressing beta-galactosidase (N. caninum-beta-Gal), wildtype N. caninum Nc-1 and N. caninum Nc-Liv (Liverpool-isolate) were cultured in Vero cells as described earlier (Hemphill et al., 1996). Intracellular parasites were harvested by trypsinization of infected Vero Cells, followed by repeated passages through a 25-gauge needle at 4 °C, and separation from cell debris on a Sephadex-G25 column (Hemphill et al., 1996). Purified tachyzoites were used to infect HFF monolayers as described below.
2.2. In vitro drug assays employing N. caninum-beta-Gal
Drugs were tested for activity against N. caninum-beta-Gal tachyzoites in 3 day growth assays employing flat-bottomed 96 well tissue culture plates (Sarstedt, Sevelen, Switzerland), essentially as described for T. gondii expressing beta-galactosidase (McFadden et al., 1997; Kropf et al., 2012). 5 × 103 HFF/well were cultured in phenol-red free DMEM medium at 37 °C and 5% CO2 until they had grown to confluency. Prior to drug treatment, the old medium was removed and 100 μl of phenol-red free DMEM medium containing 1 × 103 freshly harvested N. caninum-beta-Gal tachyzoites were distributed per well and placed at 37 °C/5% CO2. After 1 h, the drugs were added in desired amounts (1 μM for initial screening and 1 μM in 1:2 dilutions down to 0.0625 μM for IC50 determination) in 100 μl phenol-red free DMEM medium. Controls included non-drug-treated infected HFF monolayers and non-infected HFF. Eight wells were used per experimental assay, and 8 wells remained untreated in order to serve as a positive control. The plates were then incubated again at 37 °C and 5% CO2. After 72 h, the plates were centrifuged at 50g for 5 min and the medium was removed. Subsequently, 90 μl of PBS containing 0.05% of Triton X-100 were added per well and air bubbles were removed with a 25 G needle. The plate was then first test-read in a 96-well plate reader (VersaMax multiplate reader, Bucher Biotec, Basel, Switzerland). Subsequently, 10 μl of 5 mM chlorophenol red-β-d-galactopyranoside (CPRG; Roche Diagnostics, Rotkreuz, Switzerland) solved in PBS were added per well. The absorption shift was measured at (A570) at various time points. The initial velocity (ΔA570/min) was proportional to the number of tachyzoites. IC50 values were calculated after the logit-log-transformation of the relative growth (RG; control = 1) according to the formula ln [(RG/(1 − RG)] = a × ln (drug concentration) + b and subsequent regression analysis by the corresponding software tool contained in the Excel software package (Microsoft, Seattle, WA, USA).
2.3. Assessments of host cell toxicity
Flat bottomed 96 well plates (Sarstedt) were seeded with 5 × 103 HFF cells/well in phenol-red free DMEM medium and incubated at 37 °C and 5% CO2 until they had grown to confluence. Subsequently, the old medium was removed and drugs (8 wells per experimental assay) were added in desired amounts. One row of 8 wells remained untreated as a blank and another row of 8 wells was treated with 0.5% Triton X-100 as a positive control. The 96-well plates were incubated at 37 °C and 5% CO2 for 72 h. After 72 h, 10 μl of 20× resazurin were added per well and the fluorescence (A590) was measured at various time points using the Wallac Victor2 1420 multilabel counter (Perkin Elmer, Schwerzenbach, Switzerland). Resazurin is a blue indicator dye that is non toxic and nonfluorescent. Living cells however gradually convert it to resorufin, which releases very strong red fluorescence. Therefore, the cells’ metabolic activity can be directly evaluated by measuring the generated amount of fluorescence because it is directly proportional to the number of viable cells.
2.4. Pretreatment of N. caninum-beta-Gal tachyzoites with DB745 prior to invasion of HFF
Flat-bottomed 96-well tissue culture plates (Sarstedt) were seeded with 5 × 103 HFF/well and these were grown to confluency in phenol-red-free DMEM medium at 37 °C and 5% CO2. Freshly purified N. caninum-beta-Gal tachyzoites were then pre-treated with 1 μM DB745 at 37 °C for 15 min, 30 min, 1 h and 2 h, respectively, or were not treated at all. Parasites were then added to the HFF monolayers at 1 × 103 parasites/well for 1 h at 37 °C/5% CO2, then the medium and non-adherent parasites were removed, and the wells were washed once with 200 μl medium. Finally, 200 μl of fresh medium without 1 μM DB745 was added. The infected cultures were maintained at 37 °C/5% CO2 for 3 days, and relative infection intensities were quantified by adding the substrate and measuring A570 as described above. All assays were done in 8 wells in parallel, and this experiment was repeated three times with essentially identical results.
2.5. Pre-treatment of HFF monolayers with DB745 prior to infection with N. caninum-beta-Gal tachyzoites
Assays were carried out in flat-bottomed 96-well plates seeded with 5 × 103 HFF/well and grown to confluency in phenol-red free DMEM medium. Half the monolayers were pre-treated with 1 μM DB745 in fresh medium over night, the other half received DMEM without compound. Subsequently the medium was removed and the monolayers were washed twice with 200 μl DMEM before infecting them with 1 × 103 parasites/well suspended in medium without DB750. Controls received 1 μM DB745 at 1 h post-infection as described above. Cultures were maintained for 72 h at 37 °C/5% CO2 and relative parasite proliferation was measured by spectrophotometry (A570). All assays were done in 8 wells in parallel, and the experiment was repeated three times with essentially identical results.
2.6. Transmission electron microscopy (TEM)
HFF monolayers grown in 25 cm2 tissue culture flasks were infected with N. caninum Nc-1 tachyzoites. At 2 days post-infection, treatments with 1 μM DB745 were initiated. Samples were collected 24, 48 and 72 h later, respectively, by removing the medium and washing the monolayers twice with 100 mM sodium cacodylate buffer (pH 7.2). Fixation was carried out in 100 mM sodium cacodylate buffer pH 7.3 containing 2.5% glutaraldehyde for 2 h at room temperature. Then specimens were washed twice with 100 mM cacodylate buffer, were scraped off with a rubber policeman, and centrifuged at 100g for 10 min at 4 °C. Postfixation was done in cacodylate buffer containing 2% OsO4 at 22 °C for 2 h. Subsequently, specimens were washed in water, and pre-stained in 1% uranyl acetate in water for 30 min, followed by an extensive wash in water. The samples were dehydrated in a graded series of ethanol (30%, 50%, 70%, 90%, and 100%), and were embedded in Epon 820 epoxy resin as described in Kropf et al. (2012). The resin was polymerized at 65 °C for 24 h. Ultrathin sections (∼80 nm) were cut on a Reichert and Jung ultramicrotome and were loaded onto 300-mesh copper grids (Plano GmbH, Marburg, Germany), and stained with uranyle acetate and lead citrate (Hemphill et al., 2004). Grids were viewed on a Philips 400 transmission electron microscope (TEM) operating at 80 kV.
2.7. In vitro adaptation of N. caninum Nc-1 and N. caninum Nc-Liv to increased concentrations of DB745 and assessment of parasiticidal activity
Confluent HFF monolayers grown in T25 tissue culture flask (Sarstedt) were infected with N. caninum (Nc-1 and Nc-Liv, respectively) and were exposed to successively increasing DB745 drug concentrations over time, starting with 0.02 μM, and further elevated stepwise every 3–4 days by 0.02 μM. Cultures were evaluated light microscopically on a daily basis, and if no further proliferation was noted, the drug concentration was not increased. Every 6–9 days, parasites were passaged onto fresh HFF by trypsinization of infected cultures, washing them in PBS, and adding them to fresh monolayers previously grown overnight. The maximum DB745 concentration used was 0.3 μM for Nc-1 and 0.22 μM for Nc-Liv. Experiments were terminated on day 111 of drug treatment.
2.8. In vivo experiment 1: DB745-treatment of Balb/c mice experimentally infected with N. caninum Nc-1 and drug application starting prior to infection
Thirty female Balb/c mice between 8 and 9 weeks of age were purchased from Charles River Laboratories (Sulzheim, Germany) and were maintained in a common room under controlled temperature and a 14 h dark/10 h light cycle according to the standards set up by the animal welfare legislation of the Swiss Veterinary Office. At day zero, mice were randomly caged into three experimental groups of 10 mice as outlined in Table 2. Enzyme-linked immunosorbent assay (ELISA) was carried out to ensure that mice were serologically Neospora-negative (Debache et al., 2010). Mice received either DB745 or DB750 2 mg/kg/day; (Debache et al., 2011) as suspensions in 0.5% carboxymethyle-cellulose in water (CMC) by intraperitoneal injection of a volume of 100 μl. The placebo group obtained the corresponding amount of the solvent only (see Table 2). The treatments were performed on a daily basis, starting at day 1. On day 3, all mice were infected by intraperitoneal injection of 2 × 106 freshly purified N. caninum (Nc-1) tachyzoites in medium. If not indicated otherwise, treatments continued until day 18 (day 14 post-infection), after which the mice were sacrificed by CO2-euthanasia. Mice exhibiting clinical signs of neosporosis (ruffled coat, apathy, hind limb paralysis) were euthanized at the onset of these clinical signs. Blood was taken by heart puncture for serological analysis and from each animal the brain was recovered, and was processed for DNA-extraction (Debache et al., 2010; Monney et al., 2011b).
Table 2.
Summary of in vivo experiments with respect to experimental groups, numbers of mice with disease symptoms, time of death and mean animal weights during treatments with DB745. Note that in Experiment 1 (treatment starting prior to infection) the diamidine DB750 was included as a positive control.
| Experimental groups & dosage (per kg/day) | nb | Symptomatic mice | Time of death (days p-i.) | Mean weight (g) at |
||
|---|---|---|---|---|---|---|
| Beginning | End | |||||
| Exp. 1 | Placebo | 10 | 3 | 8/8/10 | 20.14 ± 0.5 | 17.83 ± 0.78a |
| 2 mg DB745 | 10 | – | – | 19.96 ± 0.52 | 19.4 ± 0.96 | |
| 2 mg DB750 | 10 | – | – | 20.13 ± 0.72 | 19.81 ± 0.74 | |
| Exp. 2 | Placebo | 8 | 5 | 13/16/26/26/26 | 19.87 ± 0.64 | 18.9 ± 0.72 |
| 2.5 mg DB745 | 10 | 2b | 16/18 | 19.96 ± 0.50 | 19.25 ± 1.07 | |
| 7.5 mg DB745 | 9 | 3 | 15/20/26 | 19.55 ± 1.01 | 19.16 ± 0.98 | |
| 7.5 mg DB745 non inf. | 9 | – | – | 19.88 ± 0.70 | 19.85 ± 0.93 | |
Indicates significant weight loss in the placebo group (P < 0.05).
Indicates that the percentage of surviving mice in the DB745 treatment group was significantly higher than in the placebo group (P < 0.05).
2.9. In vivo experiment 2: DB745-treatment of Balb/c mice with drug application starting 14 days after infection with N. caninum Nc-1 tachyzoites
Forty female Balb/c mice were purchased and housed as described above. 9 mice remained uninfected, and on day 1 the remaining 31 mice were infected by intraperitoneal injection of 2 × 106 N. caninum (Nc-1) tachyzoites. Four of these mice exhibited clinical signs during the first 2 weeks and were euthanized. On day 14 post-infection, the remaining 27 mice were allocated into different treatment groups. One group (n = 10) received intraperitoneal injections of DB745 (2.5 mg/kg/day) for 14 days as above. The second group (n = 9) received intraperitoneal injections of DB745 (7.5 mg/kg/day) for 14 days, and the third group (n = 9) received DB745 (7.5 mg/kg/day) for 14 days, but was not infected. The fourth group (n = 8) was treated with solvent only. On day 28, all mice were sacrificed by CO2-euthanasia and brain and lung tissues were collected as above.
2.10. DNA extraction and assessment of cerebral parasite burden by real time PCR
DNA purification from in vitro cell culture and cerebral tissues was performed emoploying the DNeasy® Blood & Tissue Kit (Qiagen, Basel, Switzerland) according to the standard protocol suitable for animal tissues. The DNA concentrations in all samples were determined by UV spectrophotometry (NanoDrop™, Thermo Scientific, Delaware, USA) and adjusted to 100 ng/μl with sterile DNAse free water. The assessments of N. caninum tachyzoite loads were performed using the Rotor-Gene 6000 real-time PCR machine (Corbett Research, Qiagen). The parasite counts were calculated by interpolation from a standard curve with DNA equivalents from 1000, 100 and 10 parasites included in each run (Debache et al., 2010; Monney et al., 2011b).
2.11. Data analysis
Statistical analysis to test for differences among all independent groups was performed using a one way (ANOVA) analysis of variance test (ANOVA). The ANOVA test was applied in combination with Tukey’s HSD (Honestly Significant Difference) test, P values <0.01 and <0.05 were considered statistically highly significant and significant, respectively. Survival analysis in the in vivo experiment 2 was performed according to Kaplan–Meier method and the treatment group was compared with the corresponding placebo group by Cox regression, employing the open-source software package R (2009). Cerebral parasite burdens and viable parasite load in different treatment and placebo groups were statistically assessed by Kruskal–Wallis multiple comparison, followed by Duncan’s multiple range test to compare between two particular groups (P < 0.05). Monitoring of mouse weights were statistically evaluated by Ducan’s multiple range tests and a value of P < 0.05 was taken as statistically significant. All analyses of variances employed the NCSS Quick Start 2001 software.
3. Results
3.1. In vitro screening of di-cationic pentamidine derivatives against N. caninum tachyzoites
N. caninum-beta-Gal tachyzoites were used for screening of a series of selected di-cationic pentamidine derivatives (Wang et al., 2010; Kropf et al., 2012), including 10 di-arylimidamides (DB666, DB667, DB702, DB710, DB745, DB780, DB786, DB865, DB891, DB930), 6 diamidines (DB1282, DB1341, DB1362, DB1407, DB1450, DB1479), 15 newly generated mono-arylimidamides (DB1980, DB1996, DB1997, DB2001, DB2002, DB2006, DB2007, DB2018, DB2036, DB2045, DB2048, DB2074, DB2079, DB2081, DB2083), and 10 di-guanidino analogues (DB856, DB857, DB859, DB866, DB869, DB872, DB944, DB1127, DB2086, DB2091) all at an initial concentration of 1 μM. Evaluation of beta-galactosidase activity after 72 h of exposure to these drugs revealed that the mono-arylimidamides tested did not show any adverse effects on N. caninum proliferation nor on host cells, while the di-guanidino compounds exhibited more host cell toxicity and no or only very little anti-parasitic activities (data not shown). The highest proliferation-inhibitory effect and lowest host-cell toxicity was recorded for the di-arylimidamide DB745, which is closely related to the compound DB750 previously identified by Leepin et al. (2008) (Fig. 1A and C). Dose–response studies on DB745 resulted in an IC50 of 0.08 μM (±0.012), a value that was derived from three independent experiments, which was half of what was previously reported for DB750. A virtually complete inhibition of tachyzoite proliferation was noted at 0.5 μM (Fig. 1A). Assessment of host cell toxicity by light microscopical inspection of uninfected HFF monolayers exposed to increasing concentrations of DB745 (0, 0.5, 1, 2 or 3 μM) for 2 times 3 days revealed that treatments with 0.5–2 μM did not result in visible adverse reactions in HFF monolayers, while at 3 μM a 50% reduction in viable HFF was observed (data not shown). In addition, metabolic activities of HFF were measured by Alamar-blue assay after treatments with increasing DB745 concentrations (Fig. 1B), showing that DB745 influenced host cell viability in a dose dependent manner. At low concentrations (1.25 and 0.625 μM) the HFF metabolic activity was reduced by 15% compared to the untreated control. Concentrations over 5 μM impaired the viability of the host cells by 70–90%. Altogether, these findings render DB745 a promising candidate for additional experimental testing both in vitro and in vivo.
Fig. 1.
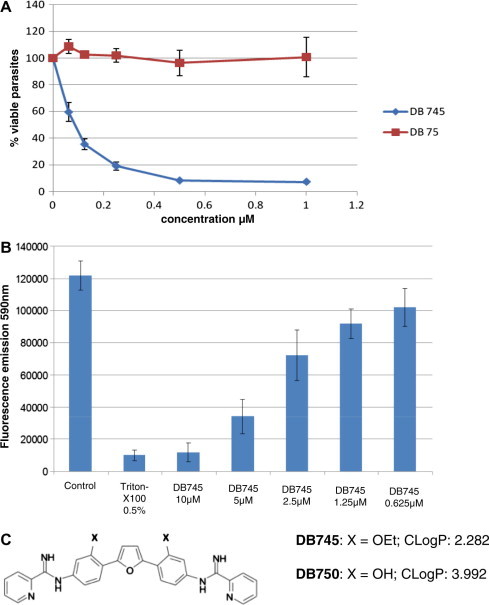
Dose–response applying DB745 and DB75 (as a negative control) for N. caninum and HFF monolayers. (A) Proliferation of transgenic N. caninum tachyzoites expressing beta-galactosidase in the presence of different concentrations of DB745 was monitored by addition of chlorophenol red-β-d-galactopyranoside and measuring the absorption shift at 570 nm. DB75 (furamidine) was included as a negative control. (B) The impact of different concentrations of DB745 on metabolic activity of HFF was measured by Alamar blue assay. Figures show the results of one out of three independent experiments with virtually identical outcome. Error bars represent standard deviations. (C) Comparison of DB745 and DB750 with regard to structure and calculated Log P values (cLog P). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. DB745-treatment of extracellular N. caninum-beta-Gal tachyzoites affected host cell invasion and proliferation of parasites
Further experiments aimed to investigate the effects of DB745 treatment on tachyzoite invasion and re-growth. N. caninum-beta-Gal tachyzoites were pre-incubated at 37 °C in the presence or absence of DB745 (1 μM) for several durations. They were then added to HFF monolayers for infection and the medium was removed after 1 h, and replaced with fresh medium without compound. After culture of 3 days, the number of viable parasites was evaluated. As can be seen in Fig. 2A, the presence of DB745 during the invasion process itself (0 min pre-treatment time and exposure to DB745 only during invasion) already had a profound inhibitory effect. This suggested that DB745 acted very efficiently on extracellular N. caninum tachyzoites and inhibited host cell invasion. Additional pretreatment of DB745 prior to invasion (30 min to 2 h) did not further decrease tachyzoite numbers, indicating that the drug exerted its action swiftly. Moreover, it was found that the parasite survival was significantly impaired (approximately 50% reduction) after only 30 min of extracellular incubation in DB745-free medium, meaning that the mere extracellular existence of N. caninum tachyzoites must already have had a harmful effect on parasite infectivity.
Fig. 2.

Effects of DB745 treatment of extracellular N. caninum tachyzoites and pretreatments of HFF monolayers with DB745 on host cell invasion and intracellular proliferation of N. caninum. (A) Extracellular N. caninum tachyzoites were pre-incubated in the presence or absence of 1 μM DB745 for different durations, were then allowed to invade HFF monolayers in the presence of the drug, and the ability to proliferate in the absence of DB745 was investigated by beta-galactosidase activity. Parasite proliferation was significantly inhibited in all DB745 pre-treated parasite samples. Significance of P < 0.01 is designated as ∗∗∗, P < 0.05 is written as ∗. (B) HFF monolayers grown to confluency were incubated with 1 μM of DB745 over night and subsequently infected with purified N. caninum tachyzoites of the Nc1 strain. Significance: P < 0.05 is marked with ∗, P < 0.01 is designated ∗∗∗. The ability to proliferate in the absence or presence of DB745 was investigated by beta-galactosidase activity.
3.3. DB745-treatment of HFF monolayers prior to infection with N. caninum tachyzoites inhibited parasite proliferation
In order to investigate potential host cell-related effects against parasite proliferation in vitro, HFF monolayers were incubated over night with 1 μM DB745, were thoroughly washed with fresh medium, and subsequently infected with N. caninum-beta-Gal tachyzoites. After 1 h, medium was changed and subsequently cultures were maintained either in the absence or presence of 1 μM DB745. The pre-treatment of HFF monolayers resulted in significantly reduced (approximately 60%) parasite proliferation in vitro even when subsequent culture during 3 days was carried out in the absence of the drug (Fig. 2B). This suggested that the host cells participated in the uptake or distribution of the compound in vitro. Moreover, additional treatment of the infected cells, starting 1 h after invasion, resulted in a cumulative inhibitory effect. Thus, DB745 treatment of HFF monolayers prior to infection severely inhibited intracellular proliferation of N. caninum tachyzoites.
3.4. In vitro adaptation of N. caninum tachyzoites to DB745
The potential of two isolates of N. caninum, Nc-1 and Nc-Liv, to adapt to DB745 treatment was explored by slowly adapting them to increasing drug concentrations. HFF monolayers grown in T25 tissue culture flasks and infected with 104 Nc-1 or Nc-Liv tachyzoites were initially subjected to 0.02 μM DB745, and the concentration was increased stepwise by 0.02 μM, typically every 3–8 days if not indicated otherwise (see Table 1). For Nc-1 tachyzoites, DB745 concentrations could be stepwise increased up to 0.30 μM until day 69. Proliferation stopped at this time point, and cultures were maintained at the same drug concentration until day 86, since no parasites could be detected anymore by light microscopy. Further maintenance of the cultures was done in medium without DB745, and recurrence of Nc-1 tachyzoites was noted between days 108 and 111. In contrast, Nc-Liv tachyzoites appeared to exhibit a lower adaptation potential compared to Nc-1, in that the DB745 concentration could be increased only to 0.22 μM until day 56, when proliferation ceased. Cultures were then further maintained in 0.22 μM DB745 for 13 days, and subsequently in medium without drug until day 111, but tachyzoites remained non-detectable by light microscopy. This indicated that DB745 acted parasiticidal for Nc-Liv, but not for Nc-1 tachyzoites in vitro.
Table 1.
Tachyzoites of the Nc-1 isolate, but not Nc-Liv tachyzoites, can adapt to stepwise increasing concentrations of DB745. N. caninum Nc-1 and Nc-Liv were inoculated into T25 tissue culture flasks containing confluent HFF monolayers, and cultured initially in the presence of 0.02 μM DB745. At indicated time points the medium was replaced with new medium containing either slightly elevated concentrations (+0.02 μM) of DB745, or (at the later time points) medium was replaced containing the drug concentration unchanged as indicated. Every 6–10 days cultures were trypsinized and seeded onto fresh HFF monolayers.
| Timepoints of treatment (days) | Nc-1 DB745 (μM) | N-Liv DB745 (μM) |
|---|---|---|
| 0 | 0.02 | 0.02 |
| 3 | 0.02 | 0.02 |
| 7 | 0.04 | 0.04 |
| 10 | 0.04 | 0.04 |
| 14 | 0.06 | 0.06 |
| 17 | 0.06 | 0.06 |
| 21 | 0.08 | 0.08 |
| 24 | 0.08 | 0.08 |
| 27 | 0.10 | 0.10 |
| 31 | 0.12 | 0.12 |
| 35 | 0.14 | 0.14 |
| 38 | 0.14 | 0.14 |
| 42 | 0.16 | 0.16 |
| 45 | 0.18 | 0.18 |
| 48 | 0.18 | 0.18 |
| 52 | 0.20 | 0.20 |
| 56 | 0.22 | 0.22 |
| 59 | 0.24 | 0.22 |
| 63 | 0.26 | 0.22 |
| 66 | 0.28 | 0.22 |
| 69 | 0.30 | 0.22 |
| 73 | 0.30 | Medium |
| 76 | 0.30 | Medium |
| 80 | 0.30 | Medium |
| 83 | 0.30 | Medium |
| 86 | Medium | Medium |
| 90 | Medium | Medium |
| 93 | Medium | Medium |
| 97 | Medium | Medium |
| 101 | Medium | Medium |
| 104 | Medium | Medium |
| 108 | Mediuma | Medium |
| 111 | Mediuma | Medium |
indicates recurrence of proliferation of Nc-1 tachyzoites between days 108 and 111.
3.5. Effects of DB745 on the ultrastructure of N. caninum tachyzoites
The effects of DB745 on N. caninum tachyzoites were visualized by TEM, through examination of specimens fixed at 24, 48 and 72 h post-treatment. In Fig. 3A and B, untreated tachyzoites are depicted, exhibiting a normal ultrastructure. The parasites are located within a parasitophorous vacuole and surrounded by a parasitophorous vacuole membrane. The hallmarks of apicomplexan parasites such as the typical secretory organelles (rhoptries, micronemes and dense granules) were clearly visible, as well as the conoid in some instances (Fig. 3B). In cultures treated for 24 h with 1 μM DB745, distinct ultrastructural alterations were visible. The cytoplasm of the parasites exhibited increased numbers of vacuoles, some seemingly empty, others filled with membranous or electron dense material of unknown origin (Fig. 3C and D). Clear alterations were also noted within the nuclear membrane, the integrity of which was altered in the drug-treated parasites compared to the untreated specimens as it appeared partially separated. In addition, most parasite nuclei were devoid of a clearly discernible nucleolus. At 72 h of treatment, alterations became more evident and more diverse (Fig. 4). On one hand, still viable intracellular tachyzoites could be observed, with increased numbers of cytoplasmic vacuoles. These parasites were often in close contact in a way that the cell membranes of two tachyzoites appeared to be fused together (Fig. 4A–C). Higher magnification views showed that parasites were clearly separated by two distinct inner membranes, but held together by the outer plasmalemma, or alternatively, the inner membrane was interrupted and ending blindly in the cytoplasm (Fig. 4B and C). In many instances, extracellular tachyzoites were noted, which exhibited a completely altered and vacuolized cytoplasm, often with fragmented nuclear material, but still an intact plasma membrane. Also intracellular parasitophorous vacuoles with a clearly delineated parasitophorous vacuole membrane and non-viable and structurally severely altered tachyzoites were found, filled with membranous vacuoles and electron dense material of unknown origin.
Fig. 3.

TEM of N. caninum tachyzoites grown in HFF and visualization of effects after 24 h of DB745 treatment. (A) and (B) represent images of control cultures at 48 h post-infection. Tachyzoites are located intracellular, within a parasitophorous vacuole. Rhoptries (Rop), micronemes (mic) and dense granules (dg) are visibile. Also visible in (B) is the conoid (con) at the anterior part of the cells. Many nuclei contain an electron-dense nucleolus (ns). Note the intact nuclear membrane (white arrows). (C) and (D) represent images of tachyzoites after treatment with 1 μM DB745 for 24 h. Separated nuclear membrane layers are pointed out with black arrows. v = vacuoles, partially filled with membranous and/or electron-dense material of unknown origin. Bar in (A) = 1 μm; (B) = 0.28 μm; (C) = 0.45 μm; (D) = 0.33 μm.
Fig. 4.

TEM of N. caninum tachyzoites after 72 h of DB745 treatment. (A) Low magnification view of a parasitophorous vacuole membrane with tachyzoites still fused together. Tachyzoites show increased vacuolization (v) and cytoplasmic lipid droplets (ld). (B) Higher magnification view of the framed portion designated B in (A) showing parasites that are clearly separated by two distinct inner membranes (im), but held together by the outer plasma membrane (om). (C) shows the framed portion on the left hand side designated C in (A) at higher magnification. Note the blind ending of the inner membrane of the upper tachyzoite (white arrow). Black arrows show the contact sites with the outer membrane fusing the parasites together. (D) Extracellular tachyzoite remnants (tachy) with distorted cytoplasm. (E) Intracellular and severely damaged tachyzoites with a clearly delineated parasitophorous vacuole membrane (pvm). Parasites are filled with membranous vacuoles and electron dense material, black arrows point towards irregular membrane structures. Bar in (A) = 0.5 μm; (B) = 0.12 μm; (C) = 0.12 μm; (D) = 1 μm; (E) = 0.4 μm.
The interference in the nucleor integrity in DB745-treated tachyzoites was further evaluated using a semiquantitative approach. By random inspection of TEM micrographs of 100 treated and untreated tachyzoites we detected severe alterations or even lack of nucleoli in 85% of all cells, while only 30% of all nuclei were devoid of a nucleolus in non-treated tachyzoites.
3.6. In vivo activity of DB745 in Balb/c mice experimentally infected with N. caninum Nc-1 tachyzoites
Two types of experiment were performed. In experiment 1, DB745 treatment by intraperitoneal injection (2 mg/kg/day) was initiated 3 days prior to infection and was repeated daily for 14 days post-infection in order to give the drug the best chance to act. The diamidine DB750 (2 mg/kg/day; (Debache et al., 2011)) was included here for comparison. The results are summarized in Table 2. In the placebo group treated with the solvent only, 3 out of 10 mice succumbed to disease between days 8 and 10 post-infection, and no fatalities were recorded in the two groups receiving DB745 and DB750, respectively. There was no significant reduction of the weight of the mice during the treatment period in any of the drug-treated groups, while in the group receiving solvent only (placebo), the mean weight of the mice diminished significantly (P < 0.05). Assessments of cerebral parasite loads by real time PCR in all three groups (Fig. 5A) demonstrated an apparent reduction in tachyzoite numbers in the two drug-treated groups, however, only the values for DB750 (P < 0.05) were statistically significant, while those for DB745 were not (P > 0.05). In terms of serological responses to infection, there were no significant differences in IgG levels directed against crude N. caninum antigen between placebo and treatment groups in sera taken at the time of necroscopy (data not shown).
Fig. 5.

Effects of DB745 treatment in experimentally infected mice with respect to parasite load in the brain and lungs. (A) Cerebral parasite burden in mice receiving treatments starting 3 days prior to infection with 2 × 106N. caninum tachyzoites. Mice were treated either by intraperitoneal application of DB745 or DB750, both at 2 mg/kg/day for a period of 14 days. The parasite burden was assessed by Neospora real time PCR. Note the significantly reduced infection load in the group treated by intraperitoneal application of DB750. ∗ indicates significant reductions in parasite numbers (P < 0.05). (B) Cerebral parasite burden in brain tissue of mice receiving intraperitoneal treatments with DB745 (2.5 mg/kg/day or 7.5 mg/kg/day) starting 14 days after infection with 2 × 106N. caninum tachyzoites. Note the significantly reduced parasite load (∗P < 0.05) in the group treated with 7.5 mg/kg/day. (C) Parasite burden in the lungs, experimental groups are identical to (B). Note the apparent reduction, (although not significant) as assessed by real time PCR.
In experiment 2, DB745 treatments were initiated at day 14 post-infection, thus at a time point at which N. caninum tachyzoites had already reached the CNS (Alaeddine et al., 2005; Collantes-Fernandez et al., 2006), and were subsequently performed daily for a period of 2 weeks (see Table 2). Dosages were 2.5 mg/kg/day and 7.5 mg/kg/day. A placebo group treated with CMC only, and another control group comprised of uninfected mice treated with the higher dosage of DB745 were included. None of the groups experienced significant weight loss during the entire treatment period. 5 out of 8 mice (62%) in the placebo group experienced severe signs of cerebral neosporosis such as walking disorders, head tilting and ruffled coat, and had to be euthanized between days 13 and 26 post-infection. In the two groups receiving DB745, 2 out of 10 (20%) and 3 out of 9 mice (33%) exhibited clinical symptoms of N. caninum infection, respectively. No adverse clinical effects were noted for the uninfected mice treated with the higher dosage of DB745 (7.5 mg/kg/day; Table 2). Quantitative real time PCR of cerebral tissues (Fig. 5B) showed that DB745 treatments reduced the overall number of tachyzoites in the brain, with the treatment at 7.5 mg DB745/kg/day providing increased efficacy (P < 0.05) compared to 2.5 mg/kg/day. The parasite load in the corresponding lung tissue samples also appeared decreased, but values were not significantly different (Fig. 5C). Assessment of IgG levels against N. caninum antigens in sera taken at necroscopy did not exhibit any differences in the treatment groups (data not shown).
4. Discussion
Neosporosis is an infectious problem affecting cattle and other intermediate hosts worldwide. There is no efficient vaccine available to date that provides complete protection against infection, and no effective means of treatment have been developed so far (Dubey and Schares, 2011). The aim of this study was to compare the in vitro efficacy of a panel of 41 di-cationic pentamidine derivatives against N. caninum tachyzoites, and to characterize potential compounds of interest. This resulted in the identification of DB745, a di-cationic arylimidamide compound that efficiently inhibited both host cell invasion and intracellular proliferation of N. caninum tachyzoites. DB745 acted parasiticidal on the Nc-Liv isolate of N. caninum, but only parasitostatic on the Nc1 isolate. Although the potency of DB745 against N. caninum tachyzoites in vitro was similar to DB750 (a previously described and closely related compound; (Debache et al., 2011; Leepin et al., 2008)), its efficacy in vivo in experimentally infected mice was slightly lower.
Di-cationic pentamidine derivatives, especially arylimidamides and related compounds, have been shown to exhibit broad-spectrum anti-parasitic properties in vitro and partially also in vivo, most notably against intracellular pathogens such as T. cruzi, N. caninum, B. besnoiti, L. donovani, and T. gondii (Batista et al., 2010a,b; Cortes et al., 2011; Kropf et al., 2012; Leepin et al., 2008; Silva et al., 2007a,b; Stephens et al., 2003). The di-cationic molecules used here have been designed to exhibit improved pharmacokinetic properties by including aryl groups on one of the amidine nitrogen atoms (Wang et al., 2010). The fact that DB745 was identified as a potentially interesting candidate due to its low IC50 (80 nM) and favorable selective toxicity (see Fig. 1) was not surprising. This compound has been previously shown to be highly active against two strains of T. gondii tachyzoites (Rh and Me49, exhibiting high and low virulence, respectively), with an IC50 of 0.03 μM in 3 days growth assays (Kropf et al., 2012). In addition, DB745 represents a lead compound for the treatment of visceral leishmaniasis caused by L. donovani, and exhibits an in vitro IC50 against intracellular L. donovani of 0.018 ± 0.007 μM (Wang et al., 2010).
Previous studies (Debache et al., 2011; Leepin et al., 2008) had shown that DB750, a compound closely related to DB745, but with hydroxyl groups on the phenyl rings instead of ethoxy groups, was active against N. caninum tachyzoites in vitro (IC50 = 0.16 μM) and in vivo in experimentally infected mice. The substitution of the DB750-hydroxyl groups on the phenyl rings by ethoxy groups in DB745 possibly resulted in increased metabolic resistance, also rendering DB745 more lipophilic (log P at pH 7.4 = 3.82 (Wang et al., 2010)), and possibly provided superior membrane permeability. This in turn could increase the chances of the drug passing through several layers of membrane (host cell, parasitophorous vacuole, parasite cell membrane, target organelle) in order to exert its anti-parasitic activity. While DB750 only inhibited intracellular proliferation of N. caninum tachyzoites in vitro and did not exert any toxicity against extracellular tachyzoites (Leepin et al., 2008), DB745 had an impact on both host cell invasion (extracellular parasites) and intracellular proliferation (see Fig. 2). The observed impairment of infectivity upon N. caninum tachyzoites incubated extracellularly in the absence of DB745 is in agreement with earlier findings indicating that N. caninum rapidly lost the capacity to invade host cells upon extracellular maintenance (Hemphill et al., 1996; Naguleswaran et al., 2003). DB745 exerted its action swiftly, which is in agreement with recent investigations on the action of arylimidamides against T. cruzi, the causative agent of Chagas disease (Da Silva et al., 2011).
In analogy to DB750, pre-incubation of HFF monolayers with DB745 also resulted in a substantial inhibition of N. caninum tachyzoite proliferation, even when infected cells were subsequently maintained in the absence of the compound. An additional inhibitory effect was noted when the drug was again added to infected cells following pretreatment of HFF. Similar results were obtained in experiments employing DB750 on T. gondii (Kropf et al., 2012). This supported the notion that compounds such as DB750 and DB745 were likely to be efficiently taken up by host cells, and then subsequently retained in the host cell cytoplasm, where they continued to exert anti-parasitic activity for extended periods of time. This argues for the presence of an active transport mechanism for drug uptake. For instance, a human organic cation transporter which uses pentamidine and furamidine as substrates identified by Ming et al. (2008) could mediate DB750 and DB745 uptake into HFF. However, furamidine (DB75) does not exhibit in vitro activity against N. caninum tachyzoites (see Fig. 1). Clearly, the current evidence does not allow definitive conclusions, and the possibility of passive uptake of these compounds through biological membranes also cannot be discarded. Further work is required to clarify this point.
The two isolates differed in their ability to adapt to DB745 treatment, when exposure to the drug was done by slow and stepwise increase of drug concentration. Nc-Liv was able to cope with a maximum concentration of 0.20 μM and subsequently ceased proliferation even in the absence of the drug, indicating parasiticidal activity of DB745. In contrast Nc-1 tachyzoites kept proliferating up to a concentration of 0.33 μM, then underwent a phase of no or only little growth, and after maintenance in medium without compound resumed proliferation towards the end of the experiment. Thus, the particular conditions in this experiment were obviously more suitable to ensure survival of Nc-1 tachyzoites. This confirmed earlier studies that demonstrated distinct genetic and biological differences of different N. caninum isolates (Atkinson et al., 1999; Schock et al., 2001). The ability to, at least partially, adapt to increased drug concentrations during in vitro culture has also been demonstrated for T. gondii tachyzoites, which readily adapted to 1.2 μM DB750 within 5–6 days of in vitro culture. This represented a 10 times higher concentration than the corresponding IC50 (=0.13 μM). In addition, T. gondii tachyzoites also readily adapted to increased concentrations (up to 0.46 μM) of DB745 (Kropf et al., 2012). Thus, N. caninum does not seem to exhibit the potential for reacting to adverse physiological conditions to the same extent as Toxoplasma, although the two species are closely related. The underlining molecular mechanisms are not known, but the lower potential of Neospora to react to environmental changes could be one factor that limited the range of potential intermediate hosts of this parasite in comparison to T. gondii (Hemphill et al., 2006; Dubey et al., 2007). It has been shown that T. gondii exhibits an outstandingly high degree of flexibility on the transcriptional and translational level (Bougdour et al., 2010), but not much is known on the regulation of gene expression in N. caninum.
The mode of action of di-cationic pentamidine derivatives is not yet completely resolved. The current view suggests that these compounds bind to AT-rich sites in the DNA minor groove, and thus inhibit transcription or the interaction with DNA-binding enzymes such as topoisomerases or nucleases (Wilson et al., 2008). This indicates that these compounds could influence gene expression, and thus many diverse cellular functions could be affected. In addition, other cellular functions could be affected directly or indirectly. In kinetoplastids such as T. cruzi, treatment with diamidines resulted in mitochondrial swelling (Batista et al., 2010b). TEM was carried out in this study to investigate the ultrastructural differences between DB745-treated and non-treated N. caninum tachyzoites. During the course of drug treatment, rather unspecific alterations were observed in DB745-treated tachyzoites such as increased vacuolization starting already after 24 h. At 72 h, increased numbers of either extracellularly located and obviously distorted tachyzoites were observed, or intracellular tachyzoites exhibiting severely aberrant and compartmentalized cytoplasmic organization filled with vesicles, membrane stacks, electron dense granules and lipid droplets. Roughly similar findings were obtained in N. caninum tachyzoites treated with toltrazuril and ponazuril (Darius et al., 2004), thiazolide compounds (Esposito et al., 2005), and with miltefosine-treated N. caninum tachyzoites (Debache and Hemphill, 2012). However, two additional and possibly more specific alterations were observed. First, at 24 h (Fig. 3C and D), the nuclear membrane of many tachyzoites exhibited a loosened appearance, indicating separation of the double-layered nuclear membrane. In addition, in many DB745-treated tachyzoites, the nucleolus was not visible anymore. This suggested that DB745 could interfere in the maintenance of the nuclear membrane integrity, possibly interfere in lipid metabolism, and/or in transcriptional activity of these parasites. Secondly, many intracellular tachyzoites visualized at 72 h of DB745 treatment were found fused together (Fig. 4A–C). Closer inspection of the contact sites at higher magnification revealed that these were probably tachyzoites caught in the process of endodyogeny, with the inner membrane layer surrounding both daughter cells and the inner membrane layer either fully present (Fig. 4B) or partially interrupted (Fig. 4C). Similar aberrant membrane structures were found on non-viable intracellular tachyzoites still located within a parasitophorous vacuole (Fig. 4E). Thus, these observations also indicated that DB745 could potentially interfere in the formation/metabolism of membranous structures within these parasites.
These promising in vitro activities lead to this study, which was performed in order to assess the efficacy of DB745 in mice experimentally infected with N. caninum Nc-1 tachyzoites. In experiment 1, the drug was given the best chance to act, and none of the mice in the DB745 treatment group exhibited signs of disease, similar to another experimental group that was treated with DB750 (Table 2; Fig. 5A; Debache et al., 2011). In experiment 2, treatment at two dosages was initiated after parasites had crossed the blood–brain border and invaded the CNS (Alaeddine et al., 2005; Collantes-Fernandez et al., 2006), and application of (7.5 mg/kg/day) resulted in benefitial outcome in 6 out of 9 mice, while only 3 out of 8 mice in the placebo group survived. In addition, DB745 treatment resulted in a significantly reduced cerebral parasite burden (Fig. 5B). This indicated that DB745, similar to DB750, most likely crossed the blood–brain barrier and exerted its action also within cerebral tissues. A similar reduction of parasite numbers due to DB745 treatment was also noted in lung tissues, however, compared to the placebo control group, these values were not significantly different (Fig. 5C).
Thus, these initial in vivo results are promising, and point out the therapeutic potential of the arylimidamide DB745 and related compounds. Wang et al. (2010) described the pharmacokinetics of the arylimidamides DB745 and DB766 in mice. DB745 exhibited improved oral bioavailability and pharmacokinetics compared to di-cationic arylimidamides such as DB750. DB766, which is one of the compounds originally identified by Leepin et al. (2008), although with a slightly higher IC50 (0.30 μM) compared to DB750 (0.23 μM) against N. caninum in vitro but with a lower toxicity profile, will also be of great interest to be evaluated for the treatment of N. caninum infection. However, these results provide evidence on the efficacy of DB745 against tachyzoites and consequently the acute phase of neosporosis. The most interesting findings would be to demonstrate effects against cyst-forming bradyzoites, which are metabolically different and are responsible for the chronic phase of infection. Only few reports have been published so far on the production of N. caninum tissue cysts in mice, such as in inbred CBA/Ca mice and outbred mice (McGuire et al., 1997; Rettigner et al., 2004). More recently, Neospora tissue cyst production, marked by positive staining with an antibody directed against the Toxoplasma bradyzoite antigen BAG5, has also been demonstrated in an experimentally infected carnivorous marsupial, the fat-tailed dunnart Sminthopsis crassicaudata (King et al., 2011). It is also very likely that N. caninum tissue cysts are not necessarily formed only in the CNS but also in other locations such as in muscle tissue (Peters et al., 2001; King et al., 2011), and this should be taken into account in future studies. .
In conclusion, we have demonstrated different aspects of the in vitro and in vivo efficacy of the arylimidamide DB745, showing that this drug exhibits the potential of a lead compound for further investigations on therapeutical approaches to neosporosis, and other diseases caused by cyst-forming apicomplexans that invade the CNS. The class of di-cationic compounds as a whole should be further exploited in order to identify potentially interesting candidate drugs. From the practical point of view on neosporosis in cattle, treatment of congenitally infected calves right after birth could potentially give rise to pathogen-free offspring (Härdi et al., 2006), provided an effective compound can be identified. As an alternative, a combined application of vaccines and chemotherapy might also lead to improved efficacy.
Acknowledgments
We are grateful to Sabrina Sonda (University of Zürich, Switzerland) and David Sibley (Washington University, St. Louis, MO, USA) for providing us with the transgenic N. caninum-beta-Gal strain, J.P. Dubey (USDA, Beltsville) for the N. caninum Nc-1 isolate, and Diana Williams (University of Liverpool) for the N. caninum Nc-Liv isolate. Bruno Gottstein and Norbert Müller are acknowledged for continuous support and comments on the manuscript. This study was financed through the Swiss National Science Foundation (grant No. 31003A_127374/1).
References
- Alaeddine F., Keller N., Leepin A., Hemphill A. Reduced infection and protection from clinical signs of cerebral neosporosis in C57BL/6 mice vaccinated with recombinant microneme antigen NcMIC1. J. Parasitol. 2005;91:657–665. doi: 10.1645/GE-401R. [DOI] [PubMed] [Google Scholar]
- Ammann P., Waldvogel A., Breyer I., Esposito M., Müller N., Gottstein B. The role of B- and T-cell immunity in toltrazuril-treated C57BL/6 WT and μMT and nude mice experimentally infected with Neospora caninum. Parasitol. Res. 2004;93:178–187. doi: 10.1007/s00436-004-1083-y. [DOI] [PubMed] [Google Scholar]
- Atkinson R., Harper P.A., Ryce C., Morrison D.A., Ellis J.T. Comparison of the biological characteristics of two isolates of Neospora caninum. Parasitology. 1999;118:363–370. doi: 10.1017/s0031182098003898. [DOI] [PubMed] [Google Scholar]
- Barber J.S., Trees A.J. Clinical aspects of 27 cases of neosporosis in dogs. Vet. Rec. 1996;139:439–443. doi: 10.1136/vr.139.18.439. [DOI] [PubMed] [Google Scholar]
- Batista D.G., Batista M.M., de Oliveira G.M., do Amaral P.B., Lannes-Vieira J., Britto C.C., Junqueira A., Lima M.M., Romanha A.J., Sales P.A., Jr, Stephens C.E., Boykin D.W., Soeiro M.N. Arylimidamide DB766, a potential chemotherapeutic candidate for Chagas’ disease treatment. Antimicrob. Agents Chemother. 2010;54:2940–2952. doi: 10.1128/AAC.01617-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista D.G., Pacheco M.G., Kumar A., Branowska D., Ismail M.A., Hu L., Boykin D.W., Soeiro M.N. Biological, ultrastructural effect and subcellular localization of aromatic diamidines in Trypanosoma cruzi. Parasitology. 2010;137:251–259. doi: 10.1017/S0031182009991223. [DOI] [PubMed] [Google Scholar]
- Bougdour A., Braun L., Cannella D., Hakimi M.A. Chromatin modifications: implications in the regulation of gene expression in Toxoplasma gondii. Cell. Microbiol. 2010;12:413–423. doi: 10.1111/j.1462-5822.2010.01446.x. [DOI] [PubMed] [Google Scholar]
- Buckner F.S., Navabi N. Advances in Chagas disease drug development: 2009–2010. Curr. Opin. Infect. Dis. 2010;23:609–616. doi: 10.1097/QCO.0b013e3283402956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collantes-Fernandez E., Lopez-Perez I., Alvarez-Garcia G., Ortega-Mora L.M. Temporal distribution and parasite load kinetics in blood and tissues during Neospora caninum infection in mice. Infect. Immun. 2006;74:2491–2494. doi: 10.1128/IAI.74.4.2491-2494.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes H.C., Muller N., Boykin D., Stephens C.E., Hemphill A. In vitro effects of arylimidamides against Besnoitia besnoiti infection in Vero cells. Parasitology. 2011;138:583–592. doi: 10.1017/S0031182011000114. [DOI] [PubMed] [Google Scholar]
- Da Silva C.F., Junqueira A., Lima M.M., Romanha A.J., Sales P.A., Jr, Stephens C.E., Som P., Boykin D.W., Soeiro Mde N. In vitro trypanocidal activity of DB745B and other novel arylimidamides against Trypanosoma cruzi. J. Antimicrob. Chemother. 2011;66:1295–1297. doi: 10.1093/jac/dkr140. [DOI] [PubMed] [Google Scholar]
- Darius A.K., Mehlhorn H., Heydorn A.O. Effects of toltrazuril and ponazuril on the fine structure and multiplication of tachyzoites of the NC-1 strain of Neospora caninum (a synonym of Hammondia heydorni) in cell cultures. Parasitol. Res. 2004;92:453–458. doi: 10.1007/s00436-003-1063-7. [DOI] [PubMed] [Google Scholar]
- De Souza E.M., da Silva P.B., Nefertiti A.S., Ismail M.A., Arafa R.K., Tao B., Nixon-Smith C.K., Boykin D.W., Soeiro M.N. Trypanocidal activity and selectivity in vitro of aromatic amidine compounds upon bloodstream and intracellular forms of Trypanosoma cruzi. Exp. Parasitol. 2011;127:429–435. doi: 10.1016/j.exppara.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Debache, K., Hemphill, A., 2012 Effects of miltefosine treatment in fibroblast cell cultures and in mice experimentally infected with Neospora caninum tachyzoites. Parasitology, (Epub ahead of print). [DOI] [PubMed]
- Debache K., Guionaud C., Alaeddine F., Hemphill A. Intraperitoneal and intra-nasal vaccination of mice with three distinct recombinant Neospora caninum antigens results in differential effects with regard to protection against experimental challenge with Neospora caninum tachyzoites. Parasitology. 2010;137:229–240. doi: 10.1017/S0031182009991259. [DOI] [PubMed] [Google Scholar]
- Debache K., Guionaud C., Kropf C., Boykin D., Stephens C.E., Hemphill A. Experimental treatment of Neospora caninum-infected mice with the arylimidamide DB750 and the thiazolide nitazoxanide. Exp. Parasitol. 2011;129:95–100. doi: 10.1016/j.exppara.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Schares G. Neosporosis in animals–the last 5 years. Vet. Parasitol. 2011;180:90–108. doi: 10.1016/j.vetpar.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Dubey J.P., Schares G., Ortega-Mora L.M. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007;20:323–367. doi: 10.1128/CMR.00031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M., Stettler R., Moores S.L., Pidathala C., Muller N., Stachulski A., Berry N.G., Rossignol J.F., Hemphill A. In vitro efficacies of nitazoxanide and other thiazolides against Neospora caninum tachyzoites reveal antiparasitic activity independent of the nitro group. Antimicrob. Agents Chemother. 2005;49:3715–3723. doi: 10.1128/AAC.49.9.3715-3723.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M., Moores S., Naguleswaran A., Muller J., Hemphill A. Induction of tachyzoite egress from cells infected with the protozoan Neospora caninum by nitro- and bromo-thiazolides, a class of broad-spectrum anti-parasitic drugs. Int. J. Parasitol. 2007;37:1143–1152. doi: 10.1016/j.ijpara.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Esposito M., Muller N., Hemphill A. Structure–activity relationships from in vitro efficacies of the thiazolide series against the intracellular apicomplexan protozoan Neospora caninum. Int. J. Parasitol. 2007;37:183–190. doi: 10.1016/j.ijpara.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Gottstein B., Razmi G.R., Ammann P., Sager H., Müller N. Toltrazuril treatment to control diaplacental Neospora caninum transmission in experimentally infected pregnant mice. Parasitology. 2005;130:41–48. doi: 10.1017/s0031182004006365. [DOI] [PubMed] [Google Scholar]
- Härdi C., Haessig M., Sager H., Greif G., Staubli D., Gottstein B. Humoral immune reaction of newborn calves congenitally infected with Neospora caninum and experimentally treated with toltrazuril. Parasitol. Res. 2006;99:534–540. doi: 10.1007/s00436-006-0199-7. [DOI] [PubMed] [Google Scholar]
- Häsler B., Regula G., Stärk K.D., Sager H., Gottstein B., Reist M. Financial analysis of various strategies for the control of Neospora caninum in dairy cattle in Switzerland. Prev. Vet. Med. 2006;77:230–253. doi: 10.1016/j.prevetmed.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Häsler B., Stärk K.D., Sager H., Gottstein B., Reist M. Simulating the impact of four control strategies on the population dynamics of Neospora caninum infection in Swiss dairy cattle. Prev. Vet. Med. 2006;77:254–283. doi: 10.1016/j.prevetmed.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Hemphill A., Gottstein B., Kaufmann H. Adhesion and invasion of bovine endothelial cells by Neospora caninum. Parasitology. 1996;112:183–197. doi: 10.1017/s0031182000084754. [DOI] [PubMed] [Google Scholar]
- Hemphill A., Vonlaufen N., Naguleswaran A., Keller N., Riesen M., Guetg N., Srinivasan S., Alaeddine F. Tissue culture and explant approaches to studying and visualizing Neospora caninum and its interactions with the host cell. Microsc. Microanal. 2004;10:602–620. doi: 10.1017/S1431927604040930. [DOI] [PubMed] [Google Scholar]
- Hemphill A., Vonlaufen N., Naguleswaran A. Cellular and immunological basis of the host-parasite relationship during infection with Neospora caninum. Parasitology. 2006;133:261–278. doi: 10.1017/S0031182006000485. [DOI] [PubMed] [Google Scholar]
- Kim J.T., Park J.Y., Seo H., Oh H.G., Noh J.W., Kim J.H., Kim D.Y., Youn H.J. In vitro anti-protozoal effects of artemisin on Neospora caninum. Vet. Parasitol. 2002;103:53–63. doi: 10.1016/s0304-4017(01)00580-5. [DOI] [PubMed] [Google Scholar]
- King J., McAllan S.B., Spielman D.S., Lindsay S.A., Hůrková-Hofmannová L., Hartigan A., Al-Qassab S.E., Ellis J.T., Slapeta J. Extensive production of Neospora caninum tissue cysts in a carnivorous marsupial succumbing to experimental neosporosis. Vet. Res. 2011;42:75. doi: 10.1186/1297-9716-42-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritzner S., Sager H., Blum J., Krebber R., Greif G., Gottstein B. An explorative study to assess the effficacy of toltrazuril-sulfone (ponazuril) in calves experimentally infected with Neospora caninum. Ann. Clin. Microbiol. Antimicrob. 2002;1:4–10. doi: 10.1186/1476-0711-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf C., Debache K., Rampa C., Barna F., Schorer M., Stephens C.E., Ismail M.A., Boykin D.W., Hemphill A. The adaptive potential of a survival artist: characterization of the in vitro interactions of Toxoplasma gondii tachyzoites with di-cationic compounds in human fibroblast cell cultures. Parasitology. 2012;139:208–220. doi: 10.1017/S0031182011001776. [DOI] [PubMed] [Google Scholar]
- Kwon H., Kim J.H., Kim M., Lee J.K., Hwang W.S., Kim D.Y. Anti-parasitic activity of depudecin on Neospora caninum via the inhibition of histone deacetylase. Vet. Parasitol. 2003;112:269–276. doi: 10.1016/s0304-4017(03)00035-9. [DOI] [PubMed] [Google Scholar]
- Leepin A., Stüdli A., Brun R., Stephens C.E., Boykin D.W., Hemphill A. Host cells participate in the in vitro effects of novel diamidine analogues against tachyzoites of the intracellular apicomplexan parasites Neospora caninum and Toxoplasma gondii. Antimicrob. Agents Chemother. 2008;52:1999–2008. doi: 10.1128/AAC.01236-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay D.S., Dubey J.P. Effects of sulfadiazine and amprolium on Neospora caninum (protozoa: apicomplexa) infections in mice. J. Parasitol. 1990;76:177–179. [PubMed] [Google Scholar]
- Lindsay D.S., Rippey N.S., Cole R.A., Parsons L.C., Dubey J.P., Tidwell R.R.B., Blagburn L. Examination of the activities of 43 chemotherapeutic agents against Neospora caninum tachyzoites in cultured cells. Am. J. Vet. Res. 1994;55:976–981. [PubMed] [Google Scholar]
- McFadden D.C., Seeber F., Boothroyd J.C. Use of Toxoplasma gondii expressing beta-galactosidase for colorimetric assessment of drug activity in vitro. Antimicrob. Agents Chemother. 1997;41:1849–1853. doi: 10.1128/aac.41.9.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire A.M., McAllister M.M., Jolley W.R., Anderson-Sprecher R.C. A protocol for the production of Neospora caninum tissue cysts in mice. J. Parasitol. 1997;83:647–651. [PubMed] [Google Scholar]
- Ming X., Ju W., Wu H., Tidwell R.R., Hall J.E., Thakker D.R. Transport of dicationic drugs pentamidine and furamidine by human organic cation transporters. Drug Metab. Dispos. 2008;37:424–430. doi: 10.1124/dmd.108.024083. [DOI] [PubMed] [Google Scholar]
- Monney T., Debache K., Hemphill A. Vaccines against a major cause of abortion in cattle, Neospora caninum infection. Animals. 2011;1:306–325. doi: 10.3390/ani1030306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monney T., Rütti D., Schorer M., Debache K., Grandgirard D., Leib S.L., Hemphill A. RecNcMIC3-1-R is a microneme- and rhoptry-based chimeric antigen that protects against acute neosporosis and limits cerebral parasite load in the mouse model for Neospora caninum infection. Vaccine. 2011;29:6967–6975. doi: 10.1016/j.vaccine.2011.07.038. [DOI] [PubMed] [Google Scholar]
- Naguleswaran A., Müller N., Hemphill A. Neospora caninum and Toxoplasma gondii: a novel adhesion/invasion assay reveals distinct differences in tachyzoite–host cell interactions. Exp. Parasitol. 2003;104:149–158. doi: 10.1016/s0014-4894(03)00137-1. [DOI] [PubMed] [Google Scholar]
- Peters M., Lütkefels E., Heckeroth A.R., Schares G. Immunohistochemical and ultrastructural evidence for Neospora caninum tissue cysts in skeletal muscles of naturally infected dogs and cattle. Int. J. Parasitol. 2001;3:1144–1148. doi: 10.1016/s0020-7519(01)00221-1. [DOI] [PubMed] [Google Scholar]
- Rettigner C., Leclipteux T., De Meerschman F., Focant C., Losson B. Survival, immune responses and tissue cyst production in outbred (Swiss white) and inbred (CBA/Ca) strains of mice experimentally infected with Neospora caninum tachyzoites. Vet. Res. 2004;35:225–232. doi: 10.1051/vetres:2004005. [DOI] [PubMed] [Google Scholar]
- Schock A., Innes E.A., Yamane I., Latham S.M., Wastling J.M. Genetic and biological diversity among isolates of Neospora caninum. Parasitology. 2001;123:13–23. doi: 10.1017/s003118200100796x. [DOI] [PubMed] [Google Scholar]
- Silva C.F., Batista M.M., Mota R.A., de Souza E.M., Stephens C.E., Som P., Boykin D.W., Soeiro M.N. Activity of “reversed” diamidines against Trypanosoma cruzi “in vitro”. Biochem. Pharmacol. 2007;15:1939–1946. doi: 10.1016/j.bcp.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Silva C.F., Meuser M.B., De Souza E.M., Meirelles M.N., Stephens C.E., Som P., Boykin D.W., Soeiro M.N. Cellular effects of reversed amidines on Trypanosoma cruzi. Antimicrob. Agents Chemother. 2007;51:3803–3809. doi: 10.1128/AAC.00047-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeiro M.N., De Souza E.M., Stephens C.E., Boykin D.W. Aromatic diamidines as antiparasitic agents. Expert Opin. Investig. Drugs. 2005;14:957–972. doi: 10.1517/13543784.14.8.957. [DOI] [PubMed] [Google Scholar]
- Stephens C.E., Brun R., Salem M.N., Werbovetz K.A., Tanious F., Wilson W.D., Boykin D.W. The activity of diguanidino and , reversed’ diamidino 2,5-diarylfurans versus Trypanosoma cruzi and Leishmania donovani. Bioorg. Med. Chem. Lett. 2003;16:2065–2069. doi: 10.1016/s0960-894x(03)00319-6. [DOI] [PubMed] [Google Scholar]
- Strohbusch M., Müller N., Hemphill A., Krebber R., Greif G., Gottstein B. Toltrazuril treatment of congenitally acquired Neospora caninum infection in newborn mice. Parasitol. Res. 2009;104:1335–1343. doi: 10.1007/s00436-009-1328-x. [DOI] [PubMed] [Google Scholar]
- Wang M.Z., Zhu X., Srivastava A., Liu Q., Sweat J.M., Pandharkar T., Stephens C.E., Riccio E., Parman T., Munde M., Mandal S., Madhubala R., Tidwell R.R., Wilson W.D., Boykin D.W., Hall J.E., Kyle D.E., Werbovetz K.A. Novel arylimidamides for treatment of visceral leishmaniasis. Antimicrob. Agents Chemother. 2010;54:2507–2516. doi: 10.1128/AAC.00250-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werbovetz K. Diamidines as antitrypanosomal, antileishmanial and antimalarial agents. Curr. Opin. Investig. Drugs. 2006;6:147–157. [PubMed] [Google Scholar]
- Wilson W.D., Tanious F.A., Mathis A., Tevis D., Hall J.E., Boykin D.W. Antiparasitic compounds that target DNA. Biochimie. 2008;90:999–1014. doi: 10.1016/j.biochi.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn H.J., Lakritz J., Rottinghaus G.E., Seo H.S., Kim D.Y., Cho M.H., Marsh A.E. Anti-protozoal efficacy of high performance liquid chromatography fractions of Torilis japonica and Sophora flavescens extracts on Neospora caninum and Toxoplasma gondii. Vet. Parasitol. 2004;125:409–414. doi: 10.1016/j.vetpar.2004.08.002. [DOI] [PubMed] [Google Scholar]


