Abstract
Background
Recently, the sigma-1 receptor has been shown to play a significant role in the neural transmission of mood by regulating N-methyl-D-aspartate receptors. Additionally, the sigma-1 receptor has been reported to influence cognitive functions including learning and memory. In this study, we measured plasma sigma-1 receptor concentrations before and after antidepressant treatment in patients with late-life major depressive disorder (MDD) and explored whether changes in depressive status are related to sigma-1 receptor concentrations.
Methods
The study participants were 12 subjects with late-life MDD diagnosed according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. All of the participants were over 60 years old. Immediately prior to and 8 weeks after the start of treatment, sigma-1 receptor concentration and mental status, including depressive symptoms (Hamilton Depression Rating Scale; HAM-D), were measured. Treatment for depression was performed according to a developed algorithm based on the choice of treatments. We examined the association between changes in sigma-1 receptor concentration and HAM-D scores during antidepressant treatment. For the measurement of plasma sigma-1 receptor concentration, blood plasma samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis. Western blots were performed using a specific antibody that acts against the sigma-1 receptor, and the net densities of each band were quantified.
Results
All participants showed improvement in depressive symptoms, which was indicated by a significant decrease in the HAM-D scores. The mean plasma sigma-1 receptor concentration also increased significantly following antidepressant treatment. However, no significant correlations were found between changes in plasma sigma-1 receptor concentration and changes in HAM-D scores.
Conclusion
In this preliminary study, we demonstrated that the sigma-1 receptor concentration in plasma increases following antidepressant treatment in patients with late-life MDD. Further studies are warranted to confirm this finding with a larger number of patients.
Keywords: sigma-1 receptor, late-life depression, depressive symptoms, antidepressant treatment
Introduction
Among various biological markers proposed for mood disorders, the recently identified sigma-1 receptor is promising, as it is known to play a significant role in neural transmission of mood by regulating N-methyl-D-aspartate (NMDA) receptors, thereby modulating glutamate activity.1 The sigma-1 receptor was originally proposed by Martin et al2 in the 1970s as an opioid receptor subtype. Subsequent pharmacological studies have demonstrated that the sigma-1 receptor is unique and distinct among opioid receptors.3,12 The sigma-1 receptor, which has a molecular weight of 25 kDa, is a protein consisting of 223 amino acids with two transmembrane regions, and it is mainly found in the endoplasmic membrane. In addition to its receptor functions, the sigma-1 receptor has recently been shown to function as a chaperone to stabilize the three-dimensional structure of inositol trisphosphate 3 receptors on the endoplasmic membrane, thereby facilitating intracellular adenosine triphosphate production.3–13 The sigma-1 receptor exists in a wide range of human tissues from the central nervous system to the peripheral organs, including the brain, liver, sexual glands, kidney, immune organs, and retina.14
In the brain, the sigma-1 receptor is specifically distributed in the hippocampus and amygdala, and it plays a particularly important role in neural formation, the induction of differentiation, and in spinal cord formation.14,15 In addition, the sigma-1 receptor has significant affinity for a wide range of pharmacological agents including antidepressants, antipsychotics, antidementia drugs, antiepileptics, and other psychotrophic drugs.10,16,17 The physiological functions of the sigma-1 receptor at the cellular level includes regulation of: 1) Ca2+ channels in NMDA receptors and endoplasmic membranes; 2) K+ channels; 3) free neurotransmitters including dopamine; 4) cellular differentiation; 5) regulation of intracellular lipid distribution; 6) behavioral sensitization to cocaine and amphetamines; and 7) cognitive functions.14 Studies at the molecular level are ongoing to further elucidate the physiological functions of sigma-1 receptors.
Regarding the pathogenesis of depression, recent neuroimaging studies have demonstrated atrophy of the hippo-campus and prefrontal cortices in patients with depression, and postmortem studies have demonstrated neuronal cell loss in the hippocampus in depressed patients, as well as a decrease in the number of glial cells in the prefrontal cortices, implicating these regions as pathognomonic substrates of depression.18–20 In addition, it has been suggested that antidepressant effects are brought about by neurogenesis or nerve growth factors, including brain-derived neurotrophic factor and insulin-like growth factor-1, and neurogenesis has been proposed as a cellular-level model for recovery from depression.21
Decreased sigma-1 receptor activity appears to play a crucial role in the pathophysiology of depression at the level of neurogenesis. In animal studies, sigma-1 receptor knockout mice showed increased immobility in a forced swimming test as a depressive like phenotype, and an agonist of the sigma-1 receptor showed antidepressant effects in animal models, including on the forced swimming test and during a tail-hanging test.22–26
Although insight into the relationship between sigma-1 receptors and depression has been gained from investigations in animals, only a limited number of studies have examined the relationship in humans. Takebayashi et al26 measured the sigma-1 receptor concentration in human plasma and demonstrated that it was decreased in adult male patients with major depressive disorder (MDD) as compared with healthy controls. However, to the best of our knowledge, there have been no human studies that have examined whether the sigma-1 receptor concentration in plasma increases following treatment with antidepressants.
In this study, we measured plasma sigma-1 receptor concentrations before and after treatment in patients with late-life MDD. The main purpose was to determine whether sigma-1 receptor concentrations change as a result of antidepressant treatment. In addition, we explored whether changes in depressive status are related to sigma-1 receptor concentrations. We hypothesized that sigma-1 receptor concentrations would be increased by antidepressant treatment, and that the change in concentration would be correlated with improvements in depressive symptoms.
Methods
Participants
The participants were 12 individuals (ten women and two men) with late-life depression who were either outpatients or hospitalized at Showa University Karasuyama Hospital in Tokyo, Japan, and who were diagnosed with MDD according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision.27 All participants were over 60 years old and the mean age was 74.9 (standard deviation [SD] =5.6) years. The mean duration of the newest episode was 13.9 (SD =12.6) months and the mean duration of the illness was 111.2 (SD =184.3) months. The cohort included eight first-episode patients and four patients who had more than two episodes. None of the subjects had been receiving antidepressants at the time of study entry. The subjects were excluded if they had any significant physical illness, neurological illness, history of drug abuse, other comorbidities, or a past history of psychiatric illness, severe cognitive decline on the Mini-Mental State Examination score (score <22),28 or comorbid personality disorders. All the participants received a head computed tomography scan that demonstrated no significant abnormality. All participants, and their family members when appropriate, gave written informed consent prior to enrollment. The study was approved by the Ethics Committee of Showa University.
Assessment of and treatment for psychiatric symptoms
Immediately prior to and 8 weeks after the start of treatment, mental status examinations – including an assessment of depressive symptoms – were performed. Depressive symptoms were measured using the 17-item Hamilton Depression Rating Scale (HAM-D).29 Treatment for depression was performed according to an algorithm that was developed in Japan for the treatment of mood disorders, which addressed the choice of antidepressant and nonpharmacological treatment.30
Measurement of plasma sigma-1 receptor concentration
The sigma-1 receptor concentration was also measured immediately prior to and 8 weeks after the start of treatment. Blood samples were drawn in ethylenediaminetetraacetic acid-coated Vacutainer® tubes (BD, Franklin Lakes, NJ, USA) and immediately transferred to cryotubes for storage at −80°C prior to being assayed. Each blood sample was diluted 30-fold with ice-cold phosphate-buffered saline and solubilized in sample buffer (100 mM Tris-HCl [pH 6.8], 20% glycerol, 4% sodium dodecyl sulfate [SDS]). The concentration of protein in the samples was standardized by dilution with sample buffer. Total amounts of proteins in each sample were measured by a bicinchoninic acid kit (Pierce Chemical Co, Rockford, IL, USA), and were adjusted to the same amount of 100 μg for all samples. The samples were then treated with 1,4-dithiothreitol and boiled for 5 minutes. Proteins in the samples were separated by SDS–polyacrylamide gel electrophoresis and transblotted to polyvinylidene difluoride membranes. The membranes were blocked with 5% (w/v) skim milk for 6 hours at 4°C, and they were incubated with a specific antibody against guinea pig sigma-1 receptor (a gift from Dr Su, National Institute on Drug Abuse/National Institutes of Health) overnight at 4°C. After washing, the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 1 hour at room temperature. Chemiluminescent detection was performed using an Immun-Star™ WesternC™ kit (Bio-Rad Laboratories, Hercules, CA, USA), and the net intensities of each band were quantified using the ChemiDoc™ XRS molecular imaging system (Bio-Rad Laboratories).
Data analysis
Analyses were performed using the Statistical Package for the Social Sciences version 17 statistical software (IBM Corporation, Armonk, NY, USA). Changes in sigma-1 receptor concentrations and HAM-D scores prior to treatment and at 8 weeks posttreatment were compared by paired t-test. Correlations between changes in sigma-1 receptor concentrations and HAM-D scores were determined using Pearson’s correlations.
Results
None of the 12 participants dropped out of the study, and all showed improvement in depressive symptoms. Eight patients received selective serotonin reuptake inhibitors, two received selective serotonin and noradrenaline reuptake inhibitors, and three patients received a noradrenergic and specific serotonergic antidepressant. A total of two patients received multiple antidepressants, and one received an antipsychotic agent. In addition, one patient received modified electroconvulsive therapy. None of the patients showed significant adverse events requiring discontinuation of pharmacotherapy, and none received nonpharmacological treatment aside from ordinary psychotherapy (Table 1).
Table 1.
Clinical data for and treatment of the individual patients
| Patient number | Age (years) | Sex | Duration of the newest episode (months) | Duration of the illness (months) | Medication |
|---|---|---|---|---|---|
| 1 | 68 | Female | 12 | 12 | Fluvoxamine 150 mg |
| 2 | 77 | Male | 3 | 3 | Mirtazapine 30 mg Electroconvulsive therapy |
| 3 | 77 | Female | 10 | 10 | Paroxetine 40 mg |
| 4 | 70 | Female | 10 | 480 | Fluvoxamine 200 mg Sulpiride 100 mg |
| 5 | 80 | Female | 20 | 240 | Mirtazapine 45 mg |
| 6 | 80 | Female | 14 | 14 | Paroxetine 40 mg |
| 7 | 81 | Female | 2 | 480 | Milnacipran 150 mg Sulpiride 150 mg Mirtazapine 30 mg Aripiprazole 6 mg |
| 8 | 79 | Female | 14 | 14 | Duloxetine hydrochloride 60 mg |
| 9 | 81 | Male | 2 | 48 | Sertraline 75 mg |
| 10 | 70 | Female | 24 | 24 | Fluvoxamine 100 mg |
| 11 | 69 | Female | 4 | 4 | Sertraline 50 mg |
| 12 | 67 | Female | 6 | 6 | Paroxetine 40 mg |
| Mean (standard deviation) | 74.9 (5.6) | 13.9 (12.6) | 111.2 (184.3) |
The mean protein band density for the sigma-1 receptor in plasma samples was 95.6 (range: 26.5–146.2; SD =33.6) before treatment and 111.4 (range: 43.9–178.3; SD =38.6) after 8 weeks of treatment; the difference between pre- and posttreatment was statistically significant (P=0.014).
Changes in the plasma sigma-1 receptor concentration for each participant are shown in Figure 1. Since the number of participants was relatively small, no significant relationship was found between individual drugs and changes in the plasma concentration of the sigma-1 receptor. The sigma-1 receptor concentration in plasma was numerically greater following treatment with each of the medications; however, none of these differences were statistically significant.
Figure 1.
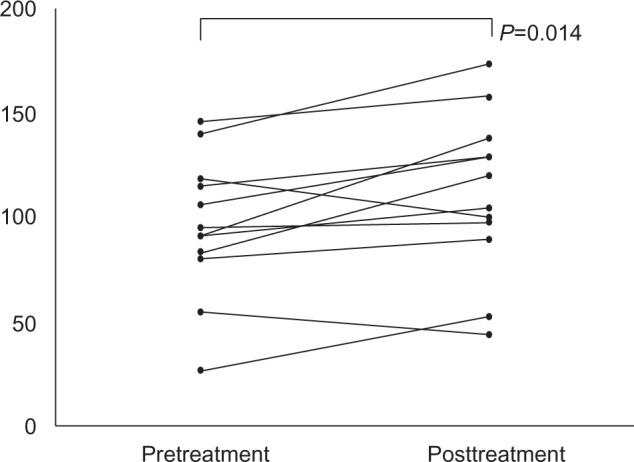
Individual values for plasma sigma-1 concentrations before and after treatment.
Notes: The mean value was 9.7 before treatment and 11.1 after treatment. There was a significant difference between the pre- and posttreatment values.
Mean HAM-D scores were 29.8 (range: 20–37; SD =5.8) before treatment and 15.3 (range: 5–30; SD =9.3) after treatment, and the difference in depressive symptoms was statistically significant (P<0.001). However, no significant correlations were found between changes in plasma sigma-1 receptor concentrations and changes in HAM-D scores (r=0.092; P=0.775).
Discussion
The present study demonstrated that plasma sigma-1 receptor concentrations increased following antidepressant treatment in patients with late-life MDD. To the best of our knowledge, this is the first study to analyze sequential changes in plasma sigma-1 receptor concentrations in the clinical setting. The results of prior studies in animals suggested the existence of a relationship between sigma-1 receptor and depression.22 Neurosteroids, including dehydroepiandrosterone, pregnenolone, and progesterone are thought to be potential endogenous ligands because neurosteroids have relatively high affinity for and act as agonists at the sigma-1 receptor.10 In addition, neurosteroids exhibit an antidepressant effect in the forced swimming test model, and that effect is blocked by NE-100, a specific sigma-1 receptor antagonist. Accordingly, neurosteroids may exert antidepressant effects via the sigma-1 receptor.25,31 Therefore, our findings may indicate that antidepressant treatments improve depressive symptoms, and that this is accompanied by an increase in the sigma-1 receptor concentration.
Although the plasma sigma-1 receptor concentration increased following antidepressant treatment, it did not show a correlation with symptoms of depression. This finding may have been due to the sample size being too small to detect a significant correlation. Further studies with larger sample sizes are needed.
The appropriate sample size for the present study, as determined by statistical power analysis using G*power version 3.1 (Heinrich Heine University, Düsseldorf, Germany) was N=27.32 Therefore, the present study should be considered as preliminary because the results may lack sufficient statistical power and reliability. In addition, because the study included more females than males, and given that the duration of the last depressive episode and the duration of the illness in males was lower, the results may be biased and not accurately representative of the effect of antidepressants on sigma-1 receptor concentration in depressed patients.
There were several additional limitations in the present study. First, treatment options were not controlled, and no conclusions could be drawn regarding the relationship between individual medications and changes in the sigma-1 receptor concentration. Second, the study examined only patients with depression and did not include a control group of healthy subjects. Previous human studies have revealed that the plasma sigma-1 receptor concentration is lower in patients with MDD than in control subjects.26 Sigma-1 receptor functioning at baseline in individuals with MDD in the present study was comparable with the results of a previous study.26 Third, depressive mood was assessed only by using HAM-D scores. Accordingly, it is not clear whether sigma-1 receptor functions affected depressive mood, concomitant anxiety, or other clinical symptoms. Fourth, the correlation between sigma-1 receptor concentration in plasma and the central nervous system is still unknown. Further studies will be needed to determine the association between sigma-1 receptor concentration in plasma and the central nervous system using another evaluation method, such as positron emission tomography. To address these points, further studies involving more participants, a controlled design, and use of detailed clinical assessment scales is warranted.
In summary, this preliminary study-demonstrated that sigma-1 receptor concentrations are increased following antidepressant treatment in patients with late-life MDD. This is the first study to analyze sequential changes in plasma sigma-1 receptor concentrations in the clinical setting. Further studies are warranted to confirm this finding in a larger cohort of patients.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bermack JE, Debonnel G. The role of sigma receptors in depression. J Pharmacol Sci. 2005;97(3):317–336. doi: 10.1254/jphs.crj04005x. [DOI] [PubMed] [Google Scholar]
- 2.Martin WR, Eades CG, Thompson JA, Huppler RE, Gilbert PE. The effects of morphine- and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976;197(3):517–532. [PubMed] [Google Scholar]
- 3.Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice KC. Sigma receptors: biology and function. Pharmacol Rev. 1990;42(4):355–402. [PubMed] [Google Scholar]
- 4.Hanner M, Moebius FF, Flandorfer A, et al. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996;93(15):8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su TP, Hayashi T. Understanding the molecular mechanism of sigma-1 receptors: towards a hypothesis that sigma-1 receptors are intracellular amplifiers for signal transduction. Curr Med Chem. 2003;10(20):2073–2080. doi: 10.2174/0929867033456783. [DOI] [PubMed] [Google Scholar]
- 6.Hayashi T, Su TP. Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther. 2003;306(2):718–725. doi: 10.1124/jpet.103.051284. [DOI] [PubMed] [Google Scholar]
- 7.Hayashi T, Su TP. Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108-15 cells. J Pharmacol Exp Ther. 2003;306(2):726–733. doi: 10.1124/jpet.103.051292. [DOI] [PubMed] [Google Scholar]
- 8.Hayashi T, Su TP. Sigma-1 receptor ligands: potential in the treatment of neuropsychiatric disorders. CNS Drugs. 2004;18(5):269–284. doi: 10.2165/00023210-200418050-00001. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi T, Su TP. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proc Natl Acad Sci U S A. 2004;101(41):14949–14954. doi: 10.1073/pnas.0402890101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takebayashi M, Hayashi T, Su TP. A perspective on the new mechanism of antidepressants: neuritogenesis through sigma-1 receptors. Pharmacopsychiatry. 2004;37(Suppl 3):S208–S213. doi: 10.1055/s-2004-832679. [DOI] [PubMed] [Google Scholar]
- 11.Hashimoto K, Ishiwata K. Sigma receptor ligands: possible application as therapeutic drugs and as radiopharmaceuticals. Curr Pharm Des. 2006;12(30):3857–3876. doi: 10.2174/138161206778559614. [DOI] [PubMed] [Google Scholar]
- 12.Maurice T, Su TP. The pharmacology of sigma-1 receptors. Pharmacol Ther. 2009;124(2):195–206. doi: 10.1016/j.pharmthera.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai SY, Hayashi T, Mori T, Su TP. Sigma-1 receptor chaperones and diseases. Cent Nerv Syst Agents Med Chem. 2009;9(3):184–189. doi: 10.2174/1871524910909030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi T, Su T. The sigma receptor: evolution of the concept in neuropsychopharmacology. Curr Neuropharmacol. 2005;3(4):267–280. doi: 10.2174/157015905774322516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alonso G, Phan V, Guillemain I, et al. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97(1):155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 16.Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240(4849):219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- 17.Bergeron R, Debonnel G, De Montigny C. Modification of the N-methyl-D-aspartate response by antidepressant sigma receptor ligands. Eur J Pharmacol. 1993;240(2–3):319–323. doi: 10.1016/0014-2999(93)90918-8. [DOI] [PubMed] [Google Scholar]
- 18.Mulkey RM, Malenka RC. Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron. 1992;9(5):967–975. doi: 10.1016/0896-6273(92)90248-c. [DOI] [PubMed] [Google Scholar]
- 19.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- 20.Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry. 2004;161(11):1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- 21.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nat Neurosci. 2007;10(9):1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 22.Sabino V, Cottone P, Parylak SL, Steardo L, Zorrilla EP. Sigma-1 receptor knockout mice display a depressive-like phenotype. Behav Brain Res. 2009;198(2):472–476. doi: 10.1016/j.bbr.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsuno K, Nakazawa M, Okamoto K, Kawashima Y, Mita S. Binding properties of SA4503, a novel and selective sigma 1 receptor agonist. Eur J Pharmacol. 1996;306(1–3):271–279. doi: 10.1016/0014-2999(96)00201-4. [DOI] [PubMed] [Google Scholar]
- 24.Ukai M, Maeda H, Nanya Y, Kameyama T, Matsuno K. Beneficial effects of acute and repeated administrations of sigma receptor agonists on behavioral despair in mice exposed to tail suspension. Pharmacol Biochem Behav. 1998;61(3):247–252. doi: 10.1016/s0091-3057(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 25.Urani A, Roman FJ, Phan VL, Su TP, Maurice T. The antidepressant-like effect induced by sigma(1)-receptor agonists and neuroactive steroids in mice submitted to the forced swimming test. J Pharmacol Exp Ther. 2001;298(3):1269–1279. [PubMed] [Google Scholar]
- 26.Takebayashi M, Hisaoka K, Maeda N, Tsuchioka M. Altered levels of whole blood sigma-1 receptor protein in patients with mood disorders; Presented at: 37th Annual Meeting of Society for Neuroscience; November 3–7, 2007; San Diego, CA. [Google Scholar]
- 27.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Text Revision. 4th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motohashi M. Algorithm for the Treatment of Mood Disorders. Tokyo, Japan: Jiho; 2003. Japanese. [Google Scholar]
- 31.Reddy DS, Kaur G, Kulkarni SK. Sigma (sigma 1) receptor mediated anti-depressant-like effects of neurosteroids in the Porsolt forced swim test. Neuroreport. 1998;9(13):3069–3073. doi: 10.1097/00001756-199809140-00028. [DOI] [PubMed] [Google Scholar]
- 32.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]


