Graphical abstract
Highlights
► New Babesia is identified in kangaroo. ► The origin is unknown. ► Caused severe anaemia and death in the infected kangaroos.
Keywords: Babesia, Kangaroo, Apicomplexan, Haematology
Abstract
The roles and epidemiological features of tick-borne protozoans are not well elicited in wildlife. Babesia spp. are documented in many domestic animals, including cattle, horses, pigs, dogs and cats. Three cases affecting eastern grey kangaroos are described. The kangaroos exhibited neurological signs, depression and marked anaemia, and microscopic examination of blood smears revealed intraerythrocytic piroplasms. One to seven intraerythrocytic spherical, oval, pyriform and irregularly-shaped parasites consistent with Babesia spp. were seen in the blood smears and the percentage of infected erythrocytes was estimated to be approximately 7% in each case. Data suggest that the tick vector for this kangaroo Babesia sp. is a Haemaphysalis species. For Case 2, ultrastructural examination of the erythrocytes of the renal capillaries showed parasites resembling Babesia spp. and 18 of 33 erythrocytes were infected. DNA sequencing of the amplified 18S rDNA confirmed that the observed intraerythrocytic piroplasms belong to the genus Babesia. The phylogenetic position of this new kangaroo Babesia sp. (de novo Babesia macropus), as a sister species to the new Australian woylie Babesia sp., suggests a close affinity to the described Afro–Eurasian species Babesia orientalis and Babesia occultans suggesting perhaps a common ancestor for the Babesia in kangaroos.
1. Introduction
Babesia species are arthropod-transmitted intraerythrocytic apicomplexan protozoans that infect a wide range of vertebrates (Ristic, 1988). Babesia infection can be subclinical or symptomatic, causing haemolytic anaemia, depression, diarrhoea, neurological signs and abortion. Babesia spp. are reported in many domestic animals, including cattle, horses, pigs, dogs and cats (Ristic, 1988).
Babesia bigemina and Babesia bovis in cattle are the most important pathogenic Babesia spp. in domestic animals (Ristic, 1988). Babesia spp. have also been detected in a wide range of wildlife, including mongooses, hyenas, weasels, polecats, raccoons, elephants, foxes and deer. Nevertheless, most of the detected wildlife Babesia spp. produce no clinical signs (Ristic, 1988). The pathogenic importance of Babesia spp. in wild animals is still uncertain (Penzhorn and Chaparro, 1994).
Babesia sp. have been described in several species of marsupials, including Babesia thylacis in the southern brown bandicoot (Isoodon obesulus) (Mackerras, 1959, Clark et al., 2004), the agile antechinus (Antechinus agilis), brown antechinus (Antechinus stuartii), Proserpine rock wallaby (Petrogale persephone) (O’Donoghue, 1997, O’Donoghue and Adlard, 2000, Clark et al., 2004) and woylies (Woylieia penicillata ogilbyi) (Paparini et al., 2012). Babesia tachyglossi and an unspeciated Babesia sp. have also been reported in the short-beaked echidna (Tachyglossus aculeatus) (Backhouse and Bolliger, 1957, Backhouse and Bolliger, 1959, Mackerras, 1959, Bolliger and Backhouse, 1960).
The current study describes a novel Babesia sp. in eastern grey kangaroos [Family Macropodidae] in Australia, supported by DNA sequencing and electronmicroscopy.
2. Material and methods
2.1. Cases
2.1.1. Case 1
In February 2006, an eastern grey kangaroo (gender unknown) tended by a carer from Fordsdale (92 km west of Brisbane, capital of the State of Queensland), South Queensland, was presented at a veterinary clinic with oedema in the cloacal region and a history of dehydrated, small faecal pellets. Blood smears and a sample of blood collected into EDTA were submitted to the Animal Disease Surveillance Laboratory, Toowoomba, Queensland for analysis. The blood smears were stained with Giemsa and examined microscopically. The kangaroo recovered after unspecified treatment. Two adult ticks were removed from the kangaroo’s skin and identified by the carer, but not formally submitted to a reference laboratory. Specimens of ticks from kangaroos being cared for by the same carer were submitted to the Biosecurity Sciences Laboratory, Coopers Plains, Queensland for identification.
2.1.2. Case 2
In March 2011, a 14 month old male eastern grey kangaroo, weighing 7.9 kg, from a fenced property at Bingie (257 km south-southwest of Sydney, capital of the State of New South Wales) on the south coast of New South Wales (NSW) was first presented to the Moruya Veterinary Hospital, Moruya, southern NSW. The kangaroo had swollen eyes with an increase in intraocular pressure. Blepharitis, conjunctivitis, and increased scleral vascularity were also noted. No corneal ulceration was present and no foreign bodies or ticks were found in the eyes. The pupillary light reflex was reduced in both eyes but menace response was normal in both eyes. Ticks were collected from the kangaroo but were not submitted for identification. Treatment with latanoprost (Xalatan®, Pfizer) and dexamethasone (Maxidex® 0.1%, Alcon) was initiated. The kangaroo was presented for a further examination one day later with acute-onset lethargy, inappetence and suspected blindness. The clinical examination revealed that the kangaroo was unable to stand; there was conjunctival and gingival haemorrhage with cutaneous ecchymoses on the groin and the inner thighs. The pupillary light reflex was normal in both eyes but menace response was absent. Respiration was laboured with an expiratory grunt. Shortly afterwards the kangaroo began to exhibit muscle tremor and seizure-like activity and it was then humanely euthanased. The cadaver was submitted to the State Veterinary Diagnostic Laboratory, Menangle, NSW, for necropsy and further investigations. Samples from the brain, spinal cord, eyes, heart, spleen, liver, kidney, and intestine were collected into 10% buffered formalin for histopathology. EDTA blood and serum samples were submitted to the Regional Laboratory Services, Benalla, Victoria for biochemical testing. Ultrastructural (electron) microscopy was undertaken from this case utilising blood and kidney.
2.1.3. Case 3
In July 2011, a 12 month old male eastern grey kangaroo, weighing 7 kg, from the same carer as for Case 2, was presented to Moruya Veterinary Hospital, Moruya, NSW. The kangaroo manifested lethargy, pale mucosal membranes, reduction of water and milk intake and swelling of the eyes. Ticks were not collected for identification. The kangaroo was treated with 0.17 ml Imidocarb dipropionate (Coopers®, 120 mg/mL subcutaneous), and 0.7 ml enrofloxacin (Baytril®, 100 mg/ml subcutaneous). A second dose of Imidocarb dipropionate was given 24 h later. Seizure-like activity and vocalisation was observed after a few hours. The pupillary light reflex was almost absent but menace response was positive. The kangaroo was humanely euthanased due to the signs of clinical deterioration. EDTA blood and serum samples were submitted to the Regional Laboratory Services, Benalla, Victoria for biochemical testing. The EDTA blood samples were also submitted to the Veterinary Teaching Hospital, Sydney University, Camden, for haematological analysis. Sufficient material for DNA analysis was not available for Case 3.
Cases 2 and 3 were received by the carer in August 2010 and had recently been moved into a newly constructed pen of approximately 40 × 50 m. They were orphans and were bottle-fed with Wombaroo formula and Impact colostrum supplement.
2.2. Electron microscopy
A 1 cm3 portion of formalin-fixed kidney from Case 2 was recut into 1–2 mm pieces and washed in phosphate buffered saline (PBS) 3 times for 5 min each time. The buffer was drained and the tissue was fixed in freshly prepared Karnovsky’s fixative for 6 h. The tissue was then washed in PBS 3 times for 5 min each and post-fixed in 1% buffered osmium tetroxide. The tissue was washed again in PBS and stained with 2% uranyl acetate for 30 min, then dehydrated in a graded series of different concentrations of ethanol and transferred through several changes of acetone. After incubation in 50:50 resin and acetone, the tissue was embedded in pure resin and polymerised at 70 °C for 10 h. Thin sections (80 nm) were placed on 300 mesh copper grids, stained with uranyl acetate and lead citrate, and examined using a Philips 208 transmission electron microscope.
2.3. DNA extraction, PCR and sequencing
DNA was extracted from blood samples collected from Cases 1 and 2, as well as lab strains of B. bigemina, B. bovis and Theileria sp. using a QIAamp DNA Mini Kit (Qiagen) following the manufacturer’s instructions. Details of the species and strains used in this study are provided in Table 1.
Table 1.
Parasite strains and isolates for DNA screening and testing in this study.
| Species | Vertebrate host | Strain | Lab accession | Origin (Australian state) | Genbank accession (this study) |
|---|---|---|---|---|---|
| Babesia bigemina | Cattle | G – vaccine strain | Calf 7720 | Queensland | JQ437264 |
| Babesia bigemina | Cattle | Bond strain | S38 | Queensland | JQ437261 |
| Babesia bovis | Cattle | Dixie - vaccine strain | Calf 8284 | Queensland | JQ437260 |
| Babesia bovis | Cattle | D’Aguilar | H81 | Queensland | JQ437262 |
| Theileria sp. | Cattle | S134 | J02 | NSW | JQ437263 |
| Unknown 1 | Kangaroo | Case 1, clone 1 | TVL 06-01974 | Queensland | JQ437265 |
| Unknown 2 | Kangaroo | Case 1, clone 2 | TVL 06-01974 | Queensland | JQ437266 |
| Unknown 3 | Kangaroo | Case 2 | M11-04231 | NSW | JQ437267 |
PCR assays for major piroplasm surface protein (MPSP) p32 of Theileria spp., (Tanaka et al. 1993), the MPSP p32 for the T. orientalis types Ikeda, Chitose and Buffeli (Zakimi et al., 2006), and the amplification of the cytochrome b genes of B. bovis and B. bigemina (Buling et al., 2007) were performed as described previously using control positive DNA samples (see Table 1).
Partial nuclear ribosomal 18S DNA sequences were amplified between primers A 5′-AACCTGGTTGATCCTGCCAGT-3′ and B 5′-TGATCCTTCTGCAGGTTCACCTAC-3′ (Medlin et al., 1988) with some DNA templates also requiring internal primers Kangabab18SF 5′-TGGTAATGGTTAATAGGAACGGT-3′ and Kangabab18SR 5′-GACGGTATCTGATCGTCTTCGA-3′ (designed by authors). Amplification reactions were carried out in 10 μl volumes containing 1 μM of each primer pair, combined with 10–100 ng of extracted DNA, 10× HotMaster Taq buffer (5 Prime, distributed by Quantum-Scientific Milton, Queensland, Australia, containing 25 mM magnesium), 1 mM dNTP, and 1 unit of HotMaster Taq DNA polymerase (5 Prime, distributed by Quantum-Scientific Milton, Queensland, Australia). Thermal cycling conditions consisted of an initial denaturation (95 °C for 3 min) followed by 30 cycles of 94 °C for 30 s, annealing at 53 °C for 30 s and extension at 72 °C for 1 min 30 s, with a final extension step of 72 °C for 7 min. Cycling was performed in a Mastercycler® pro (Eppendorf South Pacific, North Ryde, New South Wales, Australia). PCR products were viewed on a 1% agarose 1× TBE gel stained with GelRed (Biotium, USA). Prior to sequencing, PCR products were either desalted using Exosap-it® (USB Corporation distributed by GE Healthcare Bio-Sciences, Rydalmere NSW, Australia) and directly sequenced, or target bands were cut from an agarose gel and purified with a MinElute Gel Extraction Kit (Qiagen, cat # 28604, Doncaster, Victoria, Australia) then transformed into chemically competent One Shot® TOP10 cells using a TOPO® TA Cloning Kit (Invitrogen cat#K450001, Mulgrave, Victoria, Australia). Plasmid DNA was extracted from clones using a QIAprep Spin Miniprep Kit (Qiagen, cat # 27104, Doncaster, Victoria, Australia). Approximately 20 ng of PCR product or 200 ng of plasmid DNA was used in standard ABI Dye Terminator sequencing reactions using Big Dye Vers 3.1 technology (Applied Biosystems, California) and were run on an Applied Biosystems 3130xl Genetic Analyser (Griffith University DNA Sequencing Facility, School of Biomolecular and Biomedical Science, Griffith University, Qld, Australia). Forward and reverse sequences were edited and aligned using Sequencher (Vers 4.7 Gene Codes Corporation, Ann Arbor, MI, USA).
2.4. Phylogenetic analyses
Forty-one additional sequences (Table 2) were downloaded from the GenBank database (Benson et al. 2010) and were included in the phylogenetic analysis based on a nucleotide BLAST search of the kangaroo infecting Babesia-like sequence on NCBI (http://www.ncbi.nlm.nih.gov/BLAST). Strains of described B. bovis and B. bigemina were included in the analysis as well as at least one representative of all closely related Babesia species for which 18S sequences were available, including the recently described Babesia sp. isolated from a woylie (brush-tailed bettong) (Paparini et al., 2012).
Table 2.
Additional Babesia and Theileria sequences sourced from Genbank included in the phylogenetic analysis.
| Genbank accession # | Description | Vertebrate Host | Origin | Refs. |
|---|---|---|---|---|
| X59604 | Babesia bigemina gene A | Unknown | Mexico | Reddy et al. (1991) |
| X59605 | Babesia bigemina gene B | Unknown | Mexico | Reddy et al., 1991 |
| X59607 | Babesia bigemina gene C | Unknown | Mexico | Reddy et al. (1991) |
| FJ426361 | Babesia bigemina isolate BRC02 | Cattle | Brazil | Criado-Fornelio et al., 2009a, Criado-Fornelio et al., 2009b |
| AY603402 | Babesia bigemina isolate Kunming | Bovine | China | Buling et al. (2007) |
| DQ785311 | Babesia bigemina isolate Spain-1 | Cattle | Spain | Buling et al. (2007) |
| HQ840959 | Babesia bigemina strain 493 | Water buffalo | China | He et al., 2012 |
| HQ840960 | Babesia bigemina strain 563 | Water buffalo | China | He et al. (2012) |
| AY150059 | Babesia bovis Portugal | Cattle | Portugal | Criado-Fornelio et al. (2003) |
| AY309955 | Babesia caballi | Feral horse | Spain | Criado-Fornelio et al. (2004) |
| Z15104 | Babesia caballi | Horse | South Africa | Criado-Fornelio et al. (2004) |
| AY534883 | Babesia caballi strain EB1 | Equine | Spain | Nagore et al. (2004a) |
| L19079 | Babesia canis | Unknown | Unknown | Allsopp et al. (1994) |
| AY072926 | Babesia canis canis | Dog | Croatia | Cacciò et al. (2002) |
| HM590440 | Babesia canis vogeli | Dog | China | Zhang and Li (2010), not published |
| AY726009 | Babesia capreoli isolate BAB1220 | Roe deer | France | Slemenda et al. (2004), not published |
| AY572456 | Babesia divergens | Roe deer | Slovenia | Duh et al. (2004), not published |
| DQ184507 | Babesia gibsoni | Dog | USA | James et al. (2005), not published |
| EU622907 | Babesia major isolate France 1 | Cattle | France | Criado-Fornelio et al. (2009b) |
| AY260180 | Babesia motasi | Sheep | Netherlands | Schnittger et al., 2003 |
| AY533147 | Babesia motasi | Ovine | Spain | Nagore et al. (2004b) |
| EU376017 | Babesia occultans | Sable antelope | South Africa | Oosthuizen et al., 2008 |
| HQ331479 | Babesia occultans isolate 68 | Unknown | Tunisia | Ros-Garcia et al. (2011) |
| AY596279 | Babesia orientalis | Water buffalo | China | Liu et al. (2005) |
| HQ840969 | Babesia orientalis strain DaYe | Water buffalo | China | He et al. (2012) |
| AY603400 | Babesia ovata isolate Zhangjiachuan | Bovine | China | Luo et al. (2005a) |
| U09834 | Babesia sp. | Cattle | Unknown | Allsopp et al. (1994), direct submission |
| HQ437690 | Babesia sp. Anglona/AA-2011 | Wild pig | Anglona | Zobba et al. (2011) |
| GQ888709 | Babesia sp. EU1 isolate Arnhem | Reindeer | Netherlands | Kik et al., 2011 |
| DQ329138 | Babesia sp. FP44 | Florida panther | USA | Yabsley et al. (2006) |
| FJ213578 | Babesia sp. giraffe 0105 | Giraffe | South Africa | Oosthuizen et al. (2009) |
| FJ213580 | Babesia sp. giraffe 229 | Giraffe | South Africa | Oosthuizen et al., 2009 |
| FJ213577 | Babesia sp. giraffe 544 | Giraffe | South Africa | Oosthuizen et al. (2009) |
| AY726556 | Babesia sp. Kashi 1 | Bovine | China | Luo et al. (2005b) |
| DQ159071 | Babesia sp. Madang-2005 | Ovine | China | Liu et al. (2007) |
| FJ213581 | Babesia sp. roan 571 | Roan antelope | South Africa | Oosthuizen et al. (2009) |
| EU376016 | Babesia sp. sable antelope/2005 | Sable antelope | Unknown | Oosthuizen et al. (2008) |
| AB251612 | Babesia sp. SAP#131 | Raccoon | Japan | Jinnai et al., 2009 |
| DQ159073 | Babesia sp. Xinjiang-2005 | Ovine | China | Liu et al. (2007) |
| HQ730762 | Babesia sp. ZL-2011 | Sheep | China | Ma et al. (2010), not published |
| JQ682876 | Babesia sp. Woylie | Woylie | Australia | Paparini et al. (2012) |
| DQ104611 | Theileria buffeli China | Unknown | China | Liu et al. (2010) |
Sequences were aligned using ClustalX (Vers 1.81, Thompson et al. 1997) then the alignment was truncated to the shortest sequence (1560 bp alignment). The alignment was manually edited prior to export in nexus format for phylogenetic analyses. Before generating the phylogenetic trees a series of likelihood ratio tests were completed using MrModeltest (Vers 2.3, Nylander 2004) to determine the best nucleotide substitution model to use for likelihood and distance analyses. The best model predicted using the Akaike Information Criterion (AIC) was a general time reversible model (GTR) with a proportion of invariant sites (I) and among site heterogeneity (G) (summarized as GTR + I + G) with the following command block for use in Paup* (Vers 4.0b10, Swofford 2002). BEGIN PAUP; Lset Base=(0.2378 0.2057 0.2614) Nst = 6 Rmat = (0.4315 0.8650 0.6404 0.7132 1.9820) Rates = gamma shape = 0.4865 Pinvar = 0.5983; END.
Phylogenetic trees were constructed using maximum parsimony (P), maximum likelihood (L) and distance matrix analyses (D) in PAUP* (Vers 4.0b10, Swofford 2002). Unweighted trees were found using heuristic searches with random sequence addition and tree-bisection-reconnection (TBR) branch swapping. For the parsimony, analysis gaps were treated as missing data. Other settings used were Mulpars in effect, Maxtrees set to 10000 and heuristic search repetitions set to 100 (P) or one (D and L). Support for nodes was assessed using bootstrap resampling (P 1000 replicates, D 1000 replicates, L 100 replicates) and Bayesian analysis (B 935,000 generations). Bayesian analyses, using posterior probabilities, were completed in MrBayes (Huelsenbeck and Ronquis, 2001). The GTR + I + G nucleotide substitution model was used but with empirical base frequencies and invariant substitution rates built into the gamma distribution. Four chains were run for 1000,000 generations with the first 65,000 generations removed as burnin prior to constructing the consensus tree. Two representatives of the genus Theileria (Theileria spp. from Table 1 and T. buffeli, Genbank accession DQ104611 from Table 2) were included as outgroup taxa to root all trees.
3. Results
3.1. Case 1
The kangaroo had moderate to marked anaemia with a decrease in erythrocytes (2.75 × 1012/L, normal reference 5.86 × 1012/L) and haemoglobin concentration (75 g/L, normal reference 160 g/L). Babesia were observed in the blood smear mostly as paired merozoites (Fig. 1). Ticks were collected and informally identified by the carer as Haemaphysalis species. Further ticks submitted from other kangaroos from the same carer were identified by the Biosecurity Sciences Laboratory as Haemaphysalis species.
Fig. 1.
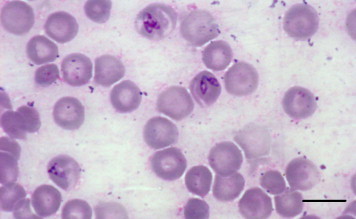
Photomicrograph of a blood smear from an Eastern Grey kangaroo stained with Giemsa and showing the presence of Babesia species merozoites. Bar = 10 μm.
3.2. Cases 2 and 3
Examination of the blood smears by light microscopy showed up to seven intraerythrocytic spherical, oval, pyriform and irregular-shaped parasites consistent with Babesia spp. (Fig. 2) and with the organisms observed in Case 1. Estimation of the percentage of infected erythrocytes in Cases 2 and 3 was 7% and the size of the parasites ranged from 2 to 6 μm in diameter. Spherical or ring-shaped trophozoites (Fig. 2B and C) were common and were mostly present singly in erythrocytes. Pyriform-shaped merozoites, however, were the next most common form found singly, in pairs (Fig. 2A) or in clusters. Rarely, free pyriform-shaped trophozoites were also observed in pairs or clusters (Fig. 2F). The blood smears from all of the cases exhibited a marked decrease in erythrocyte density, marked anisocytosis and polychromasia. A moderate increase of spherocytes and reticulocytes was noticed. Most of the infected erythrocytes appeared markedly enlarged and hypochromatic.
Fig. 2.
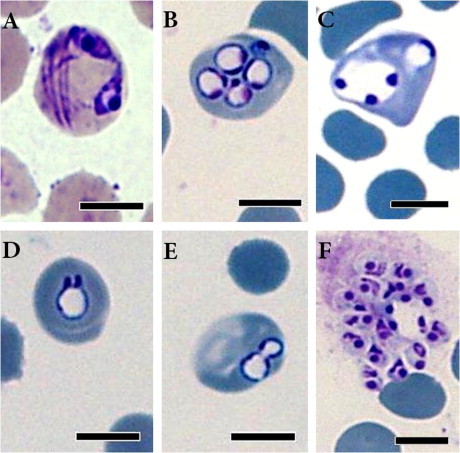
Photomicrograph of blood smears stained with Diff-Quik (A) and Giemsa (B–F) from eastern grey kangaroos (Cases 2 and 3) showing diverse forms of Babesia including paired merozoites (2A), multiple ring-shaped trophozoites with a chromatin dot (2B), a large ring-shaped trophozoite with three chromatin dots (2C), ring-shaped trophozoite with a pair of buds in early dividing stage (2D), two dividing trophozoites with a cytoplasmic bridge (2E), and a clump of diverse forms of extraerythrocytic Babesia (3F). Bar = 10 μm.
The packed–cell volume (PCV) of Cases 2 and 3 were 30% and 17%, respectively (normal reference 47%). The haematology result of kangaroo 3 also revealed a marked decrease of erythrocytes (2.44 × 1012/L, normal reference 5.86 × 1012/L), white blood cells (2.97 × 109/L, normal reference 10.13 × 109/L), and haemoglobin (66 g/L, normal reference 160 g/L).
Histologically in Case 2, the liver showed mild to moderate degeneration of the periacinar zone hepatocytes, with golden-brown granular deposits in Küpffer cells. Mild cholestasis was observed occasionally. Abundant siderophages were observed in the spleen. Haemorrhage was evident in the sclera of both eyes. Sections stained with Giemsa-eosin showed multiple dots and pyriform-like basophilic structures in the erythrocytes in the blood vessels, particularly the capillaries of the brain, kidneys and eyes.
Biochemical results from Cases 2 revealed a marked increase in the level of urea (30.3 mmol/L, normal reference 8.6 nmol/L), the urea:creatinine ratio (0.36, normal reference not found), aspartate aminotransferase (AST, 417 U/L, normal reference 60 U/L), glutamate dehydrogenase (GLDH, 130 U/L, normal reference not found) and creatine kinase (2917 U/L, normal reference 747 U/L). There was a moderate in bilirubin (4.6 μmol/L, normal reference 3 μmol/L). There was a moderate decrease in protein (41.3 g/L, normal reference 60 g/L) and globulin (17.5 g/L, normal reference 23 g/L).
3.3. Ultrastructural examination
Ultrastructural examination was undertaken using samples from Case 2 and showed that the parasites had morphology consistent with Babesia spp. Cases 1 and 3 had insufficient samples and therefore were not examined ultrastructurally. In one kidney section, 18 of the total 33 erythrocytes counted were infected with Babesia parasites (5 μm electron microscope magnification) – results not shown. The intraerythrocytic stage exhibited up to 3 morphologic types including elongated, round, and amoeboid-shaped Babesia. Trophozoites were the most common, which corresponded to what was seen by light microscopy examination. Frequently, erythrocytes contained up to 6 parasites of variable size. Trophozoites were generally surrounded by a single plasma membrane although many were doubled segmentally, with the segments separated by a clear space (Fig. 3). The nucleus was a single, round, moderately electron-dense, surrounded by multiple, prominent, irregular, double-membraned cisternae, and located at one end of the cell. No nucleolus was present. The cytoplasm contained endoplasmic reticulum, free ribosomes, mitochondria and vacuoles (Fig. 3). The endoplasmic reticulum was found very close to the nucleus. However, it was not always seen clearly in some of the developmental stages. Most trophozoites contained a large eccentric pseudoinclusion (invagination of the host cytoplasm), that was lighter in density and signicantly larger than the nucleus (Fig. 3).
Fig. 3.
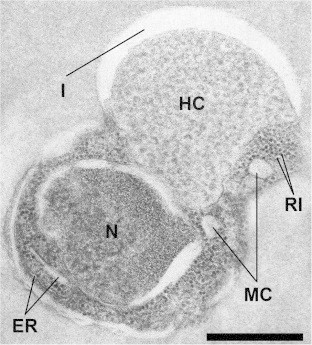
Electron micrographs of Babesia spp. (Case 2) within an erythrocyte. Nucleus (N); host cytoplasm (HC); endoplasmic reticulum (ER); ribosome (RI); mitochondria (MC), and invagination (I). Bar = 0.5 μm.
3.4. Molecular analysis and DNA sequencing
PCR assays for known piroplasms (Theileria spp.-MPSP, B. bovis, and B. bigemina-cytochrome b) did not amplify visible products from the kangaroo samples (Cases 1 and 2) confirming that known Babesia species were not co-infecting the kangaroo hosts (results not shown). A 1633 bp product of partial 18S rRNA was PCR amplified (from DNA prepared from Cases 1 and 2) and sequenced for the Babesia-like protozoan parasite found infecting the kangaroos. Two genetically distinct strains of the kangaroo Babesia were identified diverging by 0.2% (3 base changes). Both were present in Case 1, and one was present in Case 2. Samples for Case 3 were not available for DNA amplification.
The parsimony analysis of the 1560 bp alignment was based on 331 informative characters. Parsimony analysis found 96 optimal trees of length 1100 steps and consistency index 0.5455. Tree differences were largely due to taxa rearrangements at the tips. Distance analysis identified one optimal tree with minimum evolution score 0.91537 (Fig. 4). Maximum likelihood analysis retained a single tree with –Ln likelihood score 7694.24818. Branch support from all analysis methods is indicated on the distance tree by an asterisk (Fig. 4).
Fig. 4.
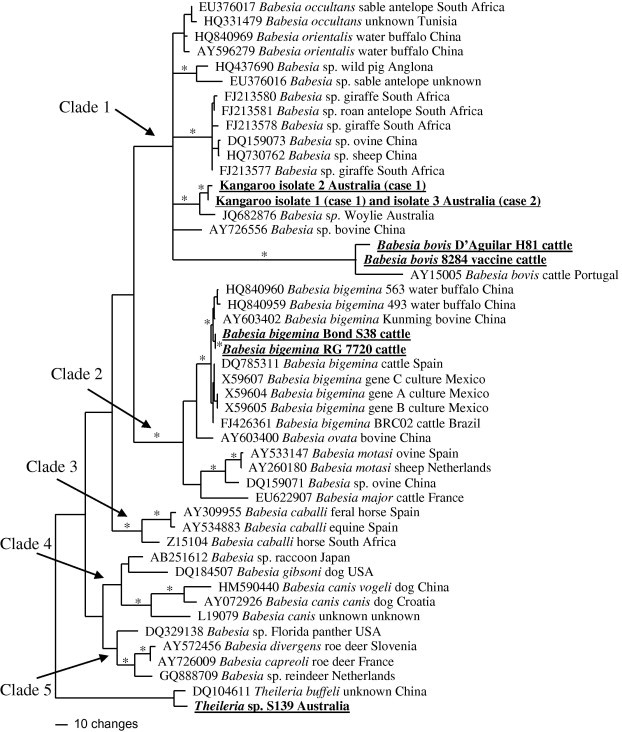
Minimum evolution phylogenetic tree based on a 1560 bp 18S rRNA alignment for representatives of the genus Babesia including the new kangaroo-infecting species (in bold and underlined) originating from Cases 1 and 2. Also underlined and in bold are other species sequenced as part of this study. Branch support is shown with a ∗ where all tree building methods (parsimony, distance and likelihood) had at least 70% bootstrap and Bayesian support.
The two strains of kangaroo Babesia sp. (Cases 1 and 2) consistently fall in a well supported clade with the woylie Babesia sp. recently characterised (Paparini et al., 2012). Lacking bootstrap support, but consistent with Paparini et al. (2012), all analyses grouped the kangaroo and woylie Babesia spp. within a clade containing B. occultans (ex antelope, South Africa), B. orientalis (ex water buffalo, China) and a number of unnamed Babesia sp. from sheep, giraffe, antelope and wild pig (Clade 1, Fig. 4). The phylogenetic position of B. bovis in Clade 1 is unclear. Distance and parsimony trees place B. bovis in a basal position (Fig. 4) while the maximum likelihood tree suggests a more derived position.
All analyses identified a well-supported clade containing B. bigemina, B. ovata, B. motasi and B. major (Clade 2, Fig. 4). Clades numbered 3, 4 and 5 form a single clade in the parsimony analysis, two clades using distance analysis (shown) and three clades based on likelihood analysis.
4. Discussion
In contrast to livestock, diseases in wild animals are not examined. For example, most sick or dead kangaroos are not investigated for haemoprotozoa thoroughly due to difficulty in collecting samples, poor sample collection or lack of appropriate samples. The eastern grey kangaroos in this study had clinical signs of acute anaemia. The laboratory findings in association with the clinical signs suggested that the kangaroos had contracted babesiosis. Abundant organisms resembling Babesia spp. were detected on examination of blood smears. Sequencing of the 18S rDNA from 2 of the 3 cases indicated that the kangaroos were infected with a novel Babesia sp. distinct from other recognised species. Most Babesia sp. species are considered to be host specific, but there is evidence of cross-species transmission, including zoonotic transmission (Ristic, 1988, Gray et al., 2010). This is the first study describing a novel Babesia species in kangaroo and the authors suggest nominating the observed species as Babesia macropus, as a potential sister species to the Babesia isolated from woylies (Paparini et al., 2012).
Subclinical infection with Babesia spp. is relatively common in wild ungulates. Clinical babesiosis in the sable antelope (Hippotragus niger), Grevy’s zebra (Equus grevyi) and black rhinoceros (Diceros bicornis) appear to be triggered by the stress of capture, restraint and translocation (Penzhorn, 2006). Among marsupials, clinical babesiosis in the male antechinus is associated with physiological stress in the post-mating period (Barker et al., 1978, Arundel, 1981). The eastern grey kangaroos in this study had a history of translocation (for Cases 2 and 3), confinement in a fenced yard and supplementary feeding. It is possible that the stress of handling, transportation and captivity contributed to the development of disease in the kangaroos (Dennig, 1966, Mclnnes et al., 1992). Alternatively, kangaroos hand-reared by carers would lack the maternal antibodies obtained passively from their doe’s milk to protect them against this Babesia sp. Furthermore hand-reared kangaroos would have less exposure to naturally occurring parasites than wild kangaroos and thus be more susceptible to infection.
It has been suggested that there is little correlation between the percentage of infected erythrocytes with Babesia and the severity of clinical signs (Ristic, 1988). For example, 72–96% of erythrocytes were infected with B. cynicti in yellow mongooses (Cynictis penicillata) without the animals showing evidence of clinical disease (Penzhorn and Chaparro, 1994). However, the pathology of the disease will also influence the parasitemia. In bovine babesiosis, B. bigemina will develop a high parasitemia causing severe haemolytic anaemia and B. bovis can result in a low parasitemia with disseminated intravascular coagulation leading to severe cerebral babesiosis (Bock et al., 2004, Allred, 2007, Hutchings et al., 2007). This is caused by the sequestering of B. bovis infected erythrocytes in the capillaries of organs not usually associated with B. bigemina pathology. In the blood smears from the kangaroos with severe clinical signs, it was estimated that 7% of the erythrocytes were infected. Importantly, examination of fixed tissue stained with Giemsa-eosin was valuable for the diagnosis of haemoprotozoans in this study. Kidney, brain and eye were the most useful organs for microscopic diagnosis demonstrating a similar pathology to B. bovis infections.
Interestingly, the Babesia in the current cases appeared larger than those recorded in previous studies in other animal species. Most of erythrocytes infected with one or more Babesia organisms were altered in shape and colour. This is inconsistent with Clark et al. (2004) who stated that one to two B. thylacis in an infected erythrocyte caused no such changes to erythrocytes in an unspecified Australian mammal. In our study, the transmission microscope images revealed some structures that are similar to those described for other Babesia spp., such as B. bovis and B. microti (Rudzinska, 1976, Todorovic et al., 1981). The number of infected erythrocytes in the capillaries in the kidney was much higher than that examined by the blood smears. This may be ascribed to the high magnification of the electron microscope which identifies the remains of autolysed Babesia and erythrocytes. In B. bovis, no food vacuole or host cytoplasm was found (Todorovic et al., 1981). However, in the Babesia sp. described here, a large portion or fragment of the host cytoplasm was found in the majority of the merozoites. The authors suggest that host cytoplasm invaginated into the protozoan and that may have participated significantly in the development of anaemia in the kangaroo by increasing the surface area of the parasites that in direct contact with the host cytoplasm, thereby increasing nutrition intake (Rudzinska, 1976). However, the exact role, importance and fate of the invaginted host cytoplasm are not fully elucidated.
The 18S rRNA phylogeny confirms that the Babesia-like blood infection found in eastern grey kangaroo is a protozoan parasite belonging to the genus Babesia. Although the kangaroo were found in close proximity to domestic cattle, the infection was neither B. bovis nor B. bigemina but represents a new species of Babesia and it has a close affinity to the recently characterised Babesia sp. isolated from woylies (brush-tailed bettongs) (Paparini et al., 2012). Woylies [Family Potoroidae] are small marsupials (commonly called ‘rat kangaroos’) whose habitat (mostly western and southern Australia) does not overlap with the habitat of the eastern grey kangaroo [Family Macropodidae] in eastern Australia. Two genetically distinct strains of the kangaroo Babesia have been identified diverging by 0.2% well within the range of strain divergence found for other species in the genus (strain divergence of up to 5% is found within B. bovis, B. canis and B. caballi). The genetic divergence observed between the kangaroo Babesia sp. and the woylie Babesia sp. (20 nucleotide differences, 1.5%) is sufficient to characterise these as distinct but sister species (Schnittger et al., 2003). A similar level of divergence separates B. occultans from B. orientalis (1.05%) and B. bigemina from B. ovata (1.6%). Geographic isolation, vector specificity and host specificity are the likely genetic isolating mechanisms maintaining the species.
The phylogenetic position of the marsupial Babesia sp. within the genus indicates a close affinity to described Afro–Eurasian species B. orientalis and B. occultans and suggests an ancestor in common with these Australian marsupial parasites. A phylogeny based on a more variable genetic marker will provide more power to resolve where this marsupial Babesia spp. falls with respect to their sister species and may help to unravel the evolutionary origins of this group.
To date, no tick species has been confirmed as the vector of Australian wildlife piroplasms. Bandicoots are known to be carriers of the native Australian Ixodes holocyclus ticks (causing tick paralysis of companion animals) which is not known to carry piroplasms but is present in the regions associated with the kangaroo studies here. Amblyomma triguttatum triguttatum ticks are described to be present in several marsupial and rodent populations but have also not been implicated as a vector of piroplasms (Waudby et al., 2007). Several species of ticks have been found on a population of kangaroos on an island off the southern coast of Australia including Bothriocroton concolor, Ixodes hirsti and Haemaphysalis bancrofti (Oorebeek and Rismiller, 2007). Our preliminary observations that Haemaphysalis spp. ticks were present in the captive area suggest that they may be the vector of the Babesia sp. to eastern grey kangaroos. However, further studies need to be undertaken to confirm this supposition.
In conclusion, to the authors’ knowledge, this is a first study describes a novel Babesia in kangaroos. The authors suggest nominating the observed Babesia as B. macropus. Eastern grey kangaroo may develop fatal babesiosis characterised by anaemia, lethargy and neurological signs following events such as capture, transportation and relocation. Examination of blood smears from anaemic kangaroos is highly recommended. Additionally, administration of prophylactic doses of anti-protozoan medication before and after translocation could potentially be a preventative measure against clinical Babesia infections.
Acknowledgements
The authors would like to thank Chris Butt (senior lecturer in English for Health Profession, Sydney) for editing the manuscript. The authors wish also to thank Leah Simmon and Cassandra Buchanan for preparing the histology and special stains, Graeme Eamens (The State Veterinary Diagnostic Laboratory, NSW) for performing the Theileria orientalis PCR technique, Peter Rolls and Bert de Vos (Biosecurity Queensland, Qld Department of Agriculture, Fisheries & Forestry) for supporting this study logistically, Grant Campbell (Biosecurity Queensland) for notifying us of Case 1 and Neil Horadagoda (Sydney University, Camden, NSW) for conducting the haematology and biochemistry.
References
- Allred D.R. Dynamics of anemia progression and recovery in Babesia bigemina infection is unrelated to initiating parasite burden. Vet. Parasitol. 2007;15:170–174. doi: 10.1016/j.vetpar.2007.02.016. [DOI] [PubMed] [Google Scholar]
- Allsopp M.T., Cavalier-Smith T., De Waal D.T., Allsopp B.A. Phylogeny and evolution of the piroplasms. Parasitology. 1994;108:147–152. doi: 10.1017/s0031182000068232. [DOI] [PubMed] [Google Scholar]
- Arundel J. In: Proceedings of the 4th International Conference of the Wildlife Disease Association. Fowler M.E., editor. Australia; Sydney: 1981. Wildlife diseases of the Pacific basin – Australia. Wildlife diseases of the Pacific basin and other Countries; pp. 18–22. [Google Scholar]
- Backhouse T., Bolliger A. A piroplasm of the echidna (Tachyglossus aculeatus) Aust. J. Sci. 1957;19:24–25. [Google Scholar]
- Backhouse T.C., Bolliger A. Babesia tachyglossi n. sp. from the echidna Tachyglossus aculeatus. J. Protozool. 1959;6:320–322. [Google Scholar]
- Barker I., Beveridge I., Bradley A., Lee A. Observations on spontaneous stress-related mortality among males of the dasyurid marsupial Antechinus stuartii Macleay. Aust. J. Zool. 1978;26:435–447. [Google Scholar]
- Benson D.A., Karsch-Mizrachi I., Lipman D.J., Ostell J., Sayers E.W. GenBank. Nucleic Acids Res. 2010;38:D46–D51. doi: 10.1093/nar/gkp1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock R., Jackson L., de Vos A., Jorgensen W. Babesiosis of cattle. Parasitology. 2004;29:247–269. doi: 10.1017/s0031182004005190. [DOI] [PubMed] [Google Scholar]
- Bolliger A., Backhouse T. Blood studies on the echidna Tachyglossus aculeatus. Proceedings of the Zoological Society of London. 1960;135:91–97. [Google Scholar]
- Buling A., Criado-Fornelio A., Asenzo G., Benitez D., Barba-Carretero J.C., Florin-Christensen M. A quantitative PCR assay for the detection and quantification of Babesia bovis and B. bigemina. Vet. Parasitol. 2007;147:16–25. doi: 10.1016/j.vetpar.2007.03.031. [DOI] [PubMed] [Google Scholar]
- Cacciò S.M., Antunovic B., Moretti A., Mangili V., Marinculic A., Baric R.R., Slemenda S.B., Pieniazek N.J. Molecular characterisation of Babesia canis canis and Babesia canis vogeli from naturally infected European dogs. Vet. Parasitol. 2002;106:285–292. doi: 10.1016/s0304-4017(02)00112-7. [DOI] [PubMed] [Google Scholar]
- Clark P., Adlard R.D., Spratt D.M. In: Haematology of Australian Mammals. Clark P., editor. CSIRO Publishing; Collingwood, Australia: 2004. Haemoparasites of Australian mammals; pp. 147–162. [Google Scholar]
- Criado-Fornelio A., Martinez-Marcos A., Buling-Sarana A., Barba-Carretero J.C. Molecular studies on Babesia, Theileria and Hepatozoon in southern Europe. Part I. Epizootiological aspects. Vet. Parasitol. 2003;113:189–201. doi: 10.1016/s0304-4017(03)00078-5. [DOI] [PubMed] [Google Scholar]
- Criado-Fornelio A., Gonzalez-del-Rio M.A., Buling-Sarana A., Barba-Carretero J.C. The “expanding universe” of piroplasms. Vet. Parasitol. 2004;119:337–345. doi: 10.1016/j.vetpar.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Criado-Fornelio A., Buling A., Asenzo G., Benitez D., Florin-Christensen M., Gonzalez-Oliva A., Henriques G., Silva M., Alongi A., Agnone A., Torina A., Madruga C.R. Development of fluorogenic probe-based PCR assays for the detection and quantification of bovine piroplasmids. Vet. Parasitol. 2009;162:200–206. doi: 10.1016/j.vetpar.2009.03.040. [DOI] [PubMed] [Google Scholar]
- Criado-Fornelio A., Buling A., Pingret J.L., Etievant M., Boucraut-Baralon C., Alongi A., Agnone A., Torina A. Hemoprotozoa of domestic animals in France. prevalence and molecular characterization. Vet. Parasitol. 2009;159:73–76. doi: 10.1016/j.vetpar.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Dennig, H., 1966. The isolation of Babesia species from wild animals. Proceedings of the First International Congress. Parasitology, 262–263.
- Gray J., Zintl A., Hildebrandt A., Hunfeldd K.P., Weisse L. Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis. 2010;1:3–10. doi: 10.1016/j.ttbdis.2009.11.003. [DOI] [PubMed] [Google Scholar]
- He L., Feng H.H., Zhang W.J., Zhang Q.L., Fang R., Wang L., Tu X., Zhou Y.Q., Zhao J.L., Oosthuizen M.C. Occurrence of Theileria and Babesia species in water buffalo (Bubalus babalis, Linnaeus, 1758) in the Hubei province, South China. Vet. Parasitol. 2012;186:490–496. doi: 10.1016/j.vetpar.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J.P., Ronquis F.T. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Hutchings C.L., Li A., Fernandez K.M., Fletcher T., Jackson L.A., Molloy J.B., Jorgensen W.K., Lim C.T., Cooke B.M. New insights into the altered adhesive and mechanical properties of red blood cells parasitized by Babesia bovis. Mol. Microbiol. 2007;65:1092–1105. doi: 10.1111/j.1365-2958.2007.05850.x. [DOI] [PubMed] [Google Scholar]
- Jinnai M., Kawabuchi-Kurata T., Tsuji M., Nakajima R., Fujisawa K., Nagata S., Koide H., Matoba Y., Asakawa M., Takahashi K., Ishihara C. Molecular evidence for the presence of new Babesia species in feral raccoons (Procyon lotor) in Hokkaido. Japan. Vet. Parasitol. 2009;162:241–247. doi: 10.1016/j.vetpar.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Kik M., Nijhof A.M., Balk J.A., Jongejan F. Babesia sp. EU1 infection in a forest reindeer. The Netherlands Emerg. Infect. Dis. 2011;17:936–938. doi: 10.3201/eid1705.101834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A.H., Yin H., Guan G.Q., Schnittger L., Liu Z.J., Ma M.L., Dang Z.S., Liu J.L., Ren Q.Y., Bai Q., Ahmed J.S., Luo J.X. At least two genetically distinct large Babesia species infective to sheep and goats in China. Vet. Parasitol. 2007;147:246–251. doi: 10.1016/j.vetpar.2007.03.032. [DOI] [PubMed] [Google Scholar]
- Liu Q., Zhao J.L., Zhou Y.Q., Liu E.Y., Yao B.A., Fu Y. Study on some molecular characterization of Babesia orientalis. Vet. Parasitol. 2005;130:191–198. doi: 10.1016/j.vetpar.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Liu Q., Zhou Y.Q., He G.S., Oosthuizen M.C., Zhou D.N., Zhao J.L. Molecular phylogenetic studies on Theileria spp. isolates (China) based on small subunit ribosomal RNA gene sequences. Trop. Anim. Health. Prod. 2010;42:109–114. doi: 10.1007/s11250-009-9392-x. [DOI] [PubMed] [Google Scholar]
- Luo J., Yin H., Guan G., Yang D., Liu A., Ma M., Liu Z., Dang Z., Liu G., Bai Q., Lu W., Chen P. A comparison of small-subunit ribosomal RNA gene sequences of bovine Babesia species transmitted by Haemaphysalis spp. in China. Parasitol. Res. 2005;95:145–149. doi: 10.1007/s00436-004-1268-4. [DOI] [PubMed] [Google Scholar]
- Luo J., Yin H., Liu Z., Yang D., Guan G., Liu A., Ma M., Dang S., Lu B., Sun C., Bai Q., Lu W., Chen P. Molecular phylogenetic studies on an unnamed bovine Babesia sp. based on small subunit ribosomal RNA gene sequences. Vet. Parasitol. 2005;133:1–6. doi: 10.1016/j.vetpar.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Mackerras M. The haematozoa of Australian mammals. Aust. J. Zool. 1959;7:105–135. [Google Scholar]
- Mclnnes E., Stewart C., Penzhorn B., Meltzer D. An outbreak of babesiosis in imported sable antelope (Hippotraguis niger) J. S. Afr. Vet. Assoc. 1992;62:30–32. [PubMed] [Google Scholar]
- Medlin L., Elwood H.J., Stickel S., Sogin M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988;7:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Nagore D., Garcia-Sanmartin J., Garcia-Perez A.L., Juste R.A., Hurtado A. Detection and identification of equine Theileria and Babesia species by reverse line blotting: epidemiological survey and phylogenetic analysis. Vet. Parasitol. 2004;123:41–54. doi: 10.1016/j.vetpar.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Nagore D., Garcia-Sanmartin J., Garcia-Perez A.L., Juste R.A., Hurtado A. Identification, genetic diversity and prevalence of Theileria and Babesia species in a sheep population from Northern Spain. Int. J. Parasitol. 2004;34:1059–1067. doi: 10.1016/j.ijpara.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Nylander, J.A.A., 2004. MrModeltest 2.3. Program distributed by the author Evolutionary Biology Centre, Uppsala University.
- O’Donoghue, P., 1997. Protozoan parasites of wildlife in south-east Queensland. Proceedings of the Australian Association of Veterinary Conservation Biologists Conference, Brisbane, Queensland, Australia, 119–135.
- O’Donoghue P., Adlard R. Catalogue of protozoan parasites recorded in Australia. Memoirs of the Queensland Museum. 2000;45:1–163. [Google Scholar]
- Oorebeek M., Rismiller P. Bothriocroton concolor (Acari: Ixodidae) on the Kangaroo Island kangaroo: a new host-parasite relationship. J. Med. Entomol. 2007;44:901–902. doi: 10.1603/0022-2585(2007)44[901:bcaiot]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Oosthuizen M.C., Zweygarth E., Collins N.E., Troskie M., Penzhorn B.L. Identification of a novel Babesia sp. from a sable antelope (Hippotragus niger Harris, 1838) J. Clin. Microbiol. 2008;46:2247–2251. doi: 10.1128/JCM.00167-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosthuizen M.C., Allsopp B.A., Troskie M., Collins N.E., Penzhorn B.L. Identification of novel Babesia and Theileria species in South African giraffe (Giraffa camelopardalis, Linnaeus, 1758) and roan antelope (Hippotragus equinus, Desmarest 1804) Vet. Parasitol. 2009;163:39–46. doi: 10.1016/j.vetpar.2009.03.045. [DOI] [PubMed] [Google Scholar]
- Paparini A., Ryan U.M., Warren K., McInnes L.M., de Tores P., Irwin P.J. Identification of novel Babesia and Theileria genotypes in the endangered marsupials, the woylie (Bettongia penicillata ogilbyi) and boodie (Bettongia lesueur) Exp. Parasitol. 2012;131:25–30. doi: 10.1016/j.exppara.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Penzhorn B., Chaparro F. Prevalence of Babesia cynicti infection in three populations of yellow mongooses (Cynictis penicillata) in the Transvaal, South Africa. J. Wildl. Dis. 1994;30:557–559. doi: 10.7589/0090-3558-30.4.557. [DOI] [PubMed] [Google Scholar]
- Penzhorn B. Babesiosis of wild carnivores and ungulates. Vet. Parasitol. 2006;138:11–21. doi: 10.1016/j.vetpar.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Reddy G.R., Chakrabarti D., Yowell C.A., Dame J.B. Sequence microheterogeneity of the three small subunit ribosomal RNA genes of Babesia bigemina: expression in erythrocyte culture. Nucleic Acids Res. 1991;19:3641–3645. doi: 10.1093/nar/19.13.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic M. CRC Press; Boca Raton, Florida, USA: 1988. Babesiosis of Domestic Animals and Man. 255. [Google Scholar]
- Ros-Garcia A., M’Ghirbi Y., Bouattour A., Hurtado A. First detection of Babesia occultans in Hyalomma ticks from Tunisia. Parasitology. 2011;138:578–582. doi: 10.1017/S0031182011000060. [DOI] [PubMed] [Google Scholar]
- Rudzinska M.A. Ultrastructure of intraerythrocytic Babesia microti with emphasis on the feeding mechanism. J. Eukaryot. Microbiol. 1976;23:224–233. doi: 10.1111/j.1550-7408.1976.tb03759.x. [DOI] [PubMed] [Google Scholar]
- Schnittger L., Yin H., Gubbels M.J., Beyer D., Niemann S., Jongejan F., Ahmed J.S. Phylogeny of sheep and goat Theileria and Babesia parasites. Parasitol. Res. 2003;91:398–406. doi: 10.1007/s00436-003-0979-2. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts: 2002. PAUP* phylogenetic analysis using parsimony (* and other methods) [Google Scholar]
- Tanaka M., Onoe S., Matsuba T., Katayama S., Yamanaka M., Yonemich H., Hiramatsu K., Baek B.K., Sugimoto C., Onuma M. Detection of Theileria sergenti infection in cattle by polymerase chain reaction amplification of parasite-specific DNA. J. Clin. Microbiol. 1993;31:2565–2569. doi: 10.1128/jcm.31.10.2565-2569.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. The CLUSTAL_X windows interface. flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic R., Wagner G., Kopf M. Ultrastructure of Babesia bovis (BABES, 1988) Vet. Parasitol. 1981;8:277–290. [Google Scholar]
- Waudby H.P., Petit S., Dixon B., Andrews R.H. Hosts of the exotic ornate kangaroo tick, Amblyomma triguttatum triguttatum Koch, on southern Yorke Peninsula, South Australia. Parasitol. Res. 2007;101:1323–1330. doi: 10.1007/s00436-007-0642-4. [DOI] [PubMed] [Google Scholar]
- Yabsley M.J., Murphy S.M., Cunningham M.W. Molecular detection and characterization of Cytauxzoon felis and a Babesia species in cougars from Florida. J. Wildl. Dis. 2006;42:366–374. doi: 10.7589/0090-3558-42.2.366. [DOI] [PubMed] [Google Scholar]
- Zakimi S., Kim J.Y., Oshiro M., Hayashida K., Fujisaki K., Sugimoto C. Genetic diversity of benign Theileria parasites of cattle in the Okinawa prefecture. J. Vet. Med. Sci. 2006;68:1335–1338. doi: 10.1292/jvms.68.1335. [DOI] [PubMed] [Google Scholar]
- Zobba R., Parpaglia M.L., Spezzigu A., Pittau M., Alberti A. First molecular identification and phylogeny of a Babesia sp. from a symptomatic sow (Sus scrofa Linnaeus 1758) J. Clin. Microbiol. 2011;49:2321–2324. doi: 10.1128/JCM.00312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


