Graphical abstract

Keywords: Arctic fox, Vulpes lagopus, Parasite, Northern, Arctic, Canada
Highlights
-
•
Few studies report the endoparasites of North American arctic foxes.
-
•
Conventional, immunological, and molecular techniques detected parasites in arctic foxes at Karrak Lake, Nunavut.
-
•
Karrak Lake foxes are infected with common parasites of foxes with a terrestrial diet.
-
•
This study provides a comparison for future studies from the central Canadian Arctic.
Abstract
The parasites of arctic foxes in the central Canadian Arctic have not been well described. Canada’s central Arctic is undergoing dramatic environmental change, which is predicted to cause shifts in parasite and wildlife species distributions, and trophic interactions, requiring that baselines be established to monitor future alterations. This study used conventional, immunological, and molecular fecal analysis techniques to survey the current gastrointestinal endoparasite fauna currently present in arctic foxes in central Nunavut, Canada. Ninety-five arctic fox fecal samples were collected from the terrestrial Karrak Lake ecosystem within the Queen Maud Gulf Migratory Bird Sanctuary. Samples were examined by fecal flotation to detect helminths and protozoa, immunofluorescent assay (IFA) to detect Cryptosporidium and Giardia, and quantitative PCR with melt-curve analysis (qPCR-MCA) to detect coccidia. Positive qPCR-MCA products were sequenced and analyzed phylogenetically. Arctic foxes from Karrak Lake were routinely shedding eggs from Toxascaris leonina (63%). Taeniid (15%), Capillarid (1%), and hookworm eggs (2%), Sarcocystis sp. sporocysts 3%), and Eimeria sp. (6%), and Cystoisospora sp. (5%) oocysts were present at a lower prevalence on fecal flotation. Cryptosporidium sp. (9%) and Giardia sp. (16%) were detected by IFA. PCR analysis detected Sarcocystis (15%), Cystoisospora (5%), Eimeria sp., and either Neospora sp. or Hammondia sp. (1%). Through molecular techniques and phylogenetic analysis, we identified two distinct lineages of Sarcocystis sp. present in arctic foxes, which probably derived from cervid and avian intermediate hosts. Additionally, we detected previously undescribed genotypes of Cystoisospora. Our survey of gastrointestinal endoparasites in arctic foxes from the central Canadian Arctic provides a unique record against which future comparisons can be made.
Introduction
Arctic foxes (Vulpes lagopus) have a circumpolar distribution and are common throughout the North American and Russian Arctic. Few reports of parasites in North American arctic fox populations currently exist, although the parasite fauna of the endangered Fennoscandian arctic fox has been well documented in recent years (Meijer et al., 2011). As environmental change in the Arctic biome continues to accelerate, parasite communities are also expected to change (Kutz et al., 2009). Already, the Canadian Arctic Tundra climate region has experienced an observed warming trend of 2.1 °C in annual temperature since observations began in 1948 (Environment Canada Annual Regional Temperature Departures, Climate Trends and Variation Bulletin 2011, http://www.ec.gc.ca/adsc-cmda/default.asp?lang=en&n=B49D9F0B-1, accessed 14.12.12).
Potential shifts in the distribution and abundance of parasites and hosts threaten our current understanding of trophic interactions in the Arctic, including host–parasite relationship (Ims and Fuglei, 2005). Parasites are also known to play a part in destabilizing some host populations (Anderson and May, 1978), and, when combined with environmental change, parasitism could have an additive negative effect on arctic fox populations. Also, red fox (Vulpes vulpes) populations continue to expand northward, creating opportunity for interspecific competition and transmission of parasites from southern canid populations (Hersteinsson and McDonald, 1982).
Early parasitological studies of arctic foxes in North America were primarily focused on detection of Echinococcus multilocularis, a cestode of public health importance in western Alaska and elsewhere in the circumpolar North. These studies led to the detection of Toxascaris leonina, Taenia crassiceps, E. multilocularis, and coccidia resembling Eimeria sp. (Rausch, 1956, Choquette et al., 1962, Eaton and Secord, 1979). Otherwise, little is known about the prevalence, distribution, and diversity of arctic fox parasites in the Canadian Arctic.
Fecal-based studies are non-invasive and logistically feasible in remote environments. Results from fecal studies are increasingly meaningful as molecular tools improve to distinguish between morphologically similar parasite species and to answer questions about the genotypes or subspecies present. In a Northern context, molecular parasitology provides information about the zoonotic potential of parasites in wildlife and the environment. For example, molecular diagnostic tools are used to identify zoonotic genotypes or canid-specific genotypes of Giardia (Thompson et al., 2009) and this knowledge can help northern residents develop safe food and water guidelines.
The objective of this study was to survey and describe helminth and protozoal parasites or endoparasite stages in feces of arctic foxes at Karrak Lake, Nunavut, Canada. Although trophic interactions in this ecosystem are well described (Samelius et al., 2007), this study provides the first regional record of arctic fox endoparasites in the central Arctic of North America based on conventional, immunological, and molecular analysis of parasites present in feces. Additionally, we used a real-time quantitative PCR with melt-curve analysis method to detect and distinguish different genera and species of coccidians (qPCR-MCA; Lalonde and Gajadhar, 2011). The results of this study serve as baseline information against which we can evaluate changes in parasite distribution and prevalence from predicted environmental change, and to better understand the wildlife and human health significance of parasites in terrestrial Arctic ecosystems.
Materials and methods
Study area
The fieldwork was conducted within the nesting colony of Lesser Snow Geese and Ross’s Geese surrounding Karrak Lake, Nunavut (67°14′N, 100°15′W) in the Queen Maud Gulf Migratory Bird Sanctuary in the central Canadian Arctic. The nearest human community is approximately 300 km and the site is only accessible by small aircraft. Karrak Lake is in the Arctic Tundra climate region, which in 2012 and 2011 experienced the 2nd and 3rd warmest summers, respectively, on record since 1948. During this period, temperatures were 2.3 °C (2012) and 2.1 °C above average (Ranked summer regional temperatures table, http://www.ec.gc.ca/adsc-cmda/default.asp?lang=En&n=30EDCA67-1, accessed 14.12.12).
The Karrak Lake ecosystem supports high arctic fox abundance and breeding density that is about 2–4 times higher than outside the goose colony (Samelius et al., 2011). Reproductive output of arctic foxes is highly correlated with small mammal abundance (Samelius et al., 2011). Grey wolves (Canis lupus), wolverines (Gulo gulo), and grizzly bears (Ursus arctos) are sporadic but common at Karrak Lake, while red foxes are very infrequent visitors to the area with only one sighting in 12 years of ongoing arctic fox study (Samelius, unpublished data). The light goose colony at Karrak Lake is one of the largest in the Arctic, spanning over 200 sq.km of contiguous nesting habitat in 2012 (Alisauskas et al., 2012), when more than 750,000 Ross’s and 450,000 Lesser Snow Geese were estimated to have nested there (Alisauskas et al., 2012).
Small mammal abundance at Karrak Lake fluctuates considerably among years (Samelius et al., 2007, Samelius et al., 2011) with collared (Dicrostonyx groenlandicus) and brown lemming (Lemmus sibiricus) abundance at a record-high of 5.7 lemming-captures per 100 trap-nights in 2011 followed by a low of 0 lemming captures per 100 trap-nights in 2012 (Alisauskas, unpublished data). Red-backed vole (Clethrionomys rutilus) numbers, in contrast, were similar among years with 0.7 and 0.5 red-backed voles captures per 100 trap-nights in 2011 and 2012, respectively (Alisauskas, unpublished data).
The landscape within the Karrak Lake ecosystem is rolling tundra, characterized by rocky outcrops and surrounding areas of lowland wetlands, sedge meadows and shallow ponds (Ryder, 1972, Didiuk and Ferguson, 2005, Alisauskas et al., 2006). Arctic foxes at Karrak Lake are known to prey on or scavenge ptarmigan, geese, bird eggs, lemmings, caribou, muskoxen, and arctic hare (Bantle and Alisauskas, 1998, Samelius et al., 2007).
Sample collection
During mid-May to mid-June in 2011 and 2012, 95 fecal samples were collected from snow cover surrounding known arctic fox den sites distributed throughout the Karrak Lake goose colony (Fig. 1). Feces were also recovered from arctic foxes captured during an ongoing population dynamics study, either from the trap or the ground after the fox was released. For each fecal sample, the location and date of collection were recorded. Samples were collected in an even distribution among sites and no sites were preferentially sampled over another, as all were visited approximately the same number of times during the study period. Fecal samples were stored in individual plastic bags at approximately 1–6 °C until shipment to the University of Saskatchewan. In the laboratory, fecal samples were held at −80 °C for 7 days to kill ova of Echinococcus sp., then at −20 °C for 1–3 weeks until analysis and permanently thereafter. The fecal samples in this study were from at least 30 individual foxes, based on capture data from these 2 years (Alisauskas and Samelius, unpublished data). However this is likely a low estimate due to the presence of unsampled transient foxes passing through the sampling area, and resident foxes that were not captured during this study. An additional limitation to scat collection from the environment is that host age remains unknown, but the age of an animal can influence the parasite fauna present. Because most of the samples were collected from the most recent snow cover, the youngest animals would have been around 11 months old when the feces were deposited.
Fig. 1.
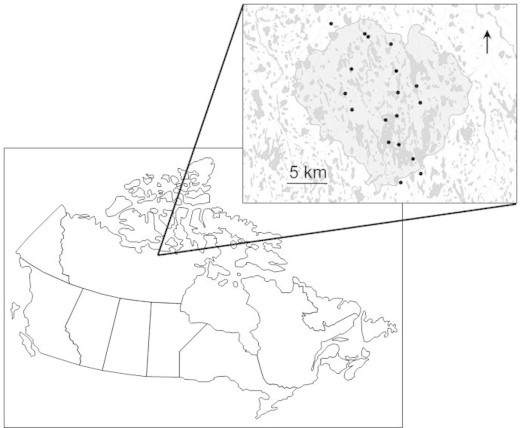
Karrak Lake goose colony within the Queen Maud Gulf Bird Sanctuary, Nunavut. Inset map: sample collection sites within the goose colony.
Fecal flotation
A modified double centrifugation Sheather’s sucrose flotation technique was performed on a known quantity (1–3 g) of feces from each sample. All ascarid eggs, observed by light microscopy, were counted and the number per gram of feces recorded. Other parasite eggs were not quantified on fecal flotation. Parasite identifications were based on morphology and morphometrics (Foreyt, 2001).
Immunofluorescent assay (IFA)
To concentrate the ova, cysts, and oocysts in the feces in preparation for the immunofluorescent assay and molecular analysis, a known quantity (0.5–3 g) of feces from each sample was mixed with 10 mL phosphate-buffered 0.9% saline (PBS). This liquid was filtered through two layers of cheesecloth into a 15 mL centrifuge tube and centrifuged for 10 min. The resulting fecal pellet was resuspended in 6 mL PBS and then centrifuged as above (Siefker et al., 2002). Finally, the washed fecal pellet was resuspended in 1 mL PBS and the fecal suspension was refrigerated at 4 °C until further analysis.
Cysts of Giardia sp. and oocysts of Cryptosporidium sp. were counted in 15 μl of fecal suspension prepared with a commercially available test kit specific for these protozoans (Cyst-a-glo; Waterborne Inc.) according to manufacturer instructions. The following formula was used to estimate the number of oocysts or cysts in each gram of feces: (1000 * number cysts/cysts counted)/# μL on IFA slide/grams feces used.
Molecular techniques
Genomic DNA was extracted from 300 μL of fecal suspension (Da Silva et al., 1999) using the Fast-DNA kit (MP Biomedicals) with Lysis Matrix E beads (MP Biomedicals), followed by the PCR Purification kit (Qiagen). Coccidian species in each fecal sample were detected using a universal coccidia primer cocktail designed to amplify a ∼315-bp region of 18S rDNA in a real-time quantitative PCR assay and differentiated using melt-curve analysis (Lalonde and Gajadhar, 2011). The PCR product melting temperature (Tm) is based on the nucleotide sequence composition, length, and G–C content, so genetically distinct members of the same genus or species can be differentiated by the melt curve shape and Tm and identified by comparison to in-run controls and/or sequencing (Lalonde and Gajadhar, 2011). All qPCR analyses were performed using the CFX96 Real-Time PCR detection system (Bio-Rad Laboratories) as described previously (Lalonde and Gajadhar, 2011) except 1X Evagreen Supermix (Bio-Rad Laboratories) was used in the final reaction mix. Each PCR reaction plate included two negative control wells (water), a standard curve consisting of plasmid DNA from Eimeria bovis (105–101 oocysts), and wells containing DNA from Toxoplasma gondii (genomic, ATCC, Virginia, USA), Cystoisospora sp. (plasmid, in house), Neospora caninum (genomic, ATCC, Virginia, USA), Sarcocystis cruzi (genomic, in house), and E. bovis (plasmid, in house) as positive controls.
Melt curves from positive samples were visually compared to the controls for preliminary identification, and amplified DNA from positive reactions were sequenced by Macrogen (Seoul, South Korea) for confirmation using original primers. Forward and reverse sequences were assembled with PreGap4 and Gap4 (Staden Package) and consensus sequences were compared with reference sequences in GenBank™ using the nucleotide Basic Local Alignment Search Tool (BLASTN). Multiple alignments of reference and sample sequences were performed with CLUSTAL X. Neighbor-joining phylogenetic trees and branch reliability bootstrap values (100 iterations) were constructed with the program PHYLIP 3.69 (Chaban and Hill, 2012).
Results
We detected eight morphologically different types of gastrointestinal endoparasites by fecal flotation in 95 fecal samples from arctic foxes in this study. Eggs of T. leonina were most common (63% prevalence), with a median shedding intensity of 33 (range 1–800) eggs per gram of feces. Taeniid, anoplocephalid, capillarid, and hookworm eggs and Sarcocystis sp., Eimeria sp., and Cystoisospora-like oocysts were observed in a lower proportion of samples (Table 1) and shedding intensity, but quantitative data from fecal flotation were only recorded for T. leonina. On immunofluorescent antibody assay, Giardia and Cryptosporidium were detected in 16% and 8% of fecal samples, respectively (Table 1). The median shedding intensity of Giardia was 162 (range 2–12,080) cysts per gram feces, while the median shedding intensity of Cryptosporidium was 77 (range 18–146) oocysts per gram feces.
Table 1.
Proportion of samples with parasites found in arctic fox feces and the frequency of agreement between detection methods.
| Parasite | Overall proportiona (n = 95) | Proportion detected by flotation | Proportion detected by qPCR-MCA | Proportion detected by IFA | Test agreementb |
|---|---|---|---|---|---|
| Toxascaris leonina | 60 (63%) | 60 (63%) | – | – | – |
| Taeniid | 14 (15%) | 14 (15%) | – | – | – |
| Anoplocephalid | 20 (21%) | 20 (21%) | – | – | – |
| Capillarid | 1 (1%) | 1 (1%) | – | – | – |
| Hookworm | 2 (2%) | 2 (2%) | – | – | – |
| Sarcocystis sp. | 16 (16%) | 3 (3%) | 14 (15%) | – | 1/16 (6.3%) |
| Cystoisospora sp. | 8 (8%) | 5 (5%) | 5 (5%) | – | 2/8 (25%) |
| Eimeria sp. | 14 (15%) | 6 (6%) | 9 (9%) | – | 1/14 (7.1%) |
| Neospora/Hammondia-like | 1 (1%) | 0 | 1 (1%) | – | 0/1 (0.0%) |
| Cryptosporidium sp. | 9 (9%) | 0 | 0 | 9 (9%) | 0 |
| Giardia sp. | 15 (16%) | 0 | – | 15 (16%) | – |
Overall prevalence reflects the total number of positive results for each parasite, all diagnostic tests combined.
Test agreement represents the number of times the parasite was detected by both methods/the number of times the parasite was detected by either method.
The qPCR-MCA analysis, subsequent sequencing, and phylogenetic analysis confirmed at least two distinct species of Sarcocystis (in 16/95 samples), two species of Cystoisospora (5/95), at least two species of Eimeria (8/95), and one sample with either Hammondia sp. or Neospora sp. (1/95). Test agreement between PCR and fecal flotation was low (0–25%) for these coccidians (Table 1). When unknown samples were visualized together, distinct melt peaks for different types of coccidia were clear (Fig. 2). Additionally, individual melt curves were visualized against the positive control for each parasite (Fig. 3), highlighting similarities and differences between the unknowns and positive controls. Phylogenetic analysis of nucleotide sequences showed at least two different lineages of Sarcocystis. One grouping included species of Sarcocystis that use cervid intermediate hosts (Lineage 2; Fig. 3; Dahlgren et al., 2007) and the other with species of Sarcocystis that use avian intermediate hosts (Lineage 1; Fig. 3; Kutkiené et al., 2012). Samples that grouped in Lineage 1 (Fig. 3) were 99–100% similar to Sarcocystis albifronsi (GenBank Accession no: EU502868) and Sarcocystis anasi (GenBank Accession no: EU553477), The second Sarcocystis lineage (Lineage 2; Fig. 4) were only 77–78% similar to S. anasi and S. albifronsi at the target region but 91–93% similar to Sarcocystis capreolicanis and 100% similar to Sarcocystis tarandivulpes. When melt curves of a representative sample from each lineage of Sarcocystis were compared, there were two distinct curves, one that was similar to the S. cruzi positive control (Lineage 2; Tm = 82.0 °C) and one that was unique (Lineage 1; Tm = 83.4 °C). Likewise, there were two separate melt curves for Eimeria sp., which differed from the E. bovis control, suggesting that at least two different species of Eimeria were present. Sequences from Cystoisospora-like oocysts in the current study were 96% (GenBank Accession nos: KC262748 and KC262749) or 98% (GenBank Accession nos: KC262746 and KC262747) similar to Cystoisospora ohioensis (GenBank Acession no: GU292305) at the target region of the 18S gene (Fig. 4). A multiple sequence alignment of the target locus showed that the reference sequences for Hammondia spp. (GenBank Accession: GQ984222) and N. caninum (GQ899206; U16159) were indistinguishable from each other and the unknown sample from the arctic fox was 99% similar to the reference sequences.
Fig. 2.
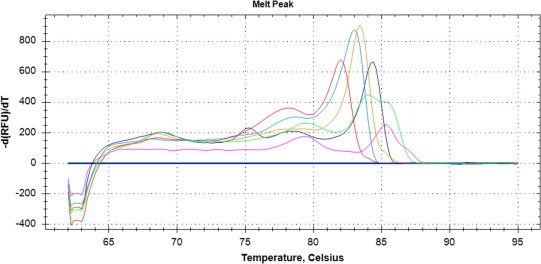
Melt curves for unknown samples. Each peak shows the melting temperature for a different coccidian species. Red: Sarcocystis (cervid), Blue: Neospora/Hammondia, Orange: Sarcocystis (avian), Black: Cystoisospora, Green: Eimeria sp., Pink: Eimeria sp. The horizontal axis indicates melting temperature (°C) and the vertical axis [−d(RFU)/dT] is related to the amount of DNA present.
Fig. 3.
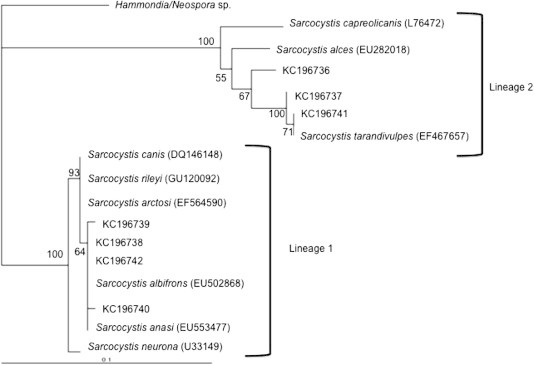
Phylogenetic tree showing relationship of Sarcocystis spp. detected in this study with existing reference sequence data in Genbank.
Fig. 4.
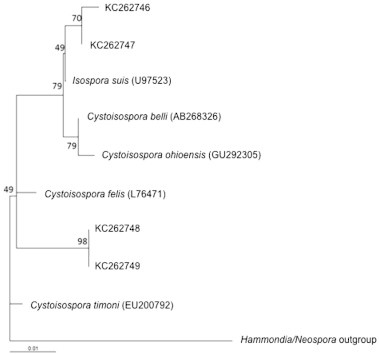
Phylogenetic tree showing relationship of Cystoisospora spp. detected in this study with existing reference sequence data in Genbank.
Discussion
These results show that arctic foxes in the Karrak Lake ecosystem host a number of potentially undescribed coccidian genotypes or species and are routinely infected with parasites common to wild canids. As in other studies of arctic foxes, T. leonina was the most abundant helminth, and in general is the most prevalent ascarid nematode in Arctic carnivores (Eaton and Secord, 1979, Kapel and Nansen, 1996, Aguirre et al., 2000, Meijer et al., 2011, Rausch and Fay, 2011). Notably absent were Toxocara canis ascarids, which are common in canids in temperate regions but have not been detected above the Arctic Circle in North America, likely due to the low freeze-tolerance of the parasite ova (O’Lorcain, 1995, Jenkins et al., 2011). The hookworm eggs observed by fecal flotation were significantly collapsed due to freezing at −80 °C. These were most likely Uncinaria sp., which has been reported occasionally in arctic foxes in Europe (Aguirre et al., 2000, Meijer et al., 2011), on St. Lawrence Island, Alaska, USA, (Rausch et al., 1990), and in domestic dogs from the Northwest Territories, Canada (Salb et al., 2008). The eggs of Ancylostoma caninum have similar morphology, but this parasite has not been reported in arctic canids. The two samples containing hookworm eggs were collected from the snow cover early in the sampling period when there was little chance of the presence of free-living nematode eggs in the soil.
We did not detect any trematodes and acanthocephalans; however, these would not necessarily have been detected with our methods. Sedimentation is a more reliable assay than flotation for detection of these species and we did not have sufficient sample quantity for both tests. Nevertheless, the absence of these parasites, which are commonly associated with marine environments, is consistent with the non-marine diet of arctic foxes at Karrak Lake, consisting mostly of birds and small mammals (Samelius et al., 2007, Meijer et al., 2011). Arctic fox in coastal regions of Greenland have a more diverse diet and parasite fauna including trematodes (Echinoparyphium sp., Plagiorchis elegans, Cryptocotyle concavum), cestodes (Diphyllobothrium dendriticum, Mesocestoides lineatus), the nematode Strongyloides stercoralis and the acanthocephalan Polymorphus sp. (Rausch et al., 1983, Kapel and Nansen, 1996).
Taeniid eggs were present in 15% of samples at low intensity on fecal flotation. However, eggs might not have been detected in either early infections due to the prepatent period or late infections in which eggs are no longer being shed (Kapel et al., 2006). On Banks Island, Northwest Territories, Canada, Eaton and Secord (1979) recovered adult T. crassiceps (78%) and E. multilocularis (2%) from the intestinal tracts of arctic foxes. Further molecular characterization to determine if the eggs in the current study were E. multilocularis or T. crassiceps was not successful; however E. multilocularis has been detected at low prevalence in the Karrak Lake fox population (Gesy, 2013).
We detected protozoa by fecal flotation, immunological, and molecular methods. The qPCR-MCA assay was especially useful in detecting protozoan species that produce small oocysts and sporocysts, which might have been missed on flotation. One exception was Cryptosporidium sp., which was detected by IFA, but not by qPCR-MCA. Alternatively, the assay might be detecting parasite DNA from prey tissue being passed through the fox in feces, and not parasite DNA that the fox is shedding as a definitive host. The ability of the qPCR-MCA to reliably detect multiple coccidia species in one sample is unknown, although the technique successfully demonstrated the amplification of multiple DNA types in validation experiments (Lalonde and Gajadhar, 2011). This might be explained by inefficient DNA extraction of oocysts, or the more pronounced DNA amplification of one parasite species over another, in which the assay was more likely to amplify the DNA of greater quantity. Although no samples with multiple melting peaks were detected by qPCR-MCA in this study, it is important to be able to differentiate one organism with multiple peaks (for example, Cryptosporidium sp. and Cyclospora sp.) with multiple peaks resulting from the presence of DNA from more than one coccidia species. This highlights the importance of including appropriate positive control DNA, against which melting temperatures can be compared. Sequencing of amplified DNA and cloning can also be important follow-up procedures to correctly identify ambiguous results.
Melt-curve analysis combined with phylogenetic analysis of sequences led to detection of multiple species of Sarcocystis, Cystoisospora, and Eimeria present in feces of arctic foxes. These results support at least two separate lineages of Sarcocystis. Samples that grouped in Lineage 1 were genetically similar to S. albifronsi (GenBank Accession no: EU502868) and S. anasi (GenBank Accession no: EU553477), which Kutkiené et al. (2012) isolated from tissue of greater white-fronted geese (Anser albifrons) and mallard ducks (Anas platyrhynchos), respectively. Samples in Lineage 2; were related to Sarcocystis spp. previously detected in cervids (Dahlgren et al., 2007). Arctic foxes are definitive hosts for both S. albifronsi and Sarcocystis capreolicanis (Gjerde, 2012, Kutkiené et al., 2012), and at Karrak Lake, foxes feed on both geese and cervids (Samelius et al., 2007).
Oocysts morphologically consistent with C. ohioensis were detected in 8% of samples. C. ohioensis oocysts are also morphologically similar to other canid coccidia, including Cystoisospora burrowsi and Cystoisospora neorivolta, and molecular diagnostic tools are necessary for species determination (Samarasinghe et al., 2008). Cystoisospora spp. detected by qPCR-MCA (Fig. 3a) could be an undescribed parasite infecting the fox, or could also be a dietary artifact from an incompletely digested prey item, such as a rodent. Additional molecular work is needed to further identify the Cystoisospora present in arctic foxes and their prey species. Infection with Cystoisospora spp. can cause diarrhea, anorexia, and weight loss in young and immunocompromised canids (Mitchell et al., 2007), which might impact fox health and survival when combined with other pathogen infections or environmental stressors.
DNA from either N. caninum or Hammondia spp. was detected in one sample in the current study. Neither N. caninum nor Hammondia spp. oocysts have been reported in North American arctic foxes, but N. caninum antibodies have been detected in a fox from Karrak Lake (Elmore, unpublished data). Other species of foxes can become infected with N. caninum and are probable definitive hosts (Hurková and Modry, 2006, Wapenaar et al., 2006); multiple fox species are known definitive hosts for Hammondia spp. (Gjerde and Dahlgren, 2011). The melting temperature of the positive sample matched the N. caninum positive control, however we did not have Hammondia sp. positive control for comparison, so diagnosis could not be made based on qPCR-MCA. To distinguish the two species, Hammondia and Neospora-specific PCR assays targeting different loci would be necessary. Because the identification was made by qPCR-MCA and oocysts were not observed, it is possible that the Neospora/Hammondia DNA could be from the passage of ingested prey containing the parasite, and not from parasite shedding by the fox.
Giardia sp. and Cryptosporidium sp. were present in arctic foxes from Karrak Lake, a novel description for this host species and region. Zoonotic genotypes of Giardia appear to be established in arctic wildlife, while Cryptosporidium is thought to be uncommon, or at least not commonly detected, at Arctic latitudes (Jenkins et al., 2013). Kutz et al. (2009) detected Giardia duodenalis Assemblage A in muskoxen from Banks Island, Northwest Territories, Canada, while Olson et al. (1997), reported the presence of Giardia cysts in ringed seals (Phoca hispida) from Holman, Northwest Territories, Canada. The few reports of Cryptosporidium in northern canids are limited to domestic dogs from British Columbia and Saskatchewan (Himsworth et al., 2010, Bryan et al., 2011, Schurer et al., 2012), but Siefker et al. (2002) detected a novel genotype of Cryptosporidium in Alaskan caribou and Dixon et al. (2008) found the parasite in the intestinal contents of ringed seals. The results of the current study suggest that IFA remains a highly sensitive and specific method of detection of these protozoan parasites compared to fecal flotation. There was no test agreement between IFA and qPCR-MCA for detection of Cryptosporidium oocysts. This could have been due to the low oocyst concentration, the small amount of sample used for the qPCR-MCA as compared to the IFA, or inefficient DNA extraction from the robust oocysts.
Agreement between the three tests used, fecal flotation, IFA, and qPCR-MCA, was low, but the combination of tests increased coccidia detection. The presence of PCR inhibitors, low oocyst counts, and inefficient DNA isolation technique are potential reasons for the failure of qPCR-MCA to detect fecal flotation positive samples. Conversely, a low oocyst quantity and loss of structural integrity after freeze/thaw cycles might lead to a positive PCR result and lack of detection on fecal flotation. PCR also offers the potential to capture free DNA in feces after parasite egg rupture.
Parasites that are not known to infect canids (Eimeria spp., Anoplocephalidae-like) were detected in fox feces in the current study. This reflects the limitations of fecal surveys in wild carnivore hosts and the need to interpret results with a broad understanding of parasite life cycles and trophic interactions. It is possible that these oocysts and eggs were present in rodent or bird prey and passed through the fox gastrointestinal tract with other undigested prey tissue (Chu et al., 2004, Stronen et al., 2011). This is supported by the qPCR-MCA and subsequent phylogenetic analysis, which demonstrated that the Eimeria spp. present in arctic fox feces were most closely related to species such as E. myoxi that infect rodents. Gastrointestinal scraping or histopathology would be necessary to determine if the Eimeria spp. were truly parasitizing the foxes. Skirnisson et al. (1993) detected either Eimeria sp. or Isospora sp. in the microvilli of the small intestine of arctic foxes.
The anoplocephalid-like tapeworm eggs, morphologically distinct from eggs from tapeworms such as Mesocestoides sp. and taeniids, for which foxes are known definitive hosts, were most likely artifacts in rodent prey species. Lemmings (Dicrostonyx and Lemmus spp.) host multiple species of Paranoplocephala (Haukisalmi et al., 2001, Haukisalmi et al., 2006), Anoplocephaloides spp., and Hymenolepis spp. (Hymenolepididae; Haukisalmi and Henttonen, 2001). Molecular techniques, in addition to parasitological studies of rodent carcasses, are needed to further characterize these tapeworm eggs in foxes from Karrak Lake.
This study broadened our understanding of parasite communities of arctic foxes from the central Canadian Arctic and serves as a record for comparison to future parasite work in this rapidly changing region. A more complete parasitological study of arctic foxes at Karrak Lake would require gastrointestinal tracts from trapped or euthanized animals, which is logistically challenging in this remote environment. Overall, the qPCR-MCA assay using a universal primer set that targets multiple coccidia species was a useful surveillance method that improved prevalence estimates and also allowed for DNA sequence analysis to determine identity and relationships of coccidian parasites. Combining traditional fecal flotation techniques with immunological and molecular assays offers more powerful methods to characterize the parasite fauna of remote and endangered wildlife species in non-invasive, fecal-based surveys, and also offer insights into trophic relationships of wild carnivores and their prey species.
Acknowledgements
The authors would like to thank D. Kellett and R. Kerbes for field logistics and assistance, D. Stern for hospitality in Cambridge Bay, B. Wagner for laboratory assistance, B. Chaban for assistance with data analysis. Funding for this Project was provided by the National Sciences and Engineering Research Council, the Western College of Veterinary Medicine Wildlife Health Research Fund, the Northern Scientific Training Program, and the University of Saskatchewan President’s NSERC Bridging Fund.
Contributor Information
Stacey A. Elmore, Email: stacey.elmore@usask.ca.
Laura F. Lalonde, Email: laura.lalonde@inspection.gc.ca.
Gustaf Samelius, Email: gustaf.samelius@slu.se.
Ray T. Alisauskas, Email: ray.alisauskas@ec.gc.ca.
Alvin A. Gajadhar, Email: alvin.gajadhar@inspection.gc.ca.
Emily J. Jenkins, Email: emily.jenkins@usask.ca.
References
- Aguirre A.A., Angerbjörn A., Tannerfeldt M., Mörner T. Health evaluation of arctic fox (Alopex lagopus) cubs in Sweden. J. Zoo. Wildl. Med. 2000;31(1):36–40. doi: 10.1638/1042-7260(2000)031[0036:HEOAFA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Alisauskas R.T., Charlwood J.W., Kellett D.K. Vegetation correlates of the history and density of nesting by Ross’s geese and lesser snow geese at Karrak Lake. Nunavut. Arctic. 2006;59:201–210. [Google Scholar]
- Alisauskas R.T., Leafloor J.O., Kellett D.K. In: Evaluation of Special Management Measures for Midcontinent Lesser Snow Geese and Ross’s Geese. Leafloor J.O., Moser T.J., Batt B.J.D., editors. Arctic Goose Joint Venture Special Publication, US Fish and Wildlife Service/Canadian Wildlife Service, Ottawa, Ontario; Washington, DC/Ottawa, Ontario: 2012. Population status of midcontinent lesser snow geese and Ross’s geese following special conservation measures. [Google Scholar]
- Anderson R.M., May R.M. Regulation and stability of host-parasite populations: I. Regulatory Processes. J. Anim. Ecol. 1978;47(1):219–247. [Google Scholar]
- Bantle J.L., Alisauskas R.T. Spatial and temporal patterns in arctic fox diets at a large goose colony. Arctic. 1998;51:231–236. [Google Scholar]
- Bryan H.M., Darimont C.T., Paquet P.C., Ellis J.A., Goji N., Gouix M., Smits J.E. Exposure to infectious agents in dogs in remote coastal British Coumbia: possible sentinels of diseases in wildlife and humans. Can. J. Vet. Res. 2011;75(1):11–17. [PMC free article] [PubMed] [Google Scholar]
- Chaban B., Hill J.E. A ‘universal’ type II chaperonin PCR detection system for the investigation of Archaea in complex microbial communities. ISME J. 2012;6:430–439. doi: 10.1038/ismej.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choquette L.P.E., MacPherson A.H., Cousineau J.G. Note on the occurrence of Echinococcus multilocularis Leuckart, 1863 in the arctic fox in Canada. Can. J. Zool. 1962;40:1167. [Google Scholar]
- Chu D.T., Sherchand J.B., Cross J.H., Orlandi P.A. Detection of Cyclospora cayetanensis in animal fecal isolates from Nepal using a FTA filter-base polymerase chain reaction method. Am. J. Trop. Med. Hyg. 2004;71(4):373–379. [PubMed] [Google Scholar]
- Dahlgren S.S., Gjerde B., Skirnisson K., Gudmundsdottir B. Morphological and molecular identification of three species of Sarcocystis in reindeer (Rangifer tarandus tarandus) in Iceland. Vet. Parasitol. 2007;149(3–4):191–198. doi: 10.1016/j.vetpar.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Da Silva A.J., Bornay-Llinares F.J., Moura I.N.S., Slemenda S.B., Tuttle J.L., Pieniazek N.J. Fast and reliable extraction of protozoan parasite DNA from fecal specimens. Mol. Diagn. 1999;4(1):57–64. doi: 10.1016/s1084-8592(99)80050-2. [DOI] [PubMed] [Google Scholar]
- Didiuk, A.B., Ferguson, R.S., 2005. Land cover mapping of the Queen Maud Gulf Migratory Bird Sanctuary. Canadian Wildlife Service Occasional Paper Number 111, Ottawa, Ontario.
- Dixon B.R., Parrington L.J., Partenteau M., Leclair D., Santin M., Fayer R. Giardia duodenalis and Cryptosporidium spp. in the intestinal contents of ringed seals (Phoca hispida) and bearded seals (Erignathus barbatus) in Nunavik, Quebec, Canada. J. Parasitol. 2008;94(5):1161–1163. doi: 10.1645/GE-1485.1. [DOI] [PubMed] [Google Scholar]
- Eaton R.D.P., Secord D.C. Some intestinal parasites of arctic fox, Banks Island, N.W.T. Can. J. Comp. Med. 1979;43:229–230. [PMC free article] [PubMed] [Google Scholar]
- Foreyt W.J., editor. Veterinary Parasitology Reference Manual. Iowa State University Press; Ames: 2001. [Google Scholar]
- Gesy, K., 2013. The geographic distribution and genetic variation of Echinococcus multilocularis in Canada. M.Sc. Thesis, University of Saskatchewan, Saskatchewan, Canada.
- Gjerde B., Dahlgren S.S. Hammondia triffittae n. of foxes (Vulpes spp.): biological and molecular characteristics and differentiation from Hammondia heydorni of dogs. Parasitology. 2011;138(3):303–321. doi: 10.1017/S0031182010001265. [DOI] [PubMed] [Google Scholar]
- Gjerde B. Morphological and molecular characterization and phylogenetic placement of Sarcocystis capreolicanis and Sarcocystis silva n. sp. from roe deer (Capreolus capreolus) in Norway. Parasitol. Res. 2012;110:1225–1237. doi: 10.1007/s00436-011-2619-6. [DOI] [PubMed] [Google Scholar]
- Haukisalmi V., Henttonen H. Biogeography of helminth parasitism in Lemmus Link (Arvicolinae), with the description of Paranoplocephala fellmani n. sp. (Cestoda: Anoplocephalidae) from the Norweigan lemming L. lemmus (Linnaeus) Syst. Parasitol. 2001;49:7–22. doi: 10.1023/a:1010778504559. [DOI] [PubMed] [Google Scholar]
- Haukisalmi V., Wickström L.M., Hantula J., Henttonen H. Taxonomy, genetic differentiation and Holarctic biogeography of Paranoplocephala spp. (Cestoda: Anoplocephalidae) in collared lemmings (Dicrostonyx; Arvicolinae) Biol. J. Linn. Soc. 2001;74:171–196. [Google Scholar]
- Haukisalmi V., Henttonen H., Hardman L.M. Taxonomy and diversity of Paranoplocephala spp. (Cestoda: Anoplocephalidae) in voles and lemmings of Beringia, with a description of three new species. Biol. J. Linn. Soc. 2006;89(2):277–299. [Google Scholar]
- Hersteinsson P., McDonald D.W. Some comparisons between red and Arctic foxes, Vulpes vulpes and Alopex lagopus, as revealed by radio-tracking. Symp. Zool. Soc. Lond. 1982;49:259–289. [Google Scholar]
- Himsworth C.G., Skinner S., Chaban B., Jenkins E.J., Wagner B.A., Harms N.J., Leighton F.A., Thompson R.C.A., Hill J.E. Multiple zoonotic pathogens identified in canine feces collected from a remote Canadian indigenous community. Am. J. Trop. Med. Hyg. 2010;83(2):338–341. doi: 10.4269/ajtmh.2010.10-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurková L., Modry D. PCR detection of Neospora caninum, Toxoplasma gondii and Encephalitozoon cuniculi in brains of wild carnivores. Vet. Parasitol. 2006;137:150–154. doi: 10.1016/j.vetpar.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Ims R.A., Fuglei E. Trophic interaction cycles in tundra ecosystems and the impact of climate change. Bioscience. 2005;55(4):311–322. [Google Scholar]
- Jenkins E.J., Schurer J.M., Gesy K.M. Old problems on a new playing field: helminth zoonoses transmitted among dogs, wildlife, and people in a changing northern climate. Vet. Parasitol. 2011;182(1):54–69. doi: 10.1016/j.vetpar.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Jenkins E.J., Castrodale L.J., de Rosemond S., Dixon B., Elmore S.A., Gesy K.M., Hoberg E. Tradition and transition: parasitic zoonoses in people and animals in northern North American and Greenland. Adv. Parasitol. 2013;82:361–362. doi: 10.1016/B978-0-12-407706-5.00002-2. [DOI] [PubMed] [Google Scholar]
- Kapel C.M.O., Nansen P. Gastrointestinal Helminths of arctic foxes (Alopex lagopus) from different bioclimatological regions in Greenland. J. Parasitol. 1996;82(1):17–24. [PubMed] [Google Scholar]
- Kapel C.M.O., Torgerson P.R., Thompson R.C.A., Deplazes P. Reproductive potential of Echinococcus multilocularis in experimentally infected foxes, dogs, raccoon dogs, and cats. Int. J. Parasitol. 2006;36:79–86. doi: 10.1016/j.ijpara.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Kutkiené L., Prakas P., Sruoga A., Butkauskas D. Description of Sarcocystis anasi sp. nov. and Sarcocystis albifronsi sp. nov. in birds of the order Anseriformes. Parasitol. Res. 2012;110:1043–1046. doi: 10.1007/s00436-011-2588-9. [DOI] [PubMed] [Google Scholar]
- Kutz S.J., Jenkins E.J., Veitch A.M., Ducroq J., Polley L., Elkin B., Lair S. The Arctic as a model for anticipating, preventing, and mitigating climate change impacts on host-paraite interactions. Vet. Parasitol. 2009;163:217–228. doi: 10.1016/j.vetpar.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Lalonde L.F., Gajadhar A.A. Detection and differentiation of coccidian oocysts by real-time PCR and melting curve analysis. J. Parasitol. 2011;97(4):725–730. doi: 10.1645/GE-2706.1. [DOI] [PubMed] [Google Scholar]
- Meijer T., Mattson R., Angerbjörn A., Osterman-Lind E., Fernández-Aguilar X., Gavier-Widén D. Endoparasites in the endangered Fennoscandian population of arctic foxes (Vulpes lagopus) Eur. J. Wildl. Res. 2011;57:923–927. [Google Scholar]
- Mitchell S.M., Zajac A.M., Charles S., Duncan R.B., Lindsay D.S. Cystoisospora canis Nemeseri, 1959 (Syn. Isospora canis), infections in dogs: Clinical signs, pathogenesis, and reproducible clinical disease in beagle dogs fed oocysts. J. Parasitol. 2007;93(2):345–352. doi: 10.1645/GE-1024R.1. [DOI] [PubMed] [Google Scholar]
- O’Lorcain P. The effects of freezing on the viability of Toxocara canis and T. cati embryonated eggs. J. Helminthol. 1995;69(2):169–171. doi: 10.1017/s0022149x00014073. [DOI] [PubMed] [Google Scholar]
- Olson M.E., Roach P.D., Stabler M., Chan W. Giardiasis in ringed seals from the western Arctic. J. Wildl. Dis. 1997;33(3):646–648. doi: 10.7589/0090-3558-33.3.646. [DOI] [PubMed] [Google Scholar]
- Rausch R.L. Studies on the helminth fauna of Alaska. XXX. The occurrence of Echinococcus multilocularis Leuckart, 1863, on the mainland of Alaska. Am. J. Trop. Med. Hyg. 1956;5(6):1086–1092. doi: 10.4269/ajtmh.1956.5.1086. [DOI] [PubMed] [Google Scholar]
- Rausch R.L., Fay F.H., Williamson F.S.L. Helminths of arctic fox, Alopex lagopus (L.), in Greenland. Can. J. Zool. 1983;61:1847–1851. [Google Scholar]
- Rausch R.L., Fay F.H., Williamson F.S. The ecology of Echinococcus multilocularis (Cestoda: Taeniidae) on St. Lawrence Island, Alaska. II. Helminth populations in the definitive host. Ann. Parasitol. Hum. Comp. 1990;65(3):131–140. doi: 10.1051/parasite/1990653131. [DOI] [PubMed] [Google Scholar]
- Rausch R.L., Fay F.H. Toxascaris leonina in rodents, and relationship to eosinophilia in a human population. Comp. Parasitol. 2011;78:236–244. [Google Scholar]
- Ryder J.P. Biology of nesting Ross’ geese. Ardea. 1972;60:185–215. [Google Scholar]
- Salb A.L., Barkema H.W., Elkin B.T., Thompson R.C.A., Whiteside D.P., Black S.R., Dubey J.P., Kutz S.J. Dogs as sources and sentinels of parasites in humans and wildlife, northern Canda. Emerg. Infect. Dis. 2008;14(1):60–63. doi: 10.3201/eid1401.071113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarasinghe B., Johnson J., Ryan U. Phylogenetic analysis of Cystoisospora species at the rRNA ITS1 locus and development of a PCR–RFLP assay. Exp. Parasitol. 2008;118(4):592–595. doi: 10.1016/j.exppara.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Samelius G., Alisauskas R.T., Hobson K.A., Lariviére S. Prolonging the arctic pulse: long-term exploitation of cached eggs by arctic foxes when lemmings are scarce. J. Anim. Ecol. 2007;76:873–880. doi: 10.1111/j.1365-2656.2007.01278.x. [DOI] [PubMed] [Google Scholar]
- Samelius G., Alisauskas R.T., Lariviére S. Seasonal pulses of migratory prey and annual variation in small mammal abundance affect abundance and reproduction by arctic foxes. Polar Biol. 2011;34:1475–1484. [Google Scholar]
- Schurer J.M., Hill J.E., Fernando C., Jenkins E.J. Sentinal surveillance for zoonotic parasites in companion animals in indigenous communities of Saskatchewan. Am. J. Trop. Med. Hyg. 2012;87(3):495–498. doi: 10.4269/ajtmh.2012.12-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefker C., Rickard L.G., Pharr T., Simmons J.S., O’Hara T.M. Molecular characterization of Cryptosporidium sp. isolated from northern Alaskan caribou (Rangifer tarandus) J. Parasitol. 2002;88(1):213–216. doi: 10.1645/0022-3395(2002)088[0213:MCOCSI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Skirnisson K., Eydal M., Gunnarsson E., Hersteinsson P. Parasites of the arctic fox (Alopex lagopus) in Iceland. J. Wildl. Dis. 1993;29(3):440–446. doi: 10.7589/0090-3558-29.3.440. [DOI] [PubMed] [Google Scholar]
- Stronen A.V., Sallows T., Forbes G.J., Wagner B., Paquet P.C. Diseases and parasites in wolves of the Riding Mountain National Park Region, Manitoba, Canada. J. Wildl. Dis. 2011;47(1):222–227. doi: 10.7589/0090-3558-47.1.222. [DOI] [PubMed] [Google Scholar]
- Thompson R.C.A., Colwell D.D., Shury T., Appelbee A.J., Read C., Njiru Z., Olson M.E. The molecular epidemiology of Cryptosporidium and Giardia infections in coyotes from Alberta, Canada, and some observations on cohabiting parasites. Vet. Parasitol. 2009;159(2):167–170. doi: 10.1016/j.vetpar.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Wapenaar W., Jenkins M.C., O’Handley R.M., Barkema H.W. Neospora caninum-like oocysts observed in the feces of free-ranging red foxes (Vulpes vulpes) and coyotes (Canis latrans) J. Parasitol. 2006;92(6):1270–1274. doi: 10.1645/GE-913R.1. [DOI] [PubMed] [Google Scholar]


